1. Introduction
The COVID-19 pandemic has transitioned from a pandemic to an endemic status in May 2023 [
1]. Despite this change, ongoing sequelae and threats pose significant challenges due to the reported mutations that can cause re-infection and subsequent consequences. The WHO report in December 2023 indicated a 53% increase in cases and a 51% increase in ICU admissions [
2]. Furthermore, several studies have shown that post-COVID conditions, also known as long COVID-19, can cause severe and debilitating symptoms such as chronic fatigue, persistent respiratory issues, persistent muscle pain, sleep disorders, and cognitive impairments like brain fog [
3]. Therefore, alertness against the potential for infection or re-infection with COVID-19 remains crucial in protecting public health. Given the uncertainty surrounding SARS-CoV-2, we must remain vigilant against the threat of this virus’s mutations.
The Omicron variant of SARS-CoV-2 has demonstrated increased transmissibility and the ability to evade immunity derived from vaccines, although the first-generation vaccines still protect against severe illness and death [
4]. The effectiveness of vaccines against Omicron infection is generally lower compared to previous variants, with protection decreasing rapidly over time [
4,
5,
6]. A new COVID-19 vaccine containing targeting omicron strain antigens has been approved in 2023 to face this problem [
7,
8,
9]
Vaccination in Indonesia started in January 2021, using two doses of Coronavac®, the whole inactivated virus vaccine, at 14-week intervals. According to the data of the Ministry of health, 86% of the population have received one dose, and 74% received the second dose. The first booster was introduced in August 2021 and reached 39% population in December 2022, while the second booster only covered 2 % of the population in early 2023 [
10]. The vaccination coverage levelled up since then; there has been no significant increase in the number of individuals receiving vaccination since May 2022 [
11]. Thus, the time gap between vaccination programs in Indonesia and the emergence of the Omicron strain can potentially reduce the level of immunity in the Indonesian population against the variant.
Continued evaluation of immune responses targeting circulating variants of SARS-CoV-2 is important to guide future vaccination strategies. Recently, at the end of 2023, the Indonesian population was struck by the XBB 1.5 variant of the Omicron strain, which caused the deaths of the elderly after a long pause in COVID-19-associated mortality [
12]. This strain -emerging as the dominant strain in several countries- is more transmissible and has a superior capability to escape immunity [
13,
14]. Thus, several questions arose regarding the immune responses against this variant, such as the persistence of the humoral response induced by vaccinations and the effectivity of booster vaccine-induced antibodies in neutralizing the virus, particularly the circulating omicron XBB 1.5 strain. In addition, will we need to introduce a vaccine containing the omicron antigen? This study was conducted to address those questions and to predict the vulnerability of the population to re-infection by emerging mutant strains of SARS-CoV-2.
2. Materials and Methods
2.1. Study Design and Study Subject
We conducted a cross-sectional study by recruiting individuals with different vaccination statuses by the number of booster shots using purposive sampling in Makassar, Indonesia’s capital city of South Sulawesi Province, from November 2023 to January 2024 (
Table 1). The inclusion criteria were those above 17 years old who had received two doses of the inactivated whole SARS-CoV-2 virus vaccine CoronaVac® from Sinovac at a 4-week interval as the primary vaccination. This study has been approved by the Ethics Committee of Hasanuddin University (Approval Number 182/UN4.6.4.5.31/PP36/2024).
Blood was withdrawn after the subjects signed the informed consent form. We also recorded the vaccination and infection history since January 2022. Collected blood was centrifuged at the Hasanuddin University Medical Research Centre (HUMRC) of Hasanuddin University Hospital for serum separation. All sera were kept at -80°C before being subjected to any experiments.
Laboratory Analysis
All samples were subjected to antibody (Ab) titer measurement of anti-RBD SARS-CoV-2 IgG and anti-RBD Omicron XBB 1.5 IgG titer by Indirect ELISA and neutralizing activity against both strains by pseudo-virus neutralizing assay.
Indirect ELISAs were done using the commercial human embryonic kidney (HEK293) HPLC-verified WT RBD protein (Sino Biological, #40591-V08H) and XBB 1.5 RBD protein (Sino Biological #40592-V08H146) as the antigens. As previously described, a similar procedure was applied in both ELISA [
15,
16]. The 96-well microplates (Corning, #3590) were coated with 0,2 μg/mL of either SARS-CoV-2 WT or the XBB 1.5 antigen and incubated overnight at 4°C. Before incubation with sera samples, the plates were blocked with 1% bovine serum albumin (BSA) in PBS (pH 7.4) for 1 hour and then washed with PBS-T. The sera were diluted 1:100 in PBS containing 1% BSA. After incubation with sera for an hour, the plates were washed and then incubated with horseradish peroxidase (HRP)-conjugated monoclonal antibodies recognizing an Fc domain of human IgG for another 1 hour. After incubation, the plates were rewashed, 100 μL/well of the substrate was added to each well, and the plates were incubated for 30 min at room temperature for colour development. The absorbance was measured at 414 nm on a microplate reader.
VSV-based neutralizing activity of the serum was examined according to a previous study [
15,
16]. We employed a VSV-based pseudovirus expressing Wuhan-Hu, generated for the previous study. In addition, we engineered the pseudo-virus for the current study to express the Omicron XBB 1.5 spike protein on the viral surface. The luciferase gene was incorporated into the viral genome. The serum was diluted with the medium on a 96-well culture plate, and the pseudo-virus of either strain was added in triplicate. The final dilution rate of the serum was 1:100. The mixture of the virus and the serum was incubated with human embryonic kidney (HEK) 293T cells that expressed human ACE2 and human TMPRSS2. The cells were examined by ONE-Glo EX
TM Luciferase Assay System (Promega, Madison, WI, USA) after 24 hours of incubation to reveal the percentage of viral internalization. Neutralization was calculated as 100% - % viral internalization.
Data Analysis
Statistical analysis was performed using GraphPad Prism version 10.0 for Mac OS. Kruskal-Wallis test with a post hoc Dunn’s multiple comparison test was used for group analysis. A comparison between the two groups was made using the Mann-Whitney U test. Spearman correlation analysis and non-linear regression analysis were used to analyze the correlations between optical densities (ODs) and the percentage of internalization. A p-value of < 0.05 was considered statistically significant.
3. Results
3.1. Characteristics of Study Subjects
We recruited 113 subjects for the study from December 2023 to January 2024. The distribution of participants according to their vaccination status is summarized in
Table 2. The proportion of participants who received two booster doses (DB group) was 41.59%, while those who received no booster dose (NB group) comprised 32.74% of the total sample. The age varied across groups, with the highest median age observed in participants with two booster doses (35.46 y.o, interval of 19.96 – 39.45 y.o). The infection status differed among the vaccination groups, with the highest percentage of confirmed infections observed in participants with two booster doses (29.78%). We recorded the confirmed breakthrough infection (BTI) in 2022 without any sequencing data.
