1. Generalities
Respiratory Syncytial Virus (RSV) is a major cause of respiratory tract infections in infants and young children, with most children being infected by RSV at least once by the age of two. In infants, RSV can cause severe lower respiratory tract infections, such as bronchiolitis, pneumonia, and other complications leading to hospitalization and, in some cases, death [
1]. In 2019, it was estimated that globally, lower respiratory tract infections caused by respiratory syncytial virus (RSV) resulted in more than 3,000,000 hospital admissions and over 100,000 overall deaths in children aged 0-5 years [
2]. RSV typically causes mild upper respiratory tract infections but can cause otalgia and acute respiratory distress in certain populations, such as the elderly and individuals with underlying health conditions. Another feature of RSV in immunocompromised patients is that viral clearance is delayed and the virus can remain in nasal secretions for a long time. [
1]
RSV, formally named as Orthopneumovirus hominis [
3], is a negative-sense, single-stranded RNA virus (Baltimore Group V) belonging to the family Pneumoviridae, genus Orthopneumovirus. Two different subtypes of RSV have been described, RSV-A and RSV-B [
4].
RSV is an enveloped virus, with a lipid bilayer derived from the host cell apical membrane. The virion is pleomorphic, ranging from nearly spherical to filamentous, a shape that is mostly found in cell cultures, with a diameter of approximately 150-300 nm. The genome of RSV is non-segmented and approximately 15.2 kb in length, with 10 ORFs, encoding 11 proteins: NS1, NS2, N, P, M, SH, G, F, M2.1, M2.2 and L. [
5]
2. Infection
RSV primarily infects ciliated epithelial cells lining most of the respiratory tract and type I alveolar pneumocytes, leading to cell death and disruption of the respiratory epithelium and often resulting in syncytia formation [
6].
The entry of RSV into host cells is initiated by the attachment of the viral glycoprotein G [
7]. RSV interacts with glycosaminoglycans on host transmembrane proteins, such as heparan sulfate proteoglycans, using its G and F glycoproteins, in addition to the CX3CR1 co-receptor (as will be detailed later). The viral F protein mediates the fusion of the viral envelope with the host cell membrane, allowing the viral ribonucleoprotein (RNP) complex (viral RNA stabilized by N and P proteins) to enter the host cytoplasm. The SH protein, functioning as a viroporin, may facilitate this process by altering membrane permeability, reducing apoptosis and inhibiting TNF-α signaling [
8].
Once inside the host cell, viral protein complexes form inclusion bodies in the cytoplasm to replicate RNA genomes and transcribe them into mRNA for protein synthesis by the host cell machinery [
9].
Besides N and P, proteins L and M2-1 are present in the nucleocapsid and are relevant for viral genome replication.[
10,
11,
12,
13,
14,
15,
16] The L and P proteins transcribe the viral genome into mRNA, to be translated by the host ribosomes. At the same time, the L protein synthesizes intermediate positive-sense RNA templates to produce new negative-sense RNA genomes.
Naked virions are assembled when the matrix protein (M) facilitates the interactions between the new RNP complexes and the viral glycoproteins G, F, and SH [
17,
18]. The assembled virions bud from the host cell membrane, acquiring their lipid envelope from the host. Glycoprotein G binds to the surface of the host cell, binding to CX3CR1 mimicking the host chemokine CX3CL1.
3. Biological Aspects of CX3CL1-CX3CR1
CX3CL1 is the only member of the CX3C chemokine subfamily [
19]. It is characterized by being the only chemokine to have three amino acid residues between the two conserved cysteines. It is expressed in activated endothelial cells, epithelial cells and neurons, among others.[
20,
21]
Of the 50 known chemokines, only CXCL16 and CX3CL1 are synthesized as membrane-bound molecules [
22]. CX3CL1 consists of a chemokine domain and a mucin stalk, both extracellular; it also has a transmembrane and a small cytoplasmic domain. [
23]. In its transmembrane form CX3CL1 is constitutively associated and forms oligomers involved in cell adhesion [
24]. Membrane-bound CX3CL1 is cleaved by several enzymes such as the metalloproteinases ADAM10, ADAM17 and MMP-3, and cathepsin S (reviewed in [
25]). The soluble CX3CL1 molecule has a chemotactic effect on various cells of the immune system. The balance between CX3CL1-mediated adhesion and chemotactic functions are finely regulated by enzymes in the cellular microenvironment that enable the release of the chemokine domain.
One of the most studied functions of CX3CL1 is its participation in the extravasation of leukocytes through the activated endothelium. [
21,
26]. In a pathophysiological context the adhesive function of CX3CL1 is relevant in atherosclerotic plaque formation, through monocyte binding [
27]. In the central nervous system, CX3CL1 is robustly expressed by neurons, and its elicited signaling are relevant in cerebral ischemia, epilepsy and in several neurodegenerative diseases [
28], although its role in these diseases remains controversial.
CX3CL1 binds with high affinity to a single receptor named CX3CR1 [
26,
29], a seven-transmembrane receptor. The binding of the chemokine to the receptor activates a heterotrimeric Gi/o protein and initiates signaling events that trigger diverse cellular responses.
On the other hand, it has been reported that the cytoplasmic domain of CX3CL1 can signal and induce its own expression [
30]; although this function has been scarcely documented, it could be of great biological relevance and increases the complexity of the system.
4. CX3CR1 and RSV Infection
It is well established that CX3CR1 is an RSV coreceptor.
