Introduction
Hand, foot and mouth disease (HFMD) is an acute febrile illness in children with a papulovesicular skin rash at the palms or soles of the feet, or both. Presentation can be with or without inclusion of mouth ulcers. HFMD can result in severe complications such as encephalitis, aseptic meningitis, pulmonary edema, myocarditis, and death [
1]. HFMD is caused by several types of Enterovirus A which includes some Coxsackievirus A (CV-A) and Enterovirus A71 (EV-A71) [
2,
3]. The EV-A71 viruses are genetically related to CV-A, and have diverged as recently as the 1920s [
4]. Both EV-A71 and CV-A infections have been associated with severe HFMD in young children, sometimes resulting in death [
1,
5,
6,
7]. The incidence of EV-71 was over 70% before the development of the anti-HFMD vaccine, which is essentially directed against this variant. This has now been reversed in favor of coxakieviruses [
8].
HFMD epidemics pose a challenge for the healthcare system in terms of caring for patients, due to 1) the symptoms and their evolution, as explained above, and 2) the sudden influx of large numbers of patients. COVID-19 showed how health systems and society itself can be completely disrupted by an epidemic. Monitoring the 2011-2012 HFMD epidemic in Vietnam has interested us in more ways than one. We have a database of almost 10,000 patients spread over the three waves that affected the Hai Phong region between late 2011 and 2012. The healthcare system, which was severely affected by the first wave, responded in the middle of the second wave with a change in patient management. We show that this change enabled at-risk individuals to be cared for without modifying the monitoring of the epidemic. Although EV-A71 was isolated for the first time in Vietnam in 2003, the first outbreak of HFMD was reported in the South in 2005 [
9]. The 2011 HFMD epidemics which caused fatal cases in South Vietnam was the first one to occur in the North [
10].
Materials and Methods
Epidemiological information and specimen collection. All HFMD cases in Hai Phong city were reported to the National Institute of Hygiene and Epidemiology (NIHE) through the national communicable disease surveillance system since 2011. HFMD patients that were present to health centers or hospitals were diagnosed and classified in 4 severity levels. The evaluation of the disease was performed according to the guidelines specifically published by the Vietnamese Ministry of Health (Supplementary Table 1) which are based on but slightly differ from WHO and Taiwanese guidelines [
1,
11].
PCR amplification and nucleotide sequencing. Molecular analyses were done on 257 throat swabs collected at the main pediatric hospital in Hai Phong city from HFMD-diagnosed patients from 14 out of the 15 districts. From February 2012 through August 2012, following authority requirements, samples were collected only on patients presenting severe symptoms (severity level 2b up). Enterovirus-positive and EV-A71-positive samples were identified according to Nix et al. using
SO,
AN and
MAS primers [
12,
13]. Samples collected in November 2011, December 2011, March 2012 and from September 2012 to December 2012 were subjected to Sanger sequencing and analyzed with the Enterovirus Genotyping Tool (
http://www.rivm.nl/mpf/enterovirus/typingtool).
Statistical analysis. Population size was estimated using 2009 census data for comparative analysis [
14]. Incomplete data (less than 5%) were excluded, leaving 9621 cases for the analysis. Each patient was described by age (date of birth was not available), severity, date of onset of the disease, date of admission to hospital, and personal address. Hierarchical classification using Gower distance was used to cluster patients as folloing: age, time from onset to admission (in days) and severity (as qualitative value). Primary Component analysis was performed using age, gender, district related to the address, time from onset to admission (in days) and severity. Hi Phong districts were numbered from 1 to 14. The severity values were considered in a continuous manner, giving the value 2 to severity 2a and 2.5 to severity 2b.Clustering and graphics were performed with R. Statistical tests on clinical data were performed using Stata 9.0 for Windows. Mean comparison was implemented by a Student’s T-test. A Chi-square test was used for proportion comparison of Hai Phong city population and a one-way ANOVA test was used for the variance analysis.
Bias and Ethics. Training session HFMD cases definition and reporting were organized for the staff of the routine surveillance system to enhance quality and consistency of case report. This work was conducted following the requirements of the Vietnamese Ministry of Health and under the Law of Communicable Diseases Prevention and Control passed in 2007.
