Submitted:
10 July 2024
Posted:
11 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
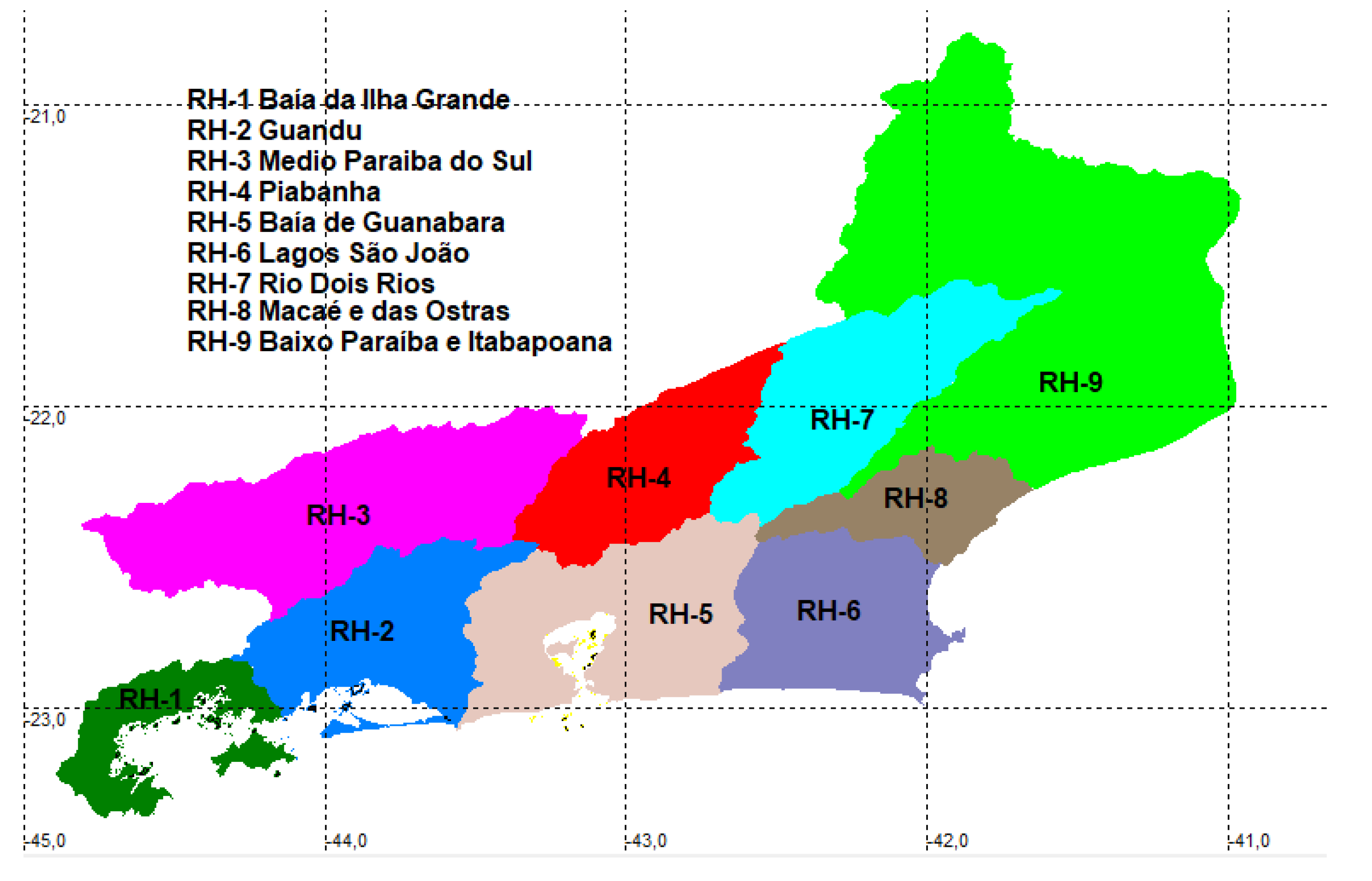
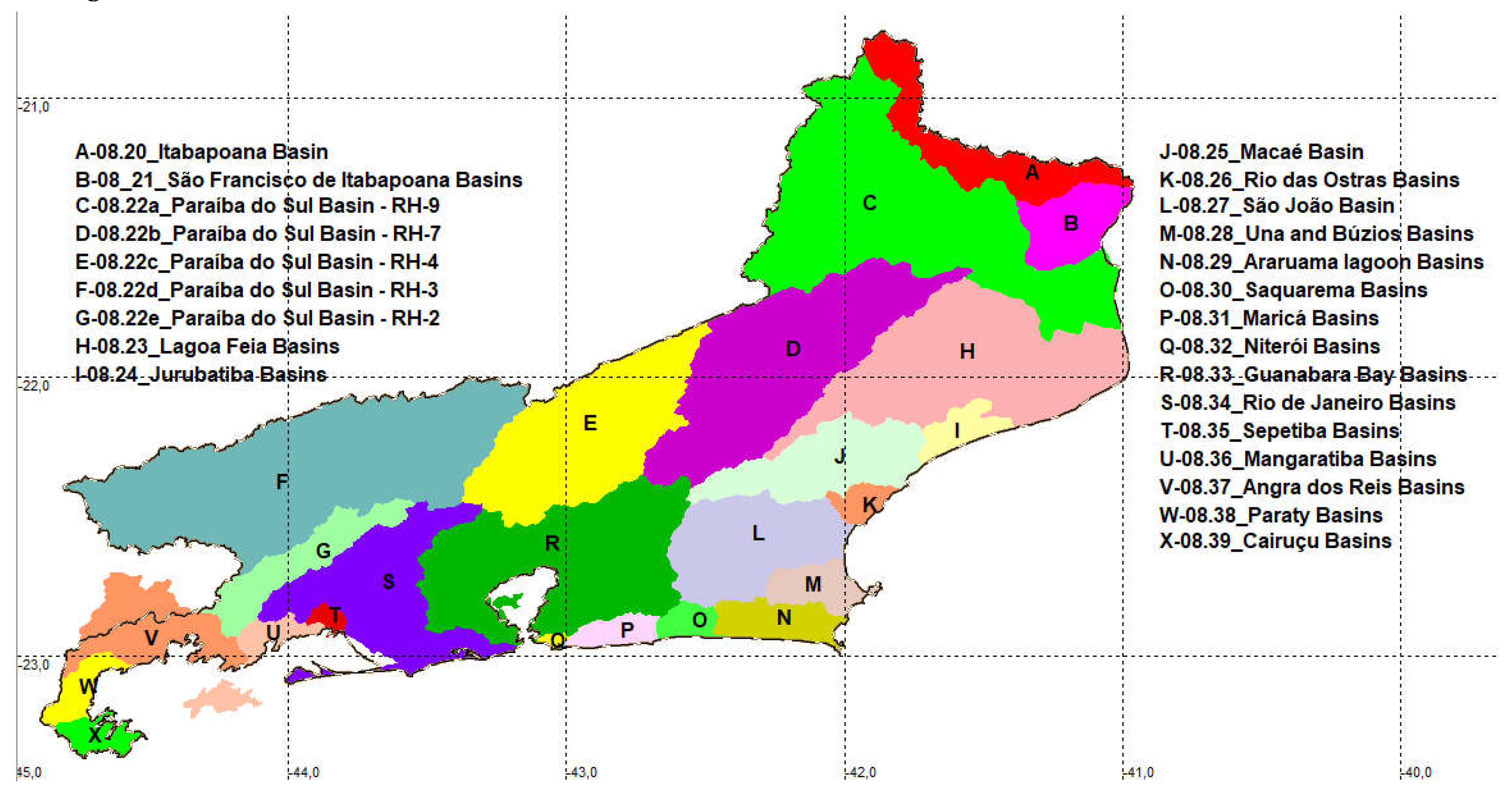
2.1. Study Area
2.2. Species Data
2.3. Geographic Data
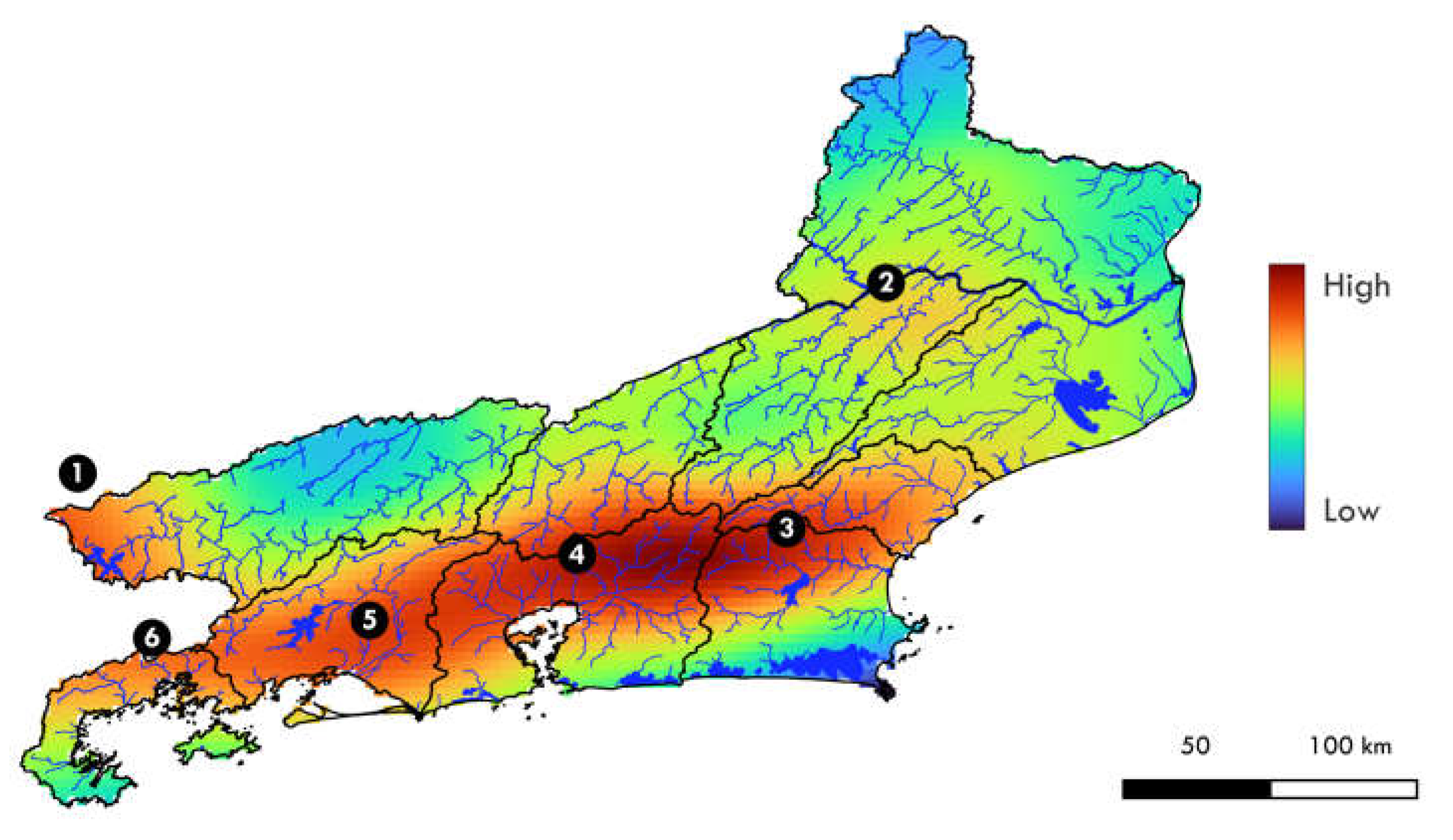
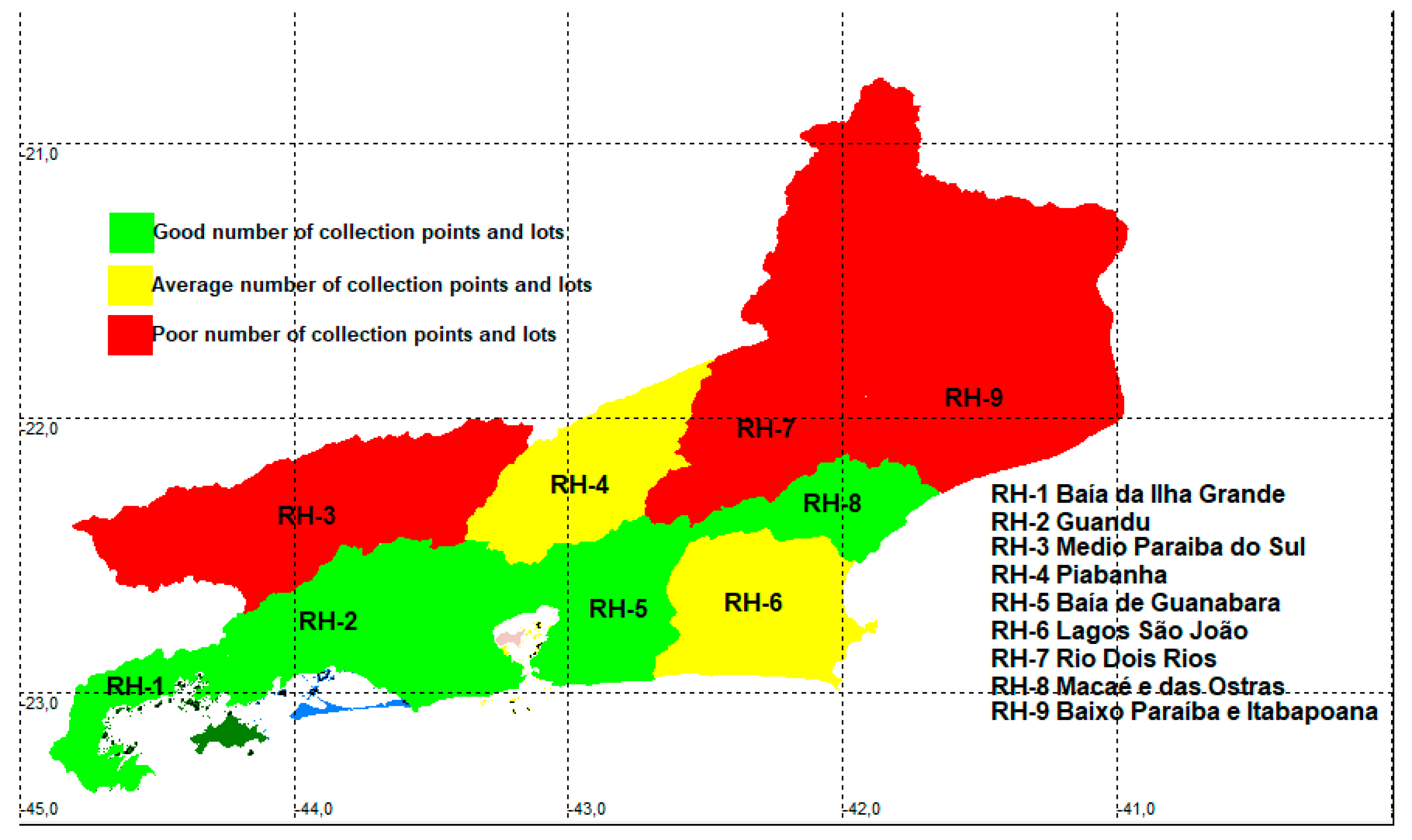
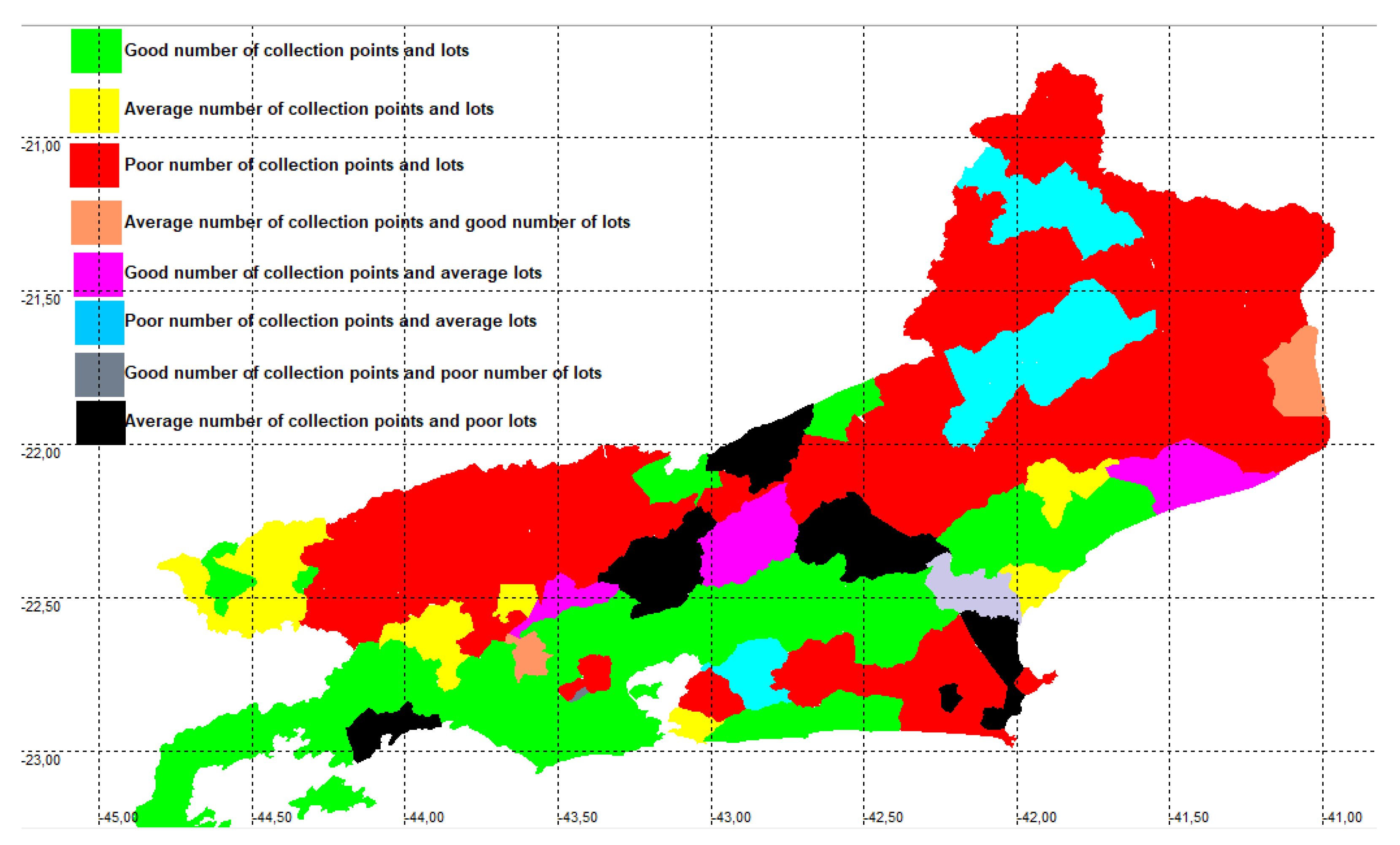
2.4. Sampling Coverage Assessment
2.5. Biogeographic and Diversity Patterns
3. Results
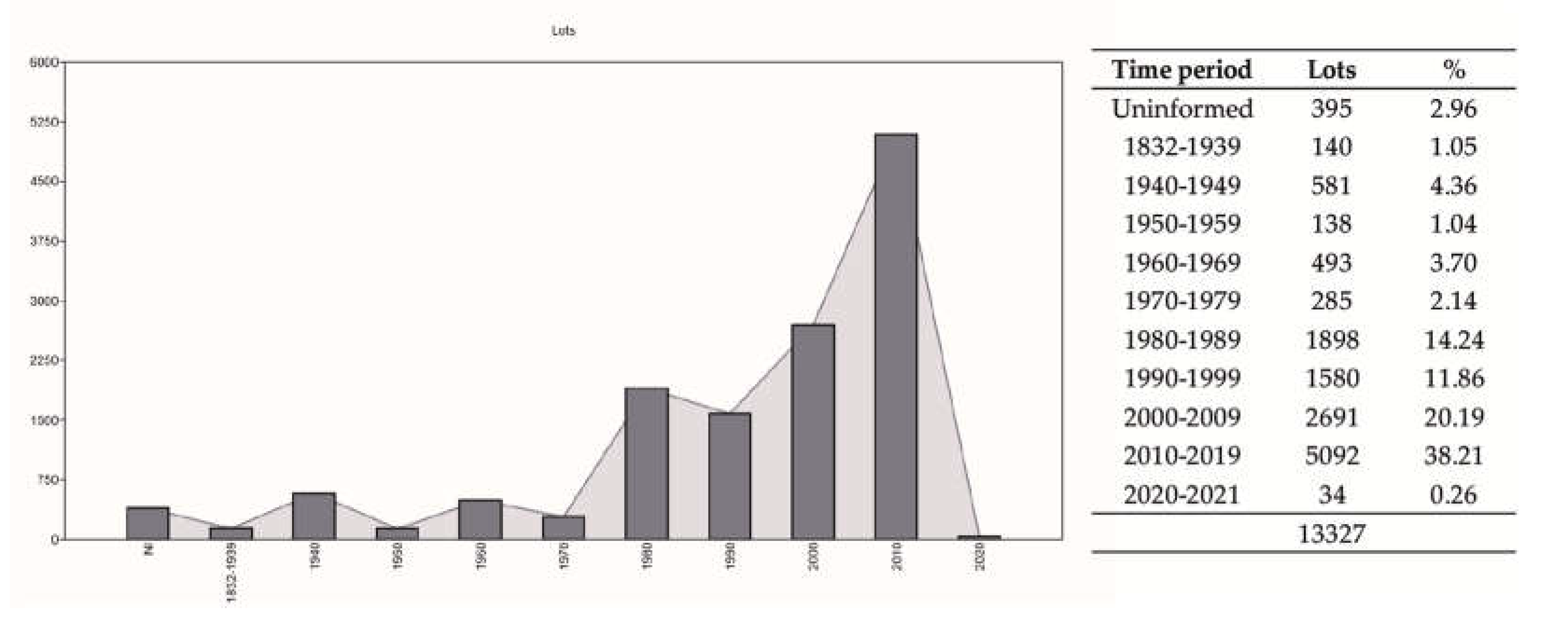
3.1. Rio de Janeiro According to the Collections - Diversity in Numbers
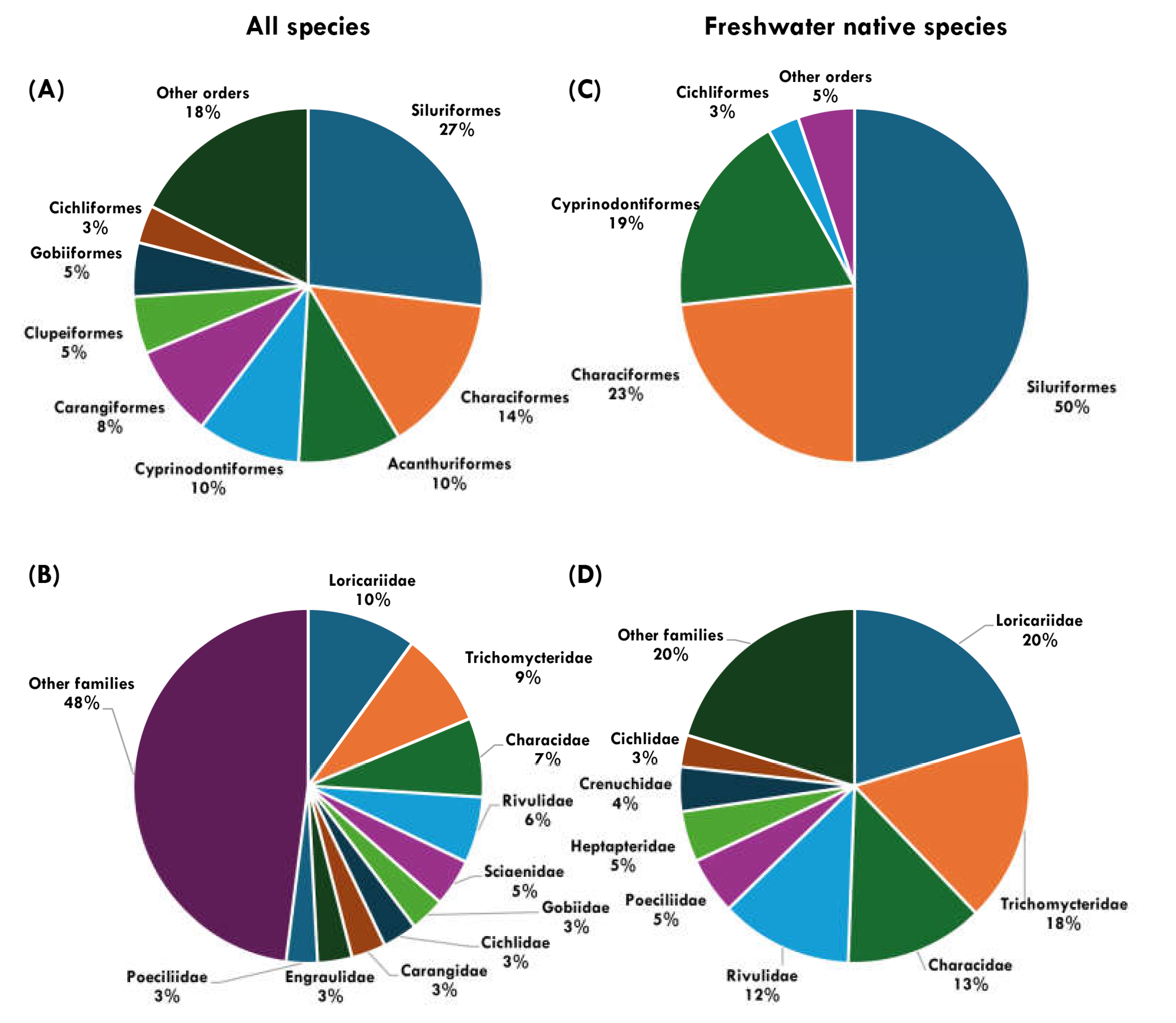
3.1.1. Taxonomic Diversity
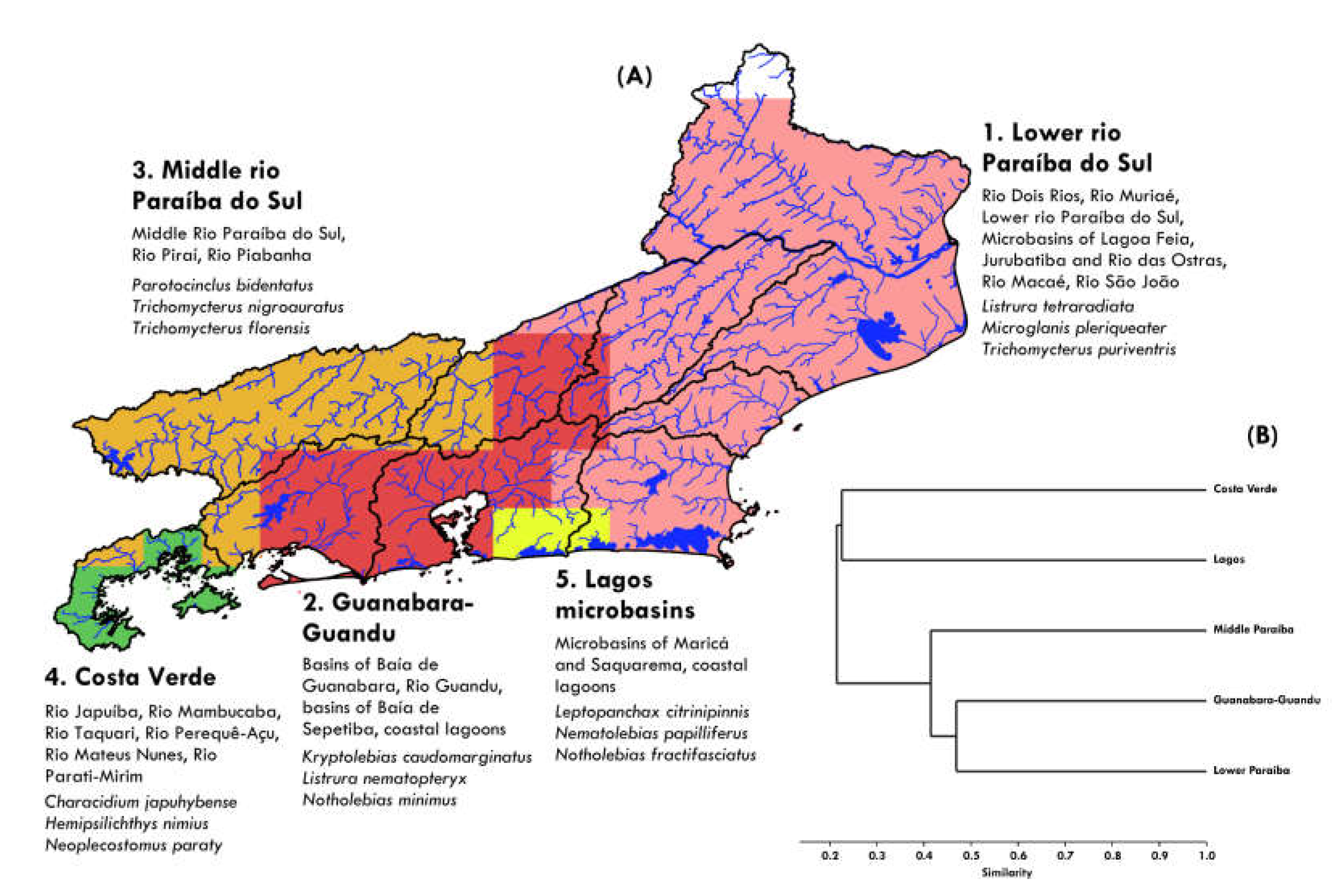
3.2. Spatial Patterns of Distribution
- Lower Rio Paraíba do Sul bioregion: The largest area in terms of territorial extension, this biogeographic unit is composed mainly of the lower Rio Paraíba do Sul basin and its tributaries, such as the Rio Muriaé, Rio Dois Rios and Rio Pomba, as well as independent basins such as the Rio Itabapoana (the geographical divide with the state of Espírito Santo), Rio Macaé and Rio São João, and coastal lagoon systems such as the Lagoa Feia. Species that delimit this bioregion include Listrura tetraradiata, Microglanis