In vitro experiments indicate that RSV G protein binds to the CX3CR1 receptor on primary human epithelial cells isolated from the airway, and promotes infection. Viral G-protein binding is specific, as treatment with an anti-CX3CR1 antibody decreases infection. Johnson
et al showed that the binding of RSV G to CX3CR1 is mediated by a CX3C motif of the G protein, since the inclusion of an alanine residue in this motif reverses the effect observed in the wild-type protein [
31]. Similar findings were found using primary epithelial cells isolated from pediatric airways and infected with RSV strain A2, in which blocking the interaction of RSV with CX3CR1 decreased viral load [
32]. Accumulating evidence from several experimental studies indicates that the conserved CX3C motif facilitates virus binding to CX3CR1
+ epithelial cells [
33], indicating that CX3CR1 is a co-receptor molecule for RSV.
The role of CX3CR1 in RSV infection becomes clinically relevant since CX3CR1 is expressed in the airway of the pediatric population [
32], at variable levels. It would be important to establish whether the biological variation found in CX3CR1 expression has any relationship with the susceptibility or outcome of RSV infection in the pediatric population.
Although CX3CR1 is a co-receptor molecule for RSV entry into epithelial cells and its expression favors infection, work with mice genetically deficient in CX3CR1 indicates that the absence of this receptor does not protect against the disease, and on the contrary, the absence of this receptor is related to more severe disease. Newborn mice genetically deficient in CX3CR1 (CX3CR1
-/-), showed higher mortality than wild-type mice, as well as increased mucus production, and increased neutrophil and γδ T-lymphocyte infiltration in the lungs. [
34]. Thus, although CX3CR1 favors the entry of the RSV into epithelial cells and its presence implies an increased viral load, the interaction of CX3CR1 with its natural ligand (or with the CX3C domain of the RSV G protein) seems to be relevant for the resolution of the infection. This indicates that the CX3CR1-CX3CL1 axis plays an important role in the immune response against the virus.
5. Immune Response against RSV
Once RSV enters the airway epithelial cell (AEC) diverse viral pathogen-associated molecular patterns (PAMPs) can be recognized by multiple host pattern recognition receptors (PRRs). RSV genomic material can be recognized by the retinoic acid inducible gene I (RIG-I) sensor in the cell cytoplasm or by toll-like receptors (TLRs) such as TLR-3 in endosome compartments leading to the activation of interferon regulatory (IRF)-3, AP1 and NF-kB transcription factors. Another TLR that is important in RSV infection is TLR-4, whose signaling is modulated by F protein, dampening pro-inflammatory cytokine production. [
35,
36]
In addition, alveolar macrophages and dendritic cells are also stimulated upon RSV infection [
37,
38]. The activation of multiple cell types induces the expression of several molecules to control viral infection. Type-I interferons (IFN-I) play a major role in restricting viral replication and dissemination by promoting an antiviral state due to the induction of several interferon stimulated genes in neighboring cells. In addition, several proinflammatory mediators are also induced, including cytokines (TNFα, IL-1β, IL-6, INF-λ1, INF-λ2 ), chemokines (CXCL6, CXCL8 (IL-8), CXCL10 and CX3CL1) [
39], and adhesion molecules (ICAM-1, VCAM-1), that in turn promote immune cell recruitment for the control of the viral infection. Moreover, major histocompatibility complex molecules (MHC)-I and II are also induced, favoring the onset of the adaptive immune response against the pathogen. [
40,
41].
Recruitment of neutrophils is an early event upon RSV infection; these cells are the most abundant leukocytes to infiltrate the lung upon infection. Neutrophil activation induces neutrophil extracellular traps (NETs) which may trap viral particles that in turn can be inactivated by a diversity of the neutrophil secreted molecular mediators such as defensins; in fact, antimicrobial peptides such as cathelicidin and defensins produced by AECs may also restrict RSV replication [
42,
43]. Viral particles can be phagocytosed by neutrophils and destroyed by distinct mechanisms including the respiratory burst (ROS production) [
44]. Nevertheless, excessive neutrophil responses may contribute to lung injury.
NK cells are relevant responders against viral infection, thus, they are also important in the control of RSV infection. These cells kill infected cells, limiting viral replication, and produce IFN, promoting a Th1 type response. Reduction of perforin secretion in RSV-infected NK cells has been demonstrated, and this might be an evasion mechanism of RSV against host immune response [
45]. Furthermore, RSV induces upregulation of inhibitory receptor in NKcells.
Dendritic cells (DCs), considered the bridge between innate and adaptive immunity, are also recruited upon RSV infection. Plasmacitoid DCs (pDCs) are a subset of DCs specialized in high production of IFN-I, a major contributor to the antiviral response; pDCs recognize viral RNA through TLR-7 and -8, leading to downstream activation of IRF-7, AP1 and NFB, which in turn initiate the massive production of IFN-I [
46,
47].
Moreover, pDCs are required for adequate RSV-specific cytotoxic T lymphocytes generation [
41]. Nevertheless, RSV infection counteracts IFN-I production by pDCs [
48]. In addition, conventional DCs (cDCs) are also recruited to the site of infection where they acquire viral antigens by direct infection or phagocytosis of infected cells. RSV sensing by TLRs and RLRs (RIG-1 like receptors, which detect RNA from viruses in the cytoplasm) in cDCs induce their maturation with the up-regulation of MCH and co-stimulatory molecules, and secretion of several Th-1 type cytokines. cDCs initiate the adaptive immune response against RSV upon antigen presentation to specific T lymphocytes. RSV infection however, induce cDC dysfunction that alter their capacity to stimulate T cells [
49].