Results
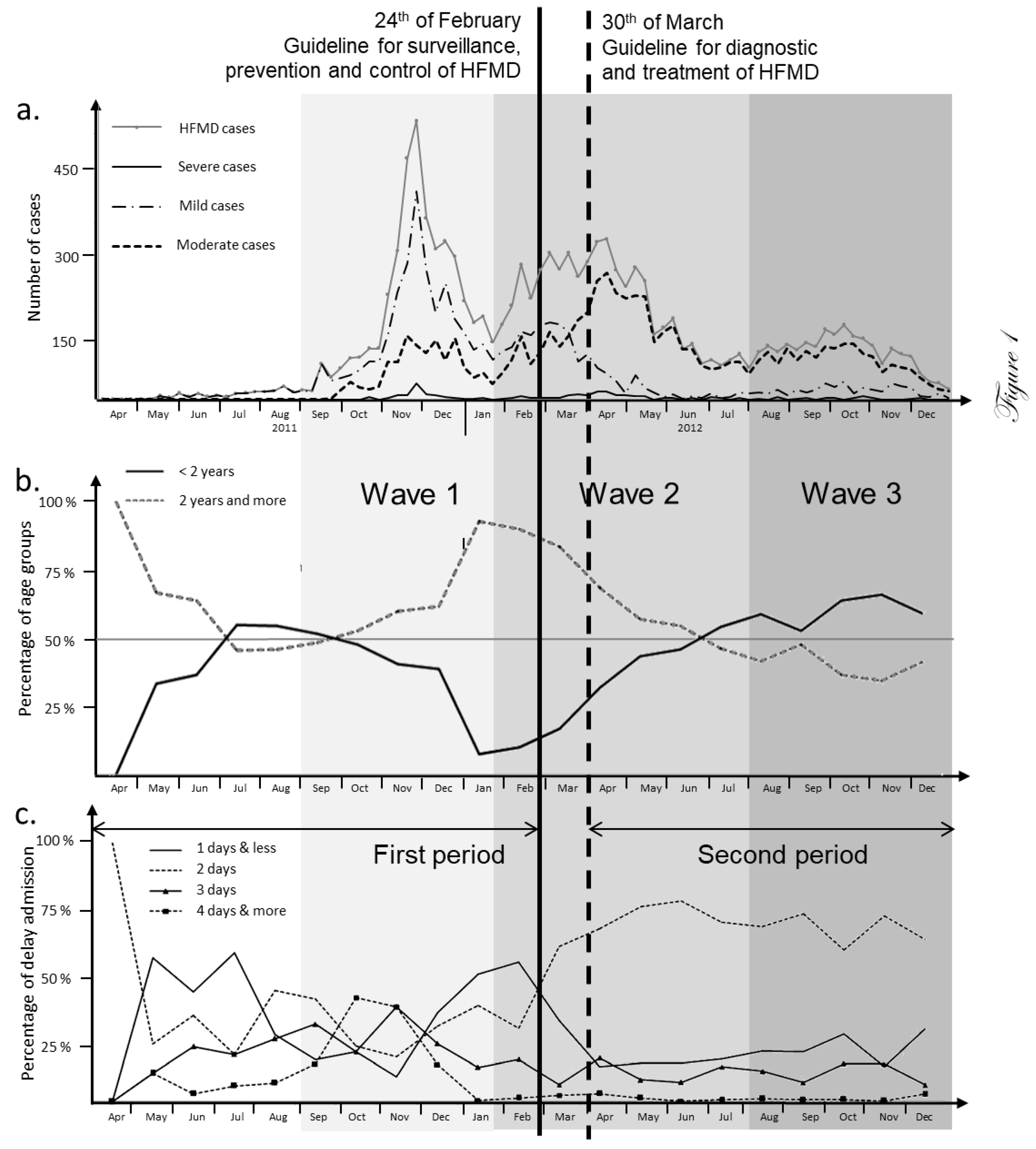
Monitoring of the HFMD burden during the 2011-2012 epidemic. The large HFMD epidemic of 2011-2012 was the first such outbreak to occur in northern Vietnam (65,039 cases). However, the number of cases was higher in the Southern part where epidemic HFMD has been observed since 2005 (157,975 cases). Hai Phong was the hardest hit among the 28 northern Vietnam provinces during the 2011-2012 HFMD epidemic with an average prevalence of 524/100,000 persons. A total of 9621 cases were collected during this period from health centers and the main pediatric hospital of Hai Phong city. The city of Hai Phong is composed of 7 urban districts, 6 countryside districts and 1 large island. HFMD cases were reported throughout the entirety of the city (Supplementary Table 2) and the epidemic was slightly delayed in 2011 when compared to the rest of northern Vietnam (
Figure 1a). The HFMD epidemic could be subdivided into three separate waves of infection: the first one stretching from August 2011 to January 2012 (Wave 1), the second from February 2012 to July 2012 (Wave 2) and the third one from August 2012 to January 2013 (Wave 3). Before the first wave started HFMD occurred sporadically in all parts of the city with low incidence (8 cases per week on average). The number of cases increased suddenly in mid-September, 2011. The outbreak peaked at 472 cases per week on early December 2011 followed by two smaller peaks in April and October of 2012 (
Figure 1a). Two periods, corresponding to different epidemiological patterns, could be distinguished: from August 2011 to March 2102 and from March 2012 to January 2013. The limit between the two periods is marked by the publication of two specific guidelines by the Ministry of Health (MoH). The first one published on the February 24, 2012 concerned surveillance, prevention and control of HFMD. The second guideline was issued on March 30, 2012 were about diagnosis and treatment. The evaluation process of the disease burden was therefore changed during wave 2. Moderate forms (severity level 2a) were reported for the majority of cases (5262 cases, 54.92%), but 218 patients were encountering severe symptoms (2.28%). Among this group, only 9 patients had severity score of 3 and no case with the highest level of 4 (Supplementary Table 2). Gender was not associated with severity. Moderate forms of HFMD were particularly pronounced in children below 2 years old (
p< 0.01, Supplementary Table 4). The level of moderate cases was significantly lower during wave 1 (
p< 0.01, Supplementary Table 5 and 6). The level of moderate cases was significantly higher during the second period. Conversely, the level of mild cases decreased notably to significantly lower after the first period of the epidemic (
Figure 1a), (
p< 0.01, Supplementary Table 7). The age of patients ranged from 24 days to 15 years (median at 2 years, IQR of 2 years, Supplementary Table 2). Out of 9142 cases, 8857 (96.9%) were under the age of 5 with the age-specific incidence highest in the 1-2 years age group (3067 cases, 33.6%) and remained very low for older children. The lowest incidence was observed in infants <5 months (1.88%) and children above 10 years old (0.4%). Boys had a significantly higher prevalence rate (59.74%). Wave 1 was associated to a higher number of children between 2 and 5. Variations in patient age after guideline release were noticeable (
Figure 1b). The proportion of cases below 2 was significantly higher to the end of second period (
p<0.01, Supplementary Table 7) and during wave 3 (
p<0.01, Supplementary Table 3). The time after onset to admission was the third epidemiological parameter followed in the present study. It varied greatly over the first period (
Figure 1c, Supplementary Table 6). The curves for time to admission after onset to admission corresponding to 1-day and 2-days crossed in March 2012 (
Figure 1c) concomitantly with those representing mild and moderate level of severity (
Figure 1a).
