Submitted:
09 July 2024
Posted:
10 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Insects and Test Oils
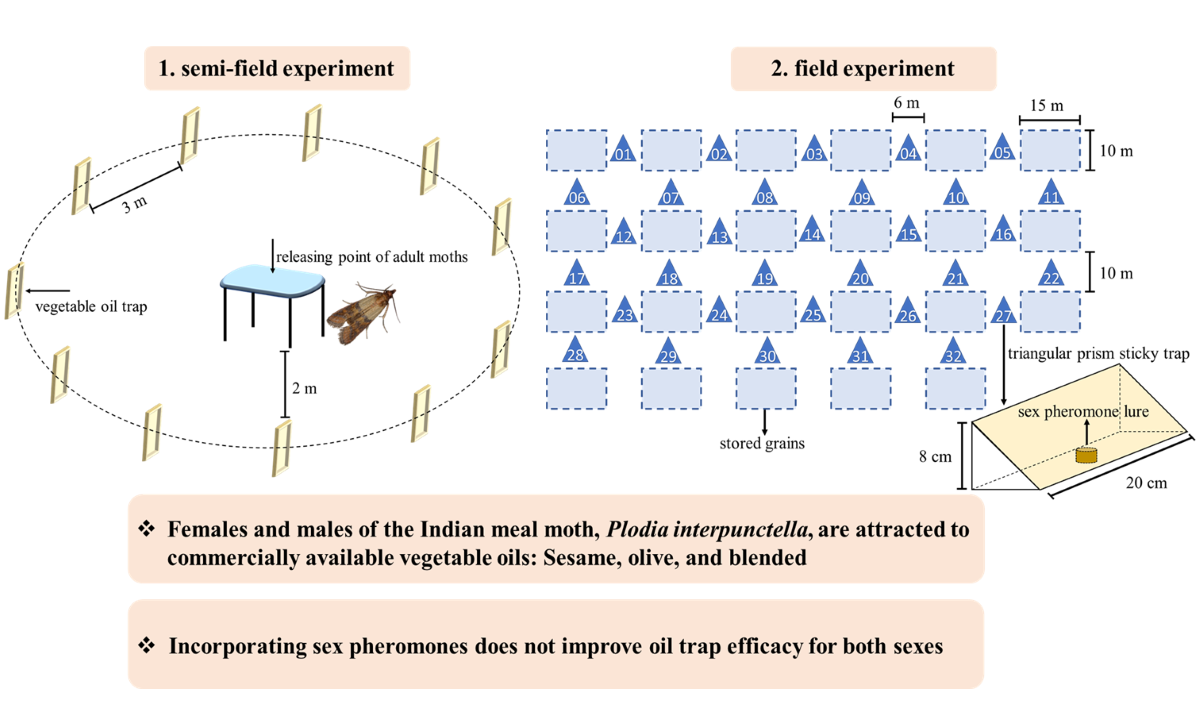
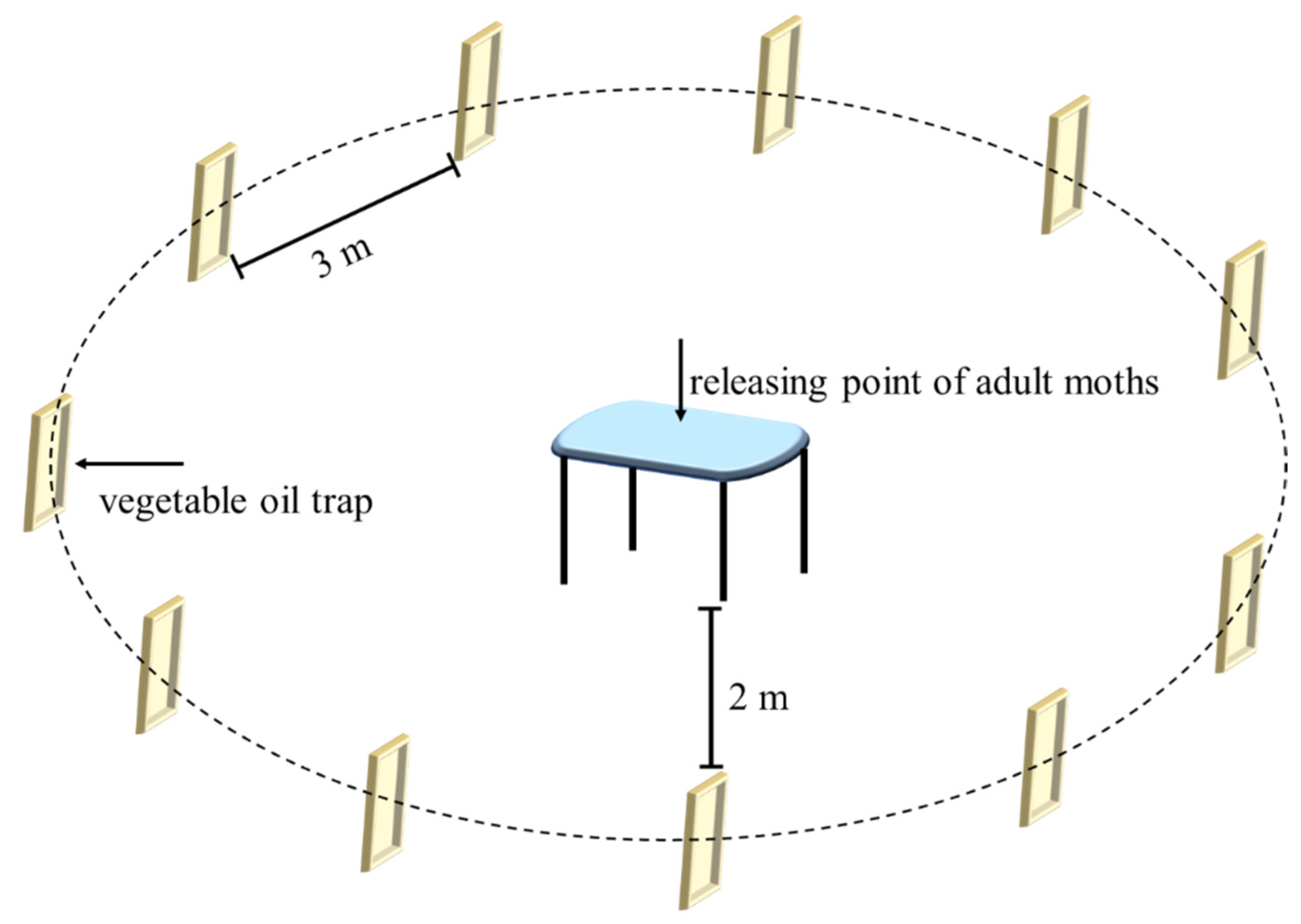
2.2. Semi-Field Experiment