pleriqueater, and Trichomycterus puriventris, present in most of the drainages that compose this area. The bioregion has 114 species, of which 26 (22.8%) occur only in this area (e.g., Atlantirivulus janeiroensis, A. jurubatibensis, Bryconamericus tenuis, Characidium litorale, Delturus parahybae, Homodiaetus banguela, Ituglanis parahybae, Trichomycterus caipora, T. fuliginosus and T. vitalbrazili). Covering a large part of the basins of the state of Rio de Janeiro, many of the species present in these basins are common to several other drainages of the Atlantic Forest, such as fish species within the genera Deuterodon, Hypostomus and Trichomycterus.
- Guanabara-Guandu bioregion: It consists mainly of the basins that drain the Baía de Guanabara and Baía de Sepetiba, such as the Rio Caceribu, Rio Guapimirim, Rio Macacu, Rio Roncador, Rio Suruí, Rio Guandu and the Jacarepaguá lagoon system. Species that delimit this bioregion include Kryptolebias caudomarginatus, Listrura nematopteryx, and Notholebias minimus. Presents 99 species, 17 of them (17.2%) occurring only in this area (e.g., Atlantirivulus guanabarensis, Australoheros macacuensis, Characidium grajahuense, Homodiaetus passarelli, Kryptolebias brasiliensis, Leptopanchax opalescens, L. sanguineus, L. splendens, Microglanis nigripinnis, Trichomycterus giganteus, and T. potschi).
- Middle Rio Paraíba do Sul bioregion: Formed by tributaries of the middle course of the Rio Paraíba do Sul, such as the Rio Piraí, Rio Paquequer, Rio Preto, Rio Piabanha, and the Rio Paraíba do Sul. This bioregion is supported by the presence of species such as Parotocinclus bidentatus, Trichomycterus nigroauratus, and Trichomycterus florensis. The area presents 81 species, of which 11 (13.6%) occur only in this bioregion, especially species of the genus Trichomycterus, such as T. itatiayae, T. macrophthalmus, T. mariamole, and T. mirissumba.
- Costa Verde bioregion: This biogeographic unit consists of small basins that flow into the Baía da Ilha Grande, as the drainages of the Rio Mambucaba, Rio Perequê-Açu, Rio Taquari and Rio Parati-Mirim. Supported by the presence of species such as Characidium japuhybense, Hemipsilichthys nimius, and Neoplecostomus paraty. The bioregion presents 40 species, of which nine (22.5%) occur only in this area (e.g., Atlantirivulus lazzarotoi, A. simplicis, Listrura costai, and Phalloceros enneaktinos).