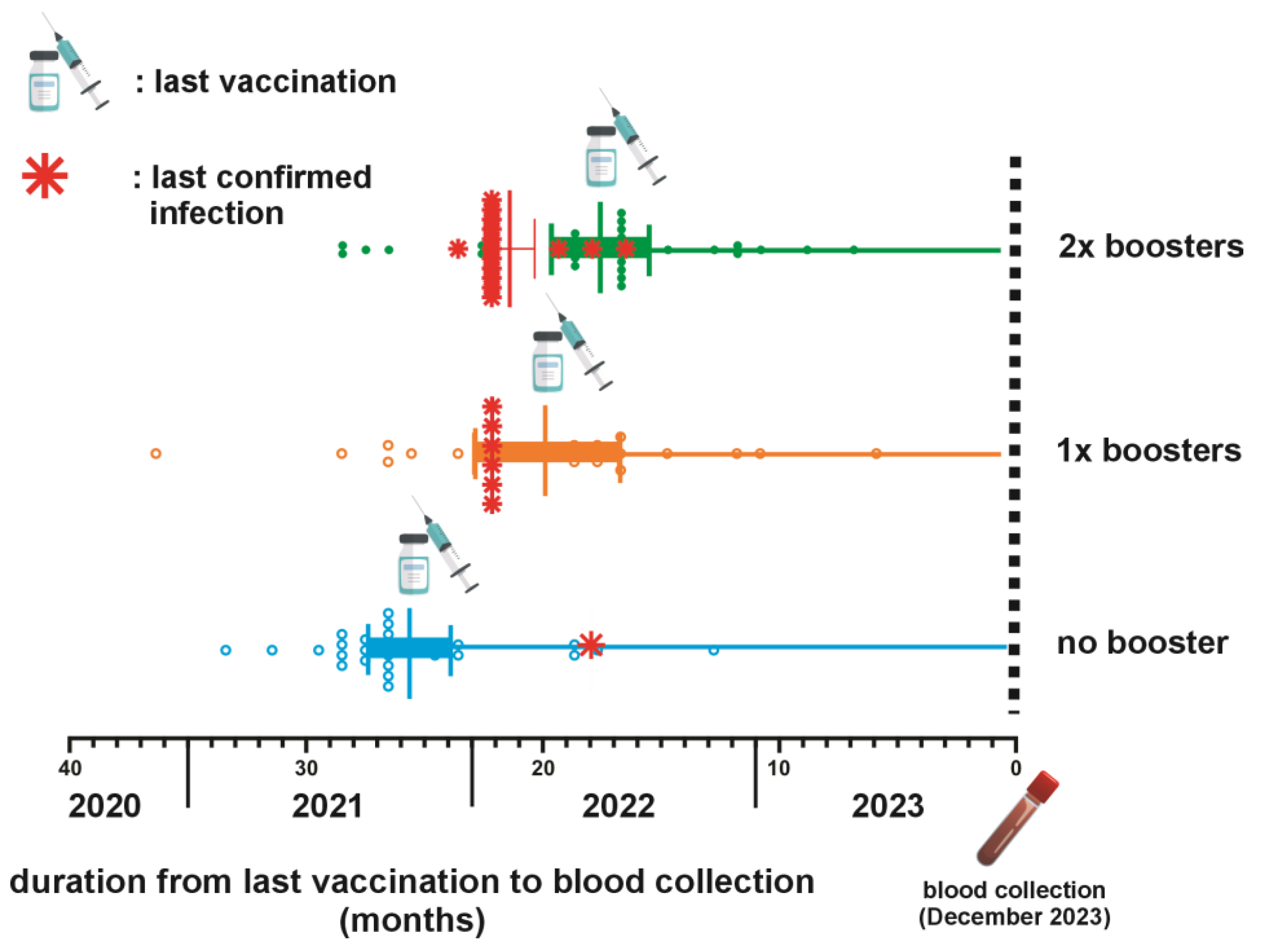
The timeline between the last vaccination and blood collection across three distinct groups categorized by their booster vaccination status is depicted in
Figure 1. The average duration from the last vaccination dose to subsequent blood sample collection was 25.11 weeks, 19.24 weeks, and 16.9 weeks for the NB, SB, and DB groups, respectively. Statistical analysis using the Kruskal-Wallis test confirmed significant differences in the mean duration among these groups (
p < 0.0001). Further examination using Dunn’s multiple comparison tests highlighted significant disparities when comparing the SB and DB groups to the NB group (
p = 0.003 and < 0.0001, respectively). Notably, while booster vaccinations contribute to variations in the timeline, the mean duration between SB and DB groups was not significantly different (
p =0.63).
Among the participants, only 22 (19.46%) were confirmed to have contracted COVID-19 beyond 2021 (
Table 2 and
Figure 1). The mean duration from the confirmation of infection status to blood sampling was 21.72 (± 1.45) months. Specifically, 81% of the confirmed cases were health workers, comprising 50% community health centre staff and 31.81% hospital staff. The remaining 18.18% worked at health service-unrelated environment. Interestingly, most confirmed infection groups (68.18%) had received a second booster dose.
Table 3.
Characteristic of study subjects Based on Infection Status.
Table 3.
Characteristic of study subjects Based on Infection Status.
| Infection Status |
N = 113 (%) |
Sex |
Age; Median (interval; y.o) |
Employment |
Vaccination status |
Duration from last infection in 2022; Mean SD (months) |
| Male; N (%) |
Female; N (%) |
Health worker N (%) |
others; N (%) |
No booster; N (%) |
1x booster; N (%) |
2x booster; N (%) |
| Confirmed infection beyond 2021 |
22 (19.46) |
1 (4.54) |
21 (95.45) |
33.23 (17-59) |
18 (81.81) |
4 (18.18) |
1 (4.54) |
6 (27.27) |
15 (68.18) |
21.72 (1.45) |
| Untested |
91 (80.53) |
30 (32.96) |
61 (67.03) |
21.83 (17-59) |
29 (31.86) |
62 (68.13) |
35 (38.46) |
23 (25.27) |
32 (35.16) |
- |
3.2. Evaluation of the Persistence of Antibody against Wildy Type SARS-CoV-2 and XBB 1.5