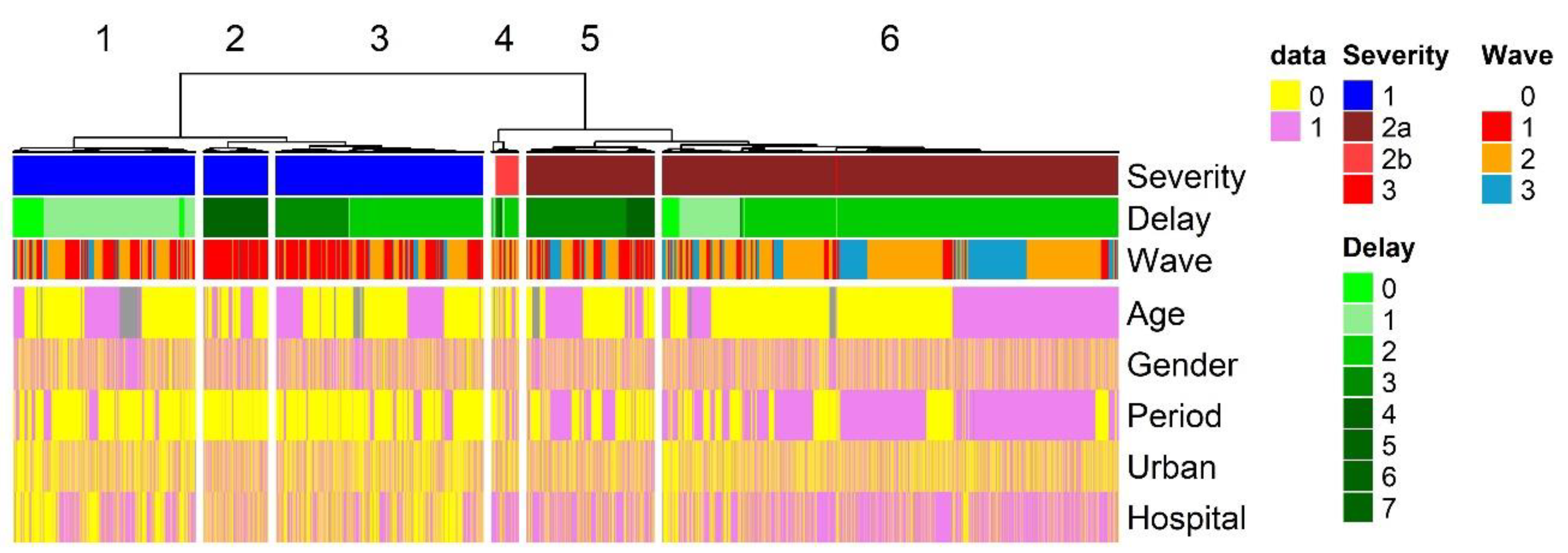
Patient categories. Severity score, age of patient and time from onset to admission were combined together in a single analysis to evaluate the effect of guidelines recommendations on surveillance and diagnosis. Six groups (clusters) of patients (clusters) were identified through hierarchical classification (
Figure 2) and each cluster could be associated to specific parameters. Severity was the main factor separating patients (see Supplemental Tables 2 to 8 & 11 for statistical analysis). Cluster 1, 2 and 3 are patients with mild symptoms. Patients are mainly found during period 1. New guidelines publication modified drastically the distribution of patients. These patients were almost absent from period 2. The health system’s communication policy was active after wave 1, encouraging parents to keep sick children with mild symptoms at home. The publication of guidelines removed these patients from the statistics, recording only cases with moderate to severe symptoms. As a result, patients in clusters 5 and 6, who are predominantly associated with moderate symptoms, are mainly associated with the second study period. Cluster 4 was associated with patient presenting severe symptoms. The delay before admission was the second vaiable associated with the clustering. Cluster 2 patients with delay over 3 days were restricted to the first period of the epidemic. Delay of admission was also separating cluster 5 from cluster 6. Patients with moderate symptoms were presenting shorter time from onset to admission during period 2. Gender and geographic aeas had no relationship with the clustering. Hai Phong city pediatric hospital could be associated with clusters 5 and 6.
