1. Introduction
There is a dramatic increase in the prevalence of obesity that has led to a marked increase in metabolic syndrome characterized by visceral adiposity, insulin resistance, elevated blood pressure (BP), elevated triglycerides (TG), and low levels of high-density lipoprotein cholesterol (HDL-C) [
1,
2]. The mere diagnosis of the metabolic syndrome appears to confer a substantial additional risk of coronary heart disease (CHD) [
3,
4,
5]. Atherogenic dyslipidemia, characterized primarily by elevated TG, low HDL-C, and accumulation of lipoprotein remnants, is a phenotype associated with increased CVD risk [
6]. As an entity or through its individual components (TG or HDL-C), atherogenic dyslipidemia has been linked to an increased risk of atherosclerotic CVD (ASCVD) even in patients with controlled LDL-C [
6,
7].
Subclinical myocardial injury (SCMI) refers to early cardiac damage that lacks clear manifestations of CHD. It’s identified by an electrocardiographic-based scoring system called the cardiac infarction/injury score (CIIS) exceeding 10 score points. SCMI has been associated with an increased risk of CVD and all-cause mortality [
8,
9]. Previous studies have linked SCMI with risk factors such as obesity and dyslipidemia [
10,
11]. However, it is unclear whether atherogenic dyslipidemia is a risk factor for SCMI. We hypothesize that atherogenic dyslipidemia is associated with an increased prevalence of SCMI independent of traditional CVD risk factors, lifestyle factors, and socioeconomic status. We tested this hypothesis in the third National Health and Nutrition Examination Survey (NHANES III).
2. Materials and Methods
NHANES, a recurring survey carried out by the National Center for Health Statistics (NCHS) under the Centers for Disease Control and Prevention (CDC), aims to evaluate the health and disease patterns among noninstitutionalized civilians in the United States. NHANES-III, conducted from 1988 to 1994, received approval from the NCHS Research Ethics Review Board. All participants provided documented informed consent. Detailed information regarding the study design and methods has been previously disseminated [
12].
For this analysis, we only included NHANES-III participants who underwent an electrocardiogram (ECG) recording (n=8,561) and with complete data on atherogenic dyslipidemia. We excluded participants with a prior history of CVD or missing key covariates. After all exclusions, a total of 7,093 participants were included in the analysis.
A resting 12-lead ECG was acquired using a Marquette MAC 12 electrocardiograph (Marquette Medical Systems, Milwaukee, Wisconsin) during a physical examination conducted in a mobile examination center (MEC). The ECG tracings were automatically processed at the Epidemiological Cardiology Research Center (EPICARE Center, Wake Forest School of Medicine, Winston-Salem, NC) after visual inspection by skilled technicians.
The methods for measuring Cardiac Infarction/Injury Score (CIIS) have been detailed in prior literature [
13]. CIIS employs a weighted scoring approach, incorporating various objective electrocardiographic waveform elements associated with myocardial injury and ischemia to establish a risk-stratified scoring system. The scoring system comprises 11 discrete and 4 continuous ECG features. These features encompass measurements of Q, R, and T waves, along with the ST segment. In the NHANES-III dataset, CIIS values underwent an initial multiplication by a factor of 10 to circumvent the use of decimal points. However, for the current analysis, CIIS values were divided by 10 to present them in their original scale. Similar to prior studies, SCMI was defined as CIIS values equal to or exceeding 10 score points [
14].
A phlebotomist collected blood samples via venipuncture. The samples were analyzed for total HDL-C, TG, glucose, and other components in the metabolic panel using laboratory procedures as reported by the National Center for Health Statistics [
15]. LDL-C was calculated using the Friedewald equation [
16]. Based on the levels of HDL-C and TG, participants were grouped into the following groups: Atherogenic dyslipidemia, defined as high TG (≥150 mg/dL) and low HDL-C (<40 mg/dL in men or <50 mg/dL in women) [
17]; high TG/normal HDL-C group; low HDL-C/normal TG; and normal HDL-C/normal TG group.
