Submitted:
10 July 2024
Posted:
10 July 2024
You are already at the latest version
Abstract
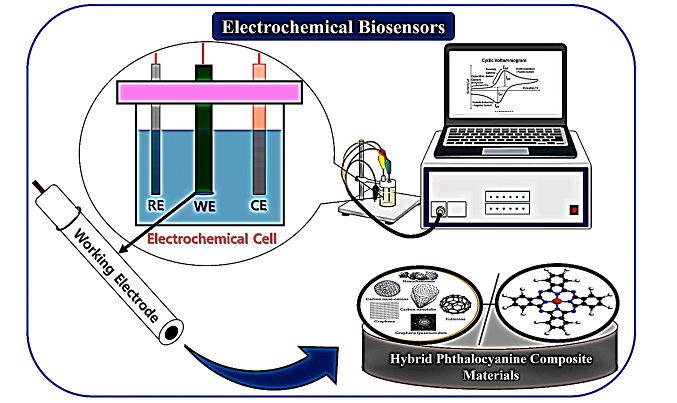
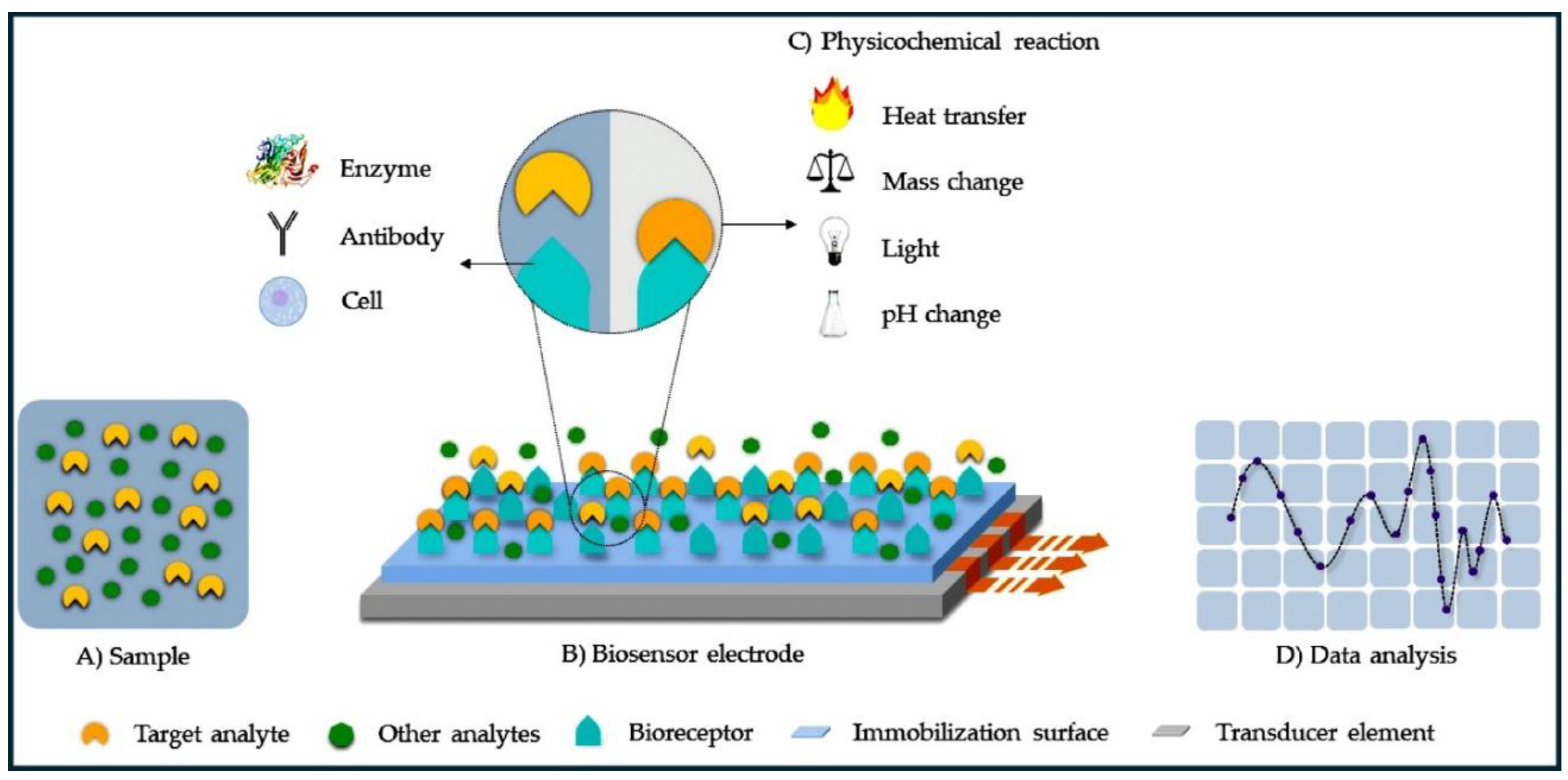
Keywords:
1. Introduction
2. Pc Composite Materials for Biosensors
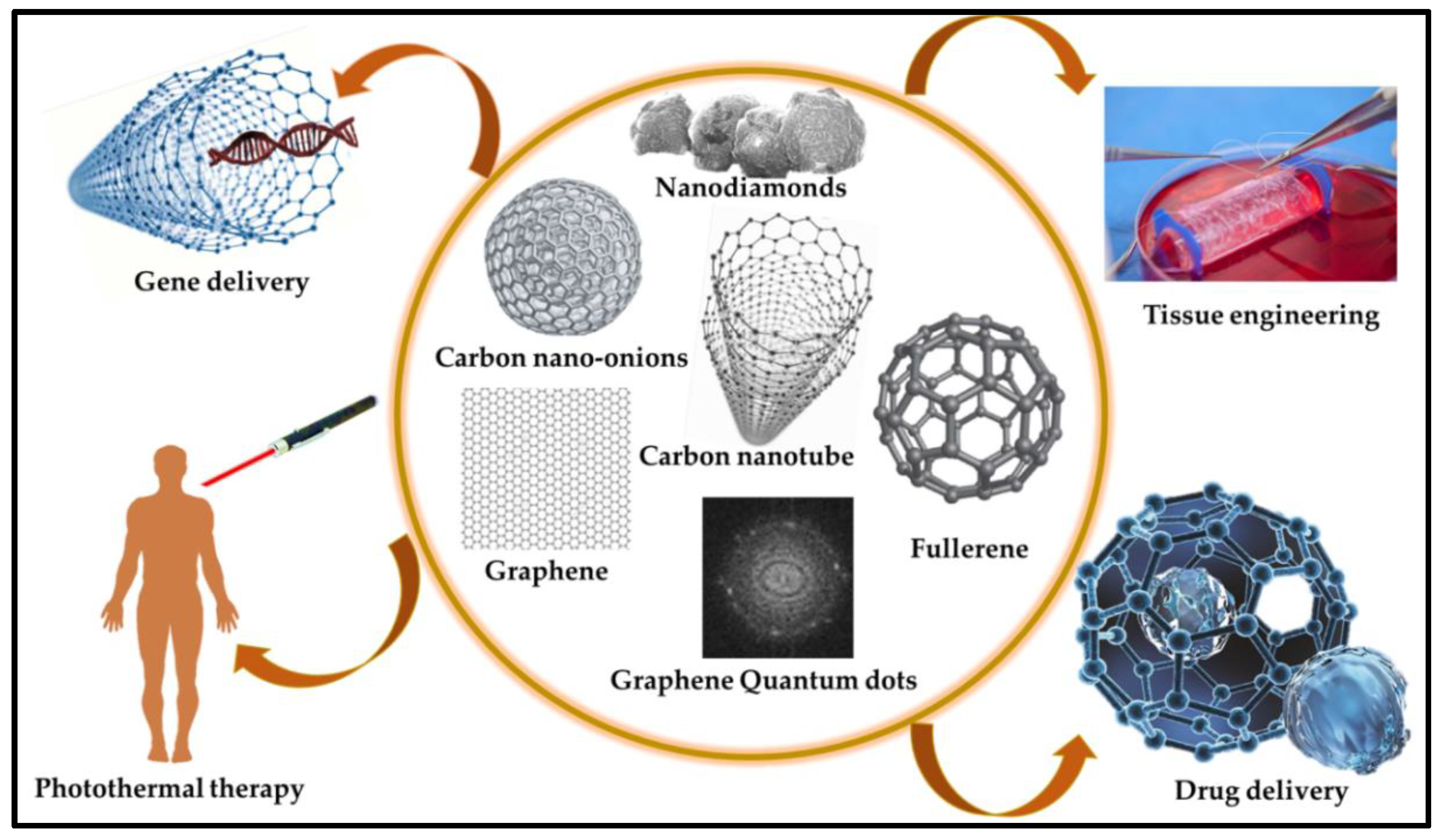
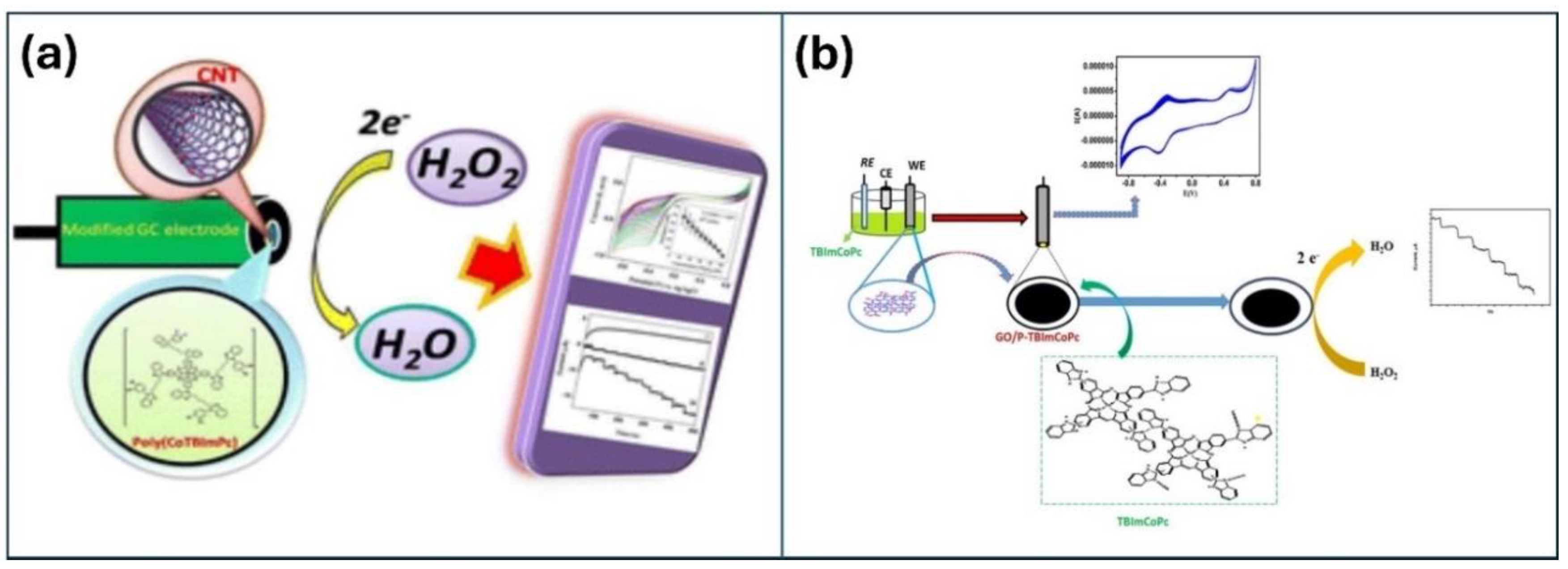
2.1. Carbonaceous Materials
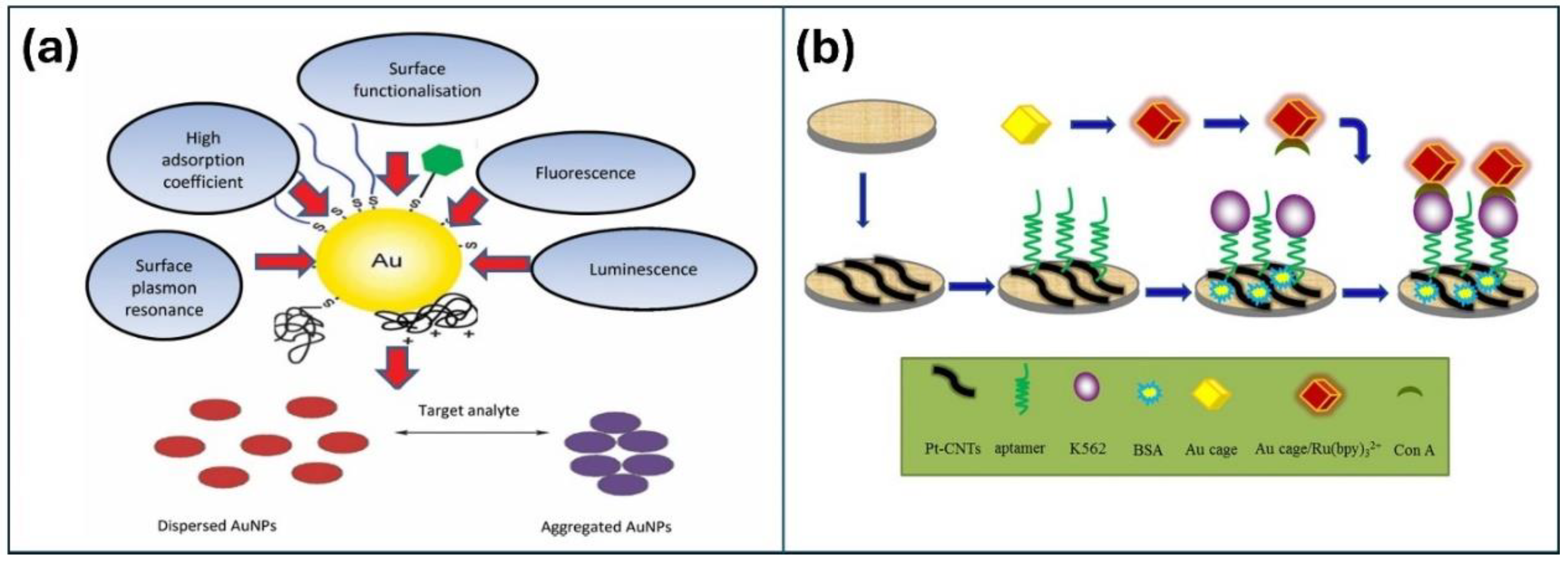
2.2. Metal and Metal Oxide NPs
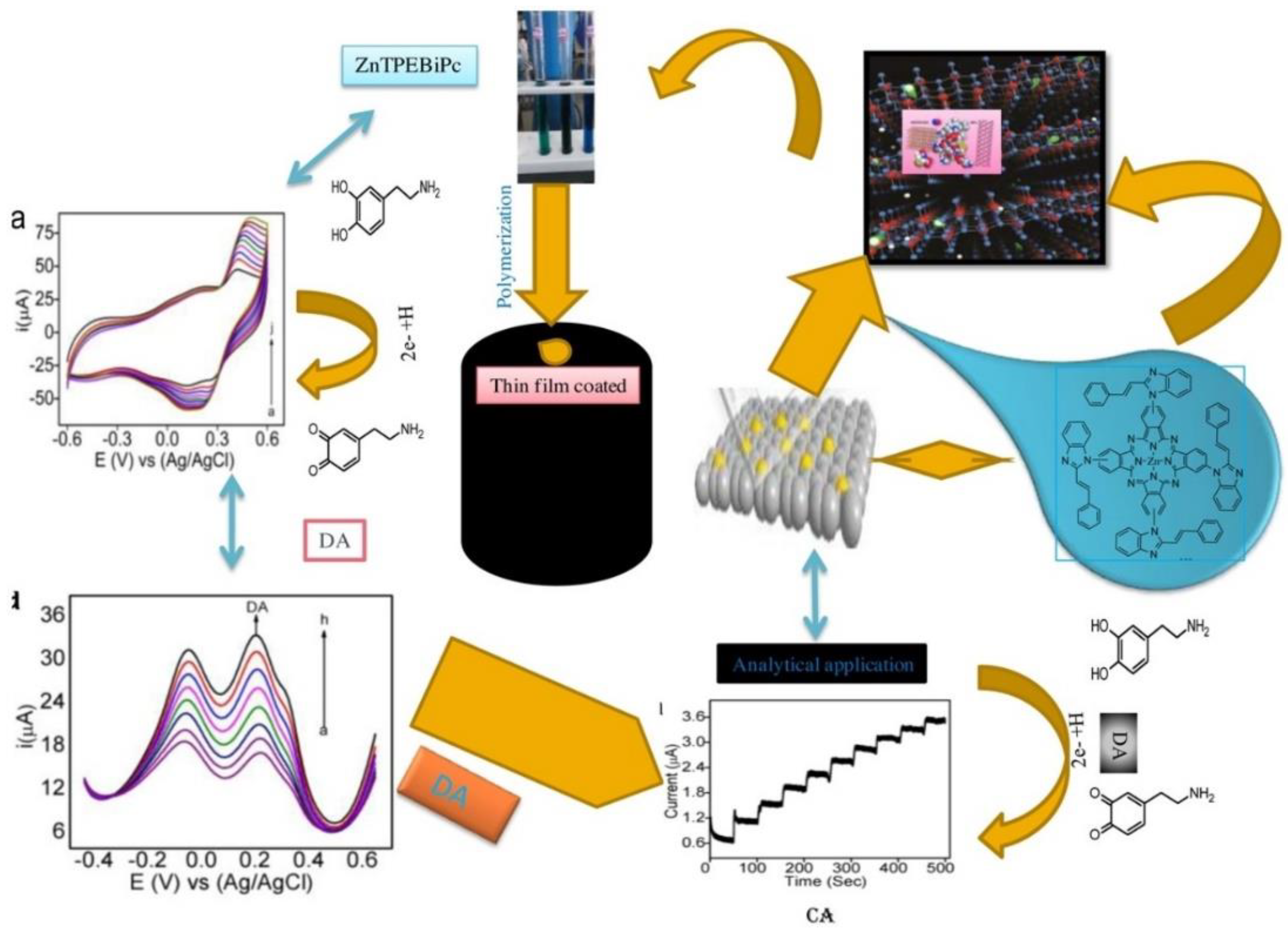
2.3. Polymeric Pc Materials
3. Pc-Based Hybrid Composites
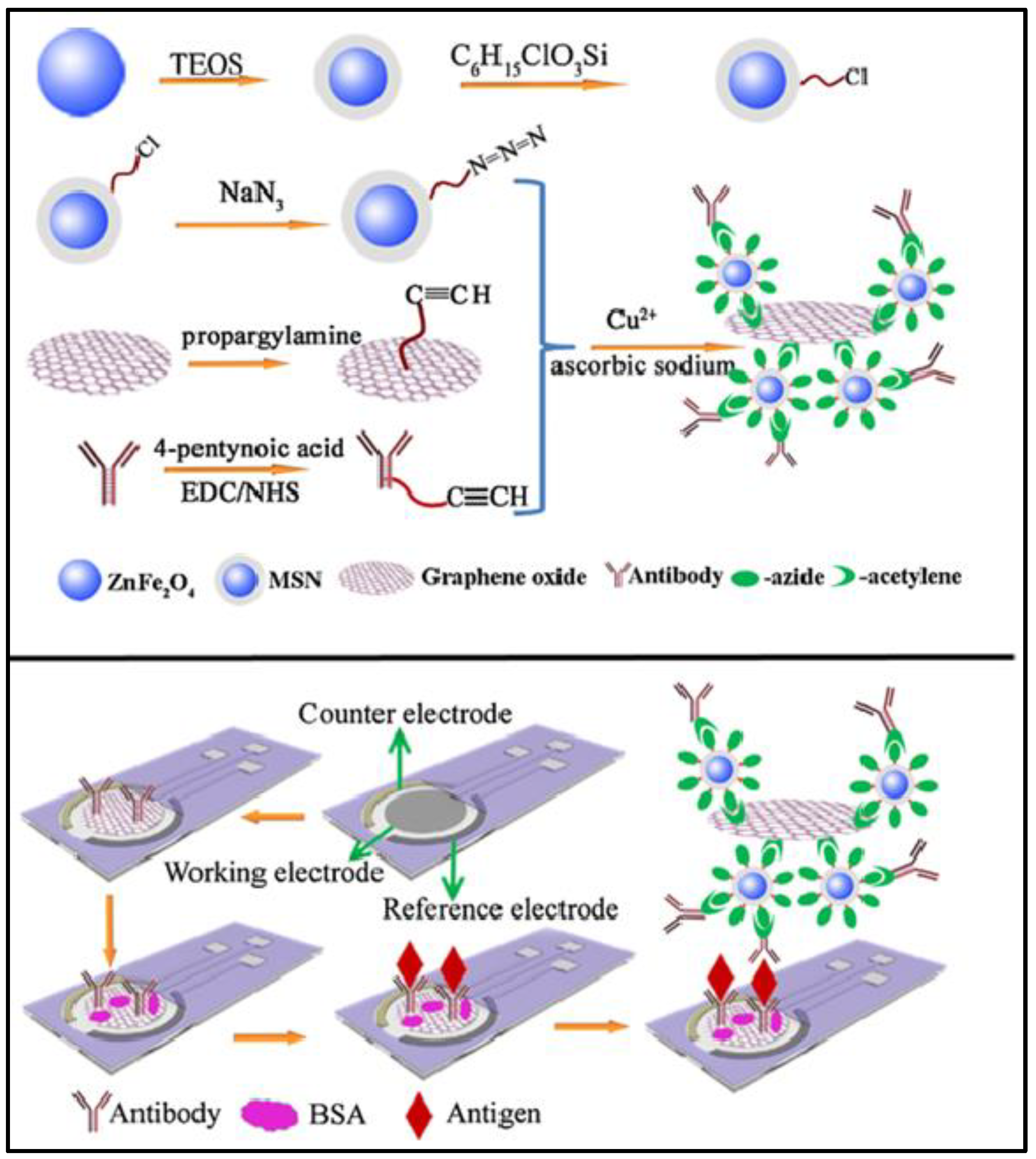
4. Fabrication of Hybrid Pc in Three-Electrode System
5. Conclusions, Challenges, and Future Perspectives
Author Contributions
Acknowledgments
References
- Naresh,V.; Lee, N.A. Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors. 2021, 21, 1109. [CrossRef]
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M. Biosensors and Microfluidic Biosensors: From Fabrication to Application. Biosensors. 2022, 12, 543. [Google Scholar] [CrossRef] [PubMed]
- Abid, H.; Mohd, J.; Ravi, P.S. R, Suman.; S, Rab. Biosensors applications in medical field: A brief review, Sensors International, 2021, 2, 202. [CrossRef]
- Mujawar, M.A.; H, Gohel.; Bhardwaj, S.K.; S. Srinivasan.; N, Hickman.; A.Kaushik, Nano-enabled biosensing systems for intelligent healthcare: towards COVID-19 management, Materials Today Chemistry, 2020, 17. [CrossRef]
- B, Katey.; Ioana, V.; Anita, N.P.; Alexandrina U. A Review of Biosensors and Their Applications, ASME Open J. Engineering. 2023, 2, 020201. [CrossRef]
- Thevenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Pure Appl. Chem. 1999, 71, 2333–2348. [Google Scholar] [CrossRef]
- Singh,A.; Sharma,A.; Ahmed, A.; Sundramoorthy, A.K.; Furukawa, H.; Arya, S.; Khosla, A. Recent Advances in Electrochemical Biosensors: Applications, Challenges, and Future Scope. Biosensors. 2021, 11, 336. [CrossRef]
- Shivalingayya ; Preeti R.K.; Sangappa, K.G.; Shashidhar.; Ashajyoti, C.; Arunkumar, L. Multifunctional Nanoparticles for Biomedical Applications, Journal of Chemical, Biological and Physical Sciences, JCBPS; Section B. 2022, 12, 410-422. [CrossRef]
- Costa, P.; Nunespereira, J.; Oliveira, JA.;, et al. High-performance graphene-based carbon nanofiller/polymer composites for piezoresistive sensor applications. Compos Sci Technol. 2017, 153, 241–252. [CrossRef]