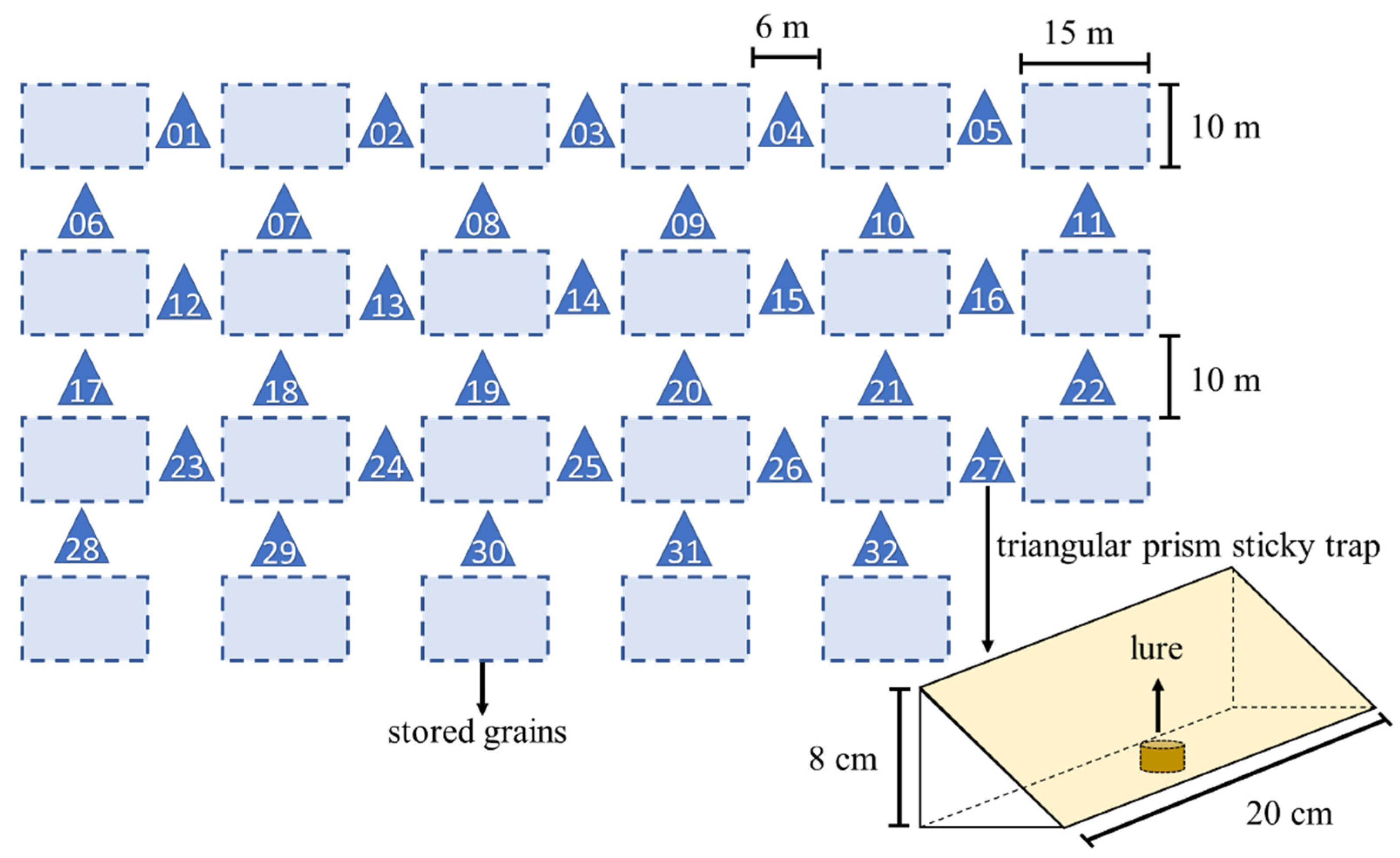
2.3. Field Experiment
2.4. Statistical Analysis
3. Results
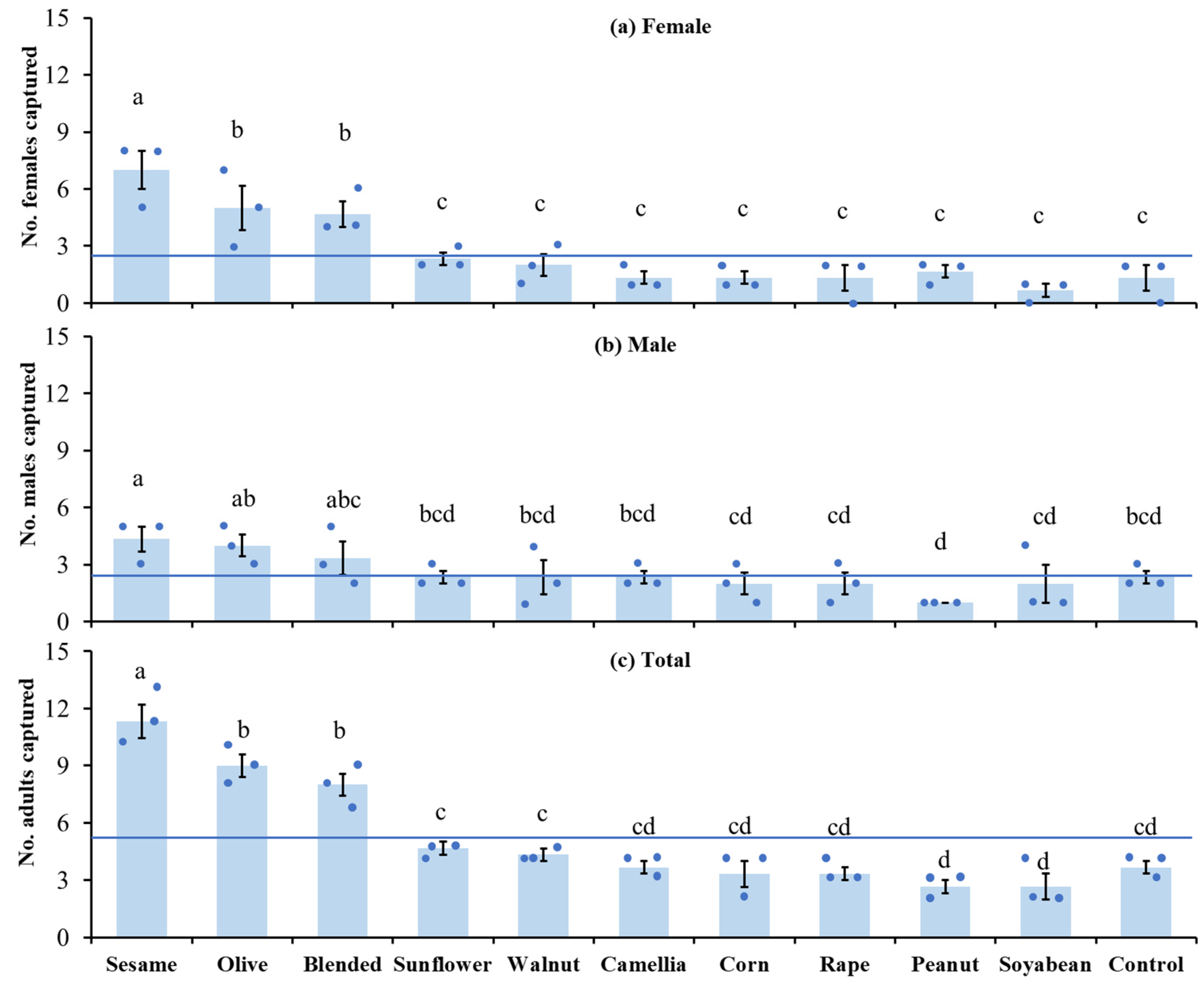
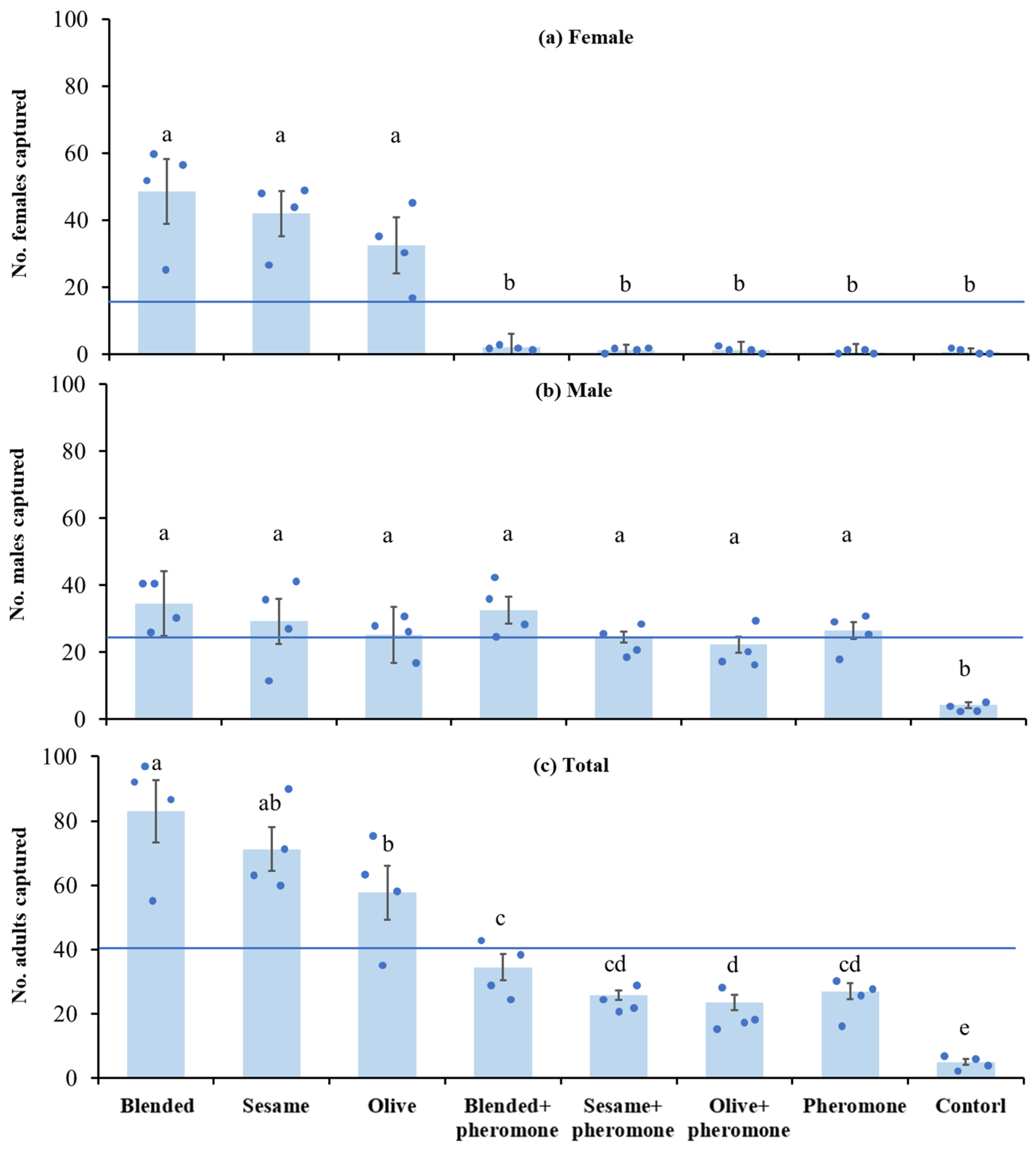
3.1. Vegetable Oils Attractiveness to P. interpunctella in Semi-Field Conditions
3.2. Combined Effects of Vegetable Oil and Sex Pheromone on Plodia interpunctella Captures in Field Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arthur, F.H., Structural pest management for stored product insects. In Recent advances in stored product protection; Athanassiou, C.G., Arthur, F.H., Eds.; Springer: Berlin, Heidelberg, Germany, 2018; pp. 65‐81.
