1. Introduction
Low birth weight (LBW), preterm and sick neonates are a small but complicated cohort for newborn screening (NBS) programs. They require additional consideration and protocols to ensure they are appropriately screened [
1,
2]. The management of this cohort in our jurisdiction (Victoria, Australia) has traditionally been by the maternity service providers for the LBW babies following our recollection guideline and for sick babies requiring a blood (red blood cell) transfusion this was managed by the NBS laboratory team. Some babies are both sick and LBW and this is complicated for the health care providers in the ward and the laboratory. Clear guidance to facilitate recollections is therefore needed. However, for this special cohort of neonates, there are gaps in evidence-based best practices to inform the re-sampling requirements.
Neonatology is a young discipline that has developed in parallel over the same period as NBS. Today neonatologists are very good at keeping preterm (20 to <37 weeks gestation) babies alive, and this cohort now makes up around 8.2% of births in Australia and globally ranges from 4 – 16% of all births, and a subset of these make up very and extremely preterm or LBW births [
3,
4]. This improvement in care coincides with the increased number of preterm babies needing follow-up by NBS programs [
3,
5]. LBW (<2500 g) can be caused by intrauterine growth restriction, prematurity or both and therefore because LBW considerably overlaps with preterm birth, and its measurement is generally considered more reliable than gestational age (GA), it has been used as the primary decision point for all preterm recollections in Australasia [
6,
7]. Noting though that being born too small is conceptually different from being born too early. Irrespective, it is the very preterm (28 to < 32 weeks), extremely preterm (<28 weeks) and very LBW neonates that provide the significant challenges for improving morbidity and interpreting laboratory test results. In addition, the sick neonate who requires a transfusion of blood products further challenges NBS programs to ensure false negative screening results do not occur.
Certainly, the smaller the baby the more immature the organ systems are and in the case of the endocrine systems this can result in false negative NBS results for thyroid screening. Thyroid hormone levels are known to decrease in the first week of life and probably reflect a transient depletion of thyroid hormone reserves. This nadir is more pronounced in preterm neonates, especially in infants < 30 weeks gestation [
8]. This also coincides with an immature hypothalamic-pituitary axis where the stimulus to produce thyroid-stimulating hormone (TSH) is blunted [
9]. As such the first measurement of TSH (and thyroxine for a few program) in the usual timeframe of 48-72 hours of life may miss cases of congenital hypothyroidism [
10]. Likewise, preterm babies have a persistent foetal adrenal zone and therefore some also require a follow-up collection when screening for congenital adrenal hyperplasia (CAH) [
11,
12]. Hence the need and routine recommendation for follow-up collection in these instances.
With the introduction of an increased number of conditions into NBS programs globally, the complexities of managing the preterm and sick neonates across all conditions are challenging. Recently we introduced CAH screening, with other conditions planned for introduction in quick succession [
13]. With more conditions comes some compromises in timing of recollections to avoid excess sample collections and iatrogenic anaemia. Given the complexities involved, we had previously received requests from our maternity service providers for a visualization of the recollection cascade for preterm and sick neonates. Hence, it was timely to review (audit) our processes and ideally set up a system to manage recollections for preterm and sick neonates that was robust, evidence-based and harmonized with other Australasian programs.
We therefore aimed to a) determine adherence to our current guidelines; and b) review and revise (if required) the guidelines with visualization to improve NBS performance.
2. Materials and Methods
2.1. Victorian NBS Process at Time of Audit Initiation
The Victorian Newborn Screening program services approximately 80,000 babies born annually. On arrival at the laboratory one 3.2mm spot is punched from the card for each first-tier assay. Commercial kits are run for TSH, 17 hydroxy progesterone (17OHP) and immunoreactive trypsin (IRT) on the Genetic Screening Processor (GSP®) from Revvity (Turku, Finland). The first-tier metabolic screening of amino acids and acyl carnitines is run by an in-house method on Waters Xevo mass spectrometry instruments. Spinal muscular atrophy (SMA) and Severe Combined Immunodeficiency (SCID) by a hyper-automated PCR procedure were being prepared for introduction in 2023. Other conditions planned for implementation were galactosaemia, X linked adrenoleukodystrophy and sickle cell disease screening in 2024, 2025 and 2026 respectively.
At the time of the audit (in 2022), the Victorian NBS program collection guideline recommended routine collections for:
All babies – between 48 to 72 hours of life. From 2020 i.e. start of COVID, samples were accepted down to 36 hours of age without the need for a recollection.
Low birthweight babies - babies with a birth weight <1500g required a regular collection and then a follow-up collection at 2 weeks (1000-<1500g at birth) or 3 weeks (<1000g at birth) of age. This was managed by the maternity service provider. Where the NBS laboratory would only follow-up for borderline or screen positive result.
Transfused neonates – babies who received any form of transfusion prior to the routine NBS collection, required an early sample (pre-transfusion), a 48-hour post-transfusion, plus a 3-weeks post-transfusion. These recollections were managed by the laboratory i.e. where electronic letters were sent out to remind providers to perform the recollection and follow-up of outstanding samples.
2.2. Audit of Current Recollection Guideline For Preterm Neonates
Babies with first samples received between January 2018 and June 2022 were included in the review. Babies with a recorded birth weight of <1500g were filtered for inclusion as the denominator and the number of babies that had more than one dried blood spot (DBS) collection was used as the numerator to give a percentage of preterm babies with a follow-up collection. As this was a quality improvement audit as part of the routine continual improvement management practices of the NBS program, ethical approval was not required.