- Campaña, A.; Florez, S.; Noguera, M.; Fuentes, O.; Ruiz, P.P.; Cruz, J.; Osma, J. Enzyme-based electrochemical biosensors for microfluidic platforms to detect pharmaceutical residues in wastewater. Biosensors. 2019, 9, 41. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Kim, S.; Min, D.H. Biosensors based on graphene oxide and its biomedical application. Adv Drug Deliv Rev. 2016, 1, 105, 275–287. [Google Scholar] [CrossRef]
- Basova, T.V.; Ray, A.K. Review—Hybrid materials based on phthalocyanines and metal nanoparticles for chemi-resistive and electrochemical sensors: A Mini-Review. ECS Journal of Solid State Science and Technology. 2020, 9, 061001. [Google Scholar] [CrossRef]
- Kuntoji, G.; Kousar, N.; Gaddimath, S.; Koodlur Sannegowda, L. Macromolecule–Nanoparticle-Based Hybrid Materials for Biosensor Applications. Biosensors. 2024, 14, 277. [Google Scholar] [CrossRef]
- Soto, D.; Orozco, J. Hybrid Nanobioengineered Nanomaterial- Based Electrochemical Biosensors. Molecules. 2022, 27, 3841. [Google Scholar] [CrossRef]
- Huai-Song, W. metal–organic frameworks for biosensing and bioimaging applications, Coordination Chemistry Reviews. 2017, 349, 139-155. [CrossRef]
- Kuntoji, G.; Kousar, N.; Gaddimath, S.; Koodlur Sannegowda, L. Macromolecule–Nanoparticle-Based Hybrid Materials for Biosensor Applications. Biosensors 2024, 14, 277. [Google Scholar] [CrossRef]
- Manjunatha, N.; Lokesh, K.S. Hybrid composites based on phthalocyanine and carbonaceous materials for sensing applications: a review, International Journal of Biosensors & Bioelectronics. 2021, 7(3), 84‒89. [CrossRef]
- Jipei, Y.; Nikolai, G.; Alexander, Eychmüller. Application of Polymer Quantum Dot-Enzyme Hybrids in the Biosensor Development and Test Paper Fabrication. Anal. Chem. 2012, 84, 11, 5047–5052. [CrossRef]
- Skladal, P.; Mascini, M. Sensitive detection of pesticides using amperometric sensors based on cobalt phthalocyanine-modified composite electrodes and immobilized choline esterases. Biosensors& Bioelectronics. 1992, 7, 335–343. [Google Scholar] [CrossRef]
- Lv, N.; Li, Q.; Zhu, H.; Mu, S.; Luo, X.; Ren, X.; Liu, X.; Li, S.; Cheng, C.; Ma, T. Electrocatalytic Porphyrin/Phthalocyanine-Based Organic Frameworks: Building Blocks, Coordination Microenvironments, Structure-Performance Relationships. Adv Sci (Weinh). 2023, 10(7), e2206239. [Google Scholar] [CrossRef] [PubMed]
- John, R.A.; Anthony, Guiseppi-Elie. Responsive Polymers in the Fabrication of Enzyme-Based Biosensors, Biomaterials Science. 2020, 1267-1286. [CrossRef]