Evolution of HFMD admission at Hai Phong city pediatric hospital. The proportion of the moderate and severe cases admitted at the pediatric hospital increased significantly during the second period (Supplementary Table 8). Concomitantly, the number of HFMD admissions increased in district hospitals. The number of outpatients (treatment at home) also increased notably with mild level of severity during the second period. The ratio between young (below 2) and old patients admitted at the pediatric hospital was reversed after March 2012, but not in district hospitals and local health stations (Supplementary Table 9). The share of patients between the pediatric hospital and the local health facilities clearly improved during the second period with more people from not urban areas going to district hospitals (Supplementary Table 10). The number of patients admited at the pediatric hospital coming from non-urban districts compared to urban ones remained the same over the two periods, but the number of non-urban district patients with severe symptoms admitted at the pediatric hospital increased (Supplementary Table 11).
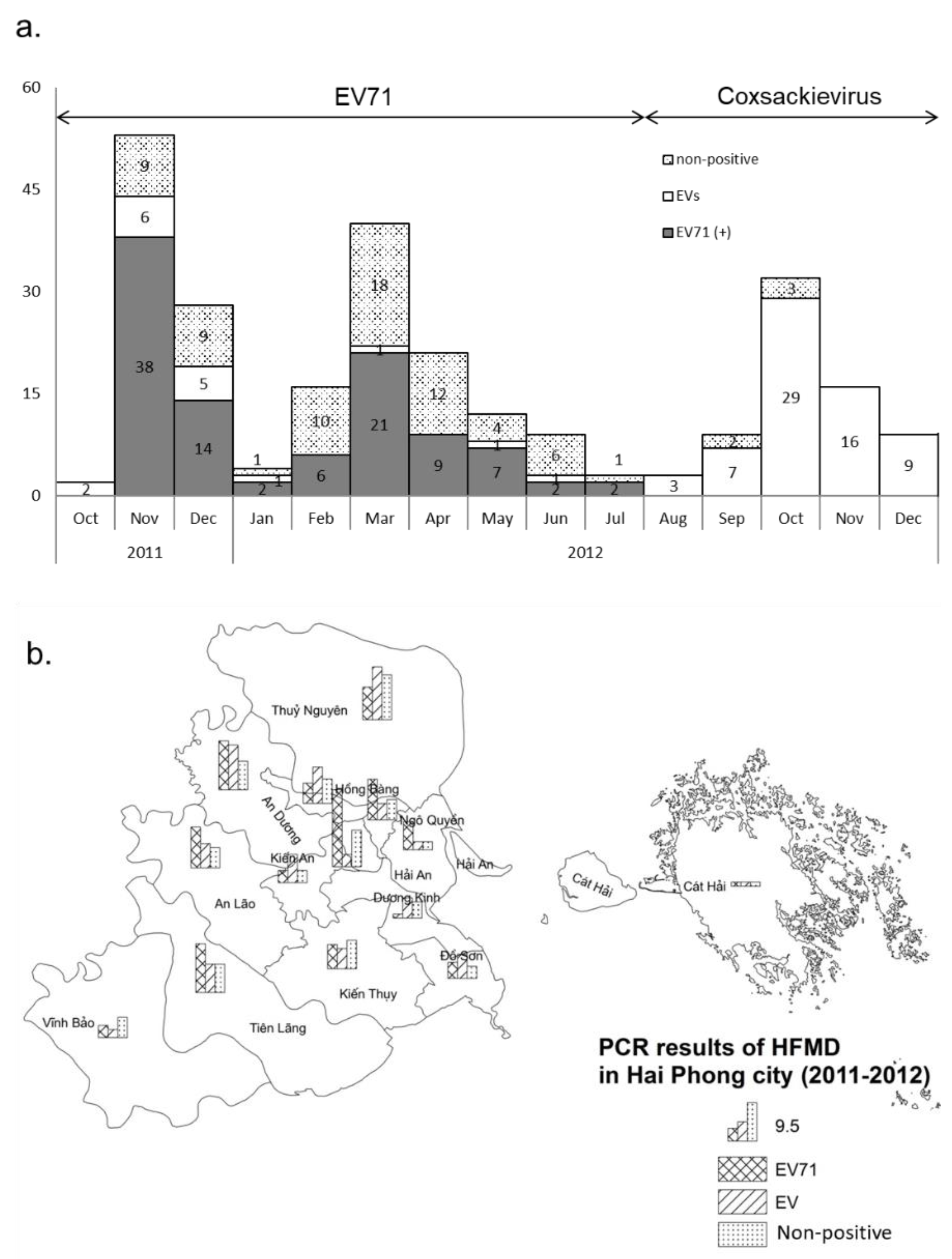
Both EV-A71 and CV-A were present during the epidemic. Molecular diagnostic confirmation was conducted by PCR on 257 samples from cases clinically identified as HFMD. Nearly 71% were positive for Human Enterovirus (182/257). Of the 182 positives, 101 (55%) were EV-A71 and 81 (45%) corresponded to other enteroviruses (EVs) (
Figure 3a). The identified EV-A71 isolates belonged to subgenogroup C4 present on northern and central city and C5 present also in northern, central and southern city (
Figure 3b). A significant part of patients diagnosed as HFMD during waves 1 and 2, i.e., 75, were not positive for enterovirus (
Figure 3a). EV-A71 coincided with wave 1 and wave 2 (
Figure 3). Wave 3 was associated with the co-circulation of CV-A6 and CV-A16 (Supplementary
Figure 2). The rate of EV negative samples started to increase in December, 2011 to reach a maximum in March, 2012 and was very low during wave 3.