As with other viral infections, adaptive immunity against RSV is essential for disease control. Both CD4
+ and CD8
+ specific T lymphocyte responses as well as high titers of virus-specific antibodies are developed and required for disease control [
37]. Regulatory T lymphocytes
(Treg) play a central role in the control of disease severity during RSV infection by limiting immunopathology [
40,
50]
RSV has evolved several mechanisms to evade the host immune response. The non-structural viral proteins NS1 and NS2 are the first to be transcribed upon infection, and both are potent IFN-I inhibitors. Deletion mutants for these proteins generate attenuated viruses, which have been proposed as vaccines [
47,
51]. Both proteins affect the RIG-I signaling pathway to inhibit IFN-I production; NS1 binds mitochondrial antiviral-signaling protein, interfering with its interaction with RIG-I, while NS2 interacts with the N-terminal caspase activation and recruitment domain of RIG-I, thus inhibiting its downstream activation pathway. Indeed, NS1 is considered a multifunctional protein of RSV that counteracts immune response in distinct ways [
52,
53,
54,
55]. Other RSV evasion mechanisms are the modulation of innate immune responses such as the activation of DCs, macrophages, and natural killer (NK) cells; induction of regulatory T cells (Tregs); and antigenic variation. RSV exhibits large antigenic and genetic diversity, particularly in the G glycoprotein, which is a target of the host immune response. This allows RSV to evade immune recognition and establish reinfections [
6]. Alterations in the CX3CL1-CX3CR1 axis are also involved in the immune evasion mechanisms elicited by RSV. The RSV G protein is involved in IFN-I antagonism. [
47]
Despite the onset of the host immune response, RSV infection commonly elicits a relatively short-lived protective immunity resulting in recurrent seasonal RSV infections [
32,
40,
56].
Recent research has highlighted the role of fractalkine (CX3CL1) in the host immune response to RSV infection.
6. CX3CR1-CX3CL1 Contribution to the Immune Response against RSV
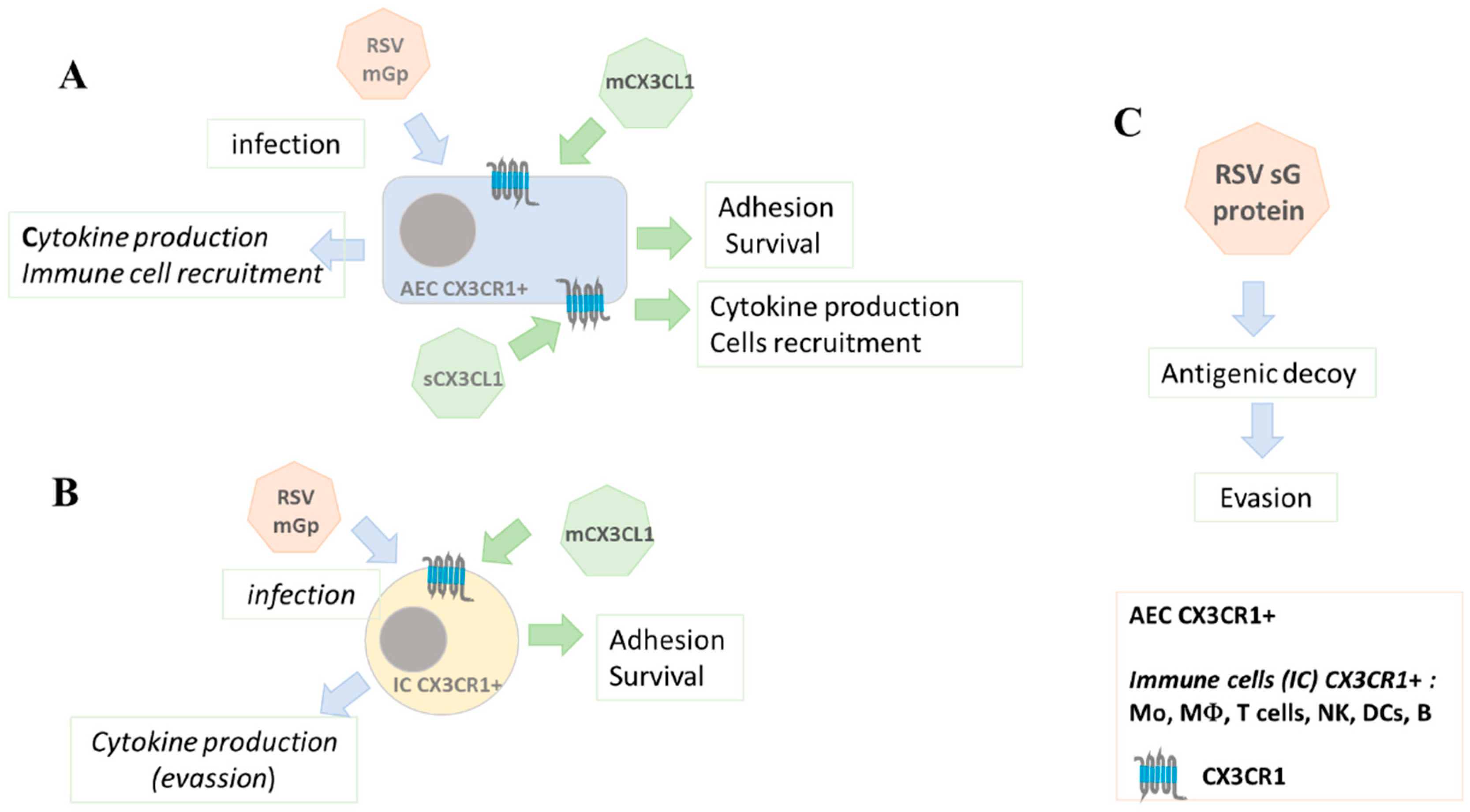
As mentioned above, infection of AECs with RSV increases the expression of several cytokines including CX3CL1. When cells are infected with a mutant RSV that has an insertion in the CX3C motif of the G protein, or when cells are treated with an anti-CX3CR1 prior to infection with WT RSV, the induction of cytokines is decreased [
39]. These results indicate that the binding of the CX3C domain of the RSV G protein to CX3CR1 influences cell activation and host immune responses.