- Mehrab, P.; Erfan, R.; Maryam, R.K.; Amirmasoud, S.; Razieh, B.; Abbas, R.; Luiz, F.R.; Ferreira. Properties and application of carbon quantum dots (CQDs) in biosensors for disease detection: A comprehensive review, Journal of Drug Delivery Science and Technology. , 2023, 80, 104156. [CrossRef]
- Zhihong, Zhang.; Yafei, Lou.; Chuanpan, Guo.; Qiaojuan, Jia.; Yingpan, Song.; Jia, Y.T.; Shuai, Z.; Minghua, W.; Linghao, He.; Miao, D. Metal–organic frameworks (MOFs) based chemosensors/biosensors for analysis of food contaminants, Trends in Food Science & Technology. 118, 2021, 569-588. [CrossRef]
- Naresh, V. .; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors. 2021, 21, 1109. [Google Scholar] [CrossRef]
- Sorokin, AB. Phthalocyanine metal complexes in catalysis. Chem Rev. 2013, 9(113), 8152–91. [Google Scholar] [CrossRef]
- Zagal, JH.; Bedioui, F.; Dodelet, JP. N4-macrocyclic Complexes. NY. Springer. 2006.
- Hong, Q.; Chen, S. Facile one-step fabrication of phthalocyanine– graphene–bacterial–cellulose nanocomposite with superior catalytic performance. Nanomaterials. 2020, 10, 1673. [Google Scholar] [CrossRef]
- Browne, MP.; Novotny, F.; Sofer, Z.; et al. 3D printed graphene electrodes’ electrochemical activation. ACS Appl Mater Interfaces. 2018, 10, 40294–40301. [CrossRef]
- Gaddimath, S.; Payamalle,S.; Channabasavana HundiPuttaningaiah, K.P.; Hur, J. RecentAdvances in pH and RedoxResponsive Polymer Nanocompositesfor Cancer Therapy. J. Compos. Sci. 024, 8, 28. [CrossRef]
- Sun, G.; Wei, X.; Zhang, D.; Huang, L.; Liu, H.; Fang, H. Immobilization of Enzyme Electrochemical Biosensors and Their Application to Food Bioprocess Monitoring. Biosensors. 2023, 13, 886. [Google Scholar] [CrossRef]
- Verma, S.; Thakur, D.; Pandey, C.M.; Kumar, D. Recent Prospects of Carbonaceous Nanomaterials-Based Laccase Biosensor for Electrochemical Detection of Phenolic Compounds. Biosensors. 2023, 13, 305. [Google Scholar] [CrossRef]
- Kamalasekaran, K.; Magesh, V.; Atchudan, R.; Arya, S.; Sundramoorthy, A.K. Development of Electrochemical Sensor Using Iron (III) Phthalocyanine/Gold Nanoparticle /Graphene Hybrid Film for Highly Selective Determination of Nicotine in HumanSalivary Samples. Biosensors. 2023, 13, 839. [Google Scholar] [CrossRef]
- Mahesh, M.S.; G, Manasa.; Ronald, J.M.; K, Mondal.; N.P, Shetti. Fundamentals of bio-electrochemical sensing, Chemical Engineering Journal Advances. 2023, 16, 100516. [CrossRef]
- Mikuła, E. Recent Advancements in Electrochemical Biosensors for Alzheimer’‘s’ Disease Biomarkers Detection. Curr Med Chem. 2021, 28(20), 4049-4073.