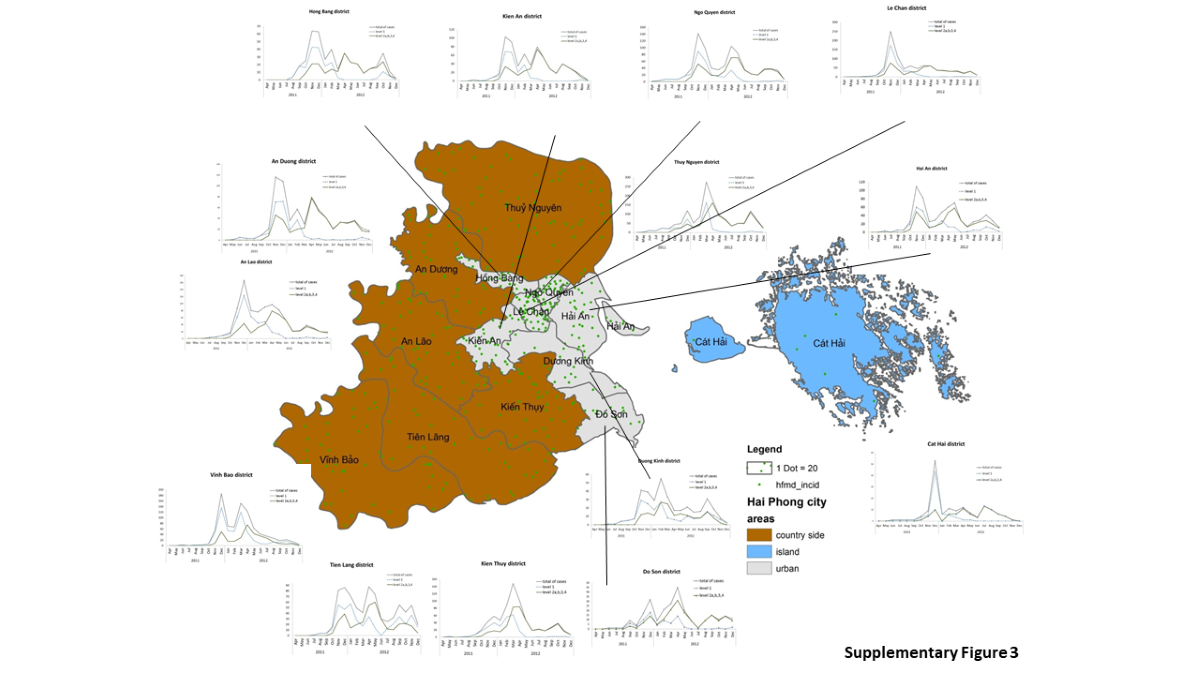
Spread of the disease in Hai Phong city. PCA analysis suggested that distribution among districts was highly variable over the three waves. Time difference in the evolution of the epidemic among district could resut from such variation. Early cases appeared in the northern and urban zone of the city (Supplementary
Figure 3) and expanded to the west and to the south (Figure. 3). Each waves displayed a different main site of emergence (
Figure 3, Supplementary Table 12). Wave 1 started in the city center whereas waves 2 and 3 emerged at the periphery. The order of occurrence of the first case for each wave defined five groups of districts (Supplementary
Figure 3): Hai Phong city center (Group 1), New urban areas in the southern part of the city and a western rural district (Group 2), peripheric districts and Do Son (Group 3), two rural districts not connected to the main road netwok (Group 4) and Cat Hai islands (Group 5). The diffusion of the disease to the south followed the axis supported by two main roads allowing to cross rivers and canals. No direct transmission of the disease was observed between the city center and these new urban areas during wave 2 and 3. Patterns of transmission among groups were similar for the three waves. Re-emergence of the disease during wave 2 and 3 shows similarity despite the presence of different etiological agent.
Discussion
The first HFMD outbreak in North Vietnam. At that time, the 2011-2012 HFMD epidemic was the largest to have ever occurred in Vietnam and the first recorded in the northern part of the country while Hai Phong city experienced the highest HFMD incidence in North Vietnam. However, no fatal cases were reported in Hai Phong unlike what was observed in South Vietnam [
10]. Age-specific incidence was the highest in the 1-2 years age group. This would be in agreement with both the persistence of maternally-derived neutralizing antibodies for up to 6 months and the kinetics of seroprevalence of EV-A71 virus neutralizing antibodies which increases with age [
15,
16]. However, this was the very first recorded outbreak of HFMD in northern Vietnam questioning thus the existence of maternally-derived neutralizing antibodies or pre-existing immunity. Children under 3 represented 85.85% of cases. They are in Vietnam traditionally cared for at home by family members. The high HFMD incidence in this population may thus have resulted from contact with adults and older children acting as asymptomatic carriers of the virus [
17,
18].