Most of the immune cells relevant in the resolution of RSV infection express the CX3CR1 receptor, such as monocytes, macrophages, DCs, NK, and CD8
+ T lymphocytes [
57]. During RSV infection, these cells are recruited to the site of infection, playing an important role in virus elimination [
59]; for example, highly-expressing-CX3CR1 non-classical monocytes, normally associated to viral sensing and clearance, are recruited to the site of infection [
58]. These cells are the main mediators of the cytotoxic response against RSV, and it has been observed that severe cases of RSV-derived bronchitis present a decrease in CD8
+ T lymphocytes [
60].
The secreted form of RSV G-protein, by binding to CX3CR1, acts as a competitive antagonist ligand, interfering with the chemotactic responses of immune cells, and preventing the recruitment of immune cells to the site [
35,
36]. As a consequence, there is a decrease in both the recruitment of CX3CR1-expressing T lymphocytes and NK cells [
61], and the concentration of cytokines such as IFN-γ, IL-4 and CX3CL1 in the pulmonary microenvironment. A deficient production of INF-γ hinders the host antiviral response [
61].
On the other hand, people carrying the polymorphic variant of CX3CR1 M280 tend to present more severe cases of bronchiolitis in response to RSV [
62]. The CX3CR1 M280 variant has been associated with decreased adhesive capacity of human monocytes [
63], hence patients homozygous for CX3CR1 M280 may have decreased transmigration of monocytes and other immune system cells to the infected lung and this may contribute to the severe cases of bronchiolitis reported. In the context of RSV pathophysiology, monocytes are recruited through chemokines secreted by infected epithelial cells in the replicative phase of infection and they are important to limit the infection [
64,
65]. In addition, human monocytes with the CX3CR1 M280 variant are known to be deficient in CX3CL1-mediated survival [
66], so individuals homozygous for the CX3CR1 M280 variant have a lower number of antiviral monocytes in the lung.
Another interesting aspect is that, in neonates, during RSV infection, the lung is infiltrated by a regulatory B-cell subtype (nBreg), that has been associated with mortality. In B cells, RSV binds to the B cell receptor via the viral F protein. This binding induces the expression of CX3CR1 and allows virus entry. During viral replication in this neonatal B cell subpopulation, there is an increase in the expression and secretion of IL-10, an anti-inflammatory cytokine that inhibits the Th1 response [
67] (
Figure 1).
Different types of vaccines have been developed against RSV infection: against different viral proteins, or immunization with attenuated viruses or with vectors encoding distinct motifs of viral proteins. However, the desired immunogenicity and safety of the vaccines has not been achieved [
68]. The RSV G protein is known to induce protective antibodies against RSV infection. In a recent report, a new vaccine was tested using the CX3C-motif mutated regions of the RSV G protein as immunogen [
68]. The mutated proteins were more immunogenic for antibody production in BALB/c mice immunized with these molecules. The anti-G protein antibodies were effective in blocking the interaction of the CX3CR1 receptor with the RSV G protein, without blocking the interaction between CX3CR1 with CX3CL1 [
68]. This experimental approach is a promising starting point for the development of an effective vaccine that generates antibodies capable of reducing RSV infection through the CX3CR1 co-receptor, but at the same time does not affect the natural functions of CX3CL1 and CX3CR1. Recently, two vaccines and a new mAb have been approved by the FDA for their use against RSV infection [
69].
7. Conclusions and Future Directions
The RSV G protein binds to the CX3CR1 receptor, which then functions as a viral co-receptor in several cell types. CX3CR1 could have clinical relevance since it is expressed in the airways in the pediatric population and its expression has been related to a higher number of infected epithelial cells. The interaction of CX3CL1 with CX3CR1 plays a crucial role in the immune response to RSV infection, because mice genetically deficient in CX3CR1 develop more severe cases of the infection. The presence of the CX3C motif of the RSV G protein during RSV infection decreases the recruitment of cells expressing CX3CR1, such as T lymphocytes and NK cells, which are involved in the clearance of the virus. Moreover, the soluble form of RSV G-protein competes with CX3CL1 for binding to CX3CR1.
On the other hand, binding of CX3CR1 to the CX3C motif of RSV can modulate the antiviral immune response inducing the anti-inflammatory cytokine IL-10, which blocks the antiviral response. If confirmed, this is a novel mechanism of immune response evasion.
Finally, mutations of the CX3C domain of the G protein increase its antigenic immunogenicity and have been used to induce antibodies in animal models, antibodies that do not prevent the binding of CX3CR1 to CX3CL1.
Further research into the role of CX3CL1 and CX3CR1 in RSV pathogenesis may lead to the development of novel treatment therapies and prevention strategies targeting the host immune response to RSV infection, as well as in the development of new vaccines.