- H.S, Hwang.; Jae, W. J.; Yoong, A.K.; Mincheol, C. Carbon Nanomaterials as Versatile Platforms for Biosensing Applications, Micromachines. 2020, 11, 814. [CrossRef]
- Joonhyub, Kim.; Gayoung, P.; Seoho, Lee.; Suk-Won, H.; Namki, M.; Kyung-Mi, Lee. Single Wall Carbon Nanotube Electrode SystemCapable of Quantitative Detection of CD4+ T Cells, Biosensors and Bioelectronic. [CrossRef]
- Lijie, Wang.; Wenke, J.; Yanju, Wu.; Fei, Wang.; Lu, Kui. Direct Fabrication of 3D Graphene–Multi Walled Carbon Nanotubes Network and Its Application for Sensitive Electrochemical Determination of Hyperin, Int. J. Electrochem. Sci., 2019, 14, 481 – 493. [CrossRef]
- Chaithra, K.P.; Akshaya, K.B.; Maiyalagan, T.; Gurumurthy, H.; Anitha, V.; Louis, G. Unique Host Matrix to Disperse Pd Nanoparticles for Electrochemical Sensing of Morin: Sustainable Engineering Approach. ACS Biomater. Sci. Eng. 2020, 6, 5264–52731. [Google Scholar] [CrossRef]
- Diao, P.; Liu, Z.; Wu, B.; Nan, X.; Zhang, J.; Wei, Z. Chemically assembled single-wall carbon nanotubes and their electrochemistry. Chemphyschem. 2002, 3, 898–901 101002/1439. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Srivastava, S.; Ali, M.A.; Gupta, T.K.; Sumana, G.; Srivastava, A.; Mathur, R.B.; Malhotra, B.D. Carboxylated multiwalled carbon nanotubes based biosensor for aflatoxin detection. Sens. Actuators Chem. 2013, 185, 258–264. [Google Scholar] [CrossRef]
- Mahor, A.; Singh, P.P.; Bharadwaj, P.; Sharma, N.; Yadav, S.; Rosenholm, J.M.; Bansal, K.K. Carbon-Based Nanomaterials for Delivery of Biologicals and Therapeutics: A Cutting-Edge Technology. 2021, 7, 19. [CrossRef]
- Ge, S.; Sun, M.; Liu, W.; Li, S.; Wang, X.; Chu, C.; Yan, M.; Yu, J. Disposable electrochemical immunosensor based on peroxidase-like magnetic silica–graphene oxide composites for detection of cancer antigen 153. Sens. Actuators B Chem. 2014, 192, 317–326. [Google Scholar] [CrossRef]
- Sumit, M.; Joginder, S.; Rohit, G.; Yajvinder, S.; Vivek, C.; Ahmad, Umar.; Ahmed, A.I.; Sheikh, A.; Sadia, Ameen.; Sotirios, B. Nanomaterials-based biosensor and their applications: A review, Heliyon. 9, 9, 2023. [CrossRef]
- Pratik, J.; Rupesh, M.; Roger, J.N. Biosensing applications of carbon-based materials, Current Opinion in Biomedical Engineering. 2021, 18, 100274. [CrossRef]
- Aoife, C.; Power, B.G.; Shaneel, C.; James, C. Carbon nanomaterials and their application to electrochemical sensors: a review, Nanotechnol Rev. 2018, 7(1), 19–41. [CrossRef]
- Keshavananda, C.P., Kenkera, R.N.; Shambhulinga A.; Shivalingayya.; Lokesh, K.S. Novel polymeric cobalt tetrabenzimidazole phthalocyanine for nanomolar detection of hydrogen peroxide, RSC Sustain. 2023,1, 128-138. [CrossRef]
- Imadadulla, M.; Manjunatha, N.; Veeresh, A.S.; Dayananda, B.P.; Lokesh, K.S. Electropolymerized film of cobalt tetrabenzimidazolephthalocyanine for the T amperometric detection of H2O2, Journal of Electroanalytical Chemistry. 2018, 826, 96–103. [CrossRef]
- Zribi, B.; Roy, E.; Pallandre, A.; Chebil, S.; Koubaa, M.; Mejri, N.; Magdinier Gomez, H.; Sola, C.; Korri-Youssoufi, H.; Haghiri-Gosnet, A.-M. A microfluidic electrochemical biosensor based on multiwall carbon nanotube/ferrocene for genomic DNA detection of Mycobacterium tuberculosis in clinical isolates. Biomicrofluidics. 2016. [CrossRef]
- Zong-hua, W.; Qiong-lin, L.; Yi-ming, W.; Guo-an, Luo. Carbon nanotube-intercalated graphite electrodes for simultaneous determination of dopamine and serotonin in the presence of ascorbic acid, Journal of Electroanalytical Chemistry. 540, 2003, 129-134. [CrossRef]
- Khan, R.; Pal, M.; Kuzikov, A.V.; Bulko, T.; Suprun, E.V.; Shumyantseva, V.V. Impedimetric immunosensor for detection of cardiovascular disorder risk biomarker. Mater. Sci. Eng. C 2016, 68, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, X.; Yan, S.; Wang, M.; Liu, P.; Dong, Y.; Zhang, C. Single-walled carbon nanotubes–carboxyl- functionalized graphene oxide-based electrochemical DNA biosensor for thermolabile hemolysin gene detection. Anal. Methods. 2015, 7, 5303–5310. [Google Scholar] [CrossRef]
- Xing, R.M.; Yang, H.T.; Li, S.N.; Yang, J.H.; Zhao, X.Y.; Wang, Q.L.; Liu, S.H.; Liu, X.H. A sensitive and reliable rutin electrochemical sensor based on palladium phthalocyanine-MWCNTs-Nafion nanocomposite. J. Solid State. Electr. 2017, 21, 1219–1228. [Google Scholar] [CrossRef]
- Yang, H.T.; Li, B.Y.; Cui, R.J.; Xing, R.M.; Liu, S.H. Electrochemical sensor for rutin detection based on Au nanoparticle-loaded helical carbon nanotubes. J. Nanopart. Res. 2017, 19, 354. [Google Scholar] [CrossRef]