Demographics (age, sex, race) and smoking status were self-reported during an in-home interview. Obesity was defined as body mass index (BMI) ≥30 kg/m2. Hypertension was defined as systolic BP ≥130 mmHg, diastolic BP ≥80 mmHg, or the use of antihypertensive medications. Diabetes was defined as fasting blood glucose levels ≥126 mg/dl or the use of glucose-lowering medications. Physical activity was assessed based on the frequency of leisure time activity and included information on types of activity, frequency, and level of activity.
Continuous variables were presented as means and standard deviation (SD). Categorical variables were presented as counts and corresponding percentages. We used the chi-square test for categorical variables, analysis of variance for normally distributed continuous variables, or Wilcoxon rank sum analysis for non-normally distributed continuous variables.
Multivariable logistic regression analysis was used to examine the cross-sectional associations of different combinations of TG and HDL-C groups, including atherogenic dyslipidemia with SCMI. The normal HDL-C/normal TG group was used as the reference group. Model 1 was adjusted for age, sex, race/ethnicity. Model 2 was adjusted for variables in model 1 plus diabetes, hypertension, serum creatinine, body mass index, lipid-lowering medications, smoking, and physical activity. In similar models, we also examined the associations of high TG (vs. normal) and low HDL-C (vs. normal) separately with SCMI.
All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC). P-values less than 0.05 were considered statistically significant.
3. Results
After exclusions, 7,093 participants (age of 59.3 ± 13.4 years, 52.8% women and 49.4% White) were included in the analysis. Approximately a quarter of the sample (n = 1,594) had prevalent SCMI. The prevalence of atherogenic dyslipidemia was 22.5%, with average LDL-C levels of 136.4 mg/dL and total cholesterol of 222.2 mg/dL. Table 1 shows the characteristics of the study population stratified by different combinations of TG and HDL-C levels. Compared to individuals with a normal lipid profile (Normal HDL-C/Normal TG), participants with atherogenic dyslipidemia were more likely to be women, Mexican Americans, with higher LDL-C, total cholesterol, and BMI, and had a higher prevalence of diabetes.