Guidelines positively influence disease management. The first guideline released related to surveillance, prevention and control and gave clear HFMD case definition, reporting procedure and strategy for collecting clinical samples. The first effect of the guideline release was a signifcant increase of the severity score. Indeed, 73.41% of patients were scored 2a after guidelines publication but only 25.59% before. The number of moderate and severe cases admitted to Hai Phong pediatric hospital increased significantly after guidelines publication while the proportion of the mild cases decreased sharply. Another positive effect was the reduced delay between onset and admission after guidelines publication. It decreased during the second period and remained very homogeneous. The presence of 9 out of 10 clusters in the first half of the outbreak supports this conclusion. The most important feature of the second guideline was the decentralization and transfer of responsibility to health care facilities. A more homogeneous spatial distribution of patients visiting pediatric hospitals was visible. Mild cases were treated at the commune level whereas districts were in charge of mild and moderate cases. At the province level, all cases were addressed. All patients recorded as severe went to province hospitals during period 2 while local health facilities hosted patients unable to go to main hospitals. Patients who remained at home only displayed mild symptoms.
Awareness and legal framework. This positive effect of guidelines is not only the consequence of the publication of guidelines but also of an increased awareness and precautious approach from parents and physicians leading to patients being majoritarily declared with severe symptoms in order to ensure a better treatment and surveillance. This could explain why a higher disease severity scores were observed in CV-A-infected patients (wave 3) than in EV-A71 cases (p< 0.01). Awareness led to the modification of guidelines but changes occurred only after publication, suggesting that the legal framework created by the guidelines is needed for implementation eventhough awareness is present. Public and professional awareness are not sufficient for implementing changes. Furthermore, emergence of CV-A (wave 3) during the second period did not lead to variation in severity and time to admission. Conversely, the publication of guidelines during phase 2 led to different patient patterns although the virus was the same. Evolution of clinical patterns should not be considered only in the light of the evolution or replacement of pathogens or host-pathogen interactions but also according to the evolution of behavior and social perception.
Shift of etiology. Improvement of molecular diagnostic was not considered by the new guidelines and they therefore had no impact on the detection of etiological agents in patients. During the Hai Phong outbreak, circulation of both EV-A71 and CV-A was recorded, a feature already reported [
9,
19,
20,
21,
22]. EV-A71 virus is considered to be the most frequent cause of severe HFMD disease although CV-A have been shown to cause severe infections with meningitis [
11,
23,
24,
25]. An uncharacterized virus might also have circulated during wave 1 and mostly wave 2. The high number of EV non-positive PCR reactions on clinically positive samples suggests that the set of primers used for enterovirus detection might not have been discriminative enough. The ratio of non-positive tests was similar to those previously reported [
26,
27,
28,
29]. EV-A71 detection with MAS primers should thus be systematically performed on SO primers products and SO222 primer should be redesigned to match with the 5′ part of the AN88 primer used for EVs detection. More attention should be therefore paid to the PCR negative patients.
Spatio-temporal dynamic and disease control. Nguyen et al., have shown the presence of HFMD in provinces west of Hai Phong after the outbreak started in South Vietnam, making the northwestern side of Hai Phong is the most likely route of entry [
10]. Despite, the main economical role of Hai Phong no early cases occurred along or at the end of the main highway linking Hai Phong to the rest of the country, indicating that major industrial and export commercial movements are not linked to the dynamic of the disease. Instead, the disease seems to have expanded following the eastbound river system to reach densely populated settlements from where it secondarily expanded through local roads. Disease expansion might thus have followed secondary local commercial routes. This commercial route allows time for the disease to be transmitted and involves a lot of favorable human to human contacts. The presence of early cases in the island and in isolated coastal localities in the southern part of the city also illustrates the role of sea transportation and role of the local trade and occupational activities in the spread of the disease. The southern part may have been affected later due to fragmentation of the territory and isolation of the communes by the complex river system. The early occurrence of the disease in northwestern communes not connected to the main local road might be related to specific occupational activities. Considering the average age of the patients, around 2, the source of contamination must be sought for within asymptomatic adults being contaminated during their occupational activities and in local and regional movements.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Figure 1. Primary component analysis. Axis 1 represented more the 95% of the variance associated with patients, based on age, gender, district related to the address, time from onset to admission (delay) and severity.
Figure 2. Spatio-temporal distribution of HFMD case in Hai Phong city.
a. Time and place of occurrence of HFMD case.
Colours represent interval of occurrence of the index case in each commune. Rivers are shown in blue and roads are shown in dark grey. Line width is proportional to the importance of the system. Numbers represent the referenced commune number.
b. Land cover and human settlements. The land cover map (GLC30, 2010) shows 6 main classes of objects detected on satellite images: cultivated land, artificial surface (settlements), forest, grassland, land water bodies and wetlands. Numbers represent the referenced communes.
Figure 3. Propagation of the HFMD epidemic among Hai Phong city districts according to median case. Rural and urban districts were differentiated (Type) and described according to major features (supplementary Table 12). Stratification (Group) was performed according to the relative order of median in the three waves. Date of the median case and relative order of the district was given for each wave. Supplementary Tables 470 Supplementary Table 1. Severity levels of HFMD cases according to guidelines from the Vietnamese Ministry of Health. Supplementary Table 2. Characteristics of reported HFMD cases in Hai Phong city, 2011-2012. Supplementary Table 3: Age of HFMD patients by epidemic waves in Hai Phong City (2011- 476 2012). Supplementary Table 4: Gender, age, living area, pathogen, delay of admission of HFMD reported cases by severity in Hai Phong city, 2011–2012. Supplementary Table 5. Age groups and severity of reported HFMD cases by epidemic outcomes in Hai Phong city between 2011 and 2012. Supplementary Table 6. Gender, Severity, Living area and Delay of admission of reported HFMD cases by epidemic waves in Hai Phong city (2011-2012). Supplementary Table 7. Age groups, severity, delay duration and living area of reported HFMD cases by epidemic period in Hai Phong city between 2011 and 2012. Supplementary Table 8. Severity of reported HFMD cases at place of admission by epidemic periods in Hai Phong city between 2011 and 2012. Supplementary Table 9. Age groups of reported HFMD cases at place of admission in Hai Phong city in 2 periods of the epidemic. Supplementary Table 10. Living area of reported HFMD cases at place of admission in Hai Phong city. Supplementary Table 11. Living area and severity of reported HFMD cases in Hai Phong pediatric hospital city. Supplementary Table 12. Propagation of the HFMD epidemic among Hai Phong city districts according to median case.