2.3. Review of Evidence and Harmonisation
For the overall review and visualization of the protocol, we sourced information from the literature and professional bodies.
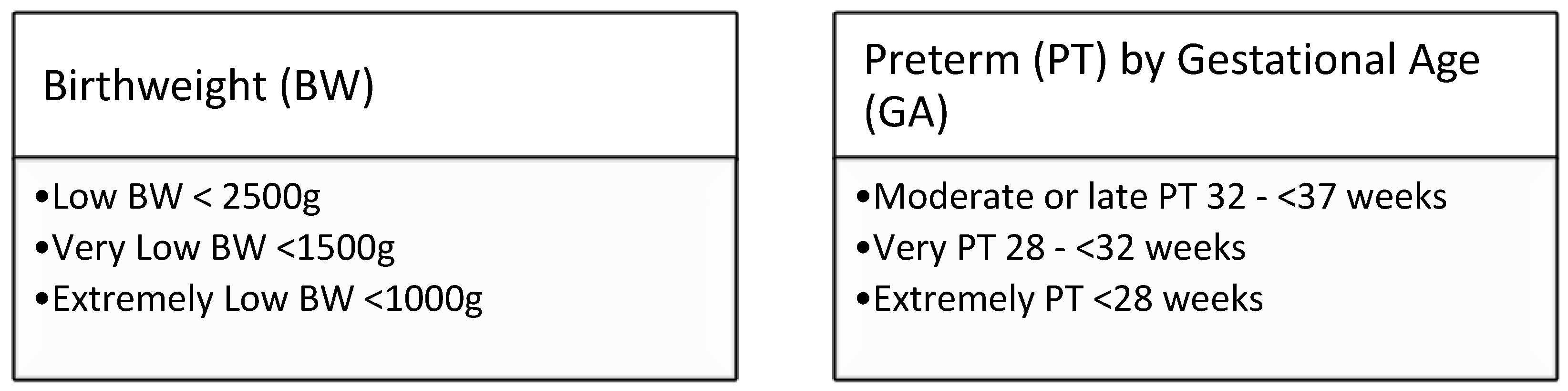
Box1: WHO definitions for low birthweight [
15] and prematurity [
16].
2.4. New Protocol Design
The initial protocol design was performed in Microsoft PowerPoint, with changes recorded as new individual slides with dates and reason for change noted. Input was also invited from Victorian tertiary hospital services that had special care nurseries.
2.5. One Year On—Review Of Impact Of New Protocol
One year of data (May 2023 to April 2024) was retrieved from our laboratory information management system to review if the change to the preterm protocol had resulted in improved second sample collection. Qualitative feedback was sort from the laboratory team on the effect of this change on their work. Anecdotal feedback was sought from the maternity service providers via our NBS Educator and review of our management system for non-conformances.
2.6. Statistical Analysis
The patient data was exported from our laboratory information system into a CSV file in Microsoft Excel. Data was sorted based on birth weight and descriptive calculations were performed in Microsoft Excel and Stata-18.0. Q-Pulse (Ideagen, Nottinghamshire United Kingdom) was used to manage the audit.
3. Results
3.1. Audit of Current Recollection Guideline For Preterm Neonates
A total of 348,584 NBS babies were screened between January 2018 and June 2022. Of these, 2647 babies had a birth weight recorded between 200 and 1499g. From this subset 2036 (77%) had a second sample collected, indicating that >1 in 5 babies were not receiving a follow-up collection.
3.2. Review of Evidence and Harmonization
The CLSI document was extensively reviewed, and this provided some recommendations for recollections. This included that transfusions were specifically whole blood transfusions, but that extra corporeal membrane oxygenation (ECMO) babies should be included (1). Representative(s) from all six Australasian jurisdictions were present at the HGSA Newborn Screening Committee meeting and responded to the questions that formed the information provided regarding the transfusion protocol. The information on LBW recollections was previously collated as part of publication on harmonization of congenital hypothyroidism screening [
7].
Table 1.
The consensus was that the evidence for the collection time frame for transfusions was not clear. Our (VIC) time of recollections for transfused babies, requiring a 3-week follow-up collection, was longer than in other Australasian jurisdictions i.e. ours was at three weeks whereas others either had one- or two-weeks post transfusion.