- Jing, S.S.; Zheng, H.J.; Zhao, L.; Qu, L.B.; Yu, L.L. A novel electrochemical sensor based on WO3 nanorods-decorated poly(sodium 4-styrenesulfonate) functionalized graphene nanocomposite modified electrode for detecting of puerarin. Talanta. 2017, 174, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; Wang, S.; Cui, F.; Zhuo, B.B.; Zhao, C.R.; Liu, W.L. Sensitive and selective detection of puerarin based on the hybrid of reduced graphene oxide and molecularly imprinted polymer. J. Pharmaceut. Biomed. 2020, 185, 113221. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Jia, W.K.; Wu, Y.J.; Wang, F.; Kui, L. Direct Fabrication of 3D Graphene-Multi Walled Carbon Nanotubes Network and Its Application for Sensitive Electrochemical Determination of Hyperin. Int. J. Electrochem. Sc. 2019, 14, 481–493. [Google Scholar] [CrossRef]
- Meng, R.Q.; Li, Q.L.; Zhang, S.J.; Tang, J.K.; Ma, C.L.; Jin, R.Y. GQDs/PEDOT Bilayer Films Modified Electrode as a Novel Electrochemical Sensing Platform for Rutin Detection. Int. J. Electrochem. Sc. 2019, 14, 11000–11011. [Google Scholar] [CrossRef]
- Zhao, P.C.; Ni, M.J.; Xu, Y.T.; Wang, C.X.; Chen, C.; Zhang, X.R.; Li, C.Y.; Xie, Y.X.; Fei, J.J. A novel ultrasensitive electrochemical quercetin sensor based on MoS2-carbon nanotube @ graphene oxide nanoribbons / HS-cyclodextrin / graphene quantum dots composite film. Sens. Actuators, 2019, 299, 126997. [Google Scholar] [CrossRef]
- Senocak, A.; Khataee, A.; Demirbas, E.; Doustkhah, E. Ultrasensitive detection of rutin antioxidant through a magnetic micro-mesoporous graphitized carbon wrapped Co nanoarchitecture. Sensor Actuat. B-Chem. 2020, 312, 127939. [Google Scholar] [CrossRef]
- Malekzad, H.; Zangabad, P.S.; Mirshekari, H.; Karimi, M.; Hamblin, M.R. Noble metal nanoparticles in biosensors: recent studies and applications. Nanotechnol Rev. 2017, 27, 6(3), 301–329. [Google Scholar] [CrossRef]
- Keshavananada prabhu C P.; Aralekallu, S.; Sajjan, V. A.; Palanna, M.; Kumar, S.; Sannegowda, L. K. Non-precious cobalt phthalocyanine-embedded iron ore electrocatalysts for hydrogen evolution reactions. Sustainable Energy & Fuels, 2021,5(5), 1448-1457. [CrossRef]
- Shivalingayya, G.; K. B, Chandrakala.; Arunkumar, L.; Santhoshkumar, Dani.; C. P, Keshavananda Prabhu.; Giddaerappa.; L.K. Sannegowda. Ilmenite-type NiTiO3 nanoparticles for oxygen evolution reaction, Journal of Applied Electrochemistry. [CrossRef]
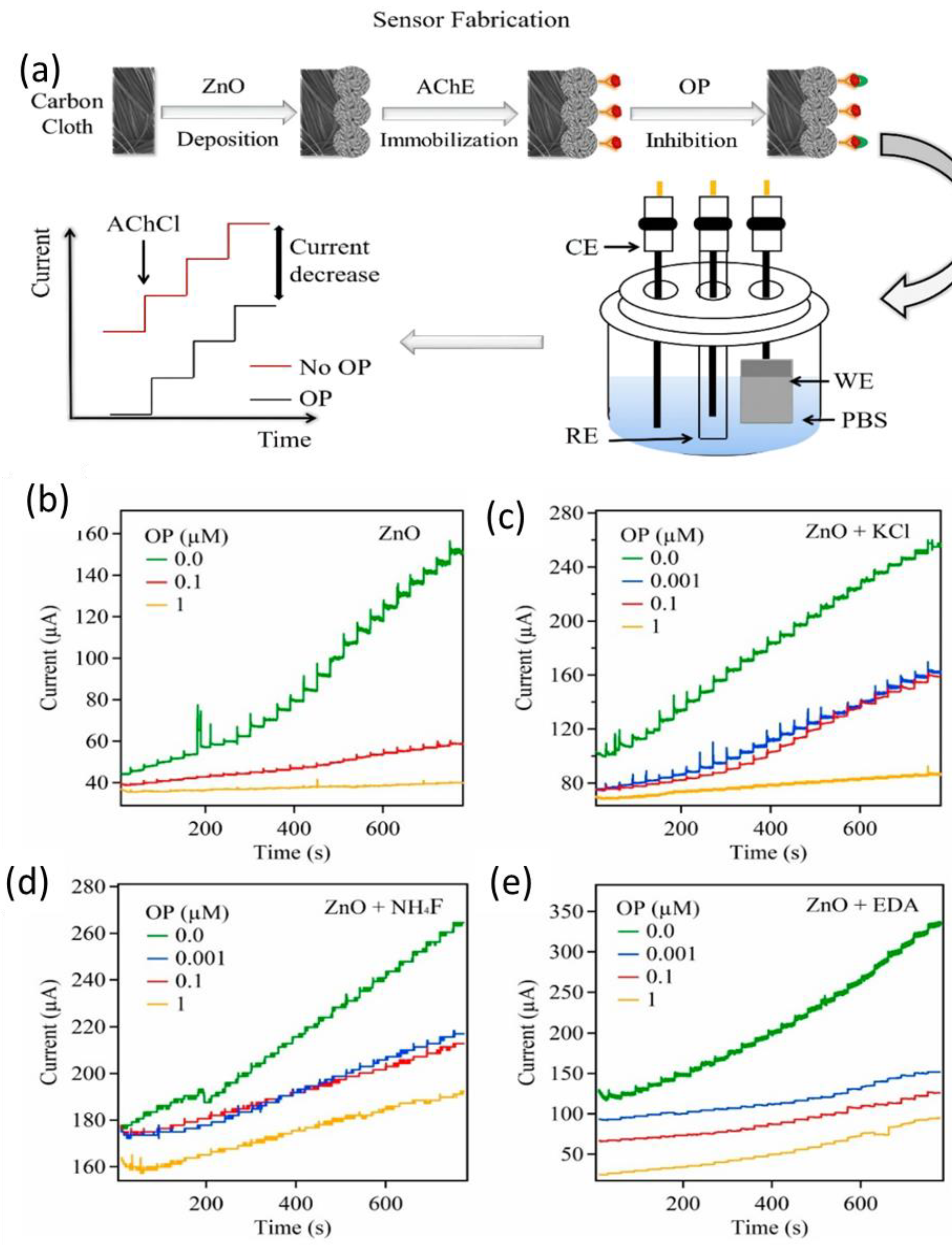
- N, Tripathy.; Deok-Ho, Kim. Metal oxide modified ZnO nanomaterials for biosensor applications, Nano Convergence. 2018, 5, 1-10. [CrossRef]
- L.K, Sannegowda.; K.R, Venugopala Reddy.; K.H, Shivaprasad. Stable nano-size copper and its oxide particles using cobalt tetraamino phthalocyanine as a stabilizer; Application to electrochemical activity, RSC Advances. 2014, 4, 11367-11374. [CrossRef]
- Guo, X.; Liang, B.; Jian, J.; Zhang, Y.; Ye, X. Glucose biosensor based on a platinum electrode modified with rhodium nanoparticles and with glucose oxidase immobilized on gold nanoparticles. Microchim Acta. 2014, 181, 519–25. [Google Scholar] [CrossRef]
- Han, W.; Yang, J.; Jiang, B.; Wang, X.; Wang, C.; Guo, L.; Sun, Y.; Liu, F.; Sun, P.; Lu, G. Conductometric ppb-Level CO Sensors Based on In2O3 Nanofibers Co-Modified with Au and Pd Species. Nanomaterials. 2022, 12, 3267. [Google Scholar] [CrossRef]
- Fallatah, A.; Kuperus, N.; Almomtan, M.; Padalkar, S. Sensitive Biosensor Based on Shape-Controlled ZnONanostructures Grown on Flexible Porous Substrate for Pesticide Detection. Sensors. 2022, 22, 3522. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, J-F.; Li, J-J.; Zhao, J-W. Tuning the fluorescence quenching properties of plasmonic Ag-coated-au triangular nanoplates: application in ultrasensitive detection of CEA. Plasmonics. 2015, 11, 565–572. [CrossRef]
- Lan, D.; Li, B.; Zhang, Z. Chemiluminescence flow biosensor for glucose based on gold nanoparticle-enhanced activities of glucose oxidase and horseradish peroxidase. Biosens Bioelectron. 2008, 24, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Su, M.; Gao, C.; Tao, X.; Ge, S. Application of Au cage/Ru (bpy) 32+ nanostructures for the electrochemiluminescence detection of K562 cancer cells based on aptamer. Sensor Actuator B. 2015, 214, 144–151. [Google Scholar] [CrossRef]
- Liang, W.; Zhuo, Y.; Xiong, C.; Zheng, Y.; Chai, Y.; Yuan, R. Ultrasensitive cytosensor based on self-enhanced electrochemiluminescent ruthenium-silica composite nanoparticles for efficient drug screening with cell apoptosis monitoring. Anal Chem. 2015, 87, 12363–12371 101021/acsanalchem5b03822. [Google Scholar] [CrossRef] [PubMed]
- Mohadeseh, Safaei.; Mohammad, M.; Foroughi.; Nasser, E.; Shohreh, J.; Ali, Omidi.; Mehrdad, Khatami. A review on metal-organic frameworks: Synthesis and applications, TrAC Trends in Analytical Chemistry. 118, 2019, 401-425. [CrossRef]
- Prasad, G.J.; Namrata, W.G.; Abdul, H.B.; Mohan, G.K. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications, Journal of Drug Delivery Science and Technology. 53, 2019, 101174. [CrossRef]
- Liu, D.; Guo, Q.; Zhang, X.; Hou, H.; You, T. PdCo alloy nanoparticle-embedded carbon nanofiber for ultrasensitive nonenzymatic detection of hydrogen peroxide and nitrite. J Colloid Interf Sci. 2015, 450, 168–173. [Google Scholar] [CrossRef]