Table 1.
Participant characteristics stratified by levels of HDL-C and TG.
Table 1.
Participant characteristics stratified by levels of HDL-C and TG.
Variable |
Overall
(n=7,093) |
Normal HDL-C,
Normal TG
(n=3,304) |
Normal HDL-C,
High TG
(n=1,134) |
Low HDL-C,
Normal TG
(n=1,061) |
Atherogenic Dyslipidemia
(n=1,594) |
P-value†
|
| Age, years |
59.3±13.4 |
59.6±13.8 |
60.9±12.4 |
57.3±13.7 |
59.1±12.8 |
<0.001 |
| Female |
3743(52.8) |
1,762(23.3) |
521(45.9) |
636(59.9) |
824(51.7) |
<0.001 |
| Race-Ethnicity |
|
|
|
|
|
<0.001 |
| Non-Hispanic White |
3506(49.4) |
1639(49.6) |
568(50.1) |
480(45.2) |
819(51.4) |
| Non-Hispanic Black |
1588(22.4) |
911(27.6) |
186(16.4) |
277(26.1) |
214(13.4) |
| Mexican-American |
1709(24.1) |
624(18.9) |
350(30.9) |
245(23.1) |
490(30.7) |
| Other |
290(4.1) |
130(3.9) |
30(2.6) |
59(5.6) |
71(4.5) |
| Income <$20k |
3205(45.2) |
1466(44.4) |
532(46.9) |
463(43.6) |
744(46.7) |
0.195 |
| Education ≥High School |
3903(55.0) |
1918(58.1) |
572(50.4) |
611(57.6) |
802(50.3) |
<0.001 |
| Ever Smoker |
3852(54.3) |
1753(53.1) |
640(56.4) |
545(51.4) |
914(57.3) |
0.0032 |
| BMI |
27.6±5.5 |
26.2±5.1 |
28.4±4.9 |
28.6±6.2 |
29.5±5.4 |
<0.001 |
| Anti-hypertensive |
1543(21.8) |
593(18.0) |
315(27.8) |
210(19.8) |
425(26.66) |
<0.001 |
| LDL, mg/dL |
136.4±38.3 |
132.7±37.6 |
142.9±42.9 |
134.1±34.5 |
142.2±38.0 |
<0.001 |
| Lipid Lowering medications |
258(4.0) |
83(2.5) |
67(5.9) |
41(3.9) |
41(5.9) |
<0.001 |
| Total Cholesterol, mg/dL |
222.2±44.2 |
217.3±40.7 |
245.8±44.4 |
197.8±38.4 |
231.5±44.4 |
<0.001 |
| SBP, mmHg, mean (SD) |
132.9±26.7 |
131±20.3 |
137.6±42.9 |
130.1±30.0 |
134.5±19.6 |
<0.001 |
| DBP, mmHg |
76.9±23.9 |
76.1±17.5 |
79.6±42.8 |
76.9±26.9 |
76.9±10.3 |
<0.001 |
| Diagnosis of Diabetes Mellitus |
1043(14.7) |
301(9.1) |
212(18.7) |
134(12.6) |