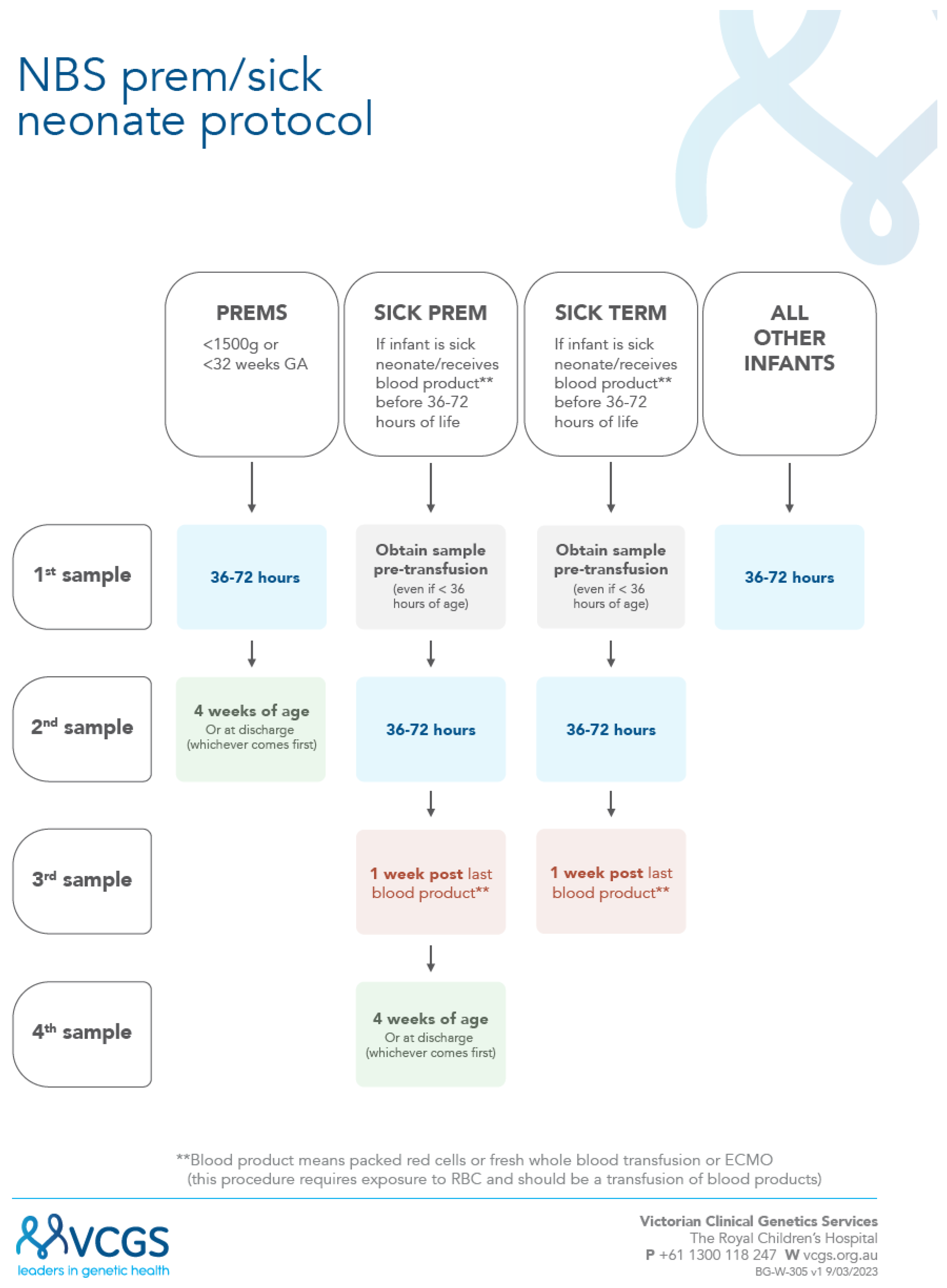
3.2. New Sick Prem (SP) Protocol
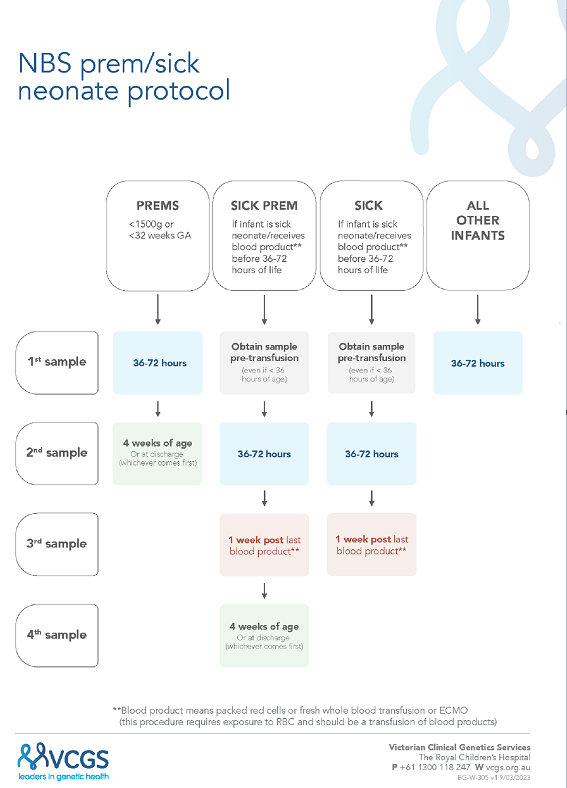
A new combined “sick-prem-protocol” was launched (17/4/2023) to support recollections;
Figure 1. In this protocol all sick and preterm neonatal recollections are now managed by the laboratory, with recollection letters electronically sent out, plus follow-up reminders. For the preterm neonates the NBS laboratory closes the recollection request (for babies with screen negative results) once a sample has been received by at least 3 weeks postnatal age. The transfusion protocol has been simplified to only require three collections instead of four (i.e. count including the pre-transfusion early sample) and to standardize the time of the second collection to reflect 36 to 72 hours. With this new protocol, it was decided to advise maternity service providers with this broader timeline for routine collections as part of the updated collection guideline [
17].
3.3. Adherence to New SP Protocol
In one year from May 2023 to April 2024 inclusive 76,403 babies were screened. Of these 668 babies (0.9%) had a recorded birth weight (BW) and or gestational age (GA) that met the SP criteria.
Table 2 and
Figure 2. This had a small organizational impact on the newborn screening program delivery for this small number (<1%) of babies.
Figure 3.
Table 2.
Comparison of time of recollection guideline per jurisdiction for preterm and sick neonates1. Note, all jurisdictions recommended a pre-transfusion sample for affected babies.
Table 2.