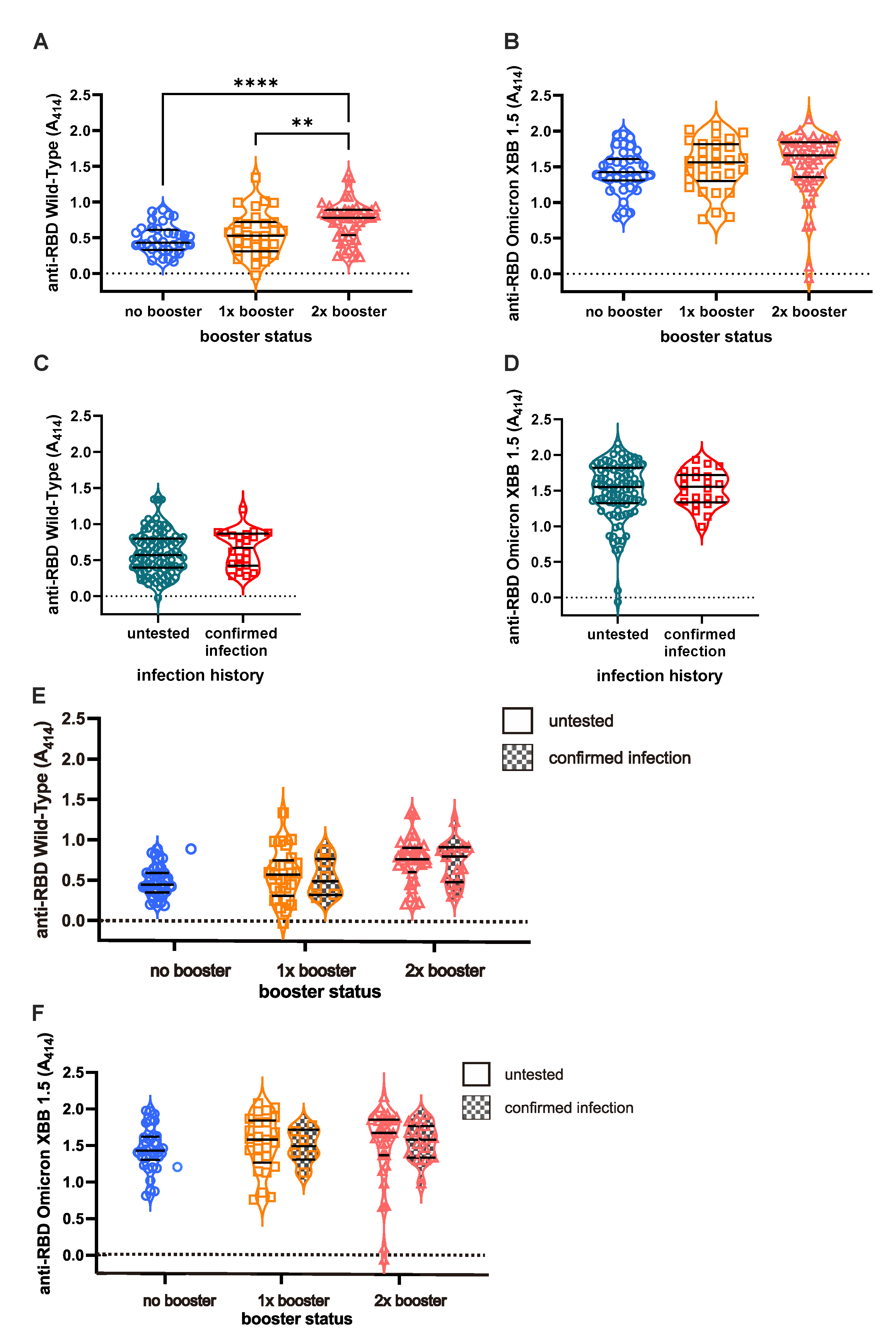
We first measured serum Ab titers against original Wild-Type (WT) and Omicron XBB 1.5 using ELISA to investigate the effect of booster shots and infection on long-term humoral immunity against COVID-19 (
Figure 2). As expected, individuals with booster shots demonstrated a higher Ab titer against the WT in a stepwise pattern (
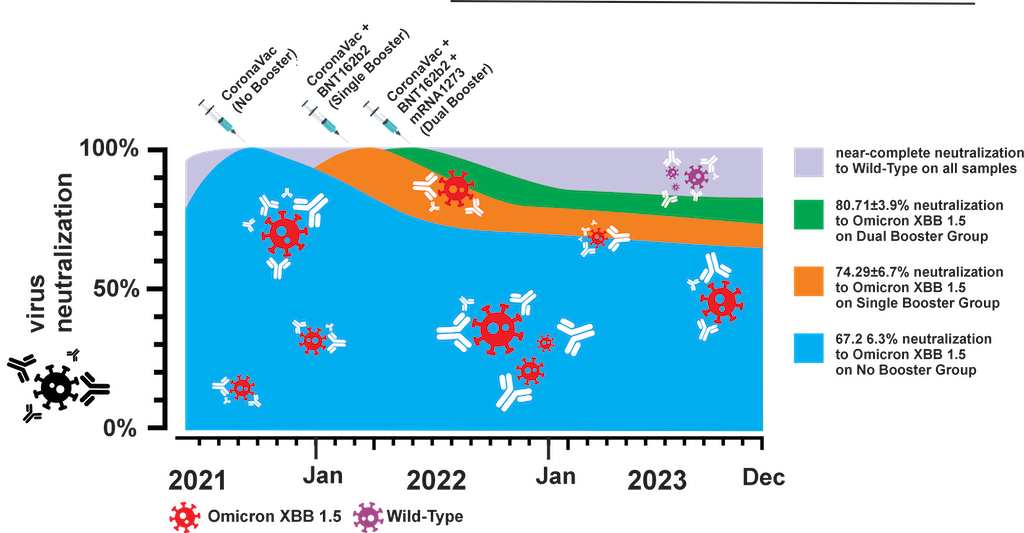
Figure 2A). The DB group showed the highest Ab titers whereas the NB group showed the lowest titers (NB vs SB vs DB = 0.48±0.03 VS 0.54±0.05 VS 0.73±0.03, respectively). Interestingly, the anti- XBB 1.5 Ab titer is higher than the WT Ab titer in all subjects, but the titer is not significantly different between booster groups (
Figure 2B). The mean of anti-XBB 1.5 Ab titer among NB, SB, and DB groups were 1.43±0.05, 1.51±0.06, and 1.52±0.06, respectively. Vaccines failed to yield a significant increase in Ab titer against both strains (
Figure 2C and 2D) in individuals with BTI. Individuals with BTI exhibited an average of 0.67±0.05 anti-WT Ab titers, while those without a BTI showed 0.59±0.02 anti-WT titer. On the other hand, the anti-XBB 1.5 Ab titer in individuals with a confirmed BTI was 1.5±0.05, and those in untested individuals were 1.49±0.04. Further analysis showed that the Ab titers among individuals with pre-infection were unsignificantly different from those without pre-infection (
Figure 2E and 2F).
The Ab titer against XBB 1.5 is higher (1.507±0.03) than that against WT in all subjects (0.606±0.02) (
p<0.0001) (Appendix
Figure 1A). Subgroup analysis based on the number of vaccines received, revealed consistently higher XBB 1.5 Ab titers relative to WT across all subgroups (Appendix
Figure 1B; NB=1.438±0.05 VS 0.48±0.03; SB= 1.513±0.06 VS 0.549±0.05; DB (1.520±0.06 VS 0.737±0.03), with p<0.0001 observed in each subgroup. Similarly, based on infection status, the level of anti-WT and anti-XBB 1.5 Ab titer is significantly different (Appendix
Figure 1C). Anti-XBB 1.5 Ab titers surpassed WT Ab titers either in pre-infected individuals (1.5±0.05 vs 0.67±0.05, p<0.0001) and in the untested group (1.49±0.04 vs 0.59±0.02, p<0.0001).