Author Contributions
Conceptualization and writing-original draft preparation, SRF.; Writing, review and editing, SRF, TS, ES and AS. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received financial support for research from the Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas (INER; protocol B15-19).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lehners, N.; Tabatabai, J.; Prifert, C.; Wedde, M.; Puthenparambil, J.; Weissbrich, B.; Biere, B.; Schweiger, B.; Egerer, G.; Schnitzler, P. Long-Term Shedding of Influenza Virus, Parainfluenza Virus, Respiratory Syncytial Virus and Nosocomial Epidemiology in Patients with Hematological Disorders. PloS One 2016, 11, e0148258. [CrossRef]
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Children Younger than 5 Years in 2019: A Systematic Analysis. Lancet Lond. Engl. 2022, 399, 2047–2064. [CrossRef]
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Alfenas-Zerbini, P.; Dempsey, D.M.; Dutilh, B.E.; García, M.L.; Curtis Hendrickson, R.; et al. Recent Changes to Virus Taxonomy Ratified by the International Committee on Taxonomy of Viruses (2022). Arch. Virol. 2022, 167, 2429–2440. [CrossRef]
- Mufson, M.A.; Orvell, C.; Rafnar, B.; Norrby, E. Two Distinct Subtypes of Human Respiratory Syncytial Virus. J. Gen. Virol. 1985, 66 ( Pt 10), 2111–2124. [CrossRef]
- Bergeron, H.C.; Tripp, R.A. Immunopathology of RSV: An Updated Review. Viruses 2021, 13, 2478. [CrossRef]
- Kaler, J.; Hussain, A.; Patel, K.; Hernandez, T.; Ray, S. Respiratory Syncytial Virus: A Comprehensive Review of Transmission, Pathophysiology, and Manifestation. Cureus 2023, 15, e36342. [CrossRef]
- Levine, S.; Klaiber-Franco, R.; Paradiso, P.R. Demonstration That Glycoprotein G Is the Attachment Protein of Respiratory Syncytial Virus. J. Gen. Virol. 1987, 68 ( Pt 9), 2521–2524. [CrossRef]
- Fuentes, S.; Tran, K.C.; Luthra, P.; Teng, M.N.; He, B. Function of the Respiratory Syncytial Virus Small Hydrophobic Protein. J. Virol. 2007, 81, 8361–8366. [CrossRef]
- Galloux, M.; Risso-Ballester, J.; Richard, C.-A.; Fix, J.; Rameix-Welti, M.-A.; Eléouët, J.-F. Minimal Elements Required for the Formation of Respiratory Syncytial Virus Cytoplasmic Inclusion Bodies In Vivo and In Vitro. mBio 2020, 11, e01202-20. [CrossRef]
- Bermingham, A.; Collins, P.L. The M2-2 Protein of Human Respiratory Syncytial Virus Is a Regulatory Factor Involved in the Balance between RNA Replication and Transcription. Proc. Natl. Acad. Sci. U. S. A. 1999, 96, 11259–11264. [CrossRef]
- Collins, P.L.; Hill, M.G.; Cristina, J.; Grosfeld, H. Transcription Elongation Factor of Respiratory Syncytial Virus, a Nonsegmented Negative-Strand RNA Virus. Proc. Natl. Acad. Sci. U. S. A. 1996, 93, 81–85. [CrossRef]
- Curran, J.; Marq, J.B.; Kolakofsky, D. An N-Terminal Domain of the Sendai Paramyxovirus P Protein Acts as a Chaperone for the NP Protein during the Nascent Chain Assembly Step of Genome Replication. J. Virol. 1995, 69, 849–855. [CrossRef]
- García-Barreno, B.; Delgado, T.; Melero, J.A. Identification of Protein Regions Involved in the Interaction of Human Respiratory Syncytial Virus Phosphoprotein and Nucleoprotein: Significance for Nucleocapsid Assembly and Formation of Cytoplasmic Inclusions. J. Virol. 1996, 70, 801–808. [CrossRef]
- Khattar, S.K.; Yunus, A.S.; Collins, P.L.; Samal, S.K. Deletion and Substitution Analysis Defines Regions and Residues within the Phosphoprotein of Bovine Respiratory Syncytial Virus That Affect Transcription, RNA Replication, and Interaction with the Nucleoprotein. Virology 2001, 285, 253–269. [CrossRef]
- Liuzzi, M.; Mason, S.W.; Cartier, M.; Lawetz, C.; McCollum, R.S.; Dansereau, N.; Bolger, G.; Lapeyre, N.; Gaudette, Y.; Lagacé, L.; et al. Inhibitors of Respiratory Syncytial Virus Replication Target Cotranscriptional mRNA Guanylylation by Viral RNA-Dependent RNA Polymerase. J. Virol. 2005, 79, 13105–13115. [CrossRef]
- Tawar, R.G.; Duquerroy, S.; Vonrhein, C.; Varela, P.F.; Damier-Piolle, L.; Castagné, N.; MacLellan, K.; Bedouelle, H.; Bricogne, G.; Bhella, D.; et al. Crystal Structure of a Nucleocapsid-like Nucleoprotein-RNA Complex of Respiratory Syncytial Virus. Science 2009, 326, 1279–1283. [CrossRef]