Author Contributions
Conceptualization: NND, EC and RF; Methodology: NND, EC and RF; Validation: NND, PR, GK, AD and LG; Formal Analysis: NND, PR, GK, AD, LG and EC; Investigation, NND, LTTH, VDT, NTHT; Resources, NND, LTTH, VDT, NTHT and LTSH; Data Curation: PR, GK, AD and LG; Writing—Original Draft Preparation: EC and RF; Writing—Review & Editing: CD, TND, THN, LG, EC and RF; Supervision: CD and RF; Project Administration: CD, TND, THN,
Funding
The authors received no funding for this work.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
No human research or human samples are used in this work.
Data Availability Statement
All data are provided in the manuscript and supplementary material.
Acknowledgments
NND PhD work was supported in part by the Erasmus Mundus project MAHEVA.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- WHO. A Guide to Clinical Management and Public Health Response for Hand, Foot and Mouth Disease (HFMD). WHO WPRO; 2011.
- Ang, L.W.; Koh, B.K.; Chan, K.P.; Chua, L.T.; James, L.; Goh, K.T. Epidemiology and Control of Hand, Foot and Mouth Disease in Singapore, 2001-2007. Ann. Acad. Med. Singap. 2009, 38, 106–112. [CrossRef]
- Chen, K.-T.; Chang, H.-L.; Wang, S.-T.; Cheng, Y.-T.; Yang, J.-Y. Epidemiologic Features of Hand-Foot-Mouth Disease and Herpangina Caused by Enterovirus 71 in Taiwan, 1998–2005. Pediatrics 2007, 120, e244–e252. [CrossRef]
- Bessaud, M.; Razafindratsimandresy, R.; Nougairède, A.; Joffret, M.-L.; Deshpande, J.M.; Dubot-Pérès, A.; Héraud, J.-M.; de Lamballerie, X.; Delpeyroux, F.; Bailly, J.-L. Molecular Comparison and Evolutionary Analyses of VP1 Nucleotide Sequences of New African Human Enterovirus 71 Isolates Reveal a Wide Genetic Diversity. PLOS ONE 2014, 9, e90624. [CrossRef]
- Abu Bakar S, Chee HY, Al-Kobaisi MF, Xiaoshan J, Bing CK, Kit LS. Identification of enterovirus 71 isolates from an outbreak of hand, foot and mouth disease (HFMD) with fatal cases of encephalomyelitis in Malaysia. Virus Research. 1999; 61:1-9.
- Ooi, M.H.; Wong, S.C.; Lewthwaite, P.; Cardosa, M.J.; Solomon, T. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 2010, 9, 1097–1105. [CrossRef]
- Zeng M, Li YF, Wang XH, et al. Epidemiology of hand, foot, and mouth disease in children in Shanghai 2007–2010. Epidemiology and infection. 2012; 140:1122-1130.
- Hu, L.; Maimaiti, H.; Zhou, L.; Gao, J.; Lu, Y. Changing serotypes of hand, foot and mouth disease in Shanghai, 2017–2019. Gut Pathog. 2022, 14, 1–9. [CrossRef]
- Van Tu, P.; Thao, N.T.T.; Perera, D.; Truong, K.H.; Thuong, T.C.; How, O.M.; Cardosa, M.J.; McMinn, P.C. Epidemiologic and Virologic Investigation of Hand, Foot, and Mouth Disease, Southern Vietnam, 2005. Emerg. Infect. Dis. 2007, 13, 1733–1741. [CrossRef]
- Nguyen, N.T.; Pham, H.V.; Hoang, C.Q.; Nguyen, T.M.; Nguyen, L.T.; Phan, H.C.; Phan, L.T.; Vu, L.N.; Minh, N.N.T. Epidemiological and clinical characteristics of children who died from hand, foot and mouth disease in Vietnam, 2011. BMC Infect. Dis. 2014, 14, 341–341. [CrossRef]
- Huang, C.-C.; Liu, C.-C.; Chang, Y.-C.; Chen, C.-Y.; Wang, S.-T.; Yeh, T.-F. Neurologic Complications in Children with Enterovirus 71 Infection. New Engl. J. Med. 1999, 341, 936–942. [CrossRef]
- Nix, W.A.; Oberste, M.S.; Pallansch, M.A. Sensitive, Seminested PCR Amplification of VP1 Sequences for Direct Identification of All Enterovirus Serotypes from Original Clinical Specimens. J. Clin. Microbiol. 2006, 44, 2698–2704. [CrossRef]
- Perera, D.; Podin, Y.; Akin, W.; Tan, C.-S.; Cardosa, M.J. Incorrect identification of recent Asian strains of Coxsackievirus A16 as human enterovirus 71: Improved primers for the specific detection of human enterovirus 71 by RT PCR. BMC Infect. Dis. 2004, 4, 11–11. [CrossRef]
- CPHCSC (Central Population and Housing Census Steering Committee). The 2009 Vietnam population and housing census: completed results. Hanoi, Vietnam893: pp; 2010.