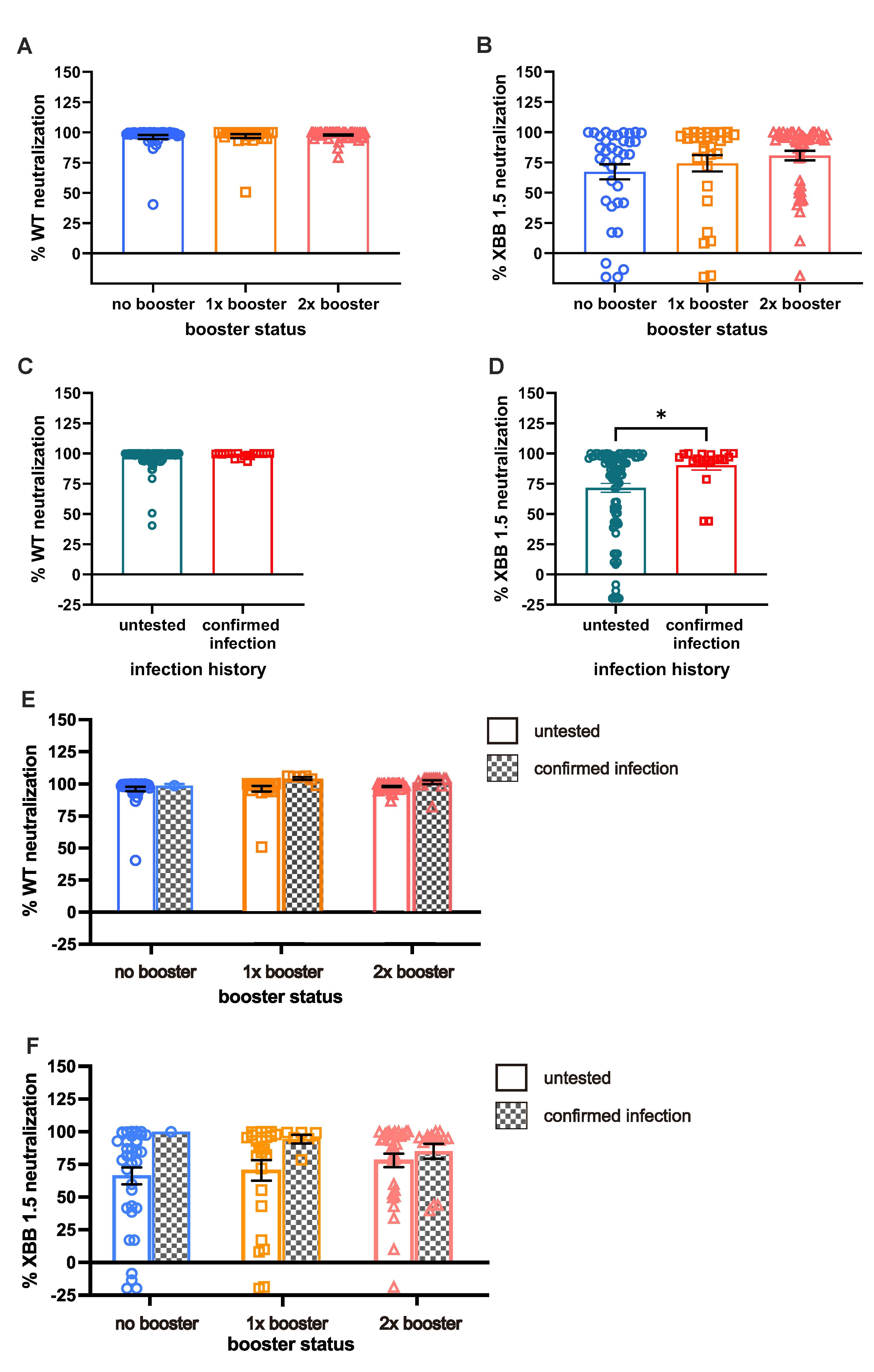
3.3. Natural Infection Results in a Better Neutralization Capacity (NC)
Next, we investigated the serum Ab neutralization capacity (NC) against WT and XBB 1.5 VSV pseudo-virus; as expected, the NC against Omicron XBB 1.5 is lower than that against the WT. While the number of booster doses did not significantly impact NC against WT (
Figure 3A), individuals with two booster doses (DB) exhibited notably higher NC against XBB 1.5 pseudo-virus as compared with those receiving one dose of booster (SB) or without a booster vaccine (NB;
Figure 3B). Across the three booster groups, NC against WT remained comparable, with nearly 100% neutralization observed (96.22±1.67% for NB, 96.88±1.64% for SB, and 97.93±0.55% for DB).
An increased NC against XBB 1.5, along with the increased number of booster doses, was observed, although the increment was not statistically significant. Specifically, the NC against XBB 1.5 among the NB, SB, and DB groups were 80.71±3.9%, 74.29±6.7%, and 67.2±6.3%, respectively.
Given that NC for WT reveals nearly 100% neutralization for the majority of the sample, the comparison of NC based on BTI history was similar (
Figure 3C).
A significant difference in NC against XBB 1.5 was demonstrated by individuals with BTI as compared with those without BTI (90.29±3.89% VS 71.75±3.63%, respectively;
Figure 3D;
p < 0.05). Individuals with more booster shots demonstrated higher NC especially those without BTI, but a different case was shown with pre-infected individuals; the second booster did not result in antibodies with better NC (
Figure 3E; 3F). The prevalence of pre-infected individuals with more than 50% neutralization of XBB 1.5 reduced from 100% to 80% after the second booster shot. On the other hand, the prevalence of untested individuals with more than 50% neutralization was 71%, 76%, and 84% in the NB, SB, and DB groups, respectively.
Appendix
Figure 2 clearly shows a comparative analysis of NC based on the strains of SARS-CoV-2. Evidently, NC against XBB 1.5 is lower than NC against WT in all subjects.
3.4. Correlation between Serum Ab Titers and Neutralization Capacity (NC) for Wild-Type and Omicron XBB 1.5 Variants
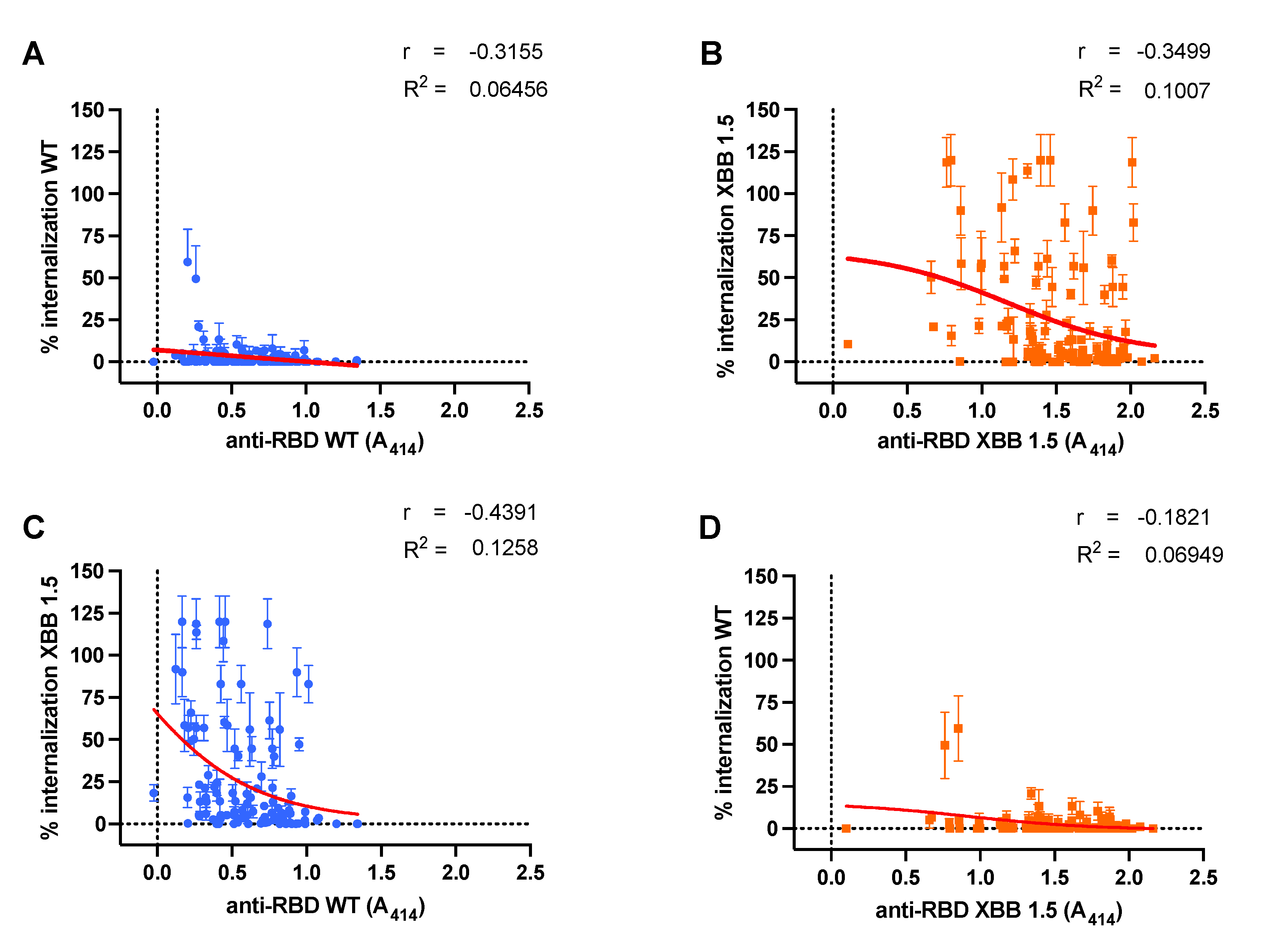
We analyzed the relationship and model fit between serum Ab titers and their corresponding NC, as illustrated in
Figure 4. Additionally, we analyzed the correlation and predictive score of Ab NC for the other variant. Ab titers against the Wild-Type (WT) variant demonstrated a moderate negative correlation with their corresponding VSV pseudo-virus internalization, as depicted in
Figure 4A (r = -0.3155, p < 0.0001). A stronger cross-neutralization correlation was observed for the Omicron XBB 1.5 variant (r = -0.439, p < 0.0001), as shown in
Figure 3C. Regression analysis revealed a minimal predictive relationship between WT Ab titers and their NC for both WT and XBB 1.5 variants (R² = 0.064 and R² = 0.125, respectively).