Comparison of time of recollection guideline per jurisdiction for preterm and sick neonates1. Note, all jurisdictions recommended a pre-transfusion sample for affected babies.
| SP criteria |
First
sample |
Second
sample |
Percentage of
second samples |
| GA <32 weeks |
566 |
546 |
96% |
| BW<1500g |
635 |
606 |
95% |
| Both BW and GA |
523 |
507 |
97% |
| GA <32 weeks & no BW |
4 |
N/A |
N/A |
| BW<1500g & no GA |
333 |
N/A |
N/A |
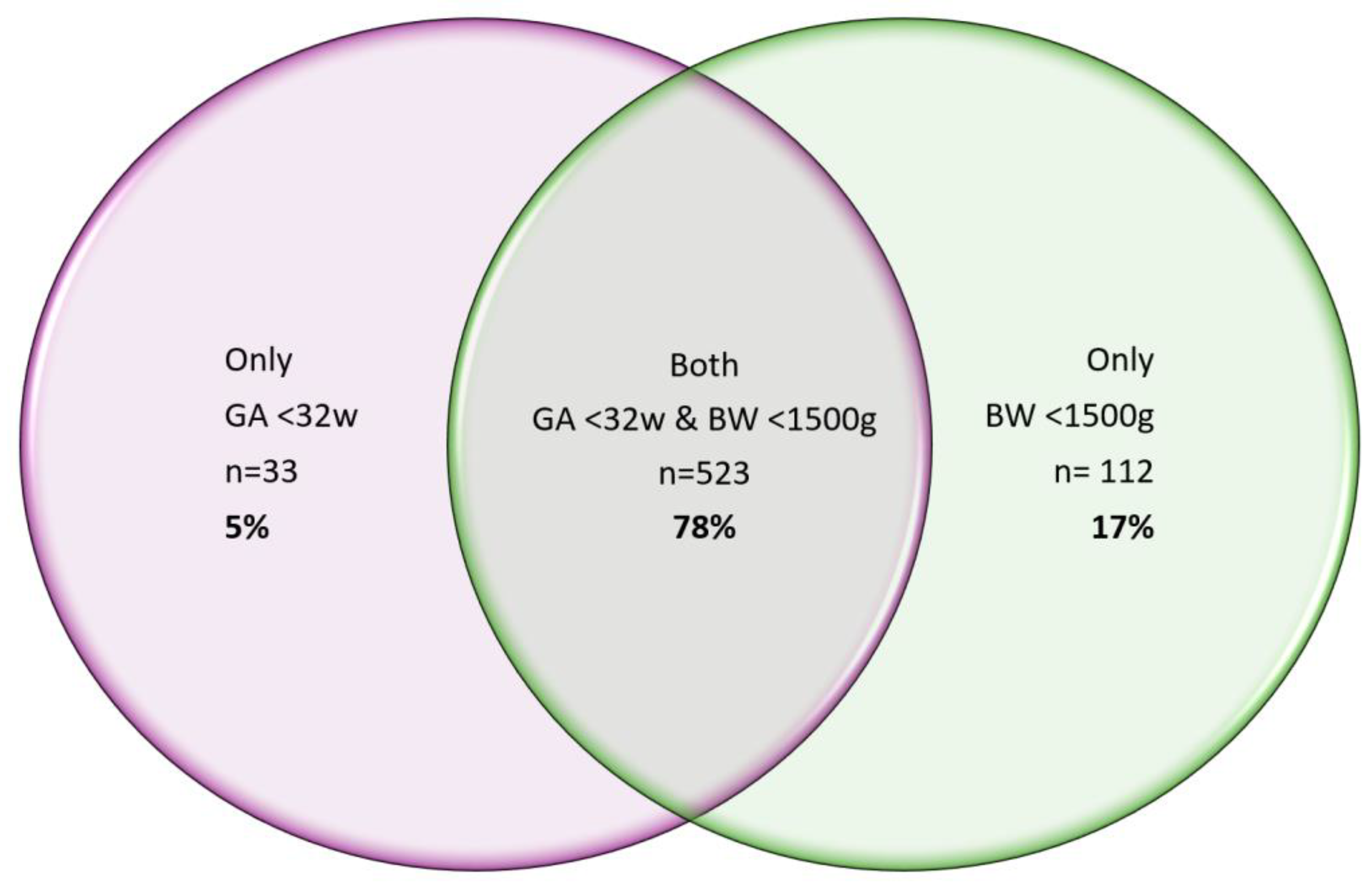
Figure 2.
a. Venn diagram showing the relationship between birthweight and gestational age for our post SP Protocol cohort of 2647 babies, where n=523 babies i.e. 78% had both a GA <32weeks & BW <1500g.
Figure 2.
a. Venn diagram showing the relationship between birthweight and gestational age for our post SP Protocol cohort of 2647 babies, where n=523 babies i.e. 78% had both a GA <32weeks & BW <1500g.