- Lagos bioregion: The smallest of the delimited areas, this bioregion consists of small drainages and lagoon systems that are part of the Região dos Lagos area, in the coast of Rio de Janeiro. It includes the drainages of the Rio Ubatiba, Rio Mato Grosso, coastal wetlands and lagoons of Maricá and Saquarema. The region is supported by the presence of species of rivulids such as Atlantirivulus maricensis, Leptopanchax citrinipinnis, Nematolebias papilliferus, and Notholebias fractifasciatus. It has 36 species, of which four (11.1%) only occur in that area.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Municipality | Area (km2) | Lots | Points | Il | Ilq | Ip | Ipq |
|---|---|---|---|---|---|---|---|
| Angra dos Reis | 813.21 | 541 | 56 | 66.5 | Good | 6.9 | Good |
| Aperibé | 94.54 | 2 | 2 | 2.1 | Poor | 2.1 | Poor |
| Araruama | 638.15 | 34 | 4 | 5.3 | Poor | 0.6 | Poor |
| Areal | 110.72 | 1 | 1 | 0.9 | Poor | 0.9 | Poor |
| Armação dos Búzios | 70.98 | 0 | 0 | 0.0 | Poor | 0.0 | Poor |
| Arraial do Cabo | 152.11 | 0 | 0 | 0.0 | Poor | 0.0 | Poor |
| Barra do Piraí | 584.61 | 41 | 8 | 7.0 | Poor | 1.4 | Poor |
| Barra Mansa | 547.13 | 40 | 7 | 7.3 | Poor | 1.3 | Poor |
| Belford Roxo | 78.99 | 0 | 0 | 0.0 | Poor | 0.0 | Poor |
| Bom Jardim | 382.43 | 19 | 5 | 5.0 | Poor | 1.3 | Poor |
| Bom Jesus do Itabapoana | 596.66 | 32 | 8 | 5.4 | Poor | 1.3 | Poor |
| Cabo Frio | 413.58 | 65 | 14 | 15.7 | Poor | 3.4 | Average |
| Cachoeiras de Macacu | 954.75 | 927 | 83 | 97.1 | Good | 8.7 | Good |
| Cambuci | 558.28 | 6 | 2 | 1.1 | Poor | 0.4 | Poor |
| Campos dos Goytacazes | 4 032.49 | 608 | 75 | 15.1 | Poor | 1.9 | Poor |
| Cantagalo | 747.21 | 48 | 6 | 6.4 | Poor | 0.8 | Poor |
| Carapebus | 304.89 | 293 | 20 | 96.1 | Good | 6.6 | Good |
| Cardoso Moreira | 522.60 | 24 | 3 | 4.6 | Poor | 0.6 | Poor |
| Carmo | 305.75 | 252 | 23 | 82.4 | Good | 7.5 | Good |
| Casimiro de Abreu | 462.92 | 343 | 32 | 74.1 | Good | 6.9 | Good |
| Comendador Levy Gasparian | 108.64 | 1 | 1 | 0.9 | Poor | 0.9 | Poor |
| Conceição de Macabu | 338.26 | 114 | 15 | 33.7 | Average | 4.4 | Average |
| Cordeiro | 113.05 | 8 | 1 | 7.1 | Poor | 0.9 | Poor |
| Duas Barras | 379.62 | 3 | 1 | 0.8 | Poor | 0.3 | Poor |
| Duque de Caxias | 467.32 | 233 | 38 | 49.9 | Good | 8.1 | Good |
| Engenheiro Paulo de Frontin | 139.38 | 31 | 4 | 22.2 | Average | 2.9 | Average |
| Guapimirim | 358.44 | 317 | 28 | 88.4 | Good | 7.8 | Good |
| Iguaba Grande | 50.98 | 7 | 2 | 13.7 | Poor | 3.9 | Average |
| Itaboraí | 429.96 | 127 | 10 | 29.5 | Average | 2.3 | Poor |
| Itaguaí | 282.61 | 167 | 16 | 59.1 | Good | 5.7 | Good |
| Italva | 291.19 | 18 | 2 | 6.2 | Poor | 0.7 | Poor |
| Itaocara | 433.18 | 118 | 8 | 27.2 | Average | 1.8 | Poor |
| Itaperuna | 1 106.69 | 319 | 16 | 28.8 | Average | 1.4 | Poor |
| Itatiaia | 241.04 | 230 | 38 | 95.4 | Good | 15.8 | Good |
| Japeri | 81.70 | 59 | 3 | 72.2 | Good | 3.7 | Average |
| Laje do Muriaé | 253.53 | 7 | 1 | 2.8 | Poor | 0.4 | Poor |
| Macaé | 1 216.99 | 886 | 82 | 72.8 | Good | 6.7 | Good |
| Macuco | 78.36 | 0 | 0 | 0.0 | Poor | 0.0 | Poor |
| Magé | 390.78 | 308 | 44 | 78.8 | Good | 11.3 | Good |
| Mangaratiba | 367.82 | 67 | 12 | 18.2 | Poor | 3.3 | Average |
| Maricá | 361.57 | 174 | 35 | 48.1 | Good | 9.7 | Good |
| Mendes | 95.32 | 15 | 2 | 15.7 | Poor | 2.1 | Poor |
| Mesquita | 41.17 | 1 | 1 | 2.4 | Poor | 2.4 | Poor |
| Miguel Pereira | 287.93 | 98 | 16 | 34.0 | Average | 5.6 | Good |
| Miracema | 303.27 | 0 | 0 | 0.0 | Poor | 0.0 | Poor |
| Natividade | 387.07 | 7 | 1 | 1.8 | Poor | 0.3 | Poor |
| Nilópolis | 19.39 | 1 | 1 | 5.2 | Poor | 5.2 | Good |
| Niterói | 133.76 | 35 | 8 | 26.2 | Average | 6.0 | Average |
| Nova Friburgo | 935.43 | 182 | 32 | 19.5 | Poor | 3.4 | Average |
| Nova Iguaçu | 520.58 | 479 | 58 | 92.0 | Good | 11.1 | Good |
| Paracambi | 190.95 | 24 | 3 | 12.6 | Poor | 1.6 | Poor |
| Paraíba do Sul | 571.12 | 15 | 4 | 2.6 | Poor | 0.7 | Poor |
| Paraty | 924.30 | 609 | 84 | 65.9 | Good | 9.1 | Good |
| Paty do Alferes | 314.34 | 8 | 2 | 2.5 | Poor | 0.6 | Poor |
| Petrópolis | 791.14 | 143 | 25 | 18.1 | Poor | 3.2 | Average |
| Pinheiral | 82.25 | 2 | 1 | 2.4 | Poor | 1.2 | Poor |
| Piraí | 490.26 | 120 | 17 | 24.5 | Average | 3.5 | Average |
| Porciúncula | 291.85 | 0 | 0 | 0.0 | Poor | 0.0 | Poor |
| Porto Real | 50.89 | 41 | 4 | 80.6 | Good | 7.9 | Good |
| Quatis | 284.83 | 51 | 8 | 17.9 | Poor | 2.8 | Poor |
| Queimados | 75.93 | 33 | 2 | 43.5 | Good | 2.6 | Average |
| Quissamã | 719.64 | 267 | 38 | 37.1 | Average | 5.3 | Good |
| Resende | 1 099.34 | 283 | 42 | 25.7 | Average | 3.8 | Average |
| Rio Bonito | 459.46 | 32 | 5 | 7.0 | Poor | 1.1 | Poor |
| Rio Claro | 846.80 | 719 | 74 | 84.9 | Good | 8.7 | Good |
| Rio das Flores | 478.78 | 10 | 2 | 2.1 | Poor | 0.4 | Poor |
| Rio das Ostras | 228.04 | 81 | 7 | 35.5 | Average | 3.1 | Average |
| Rio de Janeiro | 1 200.33 | 932 | 182 | 77.6 | Good | 15.2 | Good |
| Santa Maria Madalena | 810.96 | 84 | 19 | 10.4 | Poor | 2.3 | Poor |
| Santo Antônio de Pádua | 603.63 | 47 | 3 | 7.8 | Poor | 0.5 | Poor |
| São Fidélis | 1 034.83 | 308 | 16 | 29.8 | Average | 1.5 | Poor |
| São Francisco de Itabapoana | 1 118.04 | 212 | 18 | 19.0 | Poor | 1.6 | Poor |
| São Gonçalo | 248.16 | 2 | 1 | 0.8 | Poor | 0.4 | Poor |
| São João da Barra | 452.40 | 228 | 13 | 50.4 | Good | 2.9 | Average |
| São João de Meriti | 35.22 | 0 | 0 | 0.0 | Poor | 0.0 | Poor |
| São José de Ubá | 249.69 | 29 | 3 | 11.6 | Poor | 1.2 | Poor |
| São José do Vale do Rio Preto | 220.18 | 12 | 5 | 5.5 | Poor | 2.3 | Poor |
| São Pedro da Aldeia | 332.49 | 22 | 6 | 6.6 | Poor | 1.8 | Poor |
| São Sebastião do Alto | 397.21 | 108 | 9 | 27.2 | Average | 2.3 | Poor |
| Sapucaia | 540.67 | 98 | 14 | 18.1 | Poor | 2.6 | Average |
| Saquarema | 352.13 | 207 | 34 | 58.8 | Good | 9.7 | Good |
| Seropédica | 265.19 | 129 | 27 | 48.6 | Good | 10.2 | Good |
| Silva Jardim | 937.76 | 681 | 51 | 72.6 | Good | 5.4 | Good |
| Sumidouro | 413.41 | 18 | 3 | 4.4 | Poor | 0.7 | Poor |
| Tanguá | 143.01 | 8 | 2 | 5.6 | Poor | 1.4 | Poor |
| Teresópolis | 773.34 | 229 | 42 | 29.6 | Average | 5.4 | Good |
| Trajano de Moraes | 591.15 | 28 | 5 | 4.7 | Poor | 0.8 | Poor |
| Três Rios | 322.84 | 170 | 16 | 52.7 | Good | 5.0 | Good |
| Valença | 1 300.77 | 6 | 4 | 0.5 | Poor | 0.3 | Poor |
| Varre-Sai | 201.94 | 0 | 0 | 0.0 | Poor | 0.0 | Poor |
| Vassouras | 536.07 | 29 | 3 | 5.4 | Poor | 0.6 | Poor |
| Volta Redonda | 182.11 | 14 | 4 | 7.7 | Poor | 2.2 | Poor |
| Rio de Janeiro State | 43 750 | 13 327 | 1 623 | 30.5 | 3.7 |
| RH-1 | RH-2 | RH-3 | RH-4 | RH-5 | RH-6 | RH-7 | RH-8 | RH-9 | |
|---|---|---|---|---|---|---|---|---|---|
| Acentronichthys leptos | 109 | 20 | 0 | 0 | 171 | 17 | 36 | 5 | 7 |
| Ancistrus multispinis | 145 | 119 | 0 | 0 | 501 | 31 | 0 | 5 | 0 |
| Astyanax keronolepis | 1091 | 97 | 0 | 0 | 551 | 0 | 0 | 0 | 0 |
| Astyanax lacustris | 2 | 511 | 81 | 72 | 115 | 184 | 173 | 1950 | 1582 |
| Atlantirivulus janeiroensis | 0 | 0 | 0 | 0 | 0 | 11 | 0 | 0 | 0 |
| Atlantirivulus jurubatibensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 51 |
| Atlantirivulus guanabarensis | 0 | 0 | 0 | 0 | 73 | 0 | 0 | 0 | 0 |
| Atlantirivulus lazzarotoi | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Atlantirivulus maricensis | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Atlantirivulus simplicis | 43 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Australoheros ipatinguensis | 0 | 6 | 112 | 99 | 0 | 23 | 32 | 0 | 257 |
| Australoheros oblongus | 0 | 18 | 4 | 0 | 89 | 49 | 0 | 126 | 47 |
| Awaous tajasica | 115 | 5 | 0 | 3 | 81 | 17 | 35 | 113 | 23 |
| Brachyhypopomus janeiroensis | 0 | 0 | 0 | 1 | 1 | 82 | 12 | 11 | 49 |
| Brycon insignis | 0 | 0 | 1 | 0 | 0 | 10 | 0 | 7 | 26 |
| Brycon opalinus | 0 | 30 | 20 | 8 | 0 | 0 | 14 | 0 | 1 |
| Bryconamericus microcephalus | 919 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bryconamericus ornaticeps | 0 | 287 | 0 | 0 | 1900 | 0 | 0 | 0 | 0 |
| Bryconamericus tenuis | 0 | 0 | 0 | 20 | 0 | 128 | 88 | 287 | 40 |
| Callichthys callichthys | 0 | 47 | 12 | 3 | 198 | 19 | 11 | 56 | 165 |
| Characidium alipioi | 0 | 0 | 0 | 21 | 0 | 119 | 45 | 18 | 58 |
| Characidium grajahuense | 0 | 140 | 0 | 0 | 321 | 0 | 0 | 0 | 0 |
| Characidium interruptum | 0 | 14 | 0 | 0 | 249 | 236 | 0 | 38 | 152 |
| Characidium japuhybense | 869 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Characidium lauroi | 0 | 660 | 135 | 411 | 0 | 0 | 30 | 0 | 0 |
| Characidium litorale | 0 | 0 | 0 | 0 | 0 | 588 | 0 | 172 | 33 |
| Characidium vidali | 0 | 146 | 0 | 124 | 931 | 0 | 10 | 342 | 0 |
| Corydoras nattereri | 0 | 63 | 4 | 97 | 314 | 86 | 2 | 7 | 87 |
| Crenicichla lacustris | 0 | 19 | 36 | 7 | 0 | 43 | 60 | 71 | 597 |
| Crenicichla lepidota | 0 | 47 | 0 | 0 | 83 | 2 | 0 | 0 | 0 |
| Cyphocharax gilbert | 0 | 467 | 1 | 6 | 43 | 344 | 13 | 740 | 381 |
| Delturus parahybae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Deuterodon giton | 0 | 141 | 154 | 312 | 0 | 2217 | 243 | 677 | 1954 |
| Deuterodon hastatus | 193 | 269 | 0 | 32 | 2620 | 0 | 0 | 0 | 0 |
| Deuterodon heterostomus | 0 | 4 | 42 | 0 | 2 | 50 | 0 | 1 | 1 |
| Deuterodon intermedius | 179 | 2574 | 463 | 187 | 0 | 0 | 151 | 0 | 35 |
| Deuterodon janeiroensis | 0 | 5745 | 0 | 7 | 1384 | 512 | 17 | 0 | 0 |
| Deuterodon luetkenii | 0 | 0 | 0 | 0 | 35 | 98 | 6 | 6284 | 1450 |
| Deuterodon taeniatus | 0 | 26 | 9 | 70 | 6 | 982 | 346 | 3072 | 865 |
| Dormitator maculatus | 11 | 13 | 0 | 0 | 8 | 1 | 0 | 3 | 80 |
| Eigenmannia virescens | 0 | 0 | 7 | 17 | 7 | 42 | 125 | 14 | 17 |
| Eleotris pisonis | 31 | 9 | 0 | 0 | 28 | 26 | 5 | 26 | 23 |
| Geophagus brasiliensis | 4092 | 983 | 241 | 346 | 1809 | 200 | 364 | 875 | 1114 |
| Glanidium melanopterum | 0 | 14 | 26 | 9 | 0 | 2 | 25 | 3 | 11 |
| Gymnotus carapo | 0 | 90 | 37 | 52 | 51 | 44 | 11 | 13 | 23 |
| Gymnotus pantherinus | 38 | 10 | 0 | 0 | 61 | 47 | 5 | 41 | 5 |
| Harttia carvalhoi | 0 | 88 | 11 | 24 | 0 | 0 | 0 | 0 | 23 |
| Harttia loricariformis | 0 | 32 | 13 | 52 | 0 | 0 | 55 | 0 | 7 |
| Hemipsilichthys gobio | 0 | 9 | 0 | 10 | 1 | 0 | 8 | 0 | 0 |