Antibodies targeting the Omicron XBB 1.5 variant (
Figure 4B) also exhibited a moderate correlation with NC against their corresponding XBB 1.5 variant (r = 0.3499, p < 0.001). However, cross-NC against the Wild-Type (WT) variant showed no correlation, which might be due to the low level of viral internalization demonstrated by all sera (
Figure 3D) (r = -0.182, p = 0.053). The correlation and cross-correlation analysis displayed limited predictive value (R² = 0.1 and R² = 0.069, respectively).
Subgroup analysis assessed the in-depth correlation between serum Ab titers and NC based on vaccination status and BTI history (S
Figure 3A–E). In the booster subgroup analysis, significant correlations were observed between Omicron XBB 1.5 Ab titers and NC in the NB group (S
Figure 3A, r = -0.357, p = 0.035) and SB group (S
Figure 3B, r = -0.553, p < 0.001). Regression analysis revealed moderate relationships between Ab titers and NC in these groups (R² = 0.152 and R² = 0.311, respectively). However, no significant correlation was found in the DB group (S
Figure 3C, r = -0.146, p = 0.32), with a meager regression score (R² = 0.003).
Conversely, for the WT variant, low and non-significant correlations were observed in both the NB (S
Figure 3A, r = -0.106, p = 0.53) and SB groups (S
Figure 3B, r = -0.217, p = 0.24). However, a significant correlation was detected in the DB group (S
Figure 3C, r = -0.404, p < 0.01), accompanied by a higher regression score (R² = 0.26) compared to the Omicron XBB 1.5 variant.
Moreover, individuals with BTI beyond 2021 exhibited similar NC for both the WT and Omicron XBB 1.5 variants (S
Figure 3D), resulting in comparable correlations between serum Ab titers and NC in this group (r = -0.272, p = 0.25 and r = -0.168, p = 0.49, respectively). However, a notably better regression score was observed against Omicron XBB 1.5 (R2 = 0.476); untested individuals displayed significantly moderate correlations between Ab titer and NC against Omicron XBB 1.5 (S
Figure 3E; r = -0.387, p < 0.0001). Regression analysis was only feasible for Omicron XBB 1.5 (R2 = 0.129), as the regression for WT was exceedingly low (R2 < 0.0001), which might be due to uniformly high NC against WT.
4. Discussion
Antibody (Ab) titers against COVID-19, elicited from vaccination or infections, have been reported to decline over time and have become a growing concern [
17,
18,
19]. Several studies suggest that vaccinations, particularly in individuals with pre-existing immunity, can initially boost Ab levels, gradually decreasing and stabilizing at lower levels over an extended period [
4,
20,
21,
22,
23,
24]. Our previous study on short-term immunity against the SARS-CoV-2 WT variant revealed antibodies persisted at a high level at three months post-infection, yet no difference level between pre-infected individuals with uninfected vaccinated individuals [
15]. In the current study, we evaluated the long-term immunity, at least one year ahead of the last vaccination, based on the number of booster shots and infection history beyond December 2021. Our current study confirms that booster shots have a pronounced effect on extending long-term immunity against Wild-Type (WT) and Omicron XBB 1.5 variants, particularly in individuals without infection history, as previously reported [
25,
26]. This is evidenced by the persistent of Ab titers and neutralization capacity (NC) observed until 25 months after the last vaccination. Multiple reports suggest that breakthrough infections (BTI) post-vaccination can significantly boost Ab titers, as evidenced by cases involving the Omicron variant following booster doses [
20,
27,
28]. Recent studies indicate that individuals who received boosters and experienced BTI exhibit nearly double the Ab titers compared to those without BTI, a phenomenon observed five months after receiving the booster [
29]. However, in our study, we observed similar Ab titers between post-infected individuals and untested individuals. The Ab titer of the post-infected individuals, spanning approximately 21 months of the mean duration of the last known infections, may have spiked and decreased to eventually plateau at the same level as those of naïve vaccinated individuals when the blood sample was collected. The levelling off of Ab titers may also be attributed to asymptomatic infections [
30].
Neutralizing antibodies are pivotal in preventing SARS-CoV infection, making the assessment of neutralizing Ab (NAb) activity indispensable for addressing COVID-19 in diagnostic, therapeutic, and preventive contexts [
31,
32]. As NAb levels may decline over time and could be circumvented by viral mutations, continual monitoring of Nab activity is vital for guiding future prevention strategies [
23,
33]. Our study’s findings reveal that individuals who received booster vaccinations demonstrate enhanced long-term neutralization against the Omicron variant compared to those who did not, particularly those without BTI history. One plausible explanation for this trend is the efficacy of heterologous boosters, observed among our study participants, in bolstering neutralizing responses more effectively than homologous boosters. This approach to heterologous boosting may mitigate off-target immunity induced by different vaccine types [
22,
34,
35].
Antibodies against SARS-CoV-2 neutralize the virus in several mechanisms, including blocking the interaction of the RBD with ACE2 by binding to the RBD or binding with a co-receptor (TMPRSS2) thus blocking the subsequent steps [
36]. Therefore, natural infection may induce more complex antibodies that may be not only capable of binding to the receptor, but also to the co-receptor. Vaccines targeting only RBD-induced antibodies may not be as effective as naturally induced neutralizing antibodies as they may target the N-terminal Domain of the spike protein [
37]. Our data showed that BTI increased NC against Omicron, which is particularly evident among those who received one booster dose, similar to previous reports [
21,
38]. Cohort studies investigating booster effects have reported a 4.1-fold increase in NAb response following the third dose, rising further to 7.1 times with booster doses in BTI cases compared to primary vaccine recipients [
27]. BTI after booster administration is associated with the sustained persistence of neutralizing antibodies, observed over six months to 2 years, and decelerated rate of Nab waning [
39,
40,
41]. However, in our observation, the individuals with BTI who received the second booster dose did not show a higher NC compared to those who received the first dose. Thus, we assume that two doses of inactivated virus vaccine boosted with one dose of mRNA vaccine is sufficient for a long-term NC among individuals with a history of infection.