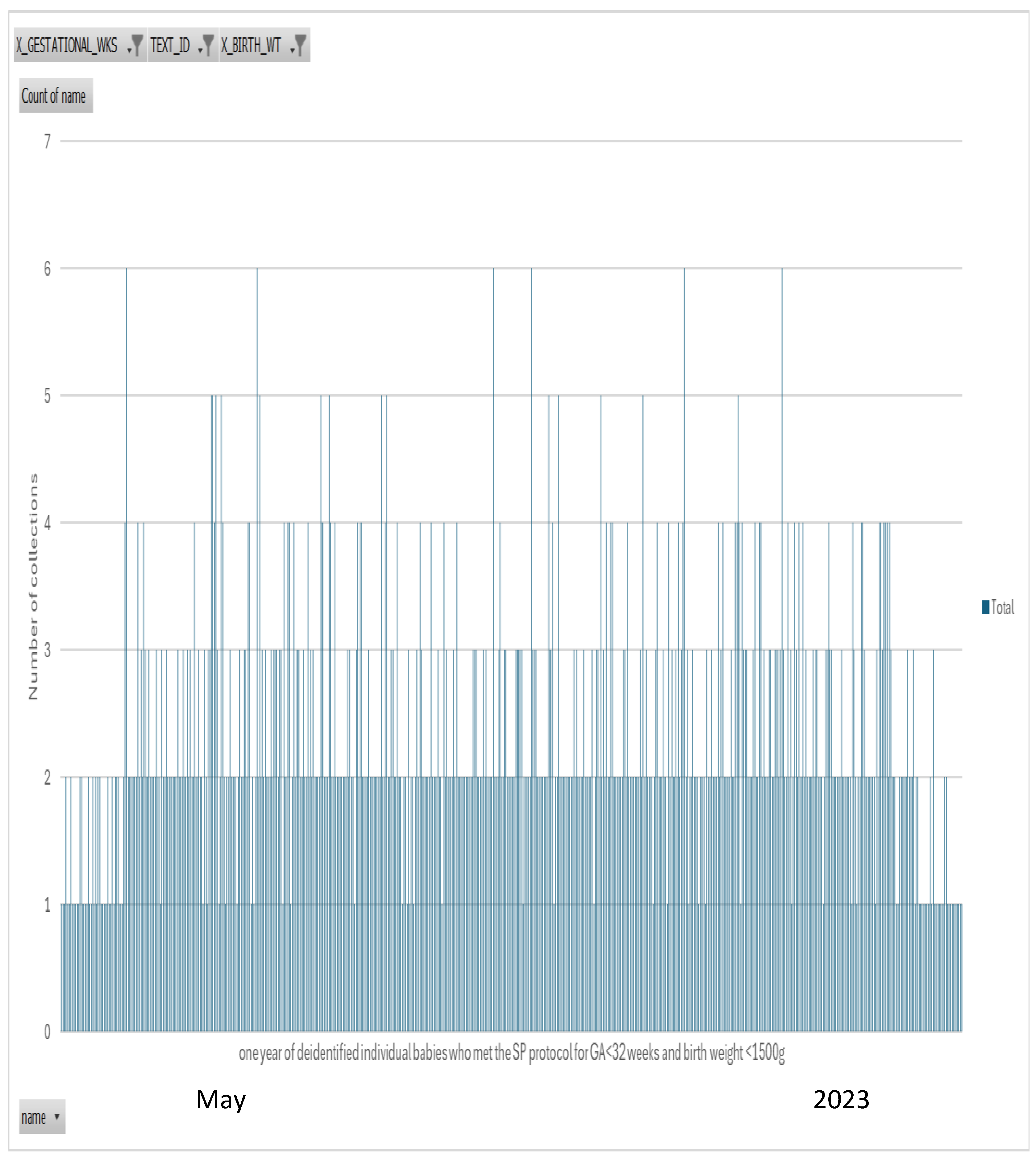
Figure 2.
b. n=523 babies who met the SP protocol for both GA<32 weeks and BW <1500g for the one year followup review. Of these n=507 or 97% of babies had a second collection. A similar percentage was achieved when comparing only GA<32 weeks (96%) or BW<1500g (95%) – data shown in Table. The start in month of May 2023 shows less second collections as the protocol was implemented and April 2024 reflects second samples yet to be collection. The months in between show excellent adherence to the SP protocol.
Figure 2.
b. n=523 babies who met the SP protocol for both GA<32 weeks and BW <1500g for the one year followup review. Of these n=507 or 97% of babies had a second collection. A similar percentage was achieved when comparing only GA<32 weeks (96%) or BW<1500g (95%) – data shown in Table. The start in month of May 2023 shows less second collections as the protocol was implemented and April 2024 reflects second samples yet to be collection. The months in between show excellent adherence to the SP protocol.
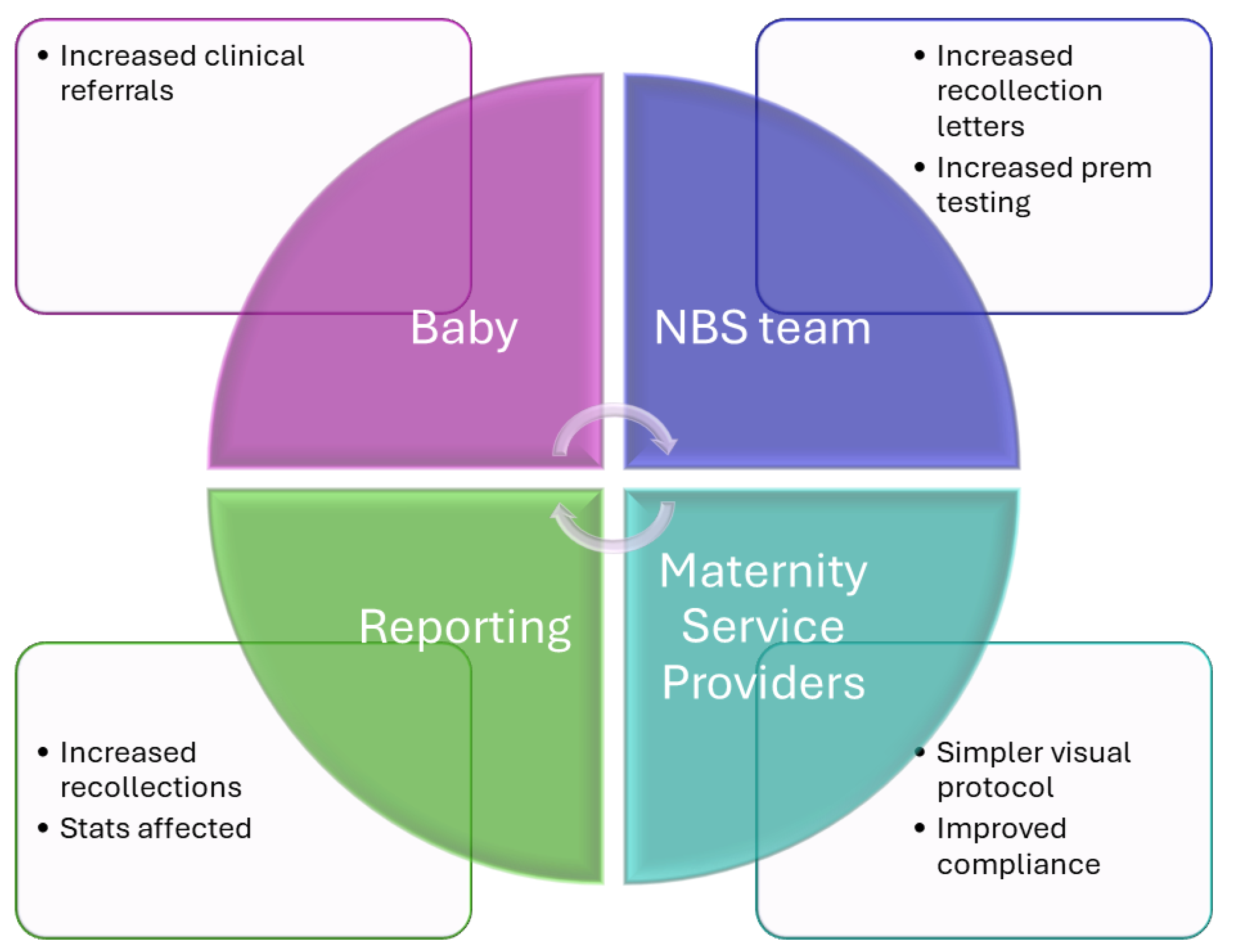
Figure 3.
Organizational impact of SP protocol – protocol live from Monday 17th April 2023. [
18]. We estimated around 160 babies in our jurisdiction received the additional collection over the 12-month follow-up audit period (May 2023 to April 2024).
Figure 3.
Organizational impact of SP protocol – protocol live from Monday 17th April 2023. [
18]. We estimated around 160 babies in our jurisdiction received the additional collection over the 12-month follow-up audit period (May 2023 to April 2024).
4. Discussion
With the introduction of an increased number of conditions, and requests for a flow-diagram visualization of a combined protocol for preterm and sick neonates, we looked to undertake a full review of our current processes. This led to the successful introduction of our new sick-prem (SP) protocol which is provided in figure 2. The successful completion of our preterm and sick recollection audit has resulted in improved and clearer processes for the management of this cohort. This is supported by the previous 76% adherence to now achieving 95% collection of second samples in this cohort. It also demonstrates the importance of data driven review for continuous improvement.
Screening for primary congenital hypothyroidism commenced in VIC and all of Australasia in the 1970’s [
7,
19,
20,
21]. At this time the mortality for extremely preterm infants was high; however with subsequent improvements in survival, consideration turned to improving morbidity and studies of thyroid function demonstrated differences in preterm infants compared to full term neonates [
10]. In preterm neonates thyroxine levels are known to decrease in the first few weeks of life and the difference between preterm and full term neonates persists until 30 – 49 days postnatally [