| Hemipsilichthys nimius | 145 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hemipsilichthys papillatus | 0 | 7 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hisonotus notatus | 0 | 205 | 4 | 3 | 689 | 24 | 31 | 0 | 113 |
| Hisonotus thayeri | 0 | 0 | 0 | 0 | 0 | 557 | 92 | 92 | 107 |
| Hollandichthys multifasciatus | 334 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Homodiaetus banguela | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 0 | 0 |
| Homodiaetus passarellii | 0 | 2 | 0 | 0 | 48 | 0 | 0 | 0 | 0 |
| Hoplerythrinus unitaeniatus | 0 | 2 | 1 | 0 | 17 | 10 | 0 | 10 | 121 |
| Hoplias malabaricus | 14 | 45 | 13 | 20 | 104 | 83 | 6 | 142 | 163 |
| Hoplosternum littorale | 0 | 96 | 23 | 8 | 38 | 1 | 4 | 2 | 31 |
| Hyphessobrycon bifasciatus | 0 | 154 | 8 | 58 | 1070 | 192 | 11 | 5063 | 2894 |
| Hyphessobrycon boulengeri | 0 | 33 | 0 | 0 | 245 | 15 | 0 | 311 | 496 |
| Hyphessobrycon flammeus | 0 | 48 | 0 | 1 | 35 | 12 | 0 | 0 | 4 |
| Hypomasticus copelandii | 0 | 10 | 2 | 13 | 13 | 13 | 19 | 19 | 132 |
| Hypomasticus mormyrops | 0 | 10 | 14 | 7 | 0 | 0 | 17 | 1 | 3 |
| Hypomasticus thayeri | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 21 |
| Hypostomus affinis | 0 | 0 | 3 | 0 | 5 | 98 | 1 | 65 | 0 |
| Hypostomus auroguttatus | 0 | 0 | 2 | 2 | 0 | 0 | 2 | 0 | 0 |
| Hypostomus luetkeni | 0 | 34 | 20 | 155 | 0 | 0 | 168 | 0 | 87 |
| Hypostomus punctatus | 0 | 95 | 0 | 0 | 244 | 2 | 0 | 0 | 0 |
| Hypostomus vermicularis | 0 | 0 | 52 | 4 | 0 | 0 | 2 | 0 | 4 |
| Imparfinis minutus | 0 | 61 | 59 | 4 | 0 | 0 | 6 | 0 | 7 |
| Imparfinis piperatus | 0 | 4 | 4 | 0 | 4 | 0 | 0 | 0 | 0 |
| Ituglanis parahybae | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 3 |
| Jenynsia darwini | 0 | 0 | 0 | 0 | 7 | 8 | 0 | 160 | 1516 |
| Jenynsia lineata | 0 | 0 | 0 | 0 | 318 | 65 | 0 | 0 | 0 |
| Knodus moenkhausii | 0 | 0 | 0 | 267 | 0 | 0 | 10 | 0 | 29 |
| Kronichthys heylandi | 664 | 167 | 0 | 1 | 151 | 4 | 0 | 0 | 0 |
| Kryptolebias brasiliensis | 0 | 54 | 0 | 0 | 164 | 16 | 0 | 0 | 0 |
| Kryptolebias caudomarginatus | 0 | 166 | 0 | 0 | 56 | 0 | 0 | 0 | 0 |
| Kryptolebias gracilis | 0 | 0 | 0 | 0 | 0 | 28 | 0 | 11 | 0 |
| Kryptolebias ocellatus | 0 | 86 | 1 | 0 | 0 | 2 | 0 | 0 | 0 |
| Leptolebias marmoratus | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 |
| Leptopanchax citrinipinnis | 184 | 0 | 0 | 0 | 0 | ||||
| Leptopanchax opalescens | 0 | 7 | 0 | 0 | 55 | 0 | 0 | 0 | 0 |
| Leptopanchax sanguineus | 0 | 0 | 0 | 0 | 15 | 0 | 0 | 0 | 0 |
| Leptopanchax splendens | 0 | 0 | 0 | 0 | 50 | 0 | 0 | 0 | 0 |
| Listrura costai | 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Listrura nematopteryx | 0 | 0 | 0 | 0 | 156 | 0 | 0 | 0 | 0 |
| Listrura tetraradiata | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 1 |
| Loricariichthys castaneus | 0 | 89 | 15 | 1 | 19 | 317 | 19 | 1 | 122 |
| Megaleporinus conirostris | 0 | 3 | 0 | 4 | 0 | 0 | 23 | 0 | 37 |
| Microcambeva barbata | 0 | 0 | 0 | 0 | 0 | 71 | 0 | 4 | 0 |
| Microcambeva bendego | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 0 |
| Microglanis nigripinnis | 0 | 0 | 0 | 0 | 21 | 0 | 0 | 0 | 0 |
| Microglanis parahybae | 0 | 31 | 0 | 0 | 0 | 0 | 44 | 0 | 61 |
| Microglanis pleriqueater | 0 | 0 | 0 | 0 | 0 | 55 | 0 | 10 | 0 |
| Mimagoniates microlepis | 808 | 255 | 0 | 637 | 1328 | 501 | 0 | 30 | 33 |
| Nematolebias papilliferus | 0 | 0 | 0 | 0 | 28 | 0 | 0 | 0 | 0 |
| Nematolebias whitei | 0 | 0 | 0 | 0 | 17 | 291 | 0 | 9 | 0 |
| Neoplecostomus microps | 0 | 825 | 225 | 135 | 82 | 0 | 118 | 219 | 1 |
| Neoplecostomus paraty | 163 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neoplecostomus variipictus | 0 | 0 | 0 | 55 | 0 | 0 | 80 | 0 | 0 |
| Notholebias cruzi | 0 | 0 | 0 | 0 | 29 | 32 | 0 | 0 | 0 |
| Notholebias fractifasciatus | 0 | 0 | 0 | 0 | 20 | 2 | 0 | 0 | 0 |
| Notholebias minimus | 0 | 181 | 0 | 0 | 8 | 0 | 0 | 0 | 0 |
| Oligosarcus hepsetus | 10 | 346 | 94 | 80 | 64 | 24 | 92 | 154 | 249 |
| Ophthalmolebias constanciae | 0 | 0 | 0 | 0 | 0 | 47 | 0 | 65 | 0 |
| Otocinclus affinis | 0 | 6 | 1 | 14 | 26 | 3 | 1 | 1 | 13 |
| Otothyris lophophanes | 2 | 24 | 4 | 3 | 45 | 135 | 3 | 69 | 41 |
| Pachyurus adspersus | 0 | 3 | 9 | 1 | 0 | 0 | 7 | 0 | 29 |
| Paragenidens grandoculis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Pareiorhaphis garbei | 0 | 0 | 0 | 0 | 232 | 13 | 22 | 54 | 0 |
| Pareiorhina brachyrhyncha | 0 | 0 | 160 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pareiorhina rudolphi | 77 | 553 | 672 | 0 | 0 | 0 | 0 | 0 | 0 |
| Parotocinclus bidentatus | 0 | 0 | 22 | 1 | 0 | 0 | 0 | 0 | 0 |
| Parotocinclus fluminense | 0 | 0 | 0 | 0 | 0 | 515 | 0 | 0 | 0 |
| Parotocinclus maculicauda | 0 | 390 | 0 | 0 | 940 | 36 | 4 | 179 | 0 |
| Parotocinclus muriaensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 |
| Phalloceros anisophallos | 3391 | 1144 | 0 | 0 | 101 | 0 | 0 | 0 | 0 |
| Phalloceros enneaktinos | 701 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Phalloceros harpagos | 468 | 3200 | 276 | 1295 | 773 | 887 | 247 | 3306 | 1148 |
| Phalloceros leptokeras | 946 | 57 | 121 | 2216 | 1419 | 56 | 0 | 0 | 0 |
| Phalloceros tupinamba | 0 | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Phalloptychus januarius | 0 | 33 | 0 | 0 | 1076 | 133 | 0 | 0 | 11100 |
| Phallotorynus fasciolatus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 47 |
| Piabina argentea | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 |
| Pimelodella lateristriga | 101 | 58 | 2 | 25 | 224 | 201 | 81 | 167 | 236 |
| Pimelodus maculatus | 0 | 11 | 57 | 11 | 0 | 0 | 8 | 0 | 13 |
| Poecilia vivipara | 120 | 378 | 3 | 58 | 2278 | 298 | 130 | 1660 | 10094 |
| Pogonopoma parahybae | 0 | 0 | 3 | 1 | 0 | 0 | 18 | 0 | 0 |
| Prochilodus lineatus | 0 | 2 | 4 | 0 | 0 | 0 | 13 | 6 | 233 |
| Prochilodus vimboides | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 11 | 170 |
| Psalidodon parahybae | 0 | 138 | 179 | 105 | 82 | 192 | 401 | 9 | 1395 |
| Pseudotothyris janeirensis | 0 | 147 | 0 | 0 | 29 | 9 | 0 | 29 | 18 |
| Rhamdia quelen | 43 | 138 | 24 | 29 | 188 | 26 | 24 | 53 | 55 |
| Rhamdioglanis frenatus | 55 | 72 | 0 | 0 | 48 | 0 | 3 | 0 | 0 |
| Rhamdioglanis transfasciatus | 0 | 1 | 0 | 0 | 63 | 108 | 1 | 131 | 0 |
| Rineloricaria nigricauda | 0 | 553 | 309 | 381 | 0 | 0 | 4 | 0 | 4 |
| Rineloricaria nudipectoris | 0 | 22 | 0 | 263 | 1249 | 62 | 0 | 145 | 0 |
| Rineloricaria steindachneri | 0 | 3 | 0 | 0 | 0 | 0 | 107 | 0 | 23 |
| Rineloricaria zawadzki | 40 | 227 | 14 | 0 | 95 | 0 | 0 | 0 | 0 |
| Schizolecis guentheri | 2592 | 622 | 0 | 20 | 1394 | 123 | 42 | 513 | 0 |
| Scleromystax barbatus | 431 | 147 | 0 | 134 | 732 | 144 | 37 | 51 | 7 |
| Scleromystax prionotos | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 7 |
| Spintherobolus broccae | 0 | 10 | 0 | 0 | 158 | 39 | 0 | 17 | 0 |
| Steindachneridion parahybae | 0 | 0 | 11 | 4 | 0 | 0 | 1 | 0 | 0 |
| Synbranchus marmoratus | 3 | 7 | 11 | 2 | 56 | 11 | 4 | 10 | 7 |
| Taunayia bifasciata | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trachelyopterus striatulus | 0 | 0 | 0 | 0 | 5 | 0 | 7 | 0 | 0 |
| Trichogenes longipinnis | 627 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trachelyopterus striatulus | 0 | 31 | 1 | 4 | 0 | 23 | 0 | 71 | 78 |
| Trichomycterus albinotatus | 0 | 0 | 94 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus auroguttatus | 0 | 11 | 142 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus caipora | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 110 | 97 |
| Trichomycterus claudiae | 0 | 83 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus florensis | 0 | 0 | 16 | 16 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus fuliginosus | 0 | 0 | 0 | 0 | 0 | 0 | 58 | 0 | 0 |
| Trichomycterus goeldii | 0 | 0 | 0 | 179 | 0 | 0 | 27 | 0 | 0 |
| Trichomycterus giganteus | 0 | 201 | 0 | 0 | 153 | 0 | 0 | 0 | 0 |
| Trichomycterus itatiayae | 0 | 0 | 197 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus jacupiranga | 452 | 30 | 0 | 0 | 77 | 0 | 0 | 0 | 0 |
| Trichomycterus largoperculatus | 0 | 0 | 0 | 33 | 0 | 0 | 28 | 0 | 1 |
| Trichomycterus macrophthalmus | 0 | 142 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus mariamole | 0 | 13 | 82 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus mirissumba | 0 | 0 | 90 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus nigricans | 0 | 0 | 0 | 0 | 972 | 16 | 0 | 0 | 0 |
| Trichomycterus nigroauratus | 0 | 215 | 24 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus paquequerense | 0 | 0 | 0 | 1012 | 80 | 0 | 0 | 0 | 0 |
| Trichomycterus potschi | 0 | 134 | 102 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichomycterus puriventris | 0 | 0 | 0 | 0 | 0 | 0 | 228 | 0 | 3 |
| Trichomycterus vitalbrazili | 0 | 0 | 0 | 0 | 0 | 0 | 60 | 0 | 0 |
References
- Thomaz, A.T.; Malabarba, L.R.; Bonatto, S.L.; Knowles, L.L. Testing the effect of palaeodrainages versus habitat stability on genetic divergence in riverine systems: Study of a Neotropical fish of the Brazilian coastal Atlantic Forest. J Biogeogr 2015, 42, 2389–2401. [Google Scholar] [CrossRef]
- Dagosta, F.C.P.; de Pinna, M.C.C. A history of the biogeography of Amazonian fishes. Neotrop Ichthyol 2018, 16, e180023. [Google Scholar] [CrossRef]
- Sarmento-Soares, L.M.; Martins-Pinheiro, R.F. A fauna de peixes nas bacias do sul do Espírito Santo, Brasil. Sitientibus. Série Ciências Biológicas 2013, 13, 1–37. [CrossRef]
- Camelier, P.; Zanata, A.M. Biogeography of freshwater fishes from the Northeastern Mata Atlântica freshwater ecoregion: Distribution, endemism, and area relationships. Neotrop Ichthyol 2014, 12, 683–698. [CrossRef]
- Vieira-Guimarães, F.; Sarmento-Soares, L.M.; Nobre, D.M.; Neiva, D.C.; Silva, J.P.; Martins-Pinheiro, R.F. Biogeographic patterns of the freshwater fishes from the state of Espírito Santo, eastern Brazil. Stud Neotrop Fauna Environ 2023, 2023, 1–20. [CrossRef]
- Bizerril, C.R.S.F.; Primo, P.B.S. Peixes de Águas Interiores do Estado do Rio de Janeiro. FEMAR—SEMADS: Rio de Janeiro, Brazil, 2001; 417 p.