- Liang, J.; Zhang, X.; Zhang, Q.; Tang, Y.; Kong, W.; Zhang, J.; An, Y. Insect‐resistant activities of Elsholtzia ciliata essential oil and its main components carvone against Tribolium castaneum in different development stages. J. Stored Prod. Res. 2024, 107, 102317. [CrossRef]
- Mohandass, S.; Arthur, F.H.; Zhu, K.Y.; Throne, J.E. Biology and management of Plodia interpunctella (Lepidoptera: Pyralidae) in stored products. J. Stored Prod. Res. 2007, 43, 302–311. [Google Scholar] [CrossRef]
- Benitez, H.A.; Salinas, C.; Hernández, J.; Contador Mejías, T.; Kim, S.; Maturana, C.S.; Rebolledo, L.; Pérez, L.M.; Câmara, P.E.A.S.; Alves Ferreira, V.; Lobos, I.; Piñeiro, A.; Convey, P. An outsider on the Antarctic peninsula: A new record of the non-native moth Plodia interpunctella (Lepidoptera: Pyralidae). Ecol. Evol. 2024, 14, e10838. [Google Scholar] [CrossRef] [PubMed]
- Câmara, P.E.A.S.; Convey, P.; Ferreira, V.A.; Togni, P.H.B.; Pujol-Luz, J.R. First record of the Indian meal moth Plodia interpunctella (Lepidoptera: Pyralidae) at a research station in Antarctica. Antarct. Sci. 2022, 34, 361–364. [Google Scholar] [CrossRef]
- Razazzian, S.; Hassani, M.R.; Imani, S.; Shojai, M. Life table parameters of Plodia interpunctella (Lepidoptera: Pyralidae) on four commercial pistachio cultivars. J. Asia Pac. Entomol. 2015, 18, 55–59. [Google Scholar] [CrossRef]
- Jaafari-Behi, V.; Ziaee, M.; Kocheili, F.; Ali Hemmati, S.; Francikowski, J. Life-table parameters of Plodia interpunctella (Lepidoptera: Pyralidae) on different stored date palm fruits under laboratory conditions. J. Insect Sci. 2023, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, C. Population dynamics of Indian meal moth in coffee bean warehouses in New Jersey. J. Stored Prod. Res. 2023, 100, 102044. [Google Scholar] [CrossRef]
- Rees, D.P. Insects of stored product. Csiro Publishing: London, UK, 2004.
- Adler, C.; Athanassiou, C.; Carvalho, M.O.; Emekci, M.; Gvozdenac, S.; Hamel, D.; Riudavets, J.; Stejskal, V.; Trdan, S.; Trematerra, P. Changes in the distribution and pest risk of stored product insects in Europe due to global warming: Need for pan-European pest monitoring and improved food-safety. J. Stored Prod. Res. 2022, 97, 101977. [Google Scholar] [CrossRef]
- Zhao, J.; Song, C.; Ma, L.; Yan, X.; Shi, J.; Hao, C. The impacts of climate change on the potential distribution of Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae) in China. Insects 2022, 13, 636. [Google Scholar] [CrossRef]
- Binder, M.; Mahler, V.; Hayek, B.; Sperr, W.R.; Schöller, M.; Prozell, S.; Wiedermann, G.; Valent, P.; Valenta, R.; Duchêne, M. Molecular and immunological characterization of arginine kinase from the Indianmeal moth, Plodia interpunctella, a novel cross-reactive invertebrate pan-allergen. J. Immunol. 2001, 167, 5470–5477. [Google Scholar] [CrossRef]
- Hallman, G.J.; Phillips, T.W. Ionizing irradiation of adults of Angoumois grain moth (Lepidoptera: Gelechiidae) and Indianmeal moth (Lepidoptera: Pyralidae) to prevent reproduction, and implications for a generic irradiation treatment for insects. J. Econ. Entomol. 2008, 101, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Sabbour, M.; Abd-El-Aziz, S.; Marwa, S. Efficacy of three entomopathogenic fungi alone or in combination with diatomaceous earth modifications for the control of three pyralid moths in stored grains. J. Plant Prot. Res. 2012, 52, 559–363. [Google Scholar] [CrossRef]
- Oh, H.W.; Yun, C.S.; Jeon, J.H.; Kim, J.A.; Park, D.S.; Ryu, H.W.; Oh, S.R.; Song, H.H.; Shin, Y.; Jung, C.S.; Shin, S.W. Conifer diterpene resin acids disrupt juvenile hormone-mediated endocrine regulation in the Indian meal moth Plodia interpunctella. J. Chem. Ecol. 2017, 43, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Small, G.J. A comparison between the impact of sulfuryl fluoride and methyl bromide fumigations on stored-product insect populations in UK flour mills. J. Stored Prod. Res. 2007, 43, 410–416. [Google Scholar] [CrossRef]