22]. In a study of preterm neonates <30weeks gestation conducted by our group a decade ago, we confirmed that serum free thyroxine levels were as low as <2.6 pmol/L for the first 28 days with the nadir at 7 days. We also identified differences in the thyroid hormone concentration in the “younger” (23-26 week) compared to the “older” (27 – 29 weeks) preterm babies i.e. fT4 levels were significantly lower compared to the older preterm neonates [
23]. Furthermore, the condition of transient hypothyroxinaemia of prematurity (THOP), is now established and affects infants born less than 30 weeks gestation. THOP is characterized by an initial rise in T4 at 24 hours post birth followed by a decline compared to cord blood levels with a nadir at 7 days of age and TSH secretion at this time sees a return of T4 levels; however, in the sickest preterm neonates this 24-hour surge is often absent. Also, an association exists between THOP and adverse outcome in the preterm neonate and this association is strongest for babies born less than 28 weeks gestation [
22]. We compared this combined evidence with the CLSI recommendations for timing of follow-up collection “at 28 days of age or discharge, whichever comes first” [
1]. This CLSI advice was the basis of our timing for follow-up collections in our preterm algorithm given the research-based evidence found for time the thyroid axis typically starts behaving as expected for a full-term neonate. Following the update of our protocol, the AAP guideline for CH screening was released and it is concordant with our decision of retesting babies who at birth where <1500g and/or <32weeks GA [
2].
Very or extremely low birth weight has been used as the decision point for our program, but some other programs also include gestational age-related decision points. In review of our data, for babies only had one of GA or BW recorded and therefore we chose to include a GA decision point to ensure all babies requiring a recollection would be captured in the future. Only, an extra 5% of babies were On reviewing the CLSI guideline cutoffs of 2000g and 34 weeks for rescreening of thyroid panel and CAH, we noted these did not coincide with the definitions of the WHO [
1,
15,
16]. Across Australasia, repeat screening is harmonized to babies with a birthweight of <1500g, which is consistent with others [
2,
7,
24] With a view to staying harmonized with other Australasian NBS Programs, and consistent with current neonatal definitions we decided to stay with the <1500g cut point for the routine recollection [
15]. However, during the audit, we noted a few babies who had a GA recorded without a birthweight and given the recommendation in the CLSI guideline to include GA, we added a GA <32 weeks for follow up collection i.e. the WHO cut point for very preterm [
16]. Overall, our decisions here are around harmonization as the evidence for appropriate cut points becomes a compromise between all the conditions screened and clinical risk.