- Gomes, J.R. Levantamento da ictiofauna do Maciço da Pedra Branca e arredores, Rio de Janeiro, estado do Rio de Janeiro. Arq Mus Nac Rio J 2006, 64, 309–320.
- Mazzoni, R.; Verani-Fenerich, N.; Caramaschi, E.P.; Iglesias-Rios, R. Stream-Dwelling Fish Communities from an Atlantic Rain Forest Drainage. Braz Arch Biol Technol 2006, 49, 249–256. [CrossRef]
- Di Dario, F.; Petry, A.C.; Pereira, M.M.S.; Mincarone, M.M.; Agostinho, L.S.; Camara, E.M.; Caramaschi, E.P.; Britto, M.R. An update on the fish composition (Teleostei) of the coastal lagoons of the Restinga de Jurubatiba National Park and the Imboassica Lagoon, northern Rio de Janeiro State. Acta Limnol Bras 2013, 25, 257–278.
- Buckup, P.A.; Britto, M.R.; Souza-Lima, R.; Pascoli, J.C.; Villa-Verde, L.; Ferraro, G.A.; Salgado, F.L.K.; Gomes, J.R. Guia de identificação das espécies de peixes da bacia do Rio das Pedras. The Nature Conservancy: Rio de Janeiro, Brazil, 2014; 79 p.
- Camilo, G.S.; Terra, B.; Araújo, F.G. Ichthyofauna from the Parque Nacional da Serra dos Órgãos and its surrounding areas, Rio de Janeiro state, Brazil. Check List 2015, 11, 1696. [CrossRef]
- De Brito, V.; Buckup, P.A. The fish fauna of the upper Piraí drainage, a transposed mountain river system in southeastern, Brazil. Check List 2019, 15, 235–247. [CrossRef]
- Guimarães, F.V.; Souza, T.M.; Rodrigues, R.R.; Souza-Lima, R. Composition and distribution of fishes from the Perequê-Açu river basin, Paraty, Rio de Janeiro, Southeastern Brazil. Biota Neotrop 2021, 21, e20201096. [CrossRef]
- Dopazo, M.; Souto-Santos, I.C.A.; Britto, M.R.; Moreira, C.R.; Buckup, P.A. The freshwater fishes from the Costa Verde Fluminense region of southeastern Brazil. Biota Neotrop 2023, 23, e20221422. [CrossRef]
- Pfafstetter, O. Classificação de bacias hidrográficas – Metodologia de classificação. Departamento Nacional de Obras de Saneamento (DNOS): Rio de Janeiro, Brazil, 1989. Unpublished manuscript.
- Sarmento-Soares, L.M.; Martins-Pinheiro, R.F.; Rodrigues, L.N. Peixes do rio Doce segundo as coleções. Bol Soc Brasil Ictiologia 2017, 123, 9-25.
- Agência Nacional de Águas (ANA). Topologia hídrica: Método de construção e modelagem da base hidrográfica para suporte à gestão de recursos hídricos: V. 1.11. Agência Nacional de Águas, Superintendência de Gestão da Informação: Brasília, Brazil, 2006.
- Conselho Estadual de Recursos Hídricos do Rio de Janeiro (CERHI-RJ). Resolução nº 107 de 22 de maio de 2013, Conselho Estadual de Recursos Hídricos: Rio de Janeiro, Brazil, 2013.
- Sabaj-Pérez, M.H. Codes for natural history collections in ichthyology and herpetology. Ichthyol Herpetol 2020, 108, 1–76. [CrossRef]
- ricke, R.; Eschmeyer, W.N.; Van Der Laan, R. Eschmeyer’s Catalog of Fishes: Genera, Species, References. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (Accessed 15 May 2024). Genera, Species.
- Ferreira Júnior, O. GPS Software for mapping and innovative solutions for asset tracking: GPS Trackmaker Pro v. 5.1. Geo Studio Tecnologia Ltda: Belo Horizonte, Brazil, 2012.
- Instituto Brasileiro de Geografia e Estatística (IBGE). Base cartográfica vetorial contínua do estado do Rio de Janeiro na escala 1:25.000 - BC25_RJ. Available online: https://www.ibge.gov.br/geociencias/downloads-geociencias.html?caminho=cartas_e_mapas/bases_cartograficas_continuas/bc250/versao2023/ (Accessed on 01 June 2024).
- Instituto Brasileiro de Geografia e Estatística (IBGE). Área territorial - Brasil, Grandes Regiões, Unidades da Federação e Municípios. Available online: https://geoftp.ibge.gov.br/organizacao_do_territorio/estrutura_territorial/areas_territoriais/2022/AR_BR_RG_UF_RGINT_MES_MIC_MUN_2022.xls (Accessed on 01 June 2024).
- Silva,T.N.; Sarmento-Soares, L.M.; Martins-Pinheiro, R.F.; Santos, A.C.A. (2021): Composition and distribution of the fish fauna in the Rio Jacuípe, northernmost tributary of the Rio Paraguaçu basin, Bahia, Brazil, Studies on Neotropical Fauna and Environment, 2021. [CrossRef]
- Dajoz, R. Ecologia Geral. 4th ed.; Vozes: Petrópolis, Brazil, 1983; 472 p.
- Harper, D.A.T. Numerical palaeobiology: Computer- based modelling and analysis of fossils and their distributions. John Wiley & Sons: Chichester, USA, 1999; 468 p.
- Tóthmérész, B. Comparison of different methods for diversity ordering. J Veg Sci 1995, 6, 283–290. [CrossRef]
- Hammer, O; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol Eletron 2001, 4, 1–9.
- Walther, B.A.; Moore, J.L. The concepts of bias, precision and accuracy, and their use in testing the performance of species richness estimators, with a literature review of estimator performance. Ecography 2005, 28, 815–829. [CrossRef]
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand J Stat 1984, 11, 265–270.
- Chao, A. Estimating the population size for capture-recapture data with unequal catchability. Biometrics 1987, 43, 783–791. [CrossRef]
- Chiu, C.H.; Wang, Y.T.; Walther, B.A.; Chao, A. An improved nonparametric lower bound of species richness via a modified Good-Turing frequency formula. Biometrics 2014, 70, 671–682. [CrossRef]
- Chao, A.; Lee, S-M. Estimating the number of classes via sample coverage. J Am Stat Assoc 1992, 87, 210–217. [CrossRef]
- Chao, A.; Hwang, W-H; Chen, Y-C.; Kuo C-Y. Estimating the number of shared species in two communities. Stat Sinica 2000, 10, 227–246.
- Alroy, J. Limits to species richness in terrestrial communities. Ecol Lett 2018, 21, 1781–1789. [CrossRef]
- Edler, D.; Guedes, T.; Zizka, A.; Rosvall, M.; Antonelli, A. Infomap bioregions: Interactive mapping of biogeographical regions from species distributions. Syst Biol 2017, 66, 197–204. [CrossRef]
- Oliveira, U; Soares-Filho, B., Leitão, R.F.M.; Rodrigues, H.O. BioDinamica: A toolkit for analyses of biodiversity and biogeography on the Dinamica-EGO modelling platform. PeerJ 2019, 7, e7213. [CrossRef]
- Ferreira, B.M.; Soares-Filho, B S.; Pereira, F.M.Q. The Dinamica EGO virtual machine. Sci Comput Program 2019, 173, 3–20. [CrossRef]
- Bizerril, C.R.S.F. A Ictiofauna da Bacia do Rio Paraíba do Sul. Biodiversidade e Padrões Biogeográficos. Braz Arch Biol Technol 1999, 42, 1–17. [CrossRef]
- Menezes, N.A.; Weitzman, S.H.; Oyakawa, O.T.; Lima, F.C.; Castro, R.M.C.; Weitzman, M.J. Peixes de água doce da Mata Atlântica. Neotrópica: São Paulo, Brazil, 2007; 407 p.
- Abell, R.; Thieme, M.L.; Revenga, C.; Bryer, M.; Kottelat, M.; Bogutskaya, N.; Coad, B.; Mandrak, N.; Balderas, S.C.; Bussing, W.; Stiassny, M.L.J.; Skelton, P.; Allen, G.R.; Unmack, P.; Naseka, A.; Ng, R.; Sindorf, N.; Robertson, J.; Armijo, E.; Higgins, J.V.; Heibel, T.J.; Wikramanayake, E.; Olson, D.; López, H.L.; Reis, R.E.; Lundberg, J.G.; Pérez, M.H.S.; Petry, P. Freshwater ecoregions of the world: A new map of biogeographic units for freshwater biodiversity conservation. BioScience 2008, 58, 403–414. [CrossRef]
- Zalán, P.V.; Oliveira, J.A.B. Origem e evolução estrutural do Sistema de Riftes Cenozóicos do Sudeste do Brasil. Bol Geoci Petrobras 2005, 13, 269–300.
- Lamego, A.R. O homem e a Guanabara, 2nd ed. IBGE- Conselho Nacional de Geografia: Rio de Janeiro, Brazil, 1964; 408 p.
- Silva, C.G.; Reis, A.T.; Goiana, L.T.C.; Ferrari, A.L. A história do preenchimento sedimentar da baía de Guanabara através da geofísica. In Baía de Guanabara: Um ambiente em transformação; Fonseca, E.M.; Neto, J.A.B.; Pompermayer, F.C.L., Eds.; Ape’Ku: Rio de Janeiro, Brazil, 2021; pp. 47–72.