Another interesting finding from our study is the near-complete neutralization against the Wild-Type (WT) strain of most samples, even of those obtained from individuals without booster. Despite the expectation of a decrease in NC over time, several reports indicate the persistence of NC against WT and even potential increases due to exposure to the mutant variants. It is reported that NAb gained from mutation may also help cross-neutralization to WT [
42]. The correlation between NAb titers and their corresponding NC offers valuable insights into the immunity against COVID-19 and the potential severity of infections [
4,
43]. Cohort studies measuring Ab titers alongside neutralization activity have shown persistent correlations, with Ab titers maintaining a solid correlation with their neutralization response for at least five months until nine months post-infection [
44,
45]. However, the applicability of this correlation may be limited over time, as Ab titers eventually reach detectable plateaus while maintaining stable neutralization activity, as elucidated above. Despite a notable correlation between NAb titers and NC in our findings, with correlations hovering around 0.3 for both metrics, it is crucial to interpret these results cautiously in the context of long-term evaluations. Hence, interpreting Ab titers and their associated neutralization activity necessitates careful consideration in long-term immunity assessments against COVID-19. In addition, a study reported that antibodies produced by B-cell clones against RBD do not always compete with ACE2 for RBD binding [
46].
It has been suggested that neither infection nor vaccination alone could induce potent cross-neutralization against Omicron [
47,
48,
49]. In this study, all subjects received vaccination; thus, we could not evaluate the impact of infection alone. A study reported that triple vaccination with an inactivated virus, even without BTI, demonstrated robust short-term cross-neutralization activity against both Delta and Omicron variants [
50]. In our study, untested individuals with no confirmed BTI showed a lower neutralization activity than those with confirmed BTI, but the NC against XBB 1.5 was more than 70% even in the naïve non-booster group although the NC may have waned due to longer duration than the other booster groups. This result is contradictive with a previous study showing almost complete XBB 1.5 evasion in 3-dose-vaccinated sera after ten weeks [
51]. Another study showed that a 2-dose vaccine produced antibodies with low avidity against the omicron variant, but an additional dose increased the avidity [
52].
This study has several limitations that warrant consideration. Firstly, its cross-sectional design, while providing rapid insights, may only partially capture the dynamics of long-term immunity. Thus, we could not evaluate the dynamic of the NC, the peak, and how long it is maintained at the same level. A prospective study would offer a more accurate assessment, not only for the dynamic of immune response but also in investigating infection history, which can sometimes go unnoticed during cross-sectional analyses. Moreover, we did not have the data on which SARS-CoV-2 strain circulated in Makassar due to limited sequencing. Another limitation of this study was that the sample size was quite small. There was only one subject had confirmed infection in the no booster group. Thus, we did not have sufficient data on comparison between uninfected individuals and infected individuals in this group. Finally, we did not consider the variability of sex, age, body mass index, and smoking status of study subjects that were reported to correlate with the Ab-neutralizing activities [
53]. Despite the limitations, this study has provided important information to guide future policies on COVID-19 vaccination in Indonesia. A two-dose of whole inactivated vaccine induced long-term immunity and sufficient cross-neutralization against mutant variants of SARS-CoV-2 for at least two years post vaccines, enhanced by booster shots in uninfected individuals.
5. Conclusions
COVID-19 vaccine induced a long-term immunity and cross-neutralization against mutant variant of SARS-CoV-2 for at least 2 years post administration in Indonesian residents. Individuals without booster shots maintain sufficient neutralizing capacity against the XBB 1.5. Booster shots enhanced the neutralization capacity especially among naïve vaccinated individuals.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, Y.Y; methodology, A.S, K.Y., M.I., S.Y; validation, K, A.A.H., A.S, M.I., Y.Y.; formal analysis, K, A.A.H, Y.Y.; investigation, K, A.A.H, K.H.Z., D.D.N.S.; resources, M.I., S.Y, Y.Y.; data curation, K, A.A.H, A.S., M.I.; writing—original draft preparation, K, Y.Y; writing— review and editing, A.A.H., I.Y., Y.Y.; visualization, K; supervision, A.S., I.Y., M.I, S.Y., Y.Y.; project administration, K.,A.A.H..; funding acquisition, A.S., M.I., S.Y., Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly funded by International Collaboration Research (I-CORE) Program Equity WCU Institute of Research and Community Services Hasanuddin University to YY (No.04966/UN4.22/PT.01.03/2023), Indonesian Directorate General of Higher Education (DGHE)-Japan Society for the Promotion of Science (JSPS) joint research projects to Y.Y (No.105/E4.4/KU/2021) and M.I (JPJSBP-120218101), a Grant-in-Aid for Scientific Research© to M.I (JSPS KAKENHI grant numbers 21K06559) and AS (JSPS KAKENHI grant numbers 21K06545). The APC was funded by Hasanuddin University.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Hasanuddin University (Approval Number 182/UN4.6.4.5.31/PP36/2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We would like to express our sincere gratitude to all volunteers involved in this study. We are also grateful for the laboratory assistance of Sri Nur Rahmi Nur Rustam at Center for Zoonotic and Emerging Diseases Laboratory. We thank Syahruddin at Tadjuddin Chalid Hospital for the support during the sample collection.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Statement on the Fifteenth Meeting of the IHR (2005) Emergency Committee on the COVID-19 Pandemic. Who.int 2023.
- COVID-19 epidemiological update – 22 December 2023. www.who.int 2023.
- Healey, Q.; Sheikh, A.; Daines, L.; Vasileiou, E. Symptoms and signs of long COVID: A rapid review and meta-analysis. Journal of Global Health 2022, 12. [Google Scholar] [CrossRef]
- Lau, E.H.Y.; Hui, D.S.C.; Tsang, O.T.Y.; Chan, W.-H.; Kwan, M.Y.W.; Chiu, S.S.; Cheng, S.M.S.; Ko, R.L.W.; Li, J.K.C.; Chaothai, S.; et al. Long-term persistence of SARS-CoV-2 neutralizing antibody responses after infection and estimates of the duration of protection. eClinicalMedicine 2021, 41, 101174. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, L.E.; Ngeh, S.; Cadby, G.; Hutcheon, K.; Effler, P.V. SARS-CoV-2 Vaccine Effectiveness against Omicron Variant in Infection-Naive Population, Australia, 2022 - Volume 29, Number 6—June 2023 - Emerging Infectious Diseases Journal - CDC. wwwnc.cdc.gov 2023, 29. [Google Scholar] [CrossRef]
- Yang, J.; Hong, W.; Lei, H.; He, C.; Lei, W.; Zhou, Y.; Zhao, T.; Alu, A.; Ma, X.; Li, J.; et al. Low levels of neutralizing antibodies against XBB Omicron subvariants after BA.5 infection. Signal Transduction and Targeted Therapy 2023, 8, 252. [Google Scholar] [CrossRef]
- Xammy Huu Nguyenla; Timothy A. Bates; Mila Trank-Greene; Mastura Wahedi; Tafesse, F.G.; Curlin, M. Evaluating Humoral Immunity Elicited by XBB.1.5 Monovalent COVID-19 Vaccine. Emerg Infect Dis. 2024, 30, 1282–1283. [Google Scholar] [CrossRef]
- Carr, E.J.; Wu, M.Y.; Gahir, J.; Harvey, R.; Townsley, H.; Bailey, C.; Fowler, A.S.; Dowgier, G.; Hobbs, A.; Herman, L.; et al. Neutralising immunity to omicron sublineages BQ.1.1, XBB, and XBB.1.5 in healthy adults is boosted by bivalent BA.1-containing mRNA vaccination and previous Omicron infection. The Lancet Infectious Diseases 2023, 23, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Zaeck, L.M.; Tan, N.H.; Rietdijk, W.J.R.; Geers, D.; Sablerolles, R.S.G.; Bogers, S.; van Dijk, L.L.A.; Gommers, L.; van Leeuwen, L.P.M.; Rugebregt, S.; et al. Original COVID-19 priming regimen impacts the immunogenicity of bivalent BA.1 and BA.5 boosters. Nature Communications 2024, 15, 4224. [Google Scholar] [CrossRef]
- Indonesia, K.K.R. Vaksinasi COVID-19 Nasional (national COVID-19 vaccination). Available online: (accessed on 2 July 2024).