The evidence around the decision and timing to recollect for transfusions was scant. In our jurisdiction we had been performing a transfusion protocol for all babies that had had any form of transfusion. On reviewing the CLSI guideline, the focus was on red blood cell (RBC) transfusions and extracorporeal life support (ECLS), indicating that a RBC transfusion invalidates multiple tests for newborn screening [
1]. Hence using the advice in this guideline, we pulled back to only request transfusion recollections related to babies who had received RBC or ECLS. We then consulted with the HGSA NBS Committee on the rational for recollections, especially timing, and we found our practice extended longer than in other jurisdictions and whilst there was limited evidence provided for the timing of follow-up collections, we moved to harmonize with the other jurisdictions in Australasia and changed from three to one week post transfusion for a follow-up collection. We note however that further transfusion related recollections may need to be considered in association with the proposed introduction of sickle cell screening [
25].
The change to the timing of collection was implemented at the start of COVID in 2020 and formalized for all babies as part of this SP introduction [
26]. This was done in the background using evidence from New Zealand and Western Australia. The aim of this change was to screen the babies before leaving the hospital. Overall, our data supports that results in the window of 36 to 72 hours are consistent with the traditional window of 48 to 72 hours and with the trend of earlier discharge from hospital this change has been seen as an advantage to the maternity service providers’ workflow. For note, other programs in Australasia do a similar expanded window, but continue to state 48-72 hours on their information [
7]. Advertising this change did result in feedback related to the hospital’s ability to be agile with updating their in-house documentation (which stated 48-72 hours). But as this timeframe was only extended the hospital’s documentation could be updated with the next review cycle.
There were a few limitations to this study and protocol review. The first one relates to the design of the preterm baby second collection review, which only looked at the receipt of a second collection and not the actual timing of the second sample. This could mean that the overall adherence rate could be lower than the 76% of babies identified in this study. The second limitation related to the unknown survival rate of this cohort, and this may have influenced the recollection numbers. Finally, whilst this review was performed in conjunction with the implementation of CAH screening, the information provided in the CLSI guideline recommended 4 weeks post birth for a follow-up collection. However, it is well established that the foetal adrenal gland persists until at least the equivalent of term despite early delivery. Hence, four weeks for extreme preterm neonates will not approach this milestone of full-term equivalent at four weeks of age and the evidence of ontogeny suggests that a longer time period for follow-up may be warranted. To date, given the rate of classical CAH approximates 1:15,000 and all preterms make up 8.2% of births, we have not yet identified a preterm baby with CAH and such babies identified should inform future practice. In the absence of evidence, harmonization of practice is the best we can achieve to ensure all babies, irrespective of the jurisdiction receive the same process.
5. Conclusions
We recommend NBS laboratories audit preterm and sick neonate recollections to ensure appropriate follow-up of babies. This should be supported with a visual process map to aid education and compliance.
Author Contributions
Conceptualization, R.G., L.C., J.N.; methodology, R.G. J.N., L.C., T.N.; software, R.G., T.N., M.T.; validation, R.G. M.T., T.N., M.OC,; formal analysis, R.G., J.P.; investigation, M.O’C.; data curation, R.G.; writing—original draft preparation, R.G.; writing—review and editing, R.G. M.O’C. J.P. L.C., M.K. T.N., N.M, J.C., J.N.; project administration, R.G. and J.C. funding acquisition, N/A. All authors have read and agreed to the published version of the manuscript.
Funding
The Victorian Newborn Bloodspot Screening Program is funded by the Department of Health Victoria.
Institutional Review Board Statement
This was a continuous improvement review of the newborn screening, that was conducted post the implementation of CAH screening. As this was a quality improvement activity Institutional Ethical approval was not required.
Informed Consent Statement
All samples tested had informed consent signed by the parent/ caregiver as part of the newborn screening program process.
Data Availability Statement
Please email the corresponding author if you would like to discuss the background data further.
Acknowledgments
We are deeply appreciative of the support and collaboration across Australia and New Zealand through participation in working groups of the HGSA, ANZPED, RCPAQAP and the Australian Government Program Management Committee. The CLSI guideline was extremely useful to look at a general recommendation and consider if it was appropriate for our jurisdiction – we thank the authors of this guideline for putting this it together. We wish to also thank our VCGS colleagues James Chi (for the IT process development) and Sara Hernandez Gutier (for the graphical design of the flowchart – figure 1).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Clinical and Laboratory Standards Institute. Newborn Screening for Preterm, Low Birth Weight and Sick Newborns. 2nd ed. CLSI guideline NBS03. Wayne, PA: CLSI; 2019. p. 82.
- Rose SR, Wassner AJ, Wintergerst KA, Yayah-Jones NH, Hopkin RJ, Chuang J, et al. Congenital Hypothyroidism: Screening and Management. Pediatrics 2023 Jan 1;151:1 as. [CrossRef]
- World Health Organization (WHO). 2020. Preterm birth. Geneva: World Health Organization.
- Australian Institute of Health and Welfare (AIHW). 2023. Australia's mothers and babies. Canberra, Australia.