- Buckup, P.A. The Eastern Brazilian Shield. In Historical Biogeography of Neotropical Freshwater Fishes; Albert, J.S.; Reis, R.E., Eds.; University of California Press, Los Angeles, USA, 2011; pp. 203–210. [CrossRef]
- Pio, N.L.; Carvalho, T.P. Evidence on the paleodrainage connectivity during Pleistocene: Phylogeography of a hypoptopomatine endemic to southeastern Brazilian coastal drainages. Neotrop Ichthyol 2021, 19, e200128. [CrossRef]
- Lima, S.M.Q.; Vasconcellos, A.V.; Berbel-Filho, W.M.; Lazoski, C.; Russo, C.A.; Sazima, I.; Solé-Cava, A.M. Effects of Pleistocene climatic and geomorphological changes on the population structure of the restricted-range catfish Trichogenes longipinnis (Siluriformes: Trichomycteridae). Syst Biodivers 2017, 14, 155–170. [CrossRef]
- Lowe-McConnell, R.H. Estudos ecológicos de comunidades de peixes tropicais. EDUSP: São Paulo, Brazil, 1999; 536 p.
- Souza-Lima, R.; Miranda, J.C.; Portugal, A.S. Ictiofauna do Rio Aldeia, São Gonçalo. In Estudos Ambientais em Regiões Metropolitanas: São Gonçalo; Santos, M.G., Ed; Ed. UERJ: Rio de Janeiro, Brazil, 2012; pp. 115–134.
- Angrizani, R.C.; Malabarba, L.R. Genetic diversity and species delimitation in Rhamdia (Siluriformes: Heptapteridae) in South America, with a redescription of R. quelen (Quoy & Gaimard, 1824). Zootaxa 2020, 4801, 85–104. [CrossRef]
- Craig, J.M; Crampton, W.G.R.; Albert, J.S. Revision of the polytypic electric fish Gymnotus carapo (Gymnotiformes, Teleostei), with descriptions of seven subspecies. Zootaxa 2017, 4318, 401–438. [CrossRef]
- Dergam, J.A., Suzuki, H.I., Shibatta, O.A., Duboc, L.F., Júlio, H.F., Jr., Giuliano-Caetano, L.; Black, W.C., IV. Molecular biogeography of the neotropical fish Hoplias malabaricus (Erythrinidae: Characiformes) in the Iguaçu, Tibagi and Paraná Rivers. Genet Mol Biol 1998, 21, 493–496. [CrossRef]
- Torres, R.A.; Roper, J.J.; Foresti, F.; Oliveira, C. Surprising genomic diversity in the Neotropical fish Synbranchus marmoratus (Teleostei: Synbranchidae): How many species? Neotrop Ichthyol 2005, 3, 277–284. [CrossRef]
- Bertaco, V.A.; Ferrer, J.; Carvalho, F.R.; Malabarba, L.R. Inventory of the freshwater fishes from a densely collected area in South America – a case study of the current knowledge of Neotropical fish diversity. Zootaxa 2016, 4138, 401–440. [CrossRef]
- Guimarães, K.L.A.; Rosso, J.J.; González-Castro, M.; Souza, M.F.B.; Díaz de Astarloa, J.M.; Rodrigues, L.R.R. A new species of Hoplias malabaricus species complex (Characiformes: Erythrinidae) from the Crepori River, Amazon basin, Brazil. J Fish Biol 2022, 100, 425–443. [CrossRef]
| Ocean | Continental | Not identified | % | |
|---|---|---|---|---|
| Marine origin | 2,890 | 985 | ----- | 7.39% |
| Allochthonous freshwater | ----- | 498 | ----- | 3.74% |
| Native freshwater | ----- | 11,724 | ----- | 87.97% |
| Not identified at the species level | ----- | 120 | ----- | 0.90% |
| Not identified at the locality level | ----- | ----- | 82 | |
| Total | 2,890 | 13,327 | 82 | 16,299 |
| % | 17.73% | 81.77% | 0.50% |
| Code | Hydrographic region | Area (km2) | Lots | Points | il | ilq | ip | ipq |
|---|---|---|---|---|---|---|---|---|
| RH-1 | Baía da Ilha Grande | 1,919.1 | 1,150 | 140 | 59.9 | Good | 7.3 | Good |
| RH-2 | Guandu | 4,087.8 | 1,789 | 234 | 43.8 | Good | 5.7 | Good |
| RH-3 | Middle Paraíba do Sul | 7,114.1 | 860 | 133 | 12.1 | Poor | 1.9 | Poor |
| RH-4 | Piabanha | 3,831.4 | 816 | 121 | 21.3 | Average | 3.2 | Average |
| RH-5 | Baía de Guanabara | 696.0 | 3,190 | 428 | 458.3 | Good | 61.5 | Good |
| RH-6 | Lagos São João | 4,030.2 | 1,353 | 143 | 33.6 | Average | 3.5 | Average |
| RH-7 | Rio Dois Rios | 4,940.9 | 780 | 81 | 15.8 | Poor | 1.6 | Poor |
| RH-8 | Macaé and das Ostras | 2,226.9 | 1,474 | 133 | 66.2 | Good | 6.0 | Good |
| RH-9 | Lower Paraíba do Sul and Itabapoana | 14,904.0 | 1,915 | 210 | 12.8 | Poor | 1.4 | Poor |
| Rio de Janeiro State | 43,750 | 13,327 | 1.623 | 30.5 | 3.7 |
| Code | Basin groups | Area (km2) | Lots | Points | Il | Ilq | Ip | Ipq |
|---|---|---|---|---|---|---|---|---|
| 08.20 | Itabapoana | 1,523.4 | 122 | 19 | 8.0 | Poor | 1.2 | Poor |
| 08.21 | São Francisco do Itabapoana | 971.3 | 38 | 6 | 3.9 | Poor | 0.6 | Poor |
| 08.22a | Rio Paraíba do Sul - RH-9 | 6,321.4 | 1012 | 63 | 16.0 | Poor | 1.0 | Poor |
| 08.22b | Rio Paraíba do Sul - RH-7 | 4,468.6 | 780 | 81 | 17.5 | Poor | 1.8 | Poor |
| 08.22c | Rio Paraíba do Sul - RH-4 | 3,469.0 | 816 | 121 | 23.5 | Average | 3.5 | Average |
| 08.22d | Rio Paraíba do Sul - RH-3 | 6,430.8 | 860 | 133 | 13.4 | Poor | 2.1 | Poor |
| 08.22e | Rio Paraíba do Sul - RH-2 | 1,014.1 | 611 | 68 | 60.3 | Good | 6.7 | Good |
| 08.23 | Lagoa Feia | 4,310.8 | 512 | 93 | 11.9 | Poor | 2.2 | Poor |
| 08.24 | Jurubatiba | 410.1 | 707 | 62 | 172.4 | Good | 15.1 | Good |
| 08.25 | Rio Macaé | 1,706.4 | 894 | 92 | 52.4 | Good | 5.4 | Good |
| 08.26 | Rio das Ostras | 249.4 | 104 | 8 | 41.7 | Good | 3.2 | Average |
| 08.27 | Rio São João | 2,155.6 | 1052 | 86 | 48.8 | Good | 4.0 | Average |
| 08.28 | Rio Una and Búzios | 541.7 | 11 | 4 | 2.0 | Poor | 0.7 | Poor |
| 08.29 | Lagoa de Araruama | 677.5 | 67 | 16 | 9.9 | Poor | 2.4 | Poor |
| 08.30 | Saquarema | 265.2 | 210 | 35 | 79.2 | Good | 13.2 | Good |
| 08.31 | Maricá | 349.5 | 167 | 33 | 47.8 | Good | 9.4 | Good |
| 08.32 | Niterói | 51.5 | 23 | 6 | 44.7 | Good | 11.7 | Good |
| 08.33 | Baía de Guanabara | 4,073.8 | 2412 | 269 | 59.2 | Good | 6.6 | Good |
| 08.34 | Rio de Janeiro | 2,636.3 | 1673 | 269 | 63.5 | Good | 10.2 | Good |
| 08.35 | Sepetiba | 107.0 | 39 | 7 | 36.4 | Average | 6.5 | Good |
| 08.36 | Mangaratiba | 289.8 | 67 | 12 | 23.1 | Average | 4.1 | Average |
| 08.37 | Angra dos Reis | 1,028.2 | 557 | 59 | 54.2 | Average | 5.7 | Average |
| 08.38 | Paraty | 376.9 | 301 | 48 | 79.9 | Good | 12.7 | Good |
| 08.38 | Cairuçu | 322.2 | 292 | 33 | 90.6 | Good | 10.2 | Good |
| Rio de Janeiro State | 43,750 | 13,327 | 1,623 | 30.5 | 3.7 |
| Environment/Origin | S | % |
|---|---|---|
| Native freshwater | 172 | 49.28% |
| Allochthonous freshwater | 22 | 6.30% |
| Marine origin | 152 | 43.55% |
| Not identified at the species level | 3 | 0.86% |
| Total | 349 |
| Order | Family | Subfamily | Suggested species | Author |
|---|---|---|---|---|
| Gymnotiformes | Sternopygidae | Eigenmannia virescens | (Valenciennes, 1836) | |
| Gymnotidae | Gymnotinae | Gymnotus carapo | Linnaeus, 1758 | |
| Gymnotus pantherinus | (Steindachner, 1908) | |||
| Hypopomidae | Brachyhypopomus janeiroensis | (Costa & Campos-da-Paz, 1992) | ||
| Characiformes | Crenuchidae | Characidiinae | Characidium alipioi | Travassos, 1955 |
| Characidium grajahuense | Travassos, 1944 | |||
| Characidium interruptum | Pellegrin, 1909 | |||
| Characidium japuhybense | Travassos, 1949 | |||
| Characidium lauroi | Travassos, 1949 | |||
| Characidium litorale | Leitão & Buckup, 2014 | |||
| Characidium vidali | Travassos, 1967 | |||
| Erythrinidae | Hoplerythrinus unitaeniatus | (Spix & Agassiz, 1829) | ||
| Hoplias malabaricus | (Bloch, 1794) | |||
| Anostomidae | Hypomasticus copelandii | (Steindachner, 1875) | ||
| Hypomasticus mormyrops | (Steindachner, 1875) | |||
| Hypomasticus thayeri | (Borodin, 1929) | |||
| Megaleporinus conirostris | (Steindachner, 1875) | |||
| Curimatidae | Cyphocharax gilbert | (Quoy & Gaimard, 1824) | ||
| Prochilodontidae | Prochilodus lineatus | (Valenciennes, 1837) | ||
| Prochilodus vimboides | Kner, 1859 | |||
| Bryconidae | Bryconinae | Brycon insignis | Steindachner, 1877 | |
| Brycon opalinus | (Cuvier, 1819) | |||
| Characidae | Stethaprioninae | Astyanax keronolepis | Silva, Malabarba & Malabarba, 2019 | |
| Astyanax lacustris | (Lütken, 1875) | |||
| Deuterodon giton | (Eigenmann, 1908) | |||
| Deuterodon hastatus | (Myers, 1928) | |||
| Deuterodon heterostomus | (Eigenmann, 1911) | |||
| Deuterodon intermedius | (Eigenmann, 1908) | |||
| Deuterodon janeiroensis | (Eigenmann, 1908) | |||
| Deuterodon luetkenii | (Boulenger, 1887) | |||
| Deuterodon taeniatus | (Jenyns, 1842) | |||
| Hollandichthys multifasciatus | (Eigenmann & Norris, 1900) | |||
| Hyphessobrycon bifasciatus | Ellis, 1911 | |||
| Hyphessobrycon boulengeri | (Eigenmann, 1907) | |||
| Hyphessobrycon flammeus | Myers, 1924 | |||
| Oligosarcus hepsetus | (Cuvier, 1829) | |||
| Psalidodon parahybae | (Eigenmann, 1908) | |||
| Spintherobolinae | Spintherobolus broccae | Myers, 1925 | ||
| Stevardiinae | Bryconamericus microcephalus | (Miranda Ribeiro, 1908) | ||
| Bryconamericus ornaticeps | Bizerril & Perez-Neto, 1995 | |||
| Bryconamericus tenuis | Bizerril & Auraujo, 1992 | |||
| Knodus moenkhausii | (Eigenmann & Kennedy, 1903) | |||
| Mimagoniates microlepis | (Steindachner, 1877) | |||
| Piabina argentea | Reinhardt, 1867 | |||
| Siluriformes | Trichomycteridae | Trichogeninae | Trichogenes longipinnis | Britski & Ortega, 1983 |