- Jenson, E.A.; Arthur, F.H.; Nechols, J.R. Methoprene and synergized pyrethrins as aerosol treatments to control Plodia interpunctella (Hübner), the Indian meal moth (Lepidoptera: Pyralidae). J. Stored Prod. Res. 2010, 46, 103–110. [Google Scholar] [CrossRef]
- Trematerra, P.; Oliviero, A.; Savoldelli, S.; Schöller, M. Controlling infestation of a chocolate factory by Plodia interpunctella by combining mating disruption and the parasitoid Habrobracon hebetor. Insect Sci. 2017, 24, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Arthur, F.H.; Phillips, T.W. Stored-product insect pest management and control. In Food Plant Sanitation; Hui, Y.H., Bruinsma, B.L., Gorham, J.R., Nip, W.K., Tong, P.S., Ventresca, P., Eds.; Marcel Dekker: New York, USA, 2003; pp. 341–358. [Google Scholar]
- Mason, L.J.; Strait, C.A., Stored product integrated pest management with extreme temperatures. In Temperature Sensitivity in Insects and Application in Integrated Pest Management; Hallman, G.J., Denlinger, D.L., Eds.; CRC Press: Boca Raton, USA, 2019; pp. 141–177. [CrossRef]
- Trematerra, P. Advances in the use of pheromones for stored-product protection. J. Pest Sci. 2012, 85, 285–299. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Casida, J.E. Quantitative analysis of the sex pheromone of several phycitid moths by electron-capture gas chromatography. Agric. Biol. Chem. 1973, 37, 681–684. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Kitamura, C.; Takashi, S.; Hara, H.; Ishii, S.; Fukami, H. Sex pheromone of the almond moth and the Indian meal moth: Cis-9,trans-12-tetradecadienyl acetate. Science 1971, 171, 801–802. [Google Scholar] [CrossRef]
- Sower, L.L.; Vick, K.W.; Tumlinson, J.H. (Z,E)-9,12-Tetradecadien-1-ol: A chemical released by female Plodia interpunctella that inhibits the sex pheromone response of male Cadra cautella. Environ. Entomol. 1974, 3, 120–122. [Google Scholar] [CrossRef]
- Yu, Z.; Deng, J.; Wang, Z.; Fu, W.; Zhou, G.; Lin, H.; Cao, Y.; Zheng, X.; Tang, J. Effect of different sex pheromone formulas, trap types and lure sources on captures of Plodia interpuntella. J. Chin. Cereals Oils. Assoc. 2018, 33, 6. [Google Scholar] [CrossRef]
- Trematerra, P.; Athanassiou, C.; Stejskal, V.; Sciarretta, A.; Kavallieratos, N.; Palyvos, N. Large-scale mating disruption of Ephestia spp. and Plodia interpunctella in Czech Republic, Greece and Italy. J. Appl. Entomol. 2011, 135, 749–762. [Google Scholar] [CrossRef]
- Campos, M.; Phillips, T.W. Attract-and-kill and other pheromone-based methods to suppress populations of the Indianmeal moth (Lepidoptera: Pyralidae). J. Econ. Entomol. 2014, 107, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Fadamiro, H.Y.; Baker, T.C. Pheromone puffs suppress mating by Plodia interpunctella and Sitotroga cerealella in an infested corn store. Entomol. Exp. Appl. 2002, 102, 239–251. [Google Scholar] [CrossRef]
- Burks, C.S.; McLaughlin, J.R.; Miller, J.R.; Brandl, D.G. Mating disruption for control of Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae) in dried beans. J. Stored Prod. Res. 2011, 47, 216–221. [Google Scholar] [CrossRef]
- Morrison III, W.R.; Scully, E.D.; Campbell, J.F. Towards developing areawide semiochemical-mediated, behaviorally-based integrated pest management programs for stored product insects. Pest Manag. Sci. 2021, 77, 2667–2682. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, C.G.; Kavallieratos, N.G.; Sciarretta, A.; Trematerra, P. Mating disruption of Ephestia kuehniella (Zeller) (Lepidoptera: Pyralidae) in a storage facility: Spatio-temporal distribution changed after long-term application. J. Stored Prod. Res. 2016, 67, 1–12. [Google Scholar] [CrossRef]
- James, D.G.; Moore, C.J.; Faulder, R.J. An improved coattractant for pheromone trapping of Carpophilus spp. (Coleoptera: Nitidulidae). Aust. J. Entomol. 1998, 37, 357–361. [Google Scholar] [CrossRef]