- F, A.; Kurmala, A. 68.57 Million Indonesians Receive Third COVID-19 Vaccine Dose. Antara News.
- HAMASY, A.I.A. Dua Warga DKI Jakarta Meninggal akibat Covid-19. Available online: https://www.kompas.id/baca/metro/2023/12/11/dua-warga-dki-jakarta-meninggal-akibat-covid-19 (accessed on 12 April 2024).
- Ao, D.; He, X.; Hong, W.; Wei, X. The rapid rise of SARS-CoV-2 Omicron subvariants with immune evasion properties: XBB.1.5 and BQ.1.1 subvariants. MedComm 2023, 4, e239. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, R.; Gilby, N.B.; Wei, G.-W. Omicron Variant (B.1.1.529): Infectivity, Vaccine Breakthrough, and Antibody Resistance. Journal of Chemical Information and Modeling 2022, 62, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Nurisyah, S.; Iyori, M.; Hasyim, A.A.; Sakamoto, A.; Hashimoto, H.; Yamagata, K.; Yamauchi, S.; Amru, K.; Zainal, K.H.; Idris, I.; et al. Comparison between Neutralization Capacity of Antibodies Elicited by COVID-19 Natural Infection and Vaccination in Indonesia: A Prospective Cohort. Antibodies 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Osawa, H.; Hashimoto, H.; Mizuno, T.; Hasyim, A.A.; Abe, Y.-i.; Okahashi, Y.; Ogawa, R.; Iyori, M.; Shida, H.; et al. A replication-competent smallpox vaccine LC16m8Δ-based COVID-19 vaccine. Emerging Microbes & Infections 2022, 11, 2359–2370. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Tang, P.; Hasan, M.R.; AlMukdad, S.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. New England Journal of Medicine 2021. [Google Scholar] [CrossRef] [PubMed]
- Chemaitelly, H.; Nagelkerke, N.; Ayoub, H.H.; Coyle, P.; Tang, P.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Hasan, M.R.; Al-Kanaani, Z.; et al. Duration of immune protection of SARS-CoV-2 natural infection against reinfection. Journal of Travel Medicine 2022, 29. [Google Scholar] [CrossRef] [PubMed]
- Tartof, S.Y.; Slezak, J.M.; Puzniak, L.; Hong, V.; Frankland, T.B.; Xie, F.; Ackerson, B.K.; Valluri, S.R.; Jodar, L.; McLaughlin, J.M. Effectiveness and durability of BNT162b2 vaccine against hospital and emergency department admissions due to SARS-CoV-2 omicron sub-lineages BA.1 and BA.2 in a large health system in the USA: a test-negative, case-control study. The Lancet Respiratory Medicine, 2022. [Google Scholar] [CrossRef]
- Bates, T.A.; Leier, H.C.; McBride, S.K.; Schoen, D.; Lyski, Z.L.; Xthona Lee, D.D.; Messer, W.B.; Curlin, M.E.; Tafesse, F.G. An extended interval between vaccination and infection enhances hybrid immunity against SARS-CoV-2 variants. JCI Insight 2023, 8. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, N.; Sasaki, A.; Kadowaki, T.; Mitsuhashi, T.; Takao, S.; Yorifuji, T. Longitudinal antibody dynamics after COVID-19 vaccine boosters based on prior infection status and booster doses. Scientific Reports 2024, 14, 4564. [Google Scholar] [CrossRef] [PubMed]
- Underwood, A.P.; Sølund, C.; Fernandez-Antunez, C.; Signe Lysemose, V.; Mikkelsen, L.S.; Ulrik, F.; Bollerup, S.; Anni Assing, W.; Uffe Vest, S.; Binderup, A.; et al. Durability and breadth of neutralisation following multiple antigen exposures to SARS-CoV-2 infection and/or COVID-19 vaccination. EBioMedicine 2023, 89, 104475–104475. [Google Scholar] [CrossRef] [PubMed]
- Ilenia, V.; Lai, A.; Fiaschi, L.; Bergna, A.; Gatti, A.; Caimi, B.; Biba, C.; Carla della, V.; Balotta, C.; Riva, A.; et al. Neutralizing antibodies response to novel SARS-CoV-2 omicron sublineages in long-term care facility residents after the fourth dose of monovalent BNT162b2 COVID-19 vaccination. Journal of infection/The Journal of infection 2023, 87, 270–272. [Google Scholar] [CrossRef]
- Varona, J.F.; Muñiz, J.; Balboa-Barreiro, V.; Peñalver, F.; Abarca, E.; Almirall, C.; Jose María, C. Persistence and Waning of Natural SARS-CoV-2 Antibodies Over 18 Months: Long-Term Durability of IgG Humoral Response in Healthcare Workers. Journal of general internal medicine 2022, 37, 2614–2616. [Google Scholar] [CrossRef] [PubMed]
- Korosec, C.S.; Dick, D.W.; Moyles, I.R.; Watmough, J. SARS-CoV-2 Booster Vaccine Dose Significantly Extends Humoral Immune Response half-life beyond the Primary Series. Scientific Reports 2024, 14, 8426. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.; Carreño, J.M.; Gleason, C.; Monahan, B.; Singh, G.; Abbad, A.; Tcheou, J.; Raskin, A.; Kleiner, G.; van Bakel, H.; et al. SARS-CoV-2-infection- and vaccine-induced antibody responses are long lasting with an initial waning phase followed by a stabilization phase. Immunity 2024, 57, 587–599.e584. [Google Scholar] [CrossRef]
- Curlin, M.E.; Bates, T.A.; Guzman, G.; Schoen, D.; McBride, S.K.; Carpenter, S.D.; Tafesse, F.G. Omicron Neutralizing Antibody Response following Booster Vaccination Compared with Breakthrough Infection. Medical Xpress 2022, 3. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, T.; Miyamatsu, Y.; Park, H.; Nakamura, N.; Yokokawa Shibata, R.; Iwami, S.; Nagasaki, Y. Modeling COVID-19 vaccine booster-elicited antibody response and impact of infection history. Vaccine 2023, 41, 7655–7662. [Google Scholar] [CrossRef] [PubMed]
- Anshari Saifuddin, H.; Sukamto, K.; Widhani, A.; Muhadi, M.; Hamzah, S.; Eka, G.; Evy, Y.; Pradana, S.; Sally Aman, N.; Samsuridjal, D.; et al. Incidence and Associated Factors of SARS-CoV-2 Infection Post-mRNA-1273 Booster Vaccination in Health-Care Workers. Vaccines 2023, 11, 481–481. [Google Scholar] [CrossRef] [PubMed]
- Glück, V.; Grobecker, S.; Köstler, J.; Tydykov, L.; Bertok, M.; Weidlich, T.; Gottwald, C.; Salzberger, B.; Wagner, R.; Zeman, F.; et al. Immunity after COVID-19 and vaccination: follow-up study over 1 year among medical personnel. Infection 2021, 38, 1–8. [Google Scholar] [CrossRef]
- Abebe, E.C.; Dejenie, T.A. Protective Roles and Protective Mechanisms of Neutralizing Antibodies against SARS-CoV-2 Infection and Their Potential Clinical Implications. Frontiers in Immunology 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.R. Antiviral Neutralizing antibodies: from in Vitro to in Vivo Activity. Nature Reviews Immunology 2023, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Harapan, H.; Hibban Ar, R.; Islam Ing, T.; Auda, N.; Irham Faraby, A.; Anwar, S.; Husnah, M.; Ichsan, I.; Agung, P.; Mudatsir, M.; et al. Waning anti-SARS-CoV-2 receptor-binding Domain Total Antibody in CoronaVac-vaccinated Individuals in Indonesia. F1000Research 2023, 11, 300–300. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.C.; Kronsteiner, B.; Longet, S.; Adele, S.; Deeks, A.S.; Liu, C.; Dejnirattisai, W.; Reyes, L.S.; Meardon, N.; Faustini, S.; et al. Evolution of long-term vaccine-induced and Hybrid Immunity in Healthcare Workers after Different COVID-19 Vaccine Regimens. Med 2023, 4, 191–215.e199. [Google Scholar] [CrossRef] [PubMed]