- Henderson, G.; Murray, J.; Yeo, R.P. Sorting of the Respiratory Syncytial Virus Matrix Protein into Detergent-Resistant Structures Is Dependent on Cell-Surface Expression of the Glycoproteins. Virology 2002, 300, 244–254. [CrossRef]
- Teng, M.N.; Collins, P.L. Identification of the Respiratory Syncytial Virus Proteins Required for Formation and Passage of Helper-Dependent Infectious Particles. J. Virol. 1998, 72, 5707–5716. [CrossRef]
- Zlotnik, A.; Yoshie, O. Chemokines: A New Classification System and Their Role in Immunity. Immunity 2000, 12, 121–127. [CrossRef]
- Lucas, A.D.; Chadwick, N.; Warren, B.F.; Jewell, D.P.; Gordon, S.; Powrie, F.; Greaves, D.R. The Transmembrane Form of the CX3CL1 Chemokine Fractalkine Is Expressed Predominantly by Epithelial Cells in Vivo. Am. J. Pathol. 2001, 158, 855–866. [CrossRef]
- Matsumiya, T.; Ota, K.; Imaizumi, T.; Yoshida, H.; Kimura, H.; Satoh, K. Characterization of Synergistic Induction of CX3CL1/Fractalkine by TNF-Alpha and IFN-Gamma in Vascular Endothelial Cells: An Essential Role for TNF-Alpha in Post-Transcriptional Regulation of CX3CL1. J. Immunol. Baltim. Md 1950 2010, 184, 4205–4214. [CrossRef]
- Murphy, P.M. International Union of Pharmacology. XXX. Update on Chemokine Receptor Nomenclature. Pharmacol. Rev. 2002, 54, 227–229. [CrossRef]
- Bazan, J.F.; Bacon, K.B.; Hardiman, G.; Wang, W.; Soo, K.; Rossi, D.; Greaves, D.R.; Zlotnik, A.; Schall, T.J. A New Class of Membrane-Bound Chemokine with a CX3C Motif. Nature 1997, 385, 640–644. [CrossRef]
- Ostuni, M.A.; Hermand, P.; Saindoy, E.; Guillou, N.; Guellec, J.; Coens, A.; Hattab, C.; Desuzinges-Mandon, E.; Jawhari, A.; Iatmanen-Harbi, S.; et al. CX3CL1 Homo-Oligomerization Drives Cell-to-Cell Adherence. Sci. Rep. 2020, 10, 9069. [CrossRef]
- Rivas-Fuentes, S.; Salgado-Aguayo, A.; Arratia-Quijada, J.; Gorocica-Rosete, P. Regulation and Biological Functions of the CX3CL1-CX3CR1 Axis and Its Relevance in Solid Cancer: A Mini-Review. J. Cancer 2021, 12, 571–583. [CrossRef]
- Imai, T.; Hieshima, K.; Haskell, C.; Baba, M.; Nagira, M.; Nishimura, M.; Kakizaki, M.; Takagi, S.; Nomiyama, H.; Schall, T.J.; et al. Identification and Molecular Characterization of Fractalkine Receptor CX3CR1, Which Mediates Both Leukocyte Migration and Adhesion. Cell 1997, 91, 521–530. [CrossRef]
- Umehara, H.; Bloom, E.T.; Okazaki, T.; Nagano, Y.; Yoshie, O.; Imai, T. Fractalkine in Vascular Biology: From Basic Research to Clinical Disease. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 34–40. [CrossRef]
- Pawelec, P.; Ziemka-Nalecz, M.; Sypecka, J.; Zalewska, T. The Impact of the CX3CL1/CX3CR1 Axis in Neurological Disorders. Cells 2020, 9, 2277. [CrossRef]
- Combadiere, C.; Salzwedel, K.; Smith, E.D.; Tiffany, H.L.; Berger, E.A.; Murphy, P.M. Identification of CX3CR1. A Chemotactic Receptor for the Human CX3C Chemokine Fractalkine and a Fusion Coreceptor for HIV-1. J. Biol. Chem. 1998, 273, 23799–23804. [CrossRef]
- Chandrasekar, B.; Mummidi, S.; Perla, R.P.; Bysani, S.; Dulin, N.O.; Liu, F.; Melby, P.C. Fractalkine (CX3CL1) Stimulated by Nuclear Factor kappaB (NF-kappaB)-Dependent Inflammatory Signals Induces Aortic Smooth Muscle Cell Proliferation through an Autocrine Pathway. Biochem. J. 2003, 373, 547–558. [CrossRef]
- Johnson, S.M.; McNally, B.A.; Ioannidis, I.; Flano, E.; Teng, M.N.; Oomens, A.G.; Walsh, E.E.; Peeples, M.E. Respiratory Syncytial Virus Uses CX3CR1 as a Receptor on Primary Human Airway Epithelial Cultures. PLoS Pathog. 2015, 11, e1005318. [CrossRef]
- Anderson, C.S.; Chu, C.-Y.; Wang, Q.; Mereness, J.A.; Ren, Y.; Donlon, K.; Bhattacharya, S.; Misra, R.S.; Walsh, E.E.; Pryhuber, G.S.; et al. CX3CR1 as a Respiratory Syncytial Virus Receptor in Pediatric Human Lung. Pediatr. Res. 2020, 87, 862–867. [CrossRef]
- Zhang, C.; Zhang, Y.; Zhuang, R.; Yang, K.; Chen, L.; Jin, B.; Ma, Y.; Zhang, Y.; Tang, K. Alterations in CX3CL1 Levels and Its Role in Viral Pathogenesis. Int. J. Mol. Sci. 2024, 25, 4451. [CrossRef]
- Das, S.; Raundhal, M.; Chen, J.; Oriss, T.B.; Huff, R.; Williams, J.V.; Ray, A.; Ray, P. Respiratory Syncytial Virus Infection of Newborn CX3CR1-Deficient Mice Induces a Pathogenic Pulmonary Innate Immune Response. JCI Insight 2017, 2, e94605, 94605. [CrossRef]