- Figueroa-Castro, P.; López-Martínez, V.; Silva-García, F.; González-Hernández, H. Food attractants to increase pheromone-baited trap performance for Scyphophorus acupunctatus (Coleoptera: Dryophthoridae) in mezcal maguey. Fla. Entomol. 2017, 100, 203–205. [Google Scholar] [CrossRef]
- Liu, J.; He, X.Z.; Wang, Q. Male larval experience of cues from adult rivals alters lifetime sperm investment patterns in a sperm heteromorphic moth, Ephestia kuehniella. Insect Sci. 2023, 30, 1773–1783. [Google Scholar] [CrossRef]
- Liu, J.; He, X.Z.; Zheng, X.L.; Zhang, Y.; Wang, Q. Juvenile socio-sexual experience determines lifetime sperm expenditure and adult survival in a polygamous moth, Ephestia kuehniella. Insect Sci. 2023, 30, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, X.Z.; Zheng, X.L.; Zhang, Y.; Wang, Q. Larval social cues influence testicular investment in an insect. Curr. Zool. 2022, 68, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, X.Z.; Zheng, X.L.; Zhang, Y.; Wang, Q. Pupal cues increase sperm production but not testis size in an insect. Insects 2021, 12, 679. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Zheng, X.L.; He, X.Z.; Wang, Q. Combined cues of male competition influence spermatozoal investment in a moth. Funct. Ecol. 2020, 34, 1223–1234. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Z.; Liu, H.; Guo, M.; Deng, J. Inhibition effect of non-host plant volatile extracts on reproductive behaviors in the diamondback moth Plutella xylostella (Linnaeus). Insects 2024, 15, 227. [Google Scholar] [CrossRef]
- Deng, J.; Lan, C.; Zhou, J.; Yao, Y.; Yin, X.; Fu, K.; Ding, X.; Guo, W.; Liu, W.; Wang, N.; Wang, F. Analysis of sex pheromone production and field trapping of the Asian corn borer (Ostrinia furnacalis Guenée) in Xinjiang, China. J. Integr. Agric. 2023, 22, 1093–1103. [Google Scholar] [CrossRef]
- Deng, J.; Shen, Z.; Wang, F.; Liu, T.; Hong, W.; Fang, M.; Wo, L.; Chu, S. Enhancement of attraction to sex pheromone of Grapholita molesta (Busck) (Lepidoptera: Tortricidae) by structurally unrelated sex pheromone compounds of Conogethes punctiferalis (Guenée) (Lepidoptera: Crambidae). J. Asia Pac. Entomol. 2022, 25, 101859. [Google Scholar] [CrossRef]
- Doud, C.W.; Phillips, T.W. Responses of red flour beetle adults, Tribolium castaneum (Coleoptera: Tenebrionidae), and other stored product beetles to different pheromone trap designs. Insects 2020, 11, 733. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, C.G.; Kavallieratos, N.G.; Campbell, J.F. Effect of the presence of live or dead insects on subsequent captures of six stored-product beetle species: The relative species matters. J. Econ. Entomol. 2017, 110, 770–775. [Google Scholar] [CrossRef]
- Doud, C.W.; Cuperus, G.W.; Kenkel, P.; Payton, M.E.; Phillips, T.W. Trapping Tribolium castaneum (Coleoptera: Tenebrionidae) and other beetles in flourmills: Evaluating fumigation efficacy and estimating population density. Insects 2021, 12, 144. [Google Scholar] [CrossRef]
- Morrison, W.R., III; Agrafioti, P.; Domingue, M.J.; Scheff, D.S.; Lampiri, E.; Gourgouta, M.; Baliota, G.V.; Sakka, M.; Myers, S.W.; Athanassiou, C.G. Comparison of different traps and attractants in 3 food processing facilities in Greece on the capture of stored product insects. J. Econ. Entomol. 2023, 116, 1432–1446. [Google Scholar] [CrossRef] [PubMed]
- Nansen, C.; Phillips, T.W. Ovipositional responses of the Indianmeal moth, Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae) to oils. Ann. Entomol. Soc. Am. 2003, 96, 524–531. [Google Scholar] [CrossRef]
- Cook, P.A.; Gage, M.J.G. Effects of risks of sperm competition on the numbers of eupyrene and apyrene sperm ejaculated by the moth Plodia interpunctella (Lepidoptera, Pyralidae). Behav. Ecol. Sociobiol. 1995, 36, 261–268. [Google Scholar] [CrossRef]
- Phelan, L. Evolution of sex pheromones and the role of asymmetric tracking. In Insect Chemical Ecology: An Evolutionary Approach; Roitberg B.D., Isman, M.B., Eds.; Chapman Hall, New York, USA, 1992; pp. 265–314.