396(24.8) |
<0.001 |
| Physically Active |
4797(67.6) |
2323(10.3) |
769(67.8) |
689(64.9) |
1016(63.4) |
<0.001 |
| Serum Creatinine |
1.1±0.4 |
1.1±0.3 |
1.1±0.3 |
1.1±0.6 |
1.1±0.4 |
0.027 |
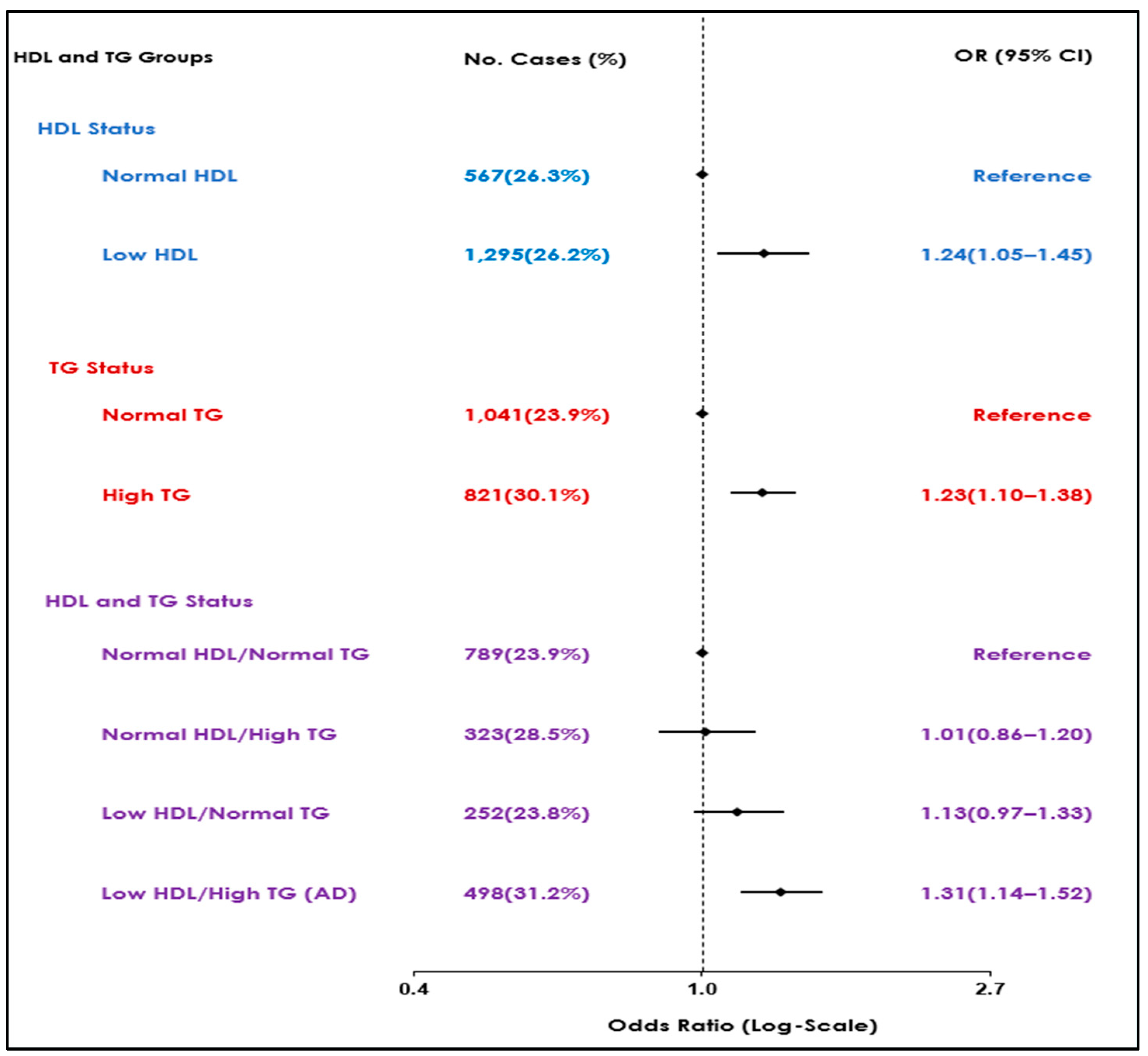
The prevalence of SCMI was highest among individuals with atherogenic dyslipidemia (31.2%), as shown in Table 2. However, a similar prevalence of SCMI was observed between individuals with normal HDL-C/normal TG and low HDL-C/normal TG (23.9% vs. 23.8%, respectively). In a multivariable logistic regression model adjusted for demographics and potential confounders, atherogenic dyslipidemia was associated with increased odds of SCMI (OR (95% CI): 1.31(1.14 – 1.52). Although similar risk patterns were observed in both the low HDL-C/normal TG and normal HDL-C/high TG groups, the results did not reach statistical significance (OR (95% CI): 1.01(0.86 – 1.20), 1.13(0.97 – 1.33), respectively).
Table 2.
Association of atherogenic dyslipidemia and other TG/HDL-C combinations with SCMI
Table 2.
Association of atherogenic dyslipidemia and other TG/HDL-C combinations with SCMI
| TG/HDL-C Group |
No. Event
(%) |
Model 1 |
Model 2 |
| OR (95% CI) |
P-value |
OR (95% CI) |
P-value |
Normal HDL-C,
Normal TG
|
789(23.9) |
Ref |
-- |
Ref. |
-- |
Low HDL-C,
Normal TG
|
252(23.8) |
1.09(0.92 – 1.29) |
0.320 |
1.01(0.86 – 1.20) |
0.877 |
Normal HDL-C,
High TG
|
323(28.5) |
1.23(1.06 – 1.44) |
0.008 |
1.13(0.97 – 1.33) |
0.120 |
| Atherogenic dyslipidemia |
498(31.2) |
1.52(1.32 – 1.74) |
<0.001 |
1.31(1.14 – 1.52) |
<0.001 |
Further analysis of individual lipid markers utilizing similar models showed increased odds of SCMI in participants with either high TG and low HDL independently compared to normal levels (OR (95% CI): 1.23(1.10 – 1.38), 1.24(1.05 – 1.45), respectively) (Table 3). Figure 1 summarizes the results of the association observed between lipid markers, either as combinations or individual markers, with the highest risk observed in the atherogenic dyslipidemia group.
Table 3.
Association of TG and HDL-C, separately, with SCMI.
Table 3.