- Bukreyev, A.; Yang, L.; Fricke, J.; Cheng, L.; Ward, J.M.; Murphy, B.R.; Collins, P.L. The Secreted Form of Respiratory Syncytial Virus G Glycoprotein Helps the Virus Evade Antibody-Mediated Restriction of Replication by Acting as an Antigen Decoy and through Effects on Fc Receptor-Bearing Leukocytes. J. Virol. 2008, 82, 12191–12204. [CrossRef]
- Tripp, R.A.; Jones, L.P.; Haynes, L.M.; Zheng, H.; Murphy, P.M.; Anderson, L.J. CX3C Chemokine Mimicry by Respiratory Syncytial Virus G Glycoprotein. Nat. Immunol. 2001, 2, 732–738. [CrossRef]
- Openshaw, P.J.M.; Chiu, C.; Culley, F.J.; Johansson, C. Protective and Harmful Immunity to RSV Infection. Annu. Rev. Immunol. 2017, 35, 501–532. [CrossRef]
- Attaianese, F.; Guiducci, S.; Trapani, S.; Barbati, F.; Lodi, L.; Indolfi, G.; Azzari, C.; Ricci, S. Reshaping Our Knowledge: Advancements in Understanding the Immune Response to Human Respiratory Syncytial Virus. Pathog. Basel Switz. 2023, 12, 1118. [CrossRef]
- Chirkova, T.; Lin, S.; Oomens, A.G.P.; Gaston, K.A.; Boyoglu-Barnum, S.; Meng, J.; Stobart, C.C.; Cotton, C.U.; Hartert, T.V.; Moore, M.L.; et al. CX3CR1 Is an Important Surface Molecule for Respiratory Syncytial Virus Infection in Human Airway Epithelial Cells. J. Gen. Virol. 2015, 96, 2543–2556. [CrossRef]
- Agac, A.; Kolbe, S.M.; Ludlow, M.; Osterhaus, A.D.M.E.; Meineke, R.; Rimmelzwaan, G.F. Host Responses to Respiratory Syncytial Virus Infection. Viruses 2023, 15, 1999. [CrossRef]
- Jung, H.E.; Kim, T.H.; Lee, H.K. Contribution of Dendritic Cells in Protective Immunity against Respiratory Syncytial Virus Infection. Viruses 2020, 12, 102. [CrossRef]
- Currie, S.M.; Gwyer Findlay, E.; McFarlane, A.J.; Fitch, P.M.; Böttcher, B.; Colegrave, N.; Paras, A.; Jozwik, A.; Chiu, C.; Schwarze, J.; et al. Cathelicidins Have Direct Antiviral Activity against Respiratory Syncytial Virus In Vitro and Protective Function In Vivo in Mice and Humans. J. Immunol. Baltim. Md 1950 2016, 196, 2699–2710. [CrossRef]
- Latsko, K.N.; Jacob, A.T.; Junod, N.A.; Haas, C.E.; Castiglia, K.R.; Kastelitz, S.R.; Huffman, E.R.; Trombley, M.P.; Stobart, C.C. Role of Differences in Respiratory Syncytial Virus F and G Glycoproteins on Susceptibility to Inactivation by Antimicrobial Peptides LL-37 and Human Beta-Defensins. Viral Immunol. 2022, 35, 559–565. [CrossRef]
- Geerdink, R.J.; Pillay, J.; Meyaard, L.; Bont, L. Neutrophils in Respiratory Syncytial Virus Infection: A Target for Asthma Prevention. J. Allergy Clin. Immunol. 2015, 136, 838–847. [CrossRef]
- van Erp, E.A.; Feyaerts, D.; Duijst, M.; Mulder, H.L.; Wicht, O.; Luytjes, W.; Ferwerda, G.; van Kasteren, P.B. Respiratory Syncytial Virus Infects Primary Neonatal and Adult Natural Killer Cells and Affects Their Antiviral Effector Function. J. Infect. Dis. 2019, 219, 723–733. [CrossRef]
- García-Sastre, A.; Biron, C.A. Type 1 Interferons and the Virus-Host Relationship: A Lesson in Détente. Science 2006, 312, 879–882. [CrossRef]
- Bergeron, H.C.; Hansen, M.R.; Tripp, R.A. Interferons-Implications in the Immune Response to Respiratory Viruses. Microorganisms 2023, 11, 2179. [CrossRef]
- Schlender, J.; Hornung, V.; Finke, S.; Günthner-Biller, M.; Marozin, S.; Brzózka, K.; Moghim, S.; Endres, S.; Hartmann, G.; Conzelmann, K.-K. Inhibition of Toll-like Receptor 7- and 9-Mediated Alpha/Beta Interferon Production in Human Plasmacytoid Dendritic Cells by Respiratory Syncytial Virus and Measles Virus. J. Virol. 2005, 79, 5507–5515. [CrossRef]
- de Graaff, P.M.A.; de Jong, E.C.; van Capel, T.M.; van Dijk, M.E.A.; Roholl, P.J.M.; Boes, J.; Luytjes, W.; Kimpen, J.L.L.; van Bleek, G.M. Respiratory Syncytial Virus Infection of Monocyte-Derived Dendritic Cells Decreases Their Capacity to Activate CD4 T Cells. J. Immunol. Baltim. Md 1950 2005, 175, 5904–5911. [CrossRef]
- Lee, D.C.P.; Harker, J.A.E.; Tregoning, J.S.; Atabani, S.F.; Johansson, C.; Schwarze, J.; Openshaw, P.J.M. CD25+ Natural Regulatory T Cells Are Critical in Limiting Innate and Adaptive Immunity and Resolving Disease Following Respiratory Syncytial Virus Infection. J. Virol. 2010, 84, 8790–8798. [CrossRef]
- Teng, M.N.; Whitehead, S.S.; Bermingham, A.; St Claire, M.; Elkins, W.R.; Murphy, B.R.; Collins, P.L. Recombinant Respiratory Syncytial Virus That Does Not Express the NS1 or M2-2 Protein Is Highly Attenuated and Immunogenic in Chimpanzees. J. Virol. 2000, 74, 9317–9321. [CrossRef]