- Bernardo-Boongaling, V.R.R.; Serrano, N.; García-Guzmán,J.J.; Palacios-Santander,J.M.; Díaz-Cruz, J.M. Screen-PrintedElectrodes Modified with Green-Synthesized Gold Nanoparticles for the Electrochemical Determination of Aminothiols. J. Electroanal. Chem. 2019, 847, 113184. [CrossRef]
- Ravalli,A.; dosSantos, G.P.; Ferroni, M.; Faglia, G.; Yamanaka, H.; Marrazza,G. New Label Free CA 125 Detection Based on Gold Nanostructured Screen-Printed Electrode. Sens. Actuators B Chem. 2013, 179. [CrossRef]
- Baradoke, A.; Jose, B.; Pauliukaite, R.; Forster, R.J. Properties of Anti-CA125 Antibody Layers on Screen-Printed Carbon Electrodes Modified by Gold and Platinum Nanostructures. Electrochim. Acta. 2019, 306, 299–306. [Google Scholar] [CrossRef]
- Phetsang, S.; Jakmunee, J.; Mungkornasawakul, P.; Laocharoensuk, R.; Ounnunkad, K. Sensitive Amperometric Biosensors for Detection of Glucose and Cholesterol Using a Platinum/Reduced Graphene Oxide/Poly(3-Aminobenzoic Acid) Film-Modified Screen-Printed Carbon Electrode. Bioelectrochemistry. 2019, 127, 125–135.
- Reitz, E.; Jia, W.; Gentile, M.; Wang, Y.; Lei, Y. CuO Nanospheres Based Nonenzymatic Glucose Sensor. Electroanalysis. 2008, 20, 2482–2486. [Google Scholar] [CrossRef]
- K.K. Naik.; C.S, Rout. Electrodeposition of ZnCo2O4 nanoparticles for biosensing applications, RSC Adv. 2015, 5, 79397–79404. [CrossRef]
- Jianjun, L. Shiwei, L.; Yue, Y.; Kai, Liu.; Wencai, Du. Highly selective and sensitive glucose sensors based on organic electrochemical transistors using TiO2 nanotube arrays-based gate electrodes, Sensors and Actuators B: Chemical. 2015, 208, 457-463. [CrossRef]
- Yuan, M.; Liu, A.; Zhao, M.; Dong, W.; Zhao, T.; Wang, J.; Tang, W. Bimetallic PdCu nanoparticle decorated three-dimensional graphene hydrogel for non-enzymatic amperometric glucose sensor. Sensor Actuator B. 2014, 19, 707–714. [Google Scholar] [CrossRef]
- Chang, G.; Shu, H.; Ji, K.; Oyama, M.; Liu, X.; He, Y. Gold nanoparticles directly modified glassy carbon electrode for non-enzymatic detection of glucose. Appl Surf Sci. 2014, 288, 524–529. [Google Scholar] [CrossRef]
- Yin, G.; Xing, L.; Ma, X-J.; Wan, J. Non-enzymatic hydrogen peroxide sensor based on a nanoporous gold electrode modified with platinum nanoparticles. Chem Pap. 2014, 68, 435–441. [CrossRef]
- Shantharaja.; Giddaerappa.; Lokesh, K.S. Phthalocyanine based metal- organic frame work with carbon nanoparticles as hybrid catalyst for oxygen reduction reaction, Electrochimica Acta. 2023, 456, 142405. [CrossRef]
- Leznoff, C.C.; Lever, A.B.P. Phthalocyanines: properties and applications vols. 1–4. New York. VCH Publishers. [CrossRef]
- Giddaerappa.; Nemakal, M.; Shantharaja.; Mirabbos, H.; Lokesh, K.S. Tetraphenolphthalein Cobalt(II) Phthalocyanine Polymer Modified with Multiwalled Carbon Nanotubes as an Efficient Catalyst for the Oxygen Reduction Reaction, ACS Omega. 2022, 7, 14291−14304. [CrossRef]
- K.I, Ozoemena.; T, Nyokong. Novel amperometric glucose biosensor based on an ether-linked cobalt(II) phthalocyanine–cobalt(II) tetraphenylporphyrin pentamer as a redox mediator, Electrochim. Acta. 2006, 51, 5131. [CrossRef]
- Liu, D.; Long, Y.T. Superior Catalytic Activity of Electrochemically Reduced Graphene Oxide Supported Iron Phthalocyanines toward Oxygen Reduction Reaction. ACS Appl Mater Interfaces. 2015, 4, 7(43), 24063-8. [CrossRef]
- Malathesh, Pari.; K.R.V, Reddy.; Fasiulla.; Chandrakala, K.B. Amperometric determination of dopamine based on an interface platform comprising tetra-substituted Zn2+ phthalocyanine film layer with embedment of reduced graphene oxide, Sensors and Actuators A: Physical. 2020, 316, 112377. [CrossRef]
- Sarvajith, M.S.; Harish, M.; Nimbegondi, K.; Mruthyunjayachari, C. D.; Fasiulla, Khan. Synthesis and Electrochemical Investigation of Tetra Amino Cobalt (II) Phthalocyanine Functionalized Polyaniline Nanofiber for the Selective Detection of Dopamine, Electroanalysis. 2020, 32(8), 1807-1817. [CrossRef]
- Veeresh, A.S.; Imadadulla, M.; Manjunatha, N.; Shambulinga, A.; Hemantha, K.K.R.; Sreenivasa, Swamy.; Lokesh, K.S. Synthesis and electropolymerization of cobalt tetraaminebenzamidephthalocyanine macrocycle for the amperometric sensing of dopamine, Journal of Electroanalytical Chemistry. 2019, 838, 33–40. [CrossRef]
- Imadadulla, M.; Manjunatha, N.; Shambhulinga, A.; Veeresh, A.S.; T.R, Divakara.; Manjunatha, P.; C.P, Keshavanandaprabu.; Lokesh, K.S. Phthalocyanine sheet polymer based amperometric sensor for the selective detection of 2,4-dichlorophenol, Journal of Electroanalytical Chemistry. 2020, 871, 114292. [CrossRef]
- Manjunatha, N.; Giddaerappa.; Shantharaja.; Veeresh, A.S.; Lokesh, K.S. Novel amide coupled phthalocyanines: Synthesis and structure-property relationship for electrocatalysis and sensing of hydroquinone, Journal of Electroanalytical Chemistry. 2021, 898, 115657. [CrossRef]