- Kannikar, I.; Suwat, C.; Kittipan, C.; Thanachol, W.; Woravut, K.; Aksara, T.; Narain, C.; Kajohnsak, N.; Krit, K.; Worachet, T.; et al. Heterologous Booster Vaccines Reduce Severity and Mortality in COVID-19 during BA.2 and BA.4/BA.5 Omicron Predominance in Thailand. Wēi-miǎn Yǔ Gǎnrǎn zázhì/Journal of microbiology, Immunology and Infection 2023, 56, 1178–1186. [Google Scholar] [CrossRef]
- Morales-Núñez, J.J.; Muñoz-Valle, J.F.; Torres-Hernández, P.C.; Hernández-Bello, J. Overview of Neutralizing Antibodies and Their Potential in COVID-19. Vaccines 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Yan, R.; Zhang, J.; Zhang, G.; Zhang, Y.; Hao, M.; Zhang, Z.; Fan, P.; Dong, Y.; Yang, Y.; et al. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science 2020, 369, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Favresse, J.; Gillot, C.; Cabo, J.; David, C.; Dogné, J.-M.; Douxfils, J. Neutralizing antibody response to XBB.1.5, BA.2.86, FL.1.5.1, and JN.1 six months after the BNT162b2 bivalent booster. International Journal of Infectious Diseases 2024, 143. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Naseer, F.; Kanani, F.; Javeria, A. Evaluating long-term Antibody Responses to Booster Doses of COVID-19 Vaccines in the Pakistani Population. Pakistan Journal of Medical Sciences 2023, 40. [Google Scholar] [CrossRef] [PubMed]
- Terbsiri, V.; Putcharoen, O.; Suwanpimolkul, G.; Jantarabenjakul, W.; Wacharapluesadee, S.; Champa, N.; Thippamom, N.; Paitoonpong, L. Long-term Immunogenicity in Previously Vaccinated Healthcare Workers with Inactivated Virus Vaccine after SARS-CoV-2 Infection or Booster Vaccination. Vaccine: X 2023, 14, 100334. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Pan, J.; Jin, M.; Wang, J.; Tung, T.-H.; Chen, S.; Bi, X.; Zhou, K.; Chen, M.; Wang, D.; et al. Efficacy of the Neutralizing Antibodies after the Booster Dose on SARS-CoV-2 Omicron Variant and a two-year Longitudinal Antibody Study on Wild Type Convalescents. International Immunopharmacology 2023, 119, 110151–110151. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Zou, J.; Kalveram, B.; Machado, R.R.G.; Ren, P.; Türeli, S.; Smith, D.J.; Weaver, S.C.; Xie, X.; et al. Cross-neutralization and cross-protection among SARS-CoV-2 Viruses Bearing Different Variant Spikes. Signal Transduction and Targeted Therapy 2022, 7. [Google Scholar] [CrossRef]
- Crawford, K.H.D.; Dingens, A.S.; Eguia, R.; Wolf, C.R.; Wilcox, N.; Logue, J.K.; Shuey, K.; Casto, A.M.; Fiala, B.; Wrenn, S.; et al. Dynamics of Neutralizing Antibody Titers in the Months after Severe Acute Respiratory Syndrome Coronavirus 2 Infection. The Journal of Infectious Diseases 2020, 223, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Wajnberg, A.; Amanat, F.; Firpo, A.; Altman, D.R.; Bailey, M.J.; Mansour, M.; McMahon, M.; Meade, P.; Mendu, D.R.; Muellers, K.; et al. Robust Neutralizing Antibodies to SARS-CoV-2 Infection Persist for Months. Science 2020, 370, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.-M.; Kim, J.-W.; Jung, S.; Jung, Y.; Woo, H.-M.; Yang, J.-S.; Kim, K.-C.; Lee, J.-Y. Persistence of the Neutralizing Antibody Response after SARS-CoV-2 Infection. Clinical Microbiology and Infection 2021. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, F.; Shen, C.; Peng, W.; Li, D.; Zhao, C.; Li, Z.; Li, S.; Bi, Y.; Yang, Y.; et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science 2020, 368, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Sheward, D.J.; Kim, C.; Ehling, R.A.; Pankow, A.; Castro Dopico, X.; Dyrdak, R.; Martin, D.P.; Reddy, S.T.; Dillner, J.; Karlsson Hedestam, G.B.; et al. Neutralisation Sensitivity of the SARS-CoV-2 Omicron (B.1.1.529) variant: a cross-sectional Study. The Lancet Infectious Diseases 2022, 22, 813–820. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.S.; Kohnen, M.; Gilson, G.; Staub, T.; Arendt, V.; Hilger, C.; Servais, J.-Y.; Charpentier, E.; Domingues, O.; Snoeck, C.J.; et al. Pre-Omicron Vaccine Breakthrough Infection Induces Superior Cross-Neutralization against SARS-CoV-2 Omicron BA.1 Compared to Infection Alone. International Journal of Molecular Sciences 2022, 23, 7675. [Google Scholar] [CrossRef] [PubMed]
- Stamatatos, L.; Czartoski, J.; Wan, Y.-H.; Homad, L.J.; Rubin, V.; Glantz, H.; Neradilek, M.; Seydoux, E.; Jennewein, M.F.; MacCamy, A.J.; et al. MRNA Vaccination Boosts cross-variant Neutralizing Antibodies Elicited by SARS-CoV-2 Infection. Science 2021. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Qi, X.; Cao, Y.; Li, P.; Lu, L.; Wang, P.; Feng, Y.; Yang, J.; Wei, H.; Guo, L.; et al. Three Doses of an inactivation-based COVID-19 Vaccine Induces cross-neutralizing Immunity against the SARS CoV-2 Omicron Variant. Emerging Microbes & Infections, 2022; 1–11. [Google Scholar] [CrossRef]
- Qu, P.; Faraone, J.N.; Evans, J.P.; Zheng, Y.-M.; Carlin, C.; Anghelina, M.; Stevens, P.; Fernandez, S.; Jones, D.; Panchal, A.R.; et al. Enhanced evasion of neutralizing antibody response by Omicron XBB.1.5, CH.1.1, and CA.3.1 variants. Cell Reports 2023, 42. [Google Scholar] [CrossRef] [PubMed]
- Dapporto, F.; Marchi, S.; Leonardi, M.; Piu, P.; Lovreglio, P.; Decaro, N.; Buonvino, N.; Stufano, A.; Lorusso, E.; Bombardieri, E.; et al. Antibody Avidity and Neutralizing Response against SARS-CoV-2 Omicron Variant after Infection or Vaccination. Journal of Immunology Research 2022, 2022, 4813199. [Google Scholar] [CrossRef] [PubMed]
- Prather, A.A.; Dutcher, E.G.; Robinson, J.; Lin, J.; Blackburn, E.; Hecht, F.M.; Mason, A.E.; Fromer, E.; Merino, B.; Frazier, R.; et al. Predictors of long-term neutralizing antibody titers following COVID-19 vaccination by three vaccine types: the BOOST study. Scientific Reports 2023, 13, 6505. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).