- Yang, X.; Wang, M.; Gu, Y.; Han, W.; Li, X.; Li, X.; Zhong, Y.; Gao, J. The oviposition preference and offspring performance of Aethina tumida (Coleoptera: Nitidulidae). J. Econ. Entomol. 2024, 117, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.R.; Agavekar, G.; Agashe, D. Fitness landscapes reveal context-dependent benefits of oviposition behavior. Evolution 2022, 77, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara-Tsujii, N.; Yasui, H.; Wakamura, S. Population differences in male responses to chemical mating cues in the white-spotted longicorn beetle, Anoplophora malasiaca. Chemoecology 2013, 23, 113–120. [Google Scholar] [CrossRef]
- Nishida, Y.; Takagi, M. Male bull-headed shrikes use food caches to improve their condition-dependent song performance and pairing success. Anim. Behav. 2019, 152, 29–37. [Google Scholar] [CrossRef]
- Gregg, P.C.; Del Socorro, A.P.; Henderson, G.S. Development of a synthetic plant volatile-based attracticide for female noctuid moths. II. Bioassays of synthetic plant volatiles as attractants for the adults of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Aust. J. Entomol. 2010, 49, 21–30. [Google Scholar] [CrossRef]
- He, W.; Zhao, X.; Ali, A.; Ge, S.; Zhang, H.; He, L.; Wu, K. Population dynamics and reproductive developmental analysis of Helicoverpa armigera (Lepidoptera: Noctuidae) trapped using food attractants in the field. J. Econ. Entomol. 2021, 114, 1533–1541. [Google Scholar] [CrossRef]
- Ogawa, Y.; Miyake, T. How do rewardless Bletilla striata flowers attract pollinators to achieve pollination? Plant Syst. Evol. 2020, 306, 78. [Google Scholar] [CrossRef]
- Saad, A.D.; Scott, D.R. Repellency of pheromones released by females of Heliothis armigera and H. Zea to females of both species. Entomol. Exp. Appl. 1981, 30, 123–127. [Google Scholar] [CrossRef]
- Pérez-Aparicio, A.; Ammagarahalli, B.; Gemeno, C. A closer look at sex pheromone autodetection in the Oriental fruit moth. Sci. Rep. 2022, 12, 7019. [Google Scholar] [CrossRef] [PubMed]
- Holdcraft, R.; Rodriguez-Saona, C.; Stelinski, L.L. Pheromone autodetection: Evidence and implications. Insects 2016, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Yasui, H.; Fukaya, M.; Wakamura, S.; Akino, T.; Yasuda, T.; Kobayashi, A.; Arakaki, N. Aggregation of the black chafer Holotrichia loochooana loochooana (Sawada) (Coleoptera: Scarabaeidae): Function of female pheromone and possible adaptive significance. Appl. Entomol. Zool. 2007, 42, 507–515. [Google Scholar] [CrossRef]
- Pearson, G.A.; Dillery, S.; Meyer, J.R. Modeling intra-sexual competition in a sex pheromone system: how much can female movement affect female mating success? J. Theor. Biol. 2004, 231, 549–555. [Google Scholar] [CrossRef]
- Stelinski, L.; Rodriguez-Saona, C.; Meyer, W. Recognition of foreign oviposition-marking pheromone in a multi-trophic context. Sci. Nat. 2009, 96, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Trematerra, P.; Battaini, F. Control of Ephestia kuehniella Zeller by mass-trapping. J. Appl. Entomol. 1987, 104, 336–340. [Google Scholar] [CrossRef]
- Walker, S.E.; Cade, W.H. A simulation model of the effects of frequency dependence, density dependence and parasitoid flies on the fitness of male field crickets. Ecol. Model. 2003, 169, 119–130. [Google Scholar] [CrossRef]
- Haverkamp, A.; Hansson, B.S.; Knaden, M. Combinatorial codes and labeled lines: How insects use olfactory cues to find and judge food, mates, and oviposition sites in complex environments. Front. Physiol. 2018, 9, 49. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Pickett, J.A. Perception of plant volatile blends by herbivorous insects – Finding the right mix. Phytochemistry 2011, 72, 1605–1611. [Google Scholar] [CrossRef]
- Riffell, J.A.; Shlizerman, E.; Sanders, E.; Abrell, L.; Medina, B.; Hinterwirth, A.J.; Kutz, J.N. Flower discrimination by pollinators in a dynamic chemical environment. Science 2014, 344, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