- Veeresh, A.S.; Shambhulinga, A.; Manjunatha, N.; Manjunatha, P.; C.P, Keshavananda Prabhu.; Lokesh, K.S. Nanomolar detection of lead using electrochemical methods based on a T novel phthalocyanine, Inorganica Chimica Acta. 2020, 506, 119564. [CrossRef]
- Shantharaja.; Manjunatha, N.; Giddaerappa.; Lokesh, K.S. Biocompatible polymeric pyrazolopyrimidinium cobalt(II) phthalocyanine: An efficient electroanalytical platform for the detection of l-arginine, Sensors and Actuators A. 2021, 324, 112690. [CrossRef]
- Veeresh, A.S.; Shambhulinga, A.; Manjunatha, N.; Manjunatha, P.; C. P, Keshavananda Prabhu.; Lokesh, K.S. Nanomolar detection of 4-nitrophenol using Schiff-base phthalocyanine, Microchemical Journal. 2021, 164, 105980. [CrossRef]
- C.P, Keshavananda Prabhu.; Nemakal, M.; Aralekallu, S.; Mohammed, I.; K.H, Shivaprasad.; M.K, Amshumali.; K.S, Lokesh. Synthesis and characterization of novel imine substituted phthalocyanine for sensing of L-cysteine, Journal of Electroanalytical Chemistry. 2019, 834, 30–137. [CrossRef]
- Gonçalo, D.; João, C.; Bruno, V.; Leticia, G.; Carina, A.; Maria, A.; João, R.; Pedro, V.B. Noble Metal Nanoparticles for Biosensing Applications, Sensors. 2012, 12, 1657-1687. [CrossRef]
- Zhibing, Liao.; Liu, Yao.; Yan, Liu.; Yaohui, Wu.; Yonghong, Wang.; Ge, Ning. Progress on nanomaterials based-signal amplification strategies for the detection of zearalenone, Biosensors and Bioelectronics. 2021, 9, 100084. [CrossRef]
- M.S, Sumitha.; T.S, Xavier. Recent advances in electrochemical biosensors – A brief review, Hybrid Advances. 2023, 2, 100023. [CrossRef]
- Duanduan, Yin.; Jian, Liu.; Xiangjie, Bo.; Mian, Li.; Liping, Guo. Porphyrinic metal-organic framework/macroporous carbon composites for electrocatalytic applications, Electrochimica Acta. 2017, 247, 41-49. [CrossRef]
- Sami, U.; Aziz, R.; Tayyaba, Najam.; Ismail, H.; Shazia, A.; Rashid, Ali.; Muhammad, U.S.; S.S, Ahmad Shah.; Muhammad, A.N. Advances in metal-organic framework@activated carbon (MOF@AC) composite materials: Synthesis, characteristics and applications, Journal of Industrial and Engineering Chemistry. 2024. [CrossRef]
- Basova, T.V.; Ray, A.K. Review—Hybrid materials based on phthalocyanines and metal nanoparticles for chemi-resistive and electrochemical sensors: A Mini-Review. ECS Journal of Solid-State Science and Technology. 2020, 9, 061001. [CrossRef]
- Gonçalves.; Josué, M; Bernardo, A.I.; Paulo, R.M.; Lúcio, A. Recent advances in electroanalytical drug detection by porphyrin/phthalocyanine macrocycles: developments and future perspectives. Analyst 146, 2021, 2, 365-381. [CrossRef]
- Nobuhle, N.; Sithi, M.; Tebello, N. Electrocatalytic Activity of Cobalt Phthalocyanines Revisited: Effect of the Number of Oxygen Atoms and Conjugation to Carbon Nanomaterials, electrocatalysis, 2021, 12, 499-515. [CrossRef]
- Costa, P.; Nunespereira, J.; Oliveira, JA.; et al. High-performance graphene-based carbon nanofiller/polymer composites for piezoresistive sensor applications. Compos Sci Technol. 2017, 153, 241–252. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Development of a Novel Electrochemical Biosensor Based on Carbon Nanofibers–Cobalt Phthalocyanine–Laccase for the Detection of p-Coumaric Acid in Phytoproducts. Int. J. Mol. Sci. 2021, 22, 9302. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, N.; Shambhulinga, A.; Imadadulla, M.; et al. Electropolymerized octabenzimidazole phthalocyanine as an amperometric sensor for hydrazine. Journal of Electroanalytical Chemistry. 2019, 839, 238–246. [Google Scholar] [CrossRef]
- Jilani, B.S.; Mounesh.; Malathesh, P.; et al. Cobalt (II) tetra methyl-quinoline oxy bridged phthalocyanine carbon nano particles modified glassy carbon electrode for sensing nitrite: A voltammetric study. Materials Chemistry and Physics. 2020, 121920. [CrossRef]
- Shambhulinga, A.; Imadadulla, M.; Manjunatha, N.; et al. Synthesis of novel azo group substituted polymeric phthalocyanine for amperometric sensing of nitrite. Sensors & Actuators: B Chemical. 2019, 282, 417–425. [Google Scholar] [CrossRef]
- Manjunatha, N.; Shambhulinga, A.; Imadadulla, M.; et al. Nanomolar detection of 4-aminophenol using amperometric sensor based on a novel phthalocyanine. Electrochimica Acta. 2019, 318, 342–353. [Google Scholar] [CrossRef]
- Manjunatha, N.; Shambhulinga, A.; Imadadulla, M.; et al. Chemisorbed palladium phthalocyanine for simultaneous determination of biomolecules. Microchemical Journal. 2018, 143, 82–91. [Google Scholar] [CrossRef]
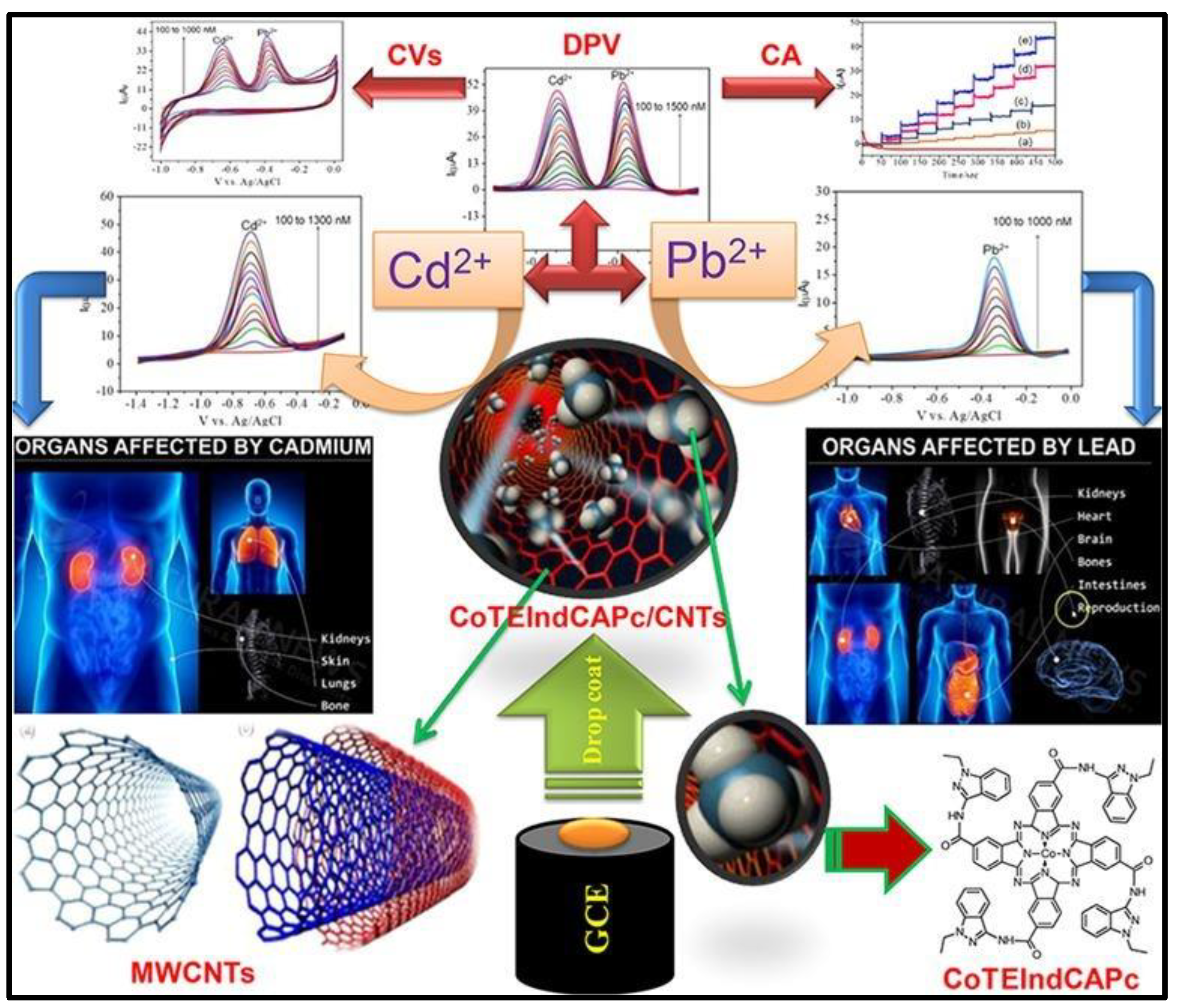
- Mounesh.; Reddy, K.R.V. Decorated CoPc with appliance of MWCNTs on GCE: Sensitive and reliable electrochemical investigation of heavy metals. Microchemical Journal. 2021, 160, 05610. [CrossRef]
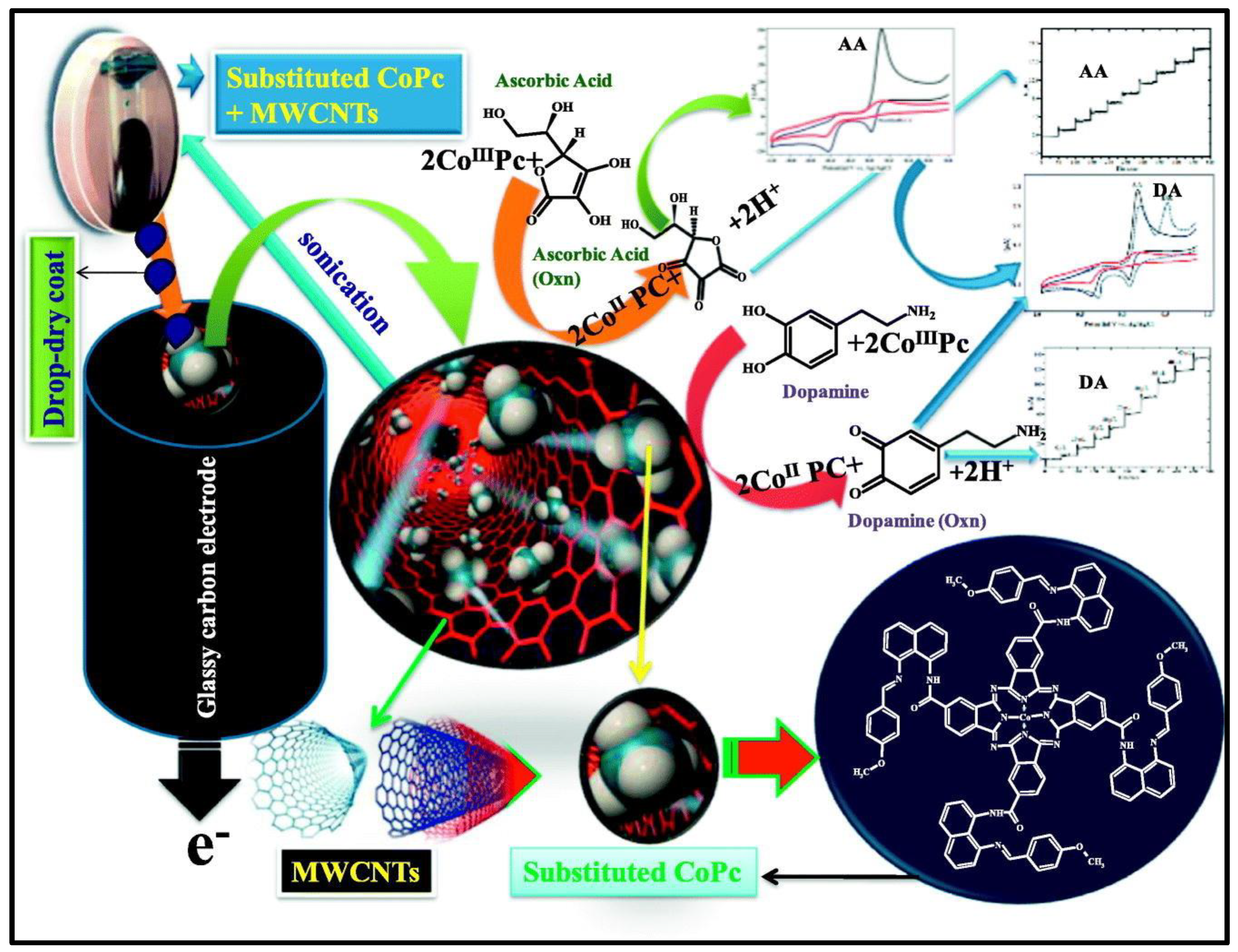