- Han, B.; Wang, Y.; Zheng, M. Inhibition of Autophagy Promotes Human RSV NS1-Induced Inflammation and Apoptosis in Vitro. Exp. Ther. Med. 2021, 22, 1054. [CrossRef]
- Li, M.; Li, J.; Zeng, R.; Yang, J.; Liu, J.; Zhang, Z.; Song, X.; Yao, Z.; Ma, C.; Li, W.; et al. Respiratory Syncytial Virus Replication Is Promoted by Autophagy-Mediated Inhibition of Apoptosis. J. Virol. 2018, 92, e02193-17. [CrossRef]
- Pei, J.; Beri, N.R.; Zou, A.J.; Hubel, P.; Dorando, H.K.; Bergant, V.; Andrews, R.D.; Pan, J.; Andrews, J.M.; Sheehan, K.C.F.; et al. Nuclear-Localized Human Respiratory Syncytial Virus NS1 Protein Modulates Host Gene Transcription. Cell Rep. 2021, 37, 109803. [CrossRef]
- Ren, J.; Liu, T.; Pang, L.; Li, K.; Garofalo, R.P.; Casola, A.; Bao, X. A Novel Mechanism for the Inhibition of Interferon Regulatory Factor-3-Dependent Gene Expression by Human Respiratory Syncytial Virus NS1 Protein. J. Gen. Virol. 2011, 92, 2153–2159. [CrossRef]
- Parsons, E.L.; Kim, J.S.; Malloy, A.M.W. Development of Innate and Adaptive Immunity to RSV in Young Children. Cell. Immunol. 2024, 399–400, 104824. [CrossRef]
- Lee, M.; Lee, Y.; Song, J.; Lee, J.; Chang, S.-Y. Tissue-Specific Role of CX3CR1 Expressing Immune Cells and Their Relationships with Human Disease. Immune Netw. 2018, 18, e5. [CrossRef]
- Cros, J.; Cagnard, N.; Woollard, K.; Patey, N.; Zhang, S.-Y.; Senechal, B.; Puel, A.; Biswas, S.K.; Moshous, D.; Picard, C.; et al. Human CD14dim Monocytes Patrol and Sense Nucleic Acids and Viruses via TLR7 and TLR8 Receptors. Immunity 2010, 33, 375–386. [CrossRef]
- Park, M.H.; Lee, J.S.; Yoon, J.H. High Expression of CX3CL1 by Tumor Cells Correlates with a Good Prognosis and Increased Tumor-Infiltrating CD8+ T Cells, Natural Killer Cells, and Dendritic Cells in Breast Carcinoma. J. Surg. Oncol. 2012, 106, 386–392. [CrossRef]
- Roe, M.F.E.; Bloxham, D.M.; White, D.K.; Ross-Russell, R.I.; Tasker, R.T.C.; O’Donnell, D.R. Lymphocyte Apoptosis in Acute Respiratory Syncytial Virus Bronchiolitis. Clin. Exp. Immunol. 2004, 137, 139–145. [CrossRef]
- Harcourt, J.; Alvarez, R.; Jones, L.P.; Henderson, C.; Anderson, L.J.; Tripp, R.A. Respiratory Syncytial Virus G Protein and G Protein CX3C Motif Adversely Affect CX3CR1+ T Cell Responses. J. Immunol. Baltim. Md 1950 2006, 176, 1600–1608. [CrossRef]
- Amanatidou, V.; Sourvinos, G.; Apostolakis, S.; Tsilimigaki, A.; Spandidos, D.A. T280M Variation of the CX3C Receptor Gene Is Associated with Increased Risk for Severe Respiratory Syncytial Virus Bronchiolitis. Pediatr. Infect. Dis. J. 2006, 25, 410–414. [CrossRef]
- McDermott, D.H.; Fong, A.M.; Yang, Q.; Sechler, J.M.; Cupples, L.A.; Merrell, M.N.; Wilson, P.W.F.; D’Agostino, R.B.; O’Donnell, C.J.; Patel, D.D.; et al. Chemokine Receptor Mutant CX3CR1-M280 Has Impaired Adhesive Function and Correlates with Protection from Cardiovascular Disease in Humans. J. Clin. Invest. 2003, 111, 1241–1250. [CrossRef]
- Becker, S.; Soukup, J.M. Airway Epithelial Cell-Induced Activation of Monocytes and Eosinophils in Respiratory Syncytial Viral Infection. Immunobiology 1999, 201, 88–106. [CrossRef]
- Soukup, J.M.; Becker, S. Role of Monocytes and Eosinophils in Human Respiratory Syncytial Virus Infection in Vitro. Clin. Immunol. Orlando Fla 2003, 107, 178–185. [CrossRef]
- Collar, A.L.; Swamydas, M.; O’Hayre, M.; Sajib, M.S.; Hoffman, K.W.; Singh, S.P.; Mourad, A.; Johnson, M.D.; Ferre, E.M.; Farber, J.M.; et al. The Homozygous CX3CR1-M280 Mutation Impairs Human Monocyte Survival. JCI Insight 2018, 3, e95417, 95417. [CrossRef]
- Zhivaki, D.; Lemoine, S.; Lim, A.; Morva, A.; Vidalain, P.-O.; Schandene, L.; Casartelli, N.; Rameix-Welti, M.-A.; Hervé, P.-L.; Dériaud, E.; et al. Respiratory Syncytial Virus Infects Regulatory B Cells in Human Neonates via Chemokine Receptor CX3CR1 and Promotes Lung Disease Severity. Immunity 2017, 46, 301–314. [CrossRef]
- Bergeron, H.C.; Murray, J.; Nuñez Castrejon, A.M.; DuBois, R.M.; Tripp, R.A. Respiratory Syncytial Virus (RSV) G Protein Vaccines With Central Conserved Domain Mutations Induce CX3C-CX3CR1 Blocking Antibodies. Viruses 2021, 13, 352. [CrossRef]
- Langedijk, A.C.; Bont, L.J. Respiratory Syncytial Virus Infection and Novel Interventions. Nat. Rev. Microbiol. 2023, 21, 734–749. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).