- Australian Institute of Health and Welfare (AIHW). Preliminary data tables: National Perinatal Data Collection annual update 2022. In: Australian Institute of Health and Welfare, editor. Canberra: Australian Government; 2022.
- UNICEF, WHO. Country Consultation on Low Birthweight and Preterm Birth Estimates - Technical Note. Geneva: 2022.
- Huynh T, Greaves R, Mawad N, Greed L, Wotton T, Wiley V, et al. Fifty years of newborn screening for congenital hypothyroidism: current status in Australasia and the case for harmonisation. Clin Chem Lab Med 2022 Sep 27;60:10:1551-61. Epub 20220824 as. [CrossRef]
- van Wassenaer AG, Kok JH, Dekker FW, de Vijlder JJ. Thyroid function in very preterm infants: influences of gestational age and disease. Pediatr Res 1997 Nov;42:5:604-9 as. [CrossRef]
- Mitchell ML, Walraven C, Rojas DA, McIntosh KF, Hermos RJ. Screening very-low-birthweight infants for congenital hypothyroidism. The Lancet 1994;343:8888:60-1 as. [CrossRef]
- Nolan B, Uy C, Stablein L, Bany-Mohammed F. Screening for Delayed Thyroid Stimulation Hormone Rise and Atypical Congenital Hypothyroidism in Infants Born Very Preterm and Infants with Very Low Birth Weight. J Pediatr 2024 Feb 22;269:113974. Epub 20240222 as. [CrossRef]
- Midgley PC, Russell K, Oates N, Shaw JC, Honour JW. Activity of the adrenal fetal zone in preterm infants continues to term. Endocr Res 1996 Nov;22:4:729-33. Epub 1996/11/01.
- Greaves RF, Jevalikar G, Hewitt JK, Zacharin MR. A guide to understanding the steroid pathway: New insights and diagnostic implications. Clinical biochemistry 2014;47:15:5-15.
- Greaves RF, Kumar M, Mawad N, Francescon A, Le C, O’Connell M, et al. Best Practice for Identification of Classical 21-Hydroxylase Deficiency Should Include 21 Deoxycortisol Analysis with Appropriate Isomeric Steroid Separation. International Journal of Neonatal Screening 2023;9:4:58.
- Human Genetics Society of Australasia (HGSA),. Newborn Screening Committee. Mascot, NSW: HGSA; 2024.
- Cutland CL, Lackritz EM, Mallett-Moore T, Bardají A, Chandrasekaran R, Lahariya C, et al. Low birth weight: Case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine 2017 Dec 4;35:48 Pt A:6492-500 as. [CrossRef]
- Ohuma EO, Moller AB, Bradley E, Chakwera S, Hussain-Alkhateeb L, Lewin A, et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: a systematic analysis. Lancet (London, England) 2023 Oct 7;402:10409:1261-71 as. [CrossRef]
- Victorian Newborn Bloodspot Screening Program. Newborn Bloodspot Screening Comprehensive Collection Guideline. In: (VCGS) VCGS, editor. 4th Ed. Victorian Clinical Genetics Services (VCGS); 2021.
- Greaves R, Kricka L, Gruson D, Ferrari M, Martin H, Loh TP, Bernardini S. Toolkit for emerging technologies in laboratory medicine. Clin Chem Lab Med 2023 Nov 27;61:12:2102-14. Epub 20230614 as. [CrossRef]
- Connelly JF, Coakley JC, Gold H, Francis I, Mathur KS, Rickards AL, et al. Newborn screening for congenital hypothyroidism, Victoria, Australia, 1977-1997. Part 1: The screening programme, demography, baseline perinatal data and diagnostic classification. Journal of pediatric endocrinology & metabolism : JPEM 2001 Nov-Dec;14:9:1597-610. Epub 2002/01/25.
- Connelly JF, Rickards AL, Coakley JC, Price GJ, Francis I, Mathur KS, Wolfe R. Newborn screening for congenital hypothyroidism, Victoria, Australia, 1977-1997. Part 2: Treatment, progress and outcome. Journal of pediatric endocrinology & metabolism : JPEM 2001 Nov-Dec;14:9:1611-34. Epub 2002/01/25.
- Coakley JC, Francis I, Gold H, Mathur K, Connelly JF. Transient primary hypothyroidism in the newborn: experience of the Victorian Neonatal Thyroid Screening Programme. Aust Paediatr J 1989 Feb;25:1:25-30 as. [CrossRef]
- Mercado M, Yu VY, Francis I, Szymonowicz W, Gold H. Thyroid function in very preterm infants. Early Hum Dev 1988 Mar;16:2-3:131-41 as. [CrossRef]
- Greaves RF, Zacharin MR, Donath SM, Inder TE, Doyle LW, Hunt RW. Establishment of hormone reference intervals for infants born< 30 weeks' gestation. Clinical biochemistry 2014;47:15:101-8.
- Ares Segura S, Casano-Sancho P, Chueca Guindulain M. Assessment of thyroid function in the preterm and/or very low birth weight newborn [10.1016/j.anpede.2021.04.003]. Anales de Pediatría (English Edition) 2021;95:4:277.e1-.e8 as. [CrossRef]
- Newborn Bloodspot Screening: What is screened in the program. In: Department of Health and Aged Care, editor. Updated 27 March 2024 Ed. Canberra, Australia: Australian Government; 2024.
- Greaves RF, Pitt J, McGregor C, Wall M, Christodoulou J. Newborn bloodspot screening in the time of COVID-19. Genet Med 2021 Jun;23:6:1143-50. Epub 2021/01/15 as. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).