- Mounesh.; Bhvimane, S.J.; Malatesh, Pari.; K.R. Venugopala Reddy.; K.S. Lokesh. Simultaneous and sensitive detection of ascorbic acid in presence of dopamine using MWCNTs-decorated cobalt (II) phthalocyanine modified GCE, Microchemical Journal. 2019, 147, 755–763. [CrossRef]
- Keshavanandaprabhu, C.P.; Manjunatha, N.; Shambhulinga, A.; Imadadulla, M.; Manjunatha, P.; Veeresh A.S.; Akshitha, D.; Lokesh, K.S. A comparative study of carboxylic acid and benzimidazole phthalocyanines and their surface modification for dopamine sensing, Journal of Electroanalytical Chemistry. 2019, 847, 113262. [CrossRef]
- Mania, V.; Huanga, S.T.; Devasenathipathy, R.; et al. Electropolymerization of cobalt tetraamino-phthalocyanine at reduced graphene oxide for electrochemical determination of cysteine and hydrazine. RSC Adv. 2016, 6(44), 38463–38469. [CrossRef]
- Tang, C.; Umasankar, Y.; Chen, S.M.s Simultaneous determination of adenine guanine and thymine at multi-walled carbon nanotubes incorporated with poly(new fuchsin) composite film. Anal Chim Acta. 2009, 636, 19–27. [CrossRef]
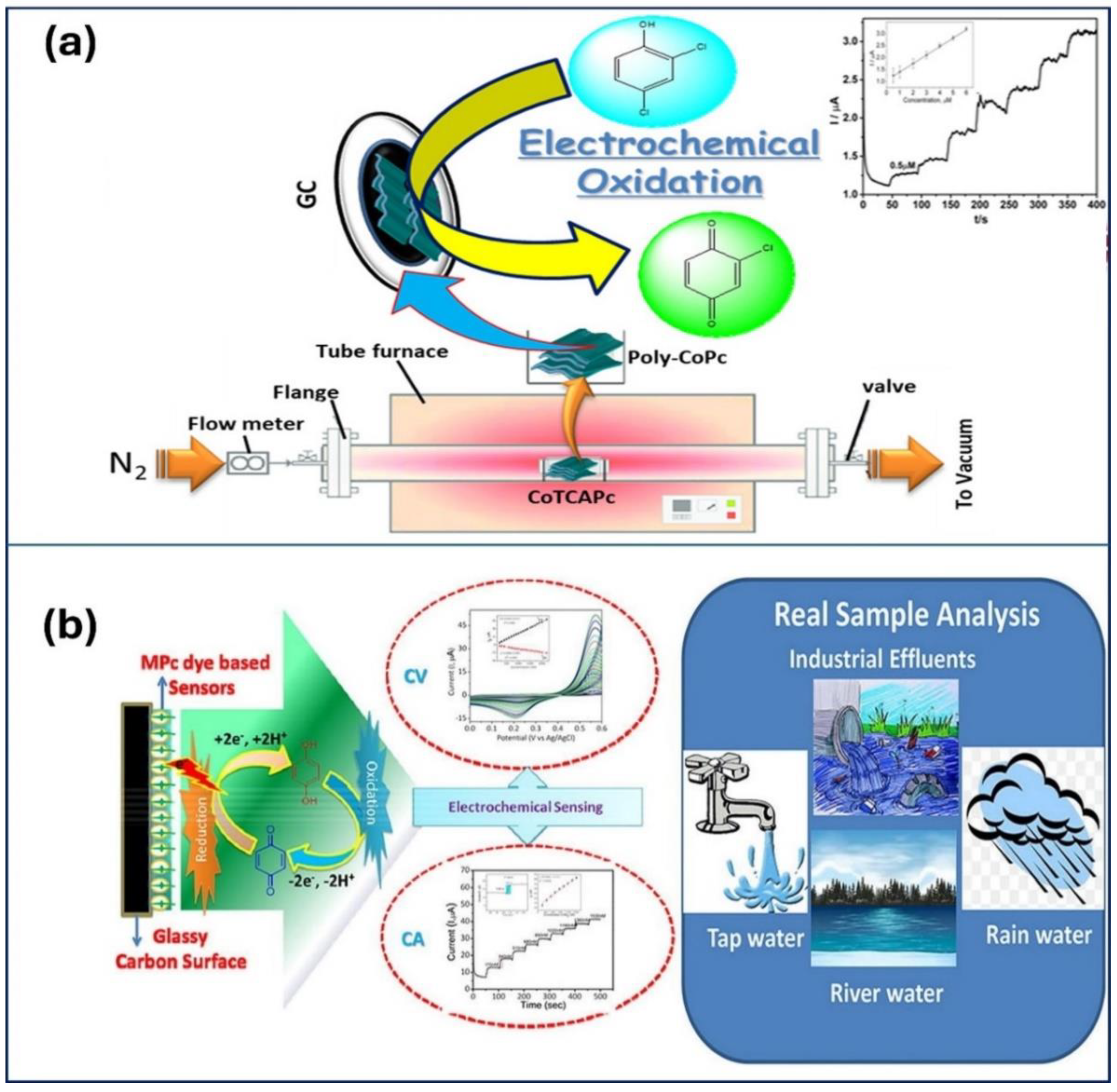
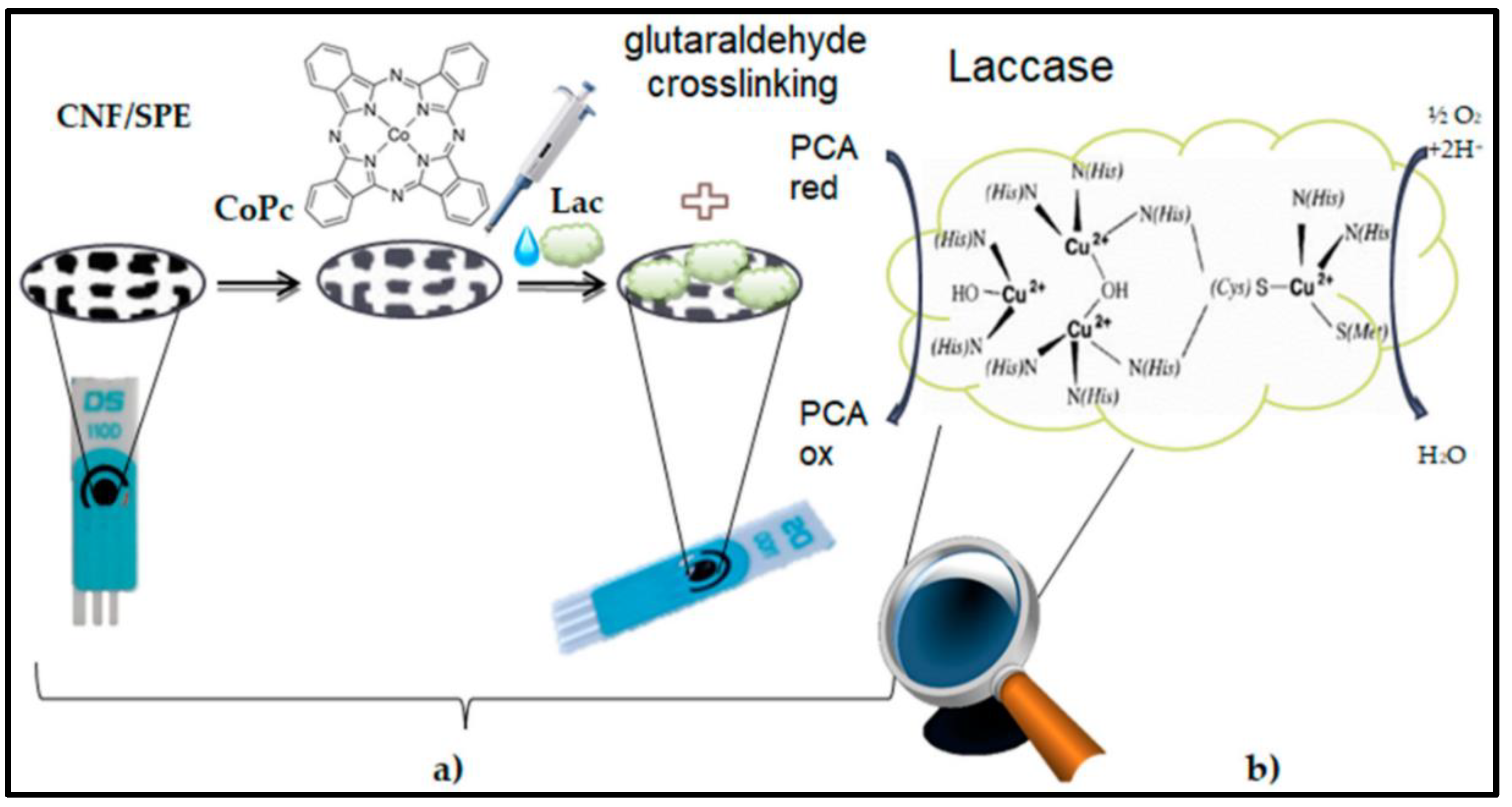
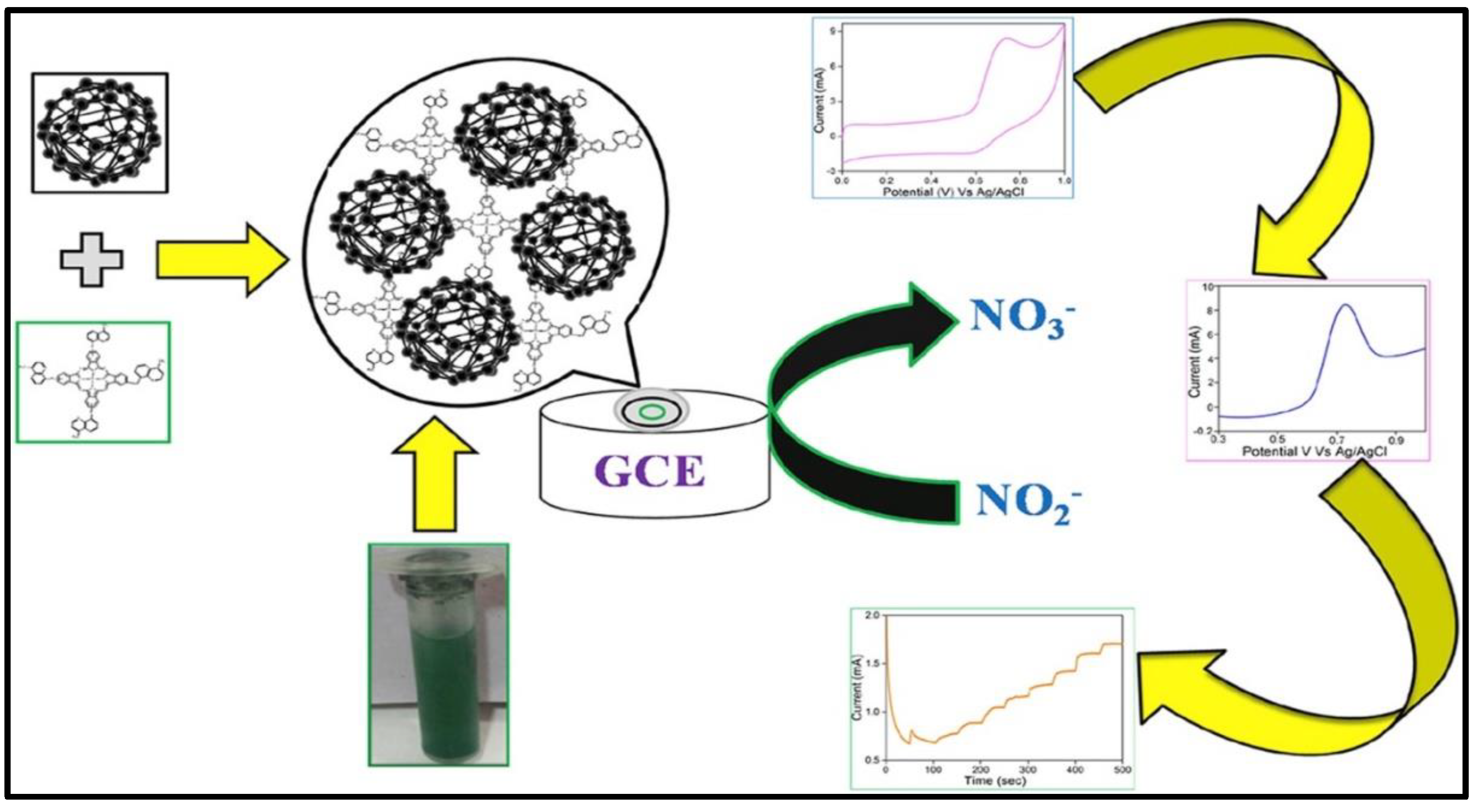
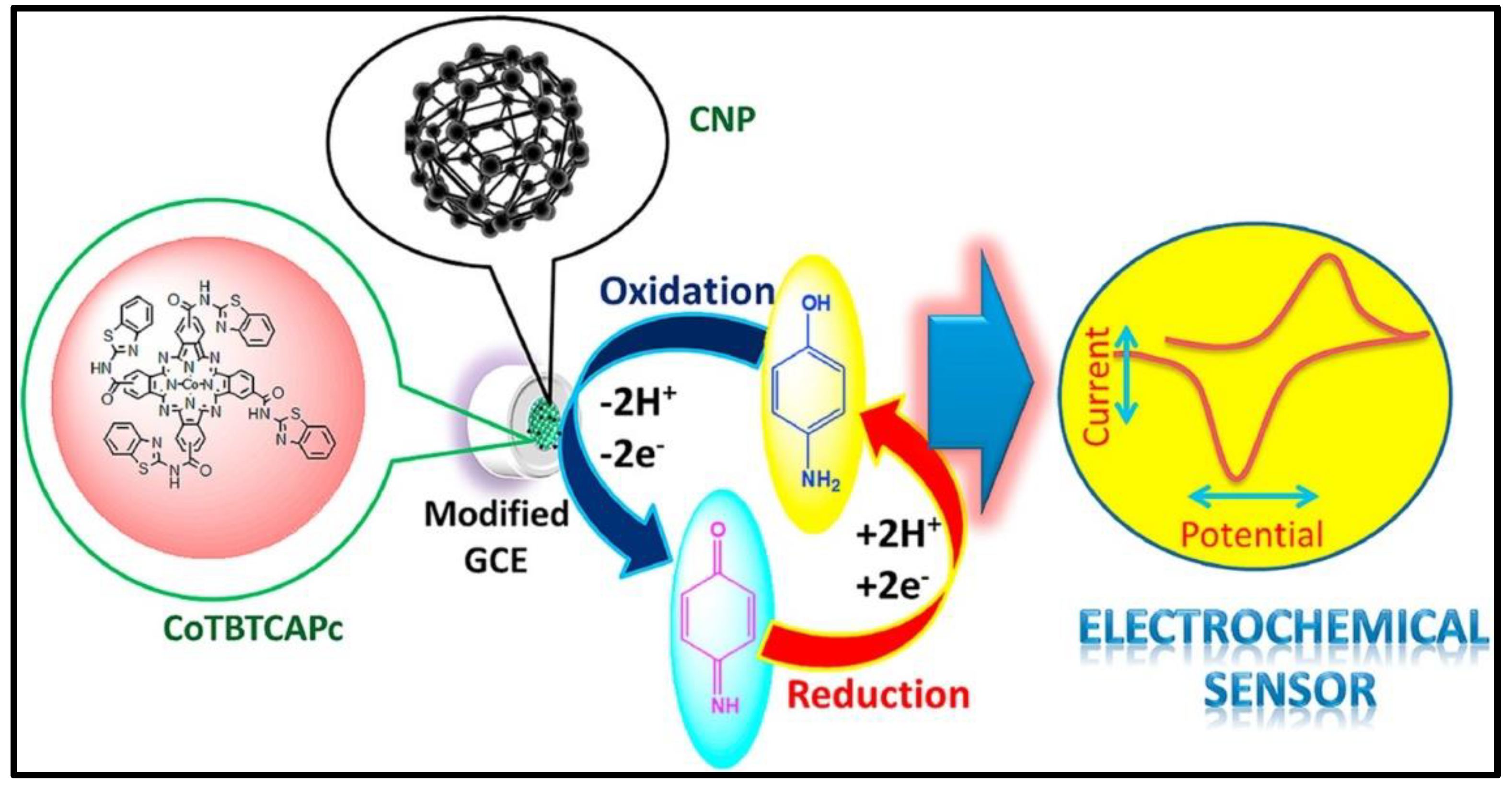
| Material | Method | Analyte | LOD | Linear Range | Ref |
|---|---|---|---|---|---|
| MSN/GO | DPV | CA 153 | 2.8 × 10-4 Um/L | 10-3–200 U/mL | 42 |
| MWCNT/ ferrocene |
CV | Hepatitis C and tuberculosis genomic DNA | 7 fM | 0.1 fM–1 pM | 48 |
| CNT | DPV | Dopamine (DA) | 0.1 μM | 0.5–10 μM | 49 |
| MWCNTs | EIS | Mb | 0.08 ng m/L | 0.1–90 ng m/L | 50 |
| SWCNT | DPV | Vibroparahaemolytics thermolabile hemolysin (tlh) gene | 7.27 uM M | 1.0 × 10-6–1 × 10-13 mol/L | 51 |
| CNTs | CV | Rutin | 0.075 uM | 0.10–51 uM | 52 |
| CNTs | CV | Rutin | 0.081 uM | 0.10–31 uM | 53 |
| Graphene | CV, LSV | Puerarin | 0.04 uM | 0.06–6.0 uM | 54 |
| Graphene | CV | Puerarin | 0.006 uM | 0.02–40 uM | 55 |
| CNTs–graphene | CV | Hyperin | 0.001 uM | 0.005–1.5 uM | 56 |
| Graphene quantum dots (GQDs) | CV | Rutin | 0.011 uM | 0.05–10 uM | 57 |
| GQD | DPV | Quercentin (Que) | 8.2 × 10-4 uM | 0.002–1.6 uM | 58 |
| Mesoporous carbon | CV | Rutin | 0.002 uM | 0.1–30 uM | 59 |
| Material | Method | Analyte | LOD | Linear Range | Ref |
|---|---|---|---|---|---|
| Au | Amperometry | Cysteine | 3.1 μM | 10–80 μM | 75 |
| Glutathione | 0.1 μM | 0.3–10 μM | |||
| Methionine | 1 μM | 3.3–39 μM | |||
| Homocysteine | 0.6 μM | 2.2–30 μM | |||
| Au | EIS | Carcinoma antigen 125 | 6.7 U/mL | 0–100 U/mL | 76 |
| Carcinoma antigen 125 | 419 ng/mL | 450 ng/mL–2.916 μg/mL | |||
| Pt | EIS | Carcinoma antigen 125 | 386 ng/mL | 450 ng/mL–2.916 μg/mL | 77 |
| Pt | Amperometry | Glucose | 44.3 μM | 0.25–6.0 mM | 78 |
| CuO nanospheres | Amperometry | Glucose | 1 μM | 2.5–20 mM | 79 |
| ZnCo2O4 | Amperometry | DA | 15.5 μM | 5–100 μM | 80 |
| TiO2 | Amperometry | Glucose | 100 nM | 100 nM–5 mM | 81 |
| Pd–Cu | - | Glucose | 20 μM | 2–18 mM | 82 |
| Au | - | Glucose | 0.05 μM | 0.1–25 mM | 83 |
| Pt | - | Glucose | 7.2 × 10-8 M | 1.0 × 10-7–2.0 × 10-5 M | 84 |
| Materials | Method | Analyte | LOD | Linear Range | Ref |
|---|---|---|---|---|---|
| Zn(II)TBPc | Amperometry | DA | 6 nM | 20 nM–1.0 μM | 90 |
| TACoPc/PANI | CV | DA | 0.064 μM | 20–200 μM | 91 |
| Poly(CoTNBAPc) |
Amperometry | DA | 20 nmol/L | 100–4000 nM/L | 92 |
| CV | 20 nmol/L | 10–1000 nM | |||
| CoTCAPc | CV | 2,4-dichlorophenol | 0.35 μM | 1–36 μM | 93 |
| CoTAMFCAPc/GCE | CV | Hydroquinone | 0.066 | 0.2-2.2 μM | 94 |
| Amperometry | 0.056 | 0.17-1.530 μM | |||
| Poly(CoTBrIMPPc) | CV | Pb | 37 nmol/L | 10–1000 nM | 95 |
| Amperometry | 180 nmol/L | 500–3000 nM/L | |||
| Poly(CoTPzPyPc) | CV | L-arginine | 2.5 uM | 10–100 uM | 96 |
| Amperometry | 0.6 uM | 2–60 uM | |||
| CoTTIMPPc | CV | 4-nitrophenol | 38 nM | 100–1000 nM | 97 |
| Amperometry | 30 nM | 100–900 nM | |||
| Poly(CoTBrImPc) | CV | L- Cysteine | 3 nM | 10–100 nM | 98 |
| Amperometry | 4 nM | 10–80 nM |
| Material | Method | Analyte | LOD | Linear Range | Ref |
|---|---|---|---|---|---|
| rGO/Poly(CoOBImPc) | CV | Hz | 0.033 uM | 0.1–0.9 uM | 109 |
| CoTM-QOPc/CNP | CV | Nitrite | 0.033 uM | 0.1–350 uM | 110 |
| Poly(TazoCoPc)/CNP | CV | Nitrite | 0.006 uM | 0.02–1 uM | 111 |
| CoTBTCAPc/CNP | CV | 4-AP | 0.030 uM | 0.1–0.9 uM | 112 |
| CoTELndCAPc/MWCNT | CV | Cd(II) | 5 nM | 100–1000nM | 113 |
| Pb(II) | 3 nM | 100–1000nM | |||
| PdTAPc/MWCNT | CV | AA | 1.0 uM | 3–24 uM | 114 |
| DA | 0.6 uM | 2–16 uM | |||
| CoTMBANAPc/MWCNT | CV | AA | 6.6 uM | 7.5–70 uM | 115 |
| Poly(FeTBImPc)/CNP | CV | DA | 20 nM | 100–1000nM | 116 |
| RGO-pTACoPc | CV | L-cysteine | 0.018 uM | 0.05–2.0 uM | 117 |
| MWCNT-PNF | CV | Adenine (AD) | 9.2 uM | 0.01–3.9 mM | 118 |
| DPV | AD | 7.9 uM | 0.01–7.9 mM | ||
| CV | Thymine (THY) | 19.3 uM | 0.02–7.7 mM | ||
| DPV | THY | 16.8 uM | 0.02–15.7 mM | ||
| CV | Guanine (GU) | 98.56 uM | 0.1–8.5 mM | ||
| DPV | GU | 96.84 uM | 0.1–3.5 mM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