| Trichomycterinae | Trichomycterus albinotatus | Costa, 1992 | ||
| Ituglanis parahybae | (Eigenmann, 1918) | |||
| Trichomycterus auroguttatus | Costa, 1992 | |||
| Trichomycterus caipora | Lima, Lazzarotto & Costa, 2008 | |||
| Trichomycterus claudiae | Barbosa & Costa, 2010 | |||
| Trichomycterus florensis | (Miranda-Ribeiro, 1943) | |||
| Trichomycterus fuliginosus | Barbosa & Costa, 2010 | |||
| Trichomycterus giganteus | Lima & Costa, 2004 | |||
| Trichomycterus goeldii | Boulenger, 1896 | |||
| Trichomycterus itatiayae | Miranda Ribeiro, 1906 | |||
| Trichomycterus jacupiranga | Wosiacki & Oyakawa, 2005 | |||
| Trichomycterus largoperculatus | Costa & Katz, 2022 | |||
| Trichomycterus macrophthalmus | Barbosa & Costa, 2012 | |||
| Trichomycterus mariamole | Barbosa & Costa, 2010 | |||
| Trichomycterus mirissumba | Costa, 1992 | |||
| Trichomycterus nigricans | Valenciennes, 1832 | |||
| Trichomycterus nigroauratus | Barbosa & Costa, 2008 | |||
| Trichomycterus paquequerense | (Miranda Ribeiro, 1943) | |||
| Trichomycterus potschi | Barbosa & Costa, 2003 | |||
| Trichomycterus puriventris | Barbosa & Costa, 2012 | |||
| Trichomycterus travassosi | (Miranda Ribeiro, 1949 | |||
| Trichomycterus vitalbrazili | Vilardo, Katz & Costa, 2020 | |||
| Microcambevinae | Listrura costai | Villa-Verde, Lazzarotto & Lima, 2012 | ||
| Listrura nematopteryx | de Pinna, 1988 | |||
| Listrura tetraradiata | Landim & Costa, 2002 | |||
| Microcambeva barbata | Costa & Bockmann, 1994 | |||
| Microcambeva bendego | Medeiros, Moreira, de Pinna & Lima, 2020 | |||
| Stegophilinae | Homodiaetus banguela | Koch, 2002 | ||
| Homodiaetus passarellii | (Miranda Ribeiro, 1944 | |||
| Callichthyidae | Callichthyinae | Callichthys callichthys | (Linnaeus, 1758) | |
| Hoplosternum littorale | (Hancock, 1828) | |||
| Corydoradinae | Corydoras nattereri | Steindachner, 1876 | ||
| Scleromystax barbatus | (Quoy & Gaimard, 1824) | |||
| Scleromystax prionotos | (Nijssen & Isbrücker, 1980) | |||
| Loricariidae | Delturinae | Delturus parahybae | Eigenmann & Eigenmann, 1889 | |
| Hemipsilichthys gobio | (Lütken, 1874) | |||
| Hemipsilichthys nimius | Pereira, Reis, Souza & Lazzarotto, 2003 | |||
| Hemipsilichthys papillatus | Pereira, Oliveira & Oyakawa, 2000 | |||
| Rhinelepinae | Pogonopoma parahybae | (Steindachner, 1877) | ||
| Loricariinae | Harttia carvalhoi | Miranda Ribeiro, 1939 | ||
| Harttia loricariformis | Steindachner, 1877 | |||
| Loricariichthys castaneus | (Castelnau, 1855) | |||
| Rineloricaria nigricauda | (Regan, 1904) | |||
| Rineloricaria nudipectoris | Mejia, Ferrar & Buckup, 2023 | |||
| Rineloricaria steindachneri | (Regan, 1904) | |||
| Rineloricaria zawadzki | Silva, Costa & Oliveira, 2022 | |||
| Hypoptopomatinae | Hisonotus notatus | Eigenmann & Eigenmann, 1889 | ||
| Hisonotus thayeri | Martins & Langeani, 2016 | |||
| Kronichthys heylandi | (Boulenger, 1900) | |||
| Neoplecostomus microps | (Steindachner, 1877) | |||
| Neoplecostomus paraty | Cherobim, Lazzarotto & Langeani, 2016 | |||
| Neoplecostomus variipictus | Bizerril, 1995 | |||
| Otocinclus affinis | Steindachner, 1877 | |||
| Otothyris lophophanes | (Eigenmann & Eigenmann, 1889) | |||
| Pareiorhaphis garbei | (Ihering, 1911) | |||
| Pareiorhina brachyrhyncha | Chamon, Aranda & Buckup, 2005 | |||
| Pareiorhina rudolphi | (Miranda Ribeiro, 1911) | |||
| Parotocinclus bidentatus | Gauger & Buckup, 2005 | |||
| Parotocinclus fluminense | Roxo, Melo, Silva & Oliveira, 2017 | |||
| Parotocinclus maculicauda | (Steindachner, 1877) | |||
| Parotocinclus muriaensis | Gauger & Buckup, 2005 | |||
| Pseudotothyris janeirensis | Britski & Garavello, 1984 | |||
| Schizolecis guentheri | (Miranda Ribeiro, 1918) | |||
| Hypostominae | Ancistrus multispinis | (Regan, 1912) | ||
| Hypostomus affinis | (Steindachner, 1877) | |||
| Hypostomus auroguttatus | Kner, 1854 | |||
| Hypostomus luetkeni | (Steindachner, 1877) | |||
| Hypostomus punctatus | Valenciennes, 1840 | |||
| Hypostomus vermicularis | (Eigenmann & Eigenmann, 1888) | |||
| Auchenipteridae | Centromochlinae | Glanidium melanopterum | Miranda Ribeiro, 1918 | |
| Auchenipterinae | Trachelyopterus striatulus | (Steindachner, 1877) | ||
| Heptapteridae | Rhamdiinae | Pimelodella lateristriga | (Lichtenstein, 1823) | |
| Rhamdia quelen | (Quoy & Gaimard, 1824) | |||
| Heptapterinae | Acentronichthys leptos | Eigenmann & Eigenmann, 1889 | ||
| Imparfinis minutus | (Lütken, 1874) | |||
| Imparfinis piperatus | Eigenmann & Norris, 1900 | |||
| Rhamdioglanis frenatus | Ihering, 1907 | |||
| Rhamdioglanis transfasciatus | Miranda Ribeiro, 1908 | |||
| Taunayia bifasciata | (Eigenmann & Norris, 1900) | |||
| Pimelodidae | Pimelodus maculatus | Lacepède, 1803 | ||
| Steindachneridion parahybae | (Steindachner, 1877) | |||
| Pseudopimelodidae | Microglanis nigripinnis | Bizerril & Perez-Neto, 1992 | ||
| Microglanis parahybae | (Steindachner, 1880) | |||
| Microglanis pleriqueater | Mattos, Ottoni & Barbosa, 2013 | |||
| Ariidae | Ariinae | Paragenidens grandoculis | (Steindachner, 1877) | |
| Gobiiformes | Eleotridae | Eleotrinae | Dormitator maculatus | (Bloch, 1792) |
| Eleotris pisonis | (Gmelin, 1789) | |||
| Oxudercidae | Gobionellinae | Awaous tajasica | (Lichtenstein, 1822) | |
| Synbranchiformes | Synbranchidae | Synbranchus marmoratus | Bloch, 1795 | |
| Cyprinodontiformes | Rivulidae | Rivulinae | Atlantirivulus guanabarensis | Costa, 2014 |
| Atlantirivulus janeiroensis | (Costa, 1991) | |||
| Atlantirivulus jurubatibensis | (Costa, 2008) | |||
| Atlantirivulus lazzarotoi | (Costa, 2007) | |||
| Atlantirivulus maricensis | Costa, 2014 | |||
| Atlantirivulus simplicis | (Costa, 2004) | |||
| Kryptolebiatinae | Kryptolebias brasiliensis | (Valenciennes, 1821) | ||
| Kryptolebias caudomarginatus | (Seegers, 1984) | |||
| Kryptolebias gracilis | Costa, 2007 | |||
| Kryptolebias ocellatus | (Hensel, 1868) | |||
| Cynolebiinae | Leptolebias marmoratus | (Ladiges, 1934) | ||
| Leptopanchax citrinipinnis | (Costa, Lacerda & Tanizaki, 1988) | |||
| Leptopanchax opalescens | (Myers, 1942) | |||
| Leptopanchax sanguineus | Costa, 2019 | |||
| Leptopanchax splendens | (Myers, 1942) | |||
| Nematolebias papilliferus | Costa, 2002 | |||
| Nematolebias whitei | (Myers, 1942) | |||
| Notholebias cruzi | (Costa, 1988) | |||
| Notholebias fractifasciatus | (Costa, 1988) | |||
| Notholebias minimus | (Myers, 1942) | |||
| Ophthalmolebias constanciae | (Myers, 1942) | |||
| Poeciliidae | Poeciliinae | Phalloceros anisophallos | Lucinda, 2008 | |
| Phalloceros enneaktinos | Lucinda, 2008 | |||
| Phalloceros harpagos | Lucinda, 2008 | |||
| Phalloceros leptokeras | Lucinda, 2008 | |||
| Phalloceros tupinamba | Lucinda, 2008 | |||
| Phalloptychus januarius | (Hensel, 1868) | |||
| Phallotorynus fasciolatus | Henn, 1916 | |||
| Poecilia reticulata | Peters, 1859 | |||
| Poecilia vivipara | Bloch & Schneider, 1801 | |||
| Anablepidae | Anablepinae | Jenynsia darwini | Amorim, 2018 | |
| Jenynsia lineata | (Jenyns, 1842) | |||
| Cichliformes | Cichlidae | Cichlinae | Australoheros ipatinguensis | Ottoni & Costa, 200 |
| Australoheros oblongus | (Castelnau 1855) | |||
| Crenicichla lacustris | (Castelnau, 1855) | |||
| Crenicichla lepidota | Heckel, 1840 | |||
| Geophagus brasiliensis | (Quoy & Gaimard, 1824) | |||
| Acanthuriformes | Sciaenidae | Pachyurus adspersus | Steindachner, 1879 |
| RH-1 | RH-2 | RH-3 | RH-4 | RH-5 | RH-6 | RH-7 | RH-8 | RH-9 | |
|---|---|---|---|---|---|---|---|---|---|
| Taxa_S | 40 | 97 | 67 | 69 | 89 | 80 | 74 | 67 | 81 |
| Individuals | 20058 | 24736 | 4581 | 9256 | 29430 | 11829 | 4243 | 27928 | 40232 |
| Dominance_D | 0.1032 | 0.0915 | 0.05703 | 0.1038 | 0.04186 | 0.06386 | 0.04392 | 0.1214 | 0.1549 |
| Shannon_H | 2.7 | 3.176 | 3.284 | 2.858 | 3.528 | 3.366 | 3.516 | 2.595 | 2.513 |
| Equitability_J | 0.732 | 0.6944 | 0.7811 | 0.675 | 0.786 | 0.7682 | 0.8169 | 0.6172 | 0.5719 |
| Chao-1 | 40 | 97.17 | 74 | 78.33 | 90.5 | 80.17 | 75.5 | 70 | 102 |
| iChao-1 | 40 | 97.17 | 78.57 | 86.75 | 93.5 | 80.4 | 77.07 | 76.5 | 103.7 |
| ACE | 40 | 97.3 | 73.45 | 74.2 | 90.16 | 81.2 | 75.69 | 68.96 | 85.34 |
| Squares | 40 | 97.09 | 70.66 | 75.65 | 89.38 | 80.26 | 74.71 | 68.94 | 88.59 |
| Variation of species richness for the non-parametric estimation indices | |||||||||
| RH-1 | RH-2 | RH-3 | RH-4 | RH-5 | RH-6 | RH-7 | RH-8 | RH-9 | |
| Chao-1 | 0.00% | 0.18% | 10.45% | 13.52% | 1.69% | 0.21% | 2.03% | 4.48% | 25.93% |
| iChao-1 | 0.00% | 0.18% | 17.27% | 25.72% | 5.06% | 0.50% | 4.15% | 14.18% | 28.02% |
| ACE | 0.00% | 0.31% | 9.63% | 7.54% | 1.30% | 1.50% | 2.28% | 2.93% | 5.36% |
| Squares | 0.00% | 0.09% | 5.46% | 9.64% | 0.43% | 0.32% | 0.96% | 2.90% | 9.37% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