Association of TG and HDL-C, separately, with SCMI.
| TG and HDL-C Status |
No. Event
(%) |
Model 1 |
Model 2 |
| OR (95%) |
P-value |
OR (95%) |
P-value |
| Normal TG |
1041(23.9) |
Ref |
-- |
Ref. |
-- |
| High TG |
821(30.1) |
1.36(1.22 – 1.52) |
<0.001 |
1.23(1.10 – 1.38) |
<0.001 |
| Normal HDL-C |
567(26.3) |
Ref |
-- |
Ref |
-- |
| Low HDL-C |
1295(26.2) |
1.34(1.14 – 1.57) |
<0.001 |
1.24(1.05 – 1.45) |
0.011 |
Figure 1.
Associations of atherogenic dyslipidemia and different combinations of HDL-C and TG with SCMI.
Figure 1.
Associations of atherogenic dyslipidemia and different combinations of HDL-C and TG with SCMI.
4. Discussion
Atherogenic dyslipidemia, characterized by abnormalities in the TG-HDL axis, is highly prevalent in patients with CHD, the leading global cause of death. Our analysis from the NHANES-III, a community-based survey, revealed that individuals with atherogenic dyslipidemia had higher odds of SCMI. This association was independent of participants’ demographics or CVD risk factors. Low levels of HDL and higher TG were also independently associated with higher odds of SCMI compared to their normal levels, with a synergistic effect when both abnormalities coexist. These results underscore the role of atherogenic dyslipidemia in the early development of CHD in the form of SCMI and its potential importance in primary prevention and risk stratification.
Except for the atherogenic dyslipidemia pattern, the associations of other different combinations of abnormal TG and HDL with SCMI did not reach statistical significance. These results indicate a preferential pattern of a stronger association between atherogenic dyslipidemia and SCMI. Notably, low HDL-C (vs. normal HDL-C) and high TG (vs. normal TG), separately, were both associated with SCMI, and their strength of association with SCMI was relatively similar but was less than that of atherogenic dyslipidemia. This further underscores the synergistic effect of the combination posed by high TG and low HDL-C (AKA atherogenic dyslipidemia) on the process of developing myocardial ischemia.
The synergistic effect of high TG and low HDL-C has been linked to an increased risk of ASCVD development and worse cardiovascular outcomes after adjusting for other lipid abnormalities [
18,
19,
20]. Our results suggest that this relationship might have been preceded by subclinical myocardial ischemia and injury before progression to ASCVD. Hence, the management of atherogenic dyslipidemia could have a bigger role in the prevention of CVD than what is currently thought. This is further underscored by the fact that atherogenic dyslipidemia commonly coexists and correlates with several CVD risk factors [
21,
22]. It is prevalent in patients with obesity, metabolic syndrome, insulin resistance, and type 2 diabetes mellitus, serving as a marker for increased CVD risk in these populations [
23,
24,
25].
The association between atherogenic dyslipidemia and SCMI may involve a broad spectrum of underlying mechanisms. The poor prognostic effect of low HDL-C/high TG appears to be an important independent risk factor for CVD. Even though the association between atherogenic dyslipidemia and SCMI attenuated after adjusting for CVD risk factors, the fact that it remained significant suggests that the association between atherogenic dyslipidemia and SCMI involves several mechanisms. Possible mechanisms may include alteration in lipoprotein lipase activity, chronic inflammation and the highly atherogenic nature of TG-rich lipoproteins, primarily due to their smaller size and high cholesterol content, which facilitates endothelial migration, acting as a substrate for atherosclerosis and myocardial injury. [
6,
26,
27,
28].
It has been suggested that HDL-C levels function more as a biological marker than a therapeutic target for ASCVD. [
29,
30,
31] We showed that lower levels of HDL-C (vs. normal) were associated with an elevated risk of SCMI. These findings reinforce the concept of utilizing low HDL-C levels as an early biomarker for myocardial injury, potentially preceding the manifestation of clinically evident ASCVD. However, the complexity of atherogenic dyslipidemia, with its intertwined components, makes it challenging to isolate and attribute the direct roles of individual components. Although our analysis revealed increased odds of SCMI with induvial components, in real life, these abnormalities rarely occur in isolates, and therapeutic modalities often provide collateral improvement of lipid biomarkers. Undoubtedly, the heightened CVD risk associated with atherogenic dyslipidemia phenotype remains not fully understood, which warrants further research to explore its effect on subclinical myocardial ischemia and injury before they manifest clinically.