- Luo, S.T.; Chiang, P.S.; Chao, A.S.; Liou, G.Y.; Lin, R.; Lin, T.Y.; Lee, M.S. Enterovirus 71 maternal antibodies in infants, Taiwan.. 2009, 15, 581–4.
- Tran, C.B.N.; Nguyen, H.T.; Phan, H.T.T.; Van Tran, N.; Wills, B.; Farrar, J.; Santangelo, J.D.; Simmons, C.P. The Seroprevalence and Seroincidence of Enterovirus71 Infection in Infants and Children in Ho Chi Minh City, Viet Nam. PLOS ONE 2011, 6, e21116. [CrossRef]
- Chang, L.-Y.; Tsao, K.-C.; Hsia, S.-H.; Shih, S.-R.; Huang, C.-G.; Chan, W.-K.; Hsu, K.-H.; Fang, T.-Y.; Huang, Y.-C.; Lin, T.-Y. Transmission and Clinical Features of Enterovirus 71 Infections in Household Contacts in Taiwan. JAMA 2004, 291, 222–227. [CrossRef]
- Witsø, E.; Palacios, G.; Rønningen, K.S.; Cinek, O.; Janowitz, D.; Rewers, M.; Grinde, B.; Lipkin, W.I. Asymptomatic circulation of HEV71 in Norway. Virus Res. 2007, 123, 19–29. [CrossRef]
- Khanh TH, Sabanathan S, Thanh TT, et al. Enterovirus 71-associated hand, foot, and mouth disease, Southern Vietnam, 2011. Emerging Infect Dis. 2012; 18:2002–2005.
- Chen, L.; Mou, X.; Zhang, Q.; Li, Y.; Lin, J.; Liu, F.; Yuan, L.; Tang, Y.; Xiang, C. Detection of human enterovirus 71 and coxsackievirus A16 in children with hand, foot and mouth disease in China. Mol. Med. Rep. 2012, 5, 1001–1004. [CrossRef]
- Yang, F.; Zhang, T.; Hu, Y.; Wang, X.; Du, J.; Li, Y.; Sun, S.; Sun, X.; Li, Z.; Jin, Q. Survey of enterovirus infections from hand, foot and mouth disease outbreak in china. Virol. J. 2009, 8, 508–508. [CrossRef]
- Wu, Y.; Yeo, A.; Phoon, M.; Tan, E.; Poh, C.; Quak, S.; Chow, V.T. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: The role of enterovirus 71 and coxsackievirus A strains. Int. J. Infect. Dis. 2010, 14, e1076–e1081. [CrossRef]
- Schmidt, N.J.; Lennette, E.H.; Ho, H.H. An Apparently New Enterovirus Isolated from Patients with Disease of the Central Nervous System. J. Infect. Dis. 1974, 129, 304–309. [CrossRef]
- Lum, L.C.; Wong, K.; Lam, S.; Chua, K.; Goh, A.; Lim, W.; Ong, B.; Paul, G.; AbuBakar, S.; Lambert, M. Fatal enterovirus 71 encephalomyelitis. J. Pediatr. 1998, 133, 795–798. [CrossRef]
- Chang, L.-Y.; Lin, T.-Y.; Huang, Y.-C.; Tsao, K.-C.; Shih, S.-R.; Kuo, M.-L.; Ning, H.-C.; Chung, P.-W.; Kang, C.-M. Comparison of enterovirus 71 and coxsackievirus A16 clinical illnesses during the Taiwan enterovirus epidemic, 1998. Pediatr. Infect. Dis. J. 1999, 18, 1092–1096. [CrossRef]
- Chiang, P.-S.; Huang, M.-L.; Luo, S.-T.; Lin, T.-Y.; Tsao, K.-C.; Lee, M.-S. Comparing Molecular Methods for Early Detection and Serotyping of Enteroviruses in Throat Swabs of Pediatric Patients. PLOS ONE 2012, 7, e48269. [CrossRef]
- Umami RN, Dhenni R, Jajuli A, Nishimura Y, Shimizu H, Utama A. Detection and Identification of Human Enteroviruses among Healthy Children in Antajaya, Bogor. Journal of Biotechnology Research in Tropical Region. 2009; 2:1-7.
- Park, K.; Lee, B.; Baek, K.; Cheon, D.; Yeo, S.; Park, J.; Soh, J.; Cheon, H.; Yoon, K.; Choi, Y. Enteroviruses isolated from herpangina and hand-foot-and-mouth disease in Korean children. Virol. J. 2012, 9, 205–205. [CrossRef]
- He SJ, Han JF, Ding XX, Wang YD, Qin CF. Characterization of enterovirus 71 and coxsackievirus A16 isolated in hand, foot, and mouth disease patients in Guangdong, 2010. International Journal of Infectious Diseases. 2013; 17:e1025–e1030.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).