Certain limitations need to be taken into consideration in the interpretation of our study. We only had a single measurement of HDL-C and TG, which may not reflect the status of the long-term lipid profile. Our study design was cross-sectional, and therefore, a causal relationship between atherogenic dyslipidemia and SCMI could not be established. Some of the measurements, like smoking and physical activity, are self-reported and thus subjected to recall bias. Finally, although we adjusted for several confounders, residual confounding remains a possibility. Our study has many strengths as well. This includes a large community-based, multiracial sample size. The ECG and laboratory data were processed in central units. All variables were ascertained using standardized approaches.
5. Conclusions
Our results revealed a strong association between AD and SCMI in a racially diverse general population. This underscores the atherogenic effect of this dyslipidemia phenotype and highlights the role of nontraditional risk factors in the development of subclinical CVD.
Author Contributions
Conceptualization, EZS and RK; methodology, RK and MAM software, RK, validation, MZS,NSE,MHS. formal analysis, RK investigation, MAM, NSE.; resources, NSE,MHS. writing—original draft preparation, NSE, MHS.; writing—review and editing, MAM, EZS visualization, RK; supervision, MZS,EZS. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Institutional Review Board Statement
Informed consent was obtained from all participants at time of data collection per NHANES III protocol.
Data Availability Statement
Acknowledgments
There were no further contributions nor grant support to this project beyond those of the listed authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ford, E.S.; Giles, W.H.; Dietz, W.H. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002, 287, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004, 110, 227–239.
- McNeill, A.M.; Rosamond, W.D.; Girman, C.J.; Golden, S.H.; Schmidt, M.I.; East, H.E.; Ballantyne, C.M.; Heiss, G. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care 2005, 28, 385–390. [Google Scholar] [CrossRef]
- McNeill, A.M.; Katz, R.; Girman, C.J.; Rosamond, W.D.; Wagenknecht, L.E.; Barzilay, J.I.; Tracy, R.P.; Savage, P.J.; Jackson, S.A. Metabolic syndrome and cardiovascular disease in older people: the cardiovascular health study. J Am Geriatr Soc 2006, 54, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Philip, S.; Granowitz, C.; Toth, P.P.; Wong, N.D. Prevalence of US Adults with Triglycerides ≥ 150 mg/dl: NHANES 2007-2014. Cardiol Ther. 2020, 9, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Alberto Lorenzatti, Peter P Toth, New Perspectives on Atherogenic Dyslipidaemia and Cardiovascular Disease, European Cardiology Review 2020; 15.
- Valensi, P. , Avignon, A., Sultan, A. et al. Atherogenic dyslipidemia and risk of silent coronary artery disease in asymptomatic patients with type 2 diabetes: a cross-sectional study. Cardiovasc Diabetol, 2016; 15; 104.
- Rautaharju, P.M.; Warren, J.W.; Jain, U.; Wolf, H.K.; Nielsen, C.L. Cardiac infarction injury score: an electrocardiographic coding scheme for ischemic heart disease. Circulation. 1981, 64, 249–256. [Google Scholar] [CrossRef]
- O’Neal, W.T.; Shah, A.J.; Efird, J.T.; Rautaharju, P.M.; Soliman, E.Z. Subclinical myocardial injury identified by cardiac infarction/injury score and the risk of mortality in men and women free of cardiovascular disease. Am J Cardiol. 2014, 114, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Vasim, I.; Ahmad, M.I.; Mongraw-Chaffin, M.; Soliman, E.Z. Association of Obesity Phenotypes With Electrocardiographic Subclinical Myocardial Injury in the General Population. Clin Cardiol 2019, 42, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, M.; Xu, J.; Sha, D.; Xu, B.; Kang, L. Association Between Triglyceride and Glycose (TyG) Index and Subclinical Myocardial Injury. Nutr Metab Cardiovasc Dis 2020, 30, 2072–2076. [Google Scholar] [CrossRef]
- Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–1994. Series 1: programs and collection procedures. Vital and health statistics Ser 1, Programs and collection procedures. 1994, 32, 1–407.
- Rautaharju PM, Warren JW, Jain U, et al. Cardiac infarction injury score: an electrocardiographic coding scheme for ischemic heart disease. Circulation. 1981, 64, 249–256.
- O’Neal WT, Shah AJ, Efird JT, et al. Subclinical myocardial injury identified by cardiac infarction/injury score and the risk of mortality in men and women free of cardiovascular disease. Am J Cardiol. 2014, 114, 1018–1023.
- US Department of Health and Human Services. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–1994. Series 1: programs and collection procedures. Vital and Health Statistics, no, 32. Hyattsville, MD: National Center for Health Statistics; 1994.
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Arca, M.; Montali, A.; Valiante, S.; Campagna, F.; Pigna, G.; Paoletti, V.; Antonini, R.; Barillà, F.; Tanzilli, G.; Vestri, A.; Gaudio, C. Usefulness of atherogenic dyslipidemia for predicting cardiovascular risk in patients with angiographically defined coronary artery disease. A J Cardiol. 2007, 100, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Guyton JR, Slee AE, Anderson T, et al. Relationship of lipoproteins to cardiovascular events: the AIM-HIGH Trial (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides and Impact on Global Health Outcomes). J Am Coll Cardiol 2013, 62, 1580–1584.
- Athyros, V.G.; Tziomalos, K.; Karagiannis, A.; Mikhailidis, D.P. Dyslipidaemia of obesity, metabolic syndrome and type 2 diabetes mellitus: the case for residual risk reduction after statin treatment. Open Cardiovasc Med J 2011, 5, 24–34. [Google Scholar] [CrossRef]
- Banegas JR, Lopez-Garcia E, Dallongeville J, Guallar E, Halcox JP, Borghi C, et al. Achievement of treatment goals for primary prevention of cardiovascular disease in clinical practice across Europe: the EURIKA study. Eur Heart J. 2011, 32, 2143–2152.
- Austin, M.A.; King, M.C.; Vranizan, K.M.; Krauss, R.M. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation. 1990, 82, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, C N et al. “Atherogenic dyslipidemia.” Indian journal of endocrinology and metabolismvol. 2013, 17, 969–976.
- Reaven, G.M.; Chen, Y.D.; Jeppesen, J.; Maheux, P.; Krauss, R.M. Insulin resistance and hyperinsulinemia in individuals with small, dense low density lipoprotein particles. J Clin Invest. 1993, 92, 141–146. [Google Scholar] [CrossRef]
- Musunuru, K. Atherogenic dyslipidemia: Cardiovascular risk and dietary intervention. Lipids. 2010, 45, 907–914. [Google Scholar] [CrossRef]
- Cooney MT, Dudina A, De Bacquer D, et al. HDL cholesterol protects against cardiovascular disease in both genders, at all ages and at all levels of risk. Atherosclerosis. 2009, 206, 611–616. [Google Scholar] [CrossRef] [PubMed]
- TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med 2014, 371, 22–31.
- McPherson, R. Remnant cholesterol “Non-(HDL-C + LDL-C)” as a coronary artery disease risk factor. J Am Coll Cardiol. 2013, 61, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Varbo, A.; Benn, M.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation 2013, 128, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Castelli, W.P. Cholesterol and lipids in the risk of coronary artery disease – the Framingham Heart Study. Can J Cardiol 1988, 4, 5A–10A. [Google Scholar]
- Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989, 79, 8–15.
- Bartlett J, Predazzi IM, Williams SM, et al. Is isolated low high-density lipoprotein cholesterol a cardiovascular disease risk factor? New insights from the Framingham Offspring Study. Circ Cardiovasc Qual Outcomes 2016, 9, 206–212.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).