Submitted:
10 July 2024
Posted:
11 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
2.1. Preparing the Animal Groups
2.2. Behavior Tests
2.3. Tissue Collection
2.4. Total RNA and Hybrid DNA/RNA Isolation from Hippocampal Tissue
2.5. RNA and Hybrid DNA/RNA Isolation from Sperm Tissue
2.6. Complementary DNA (cDNA) Synthesis and miRNA Profiling
2.7. Statistical Analysis
3. Results
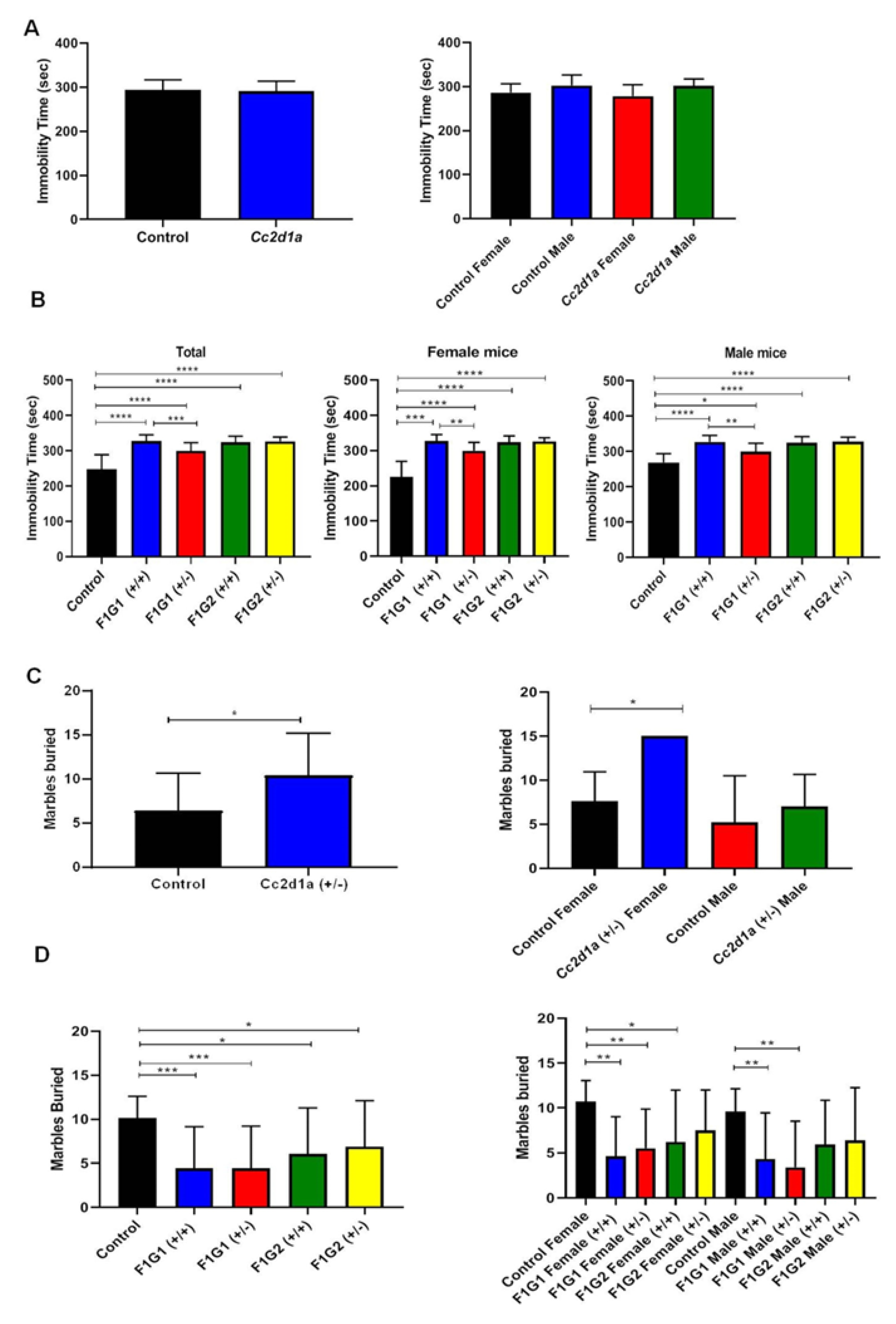
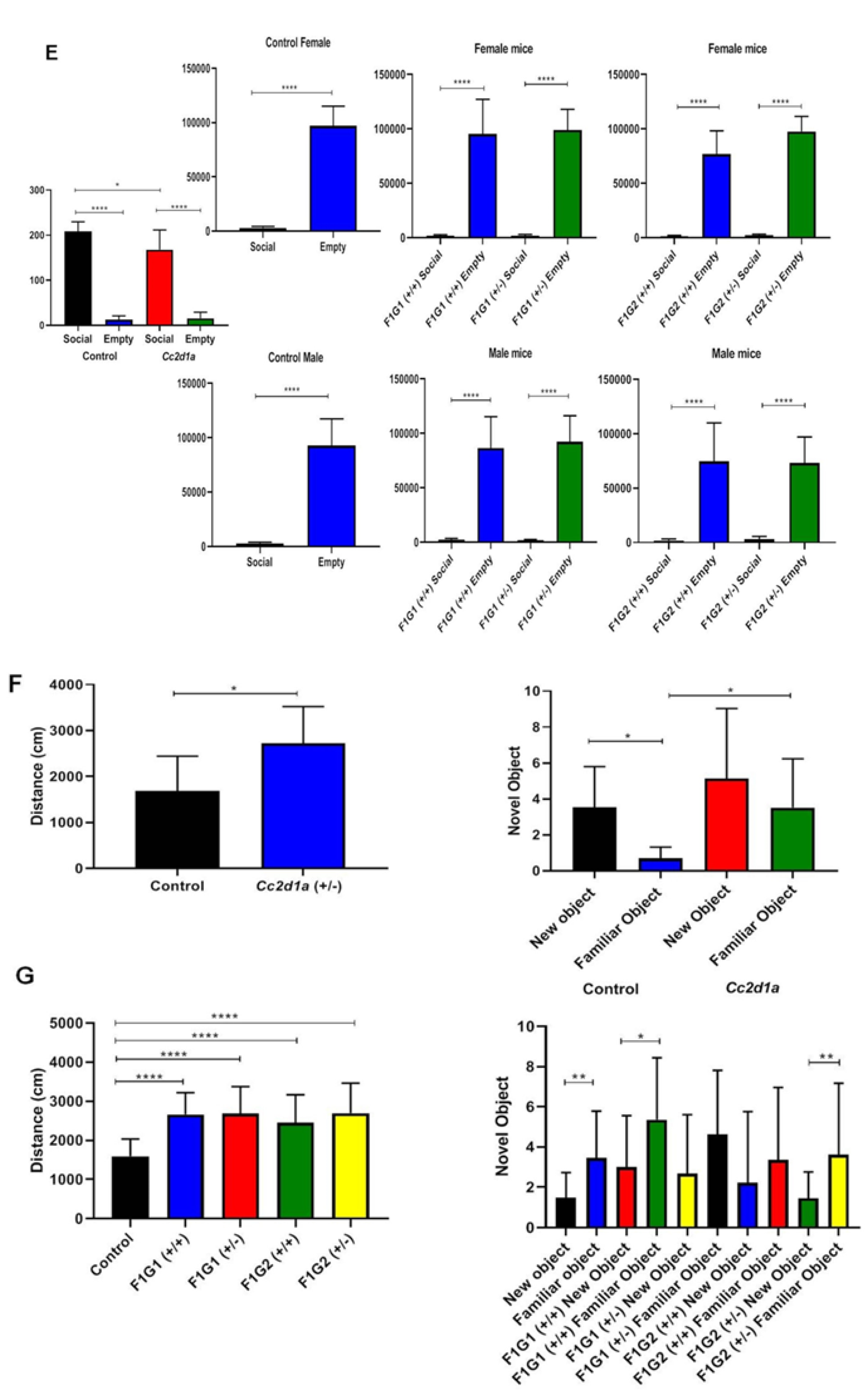
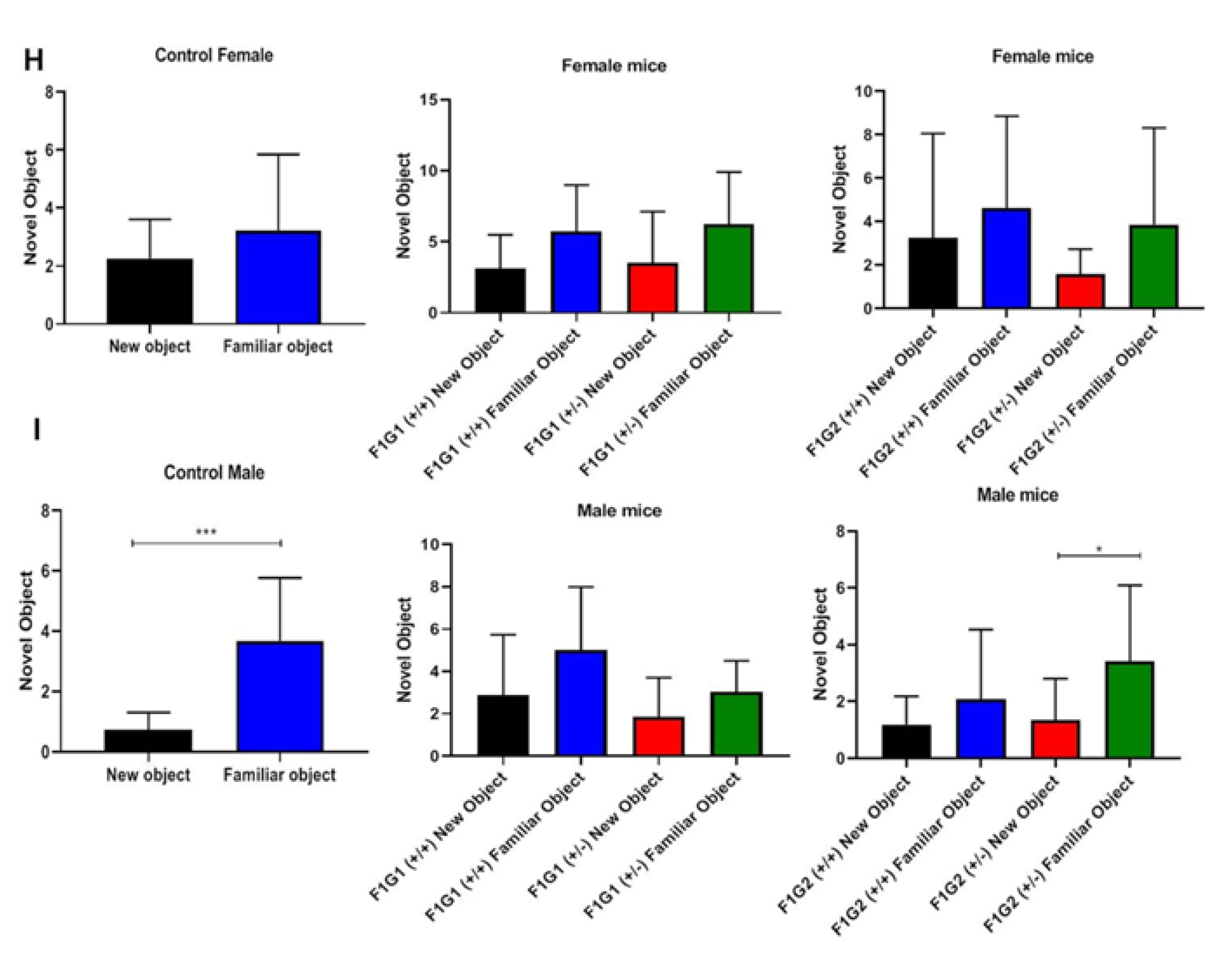
3.1. Behavioral Gender Differences Are greater in Autism
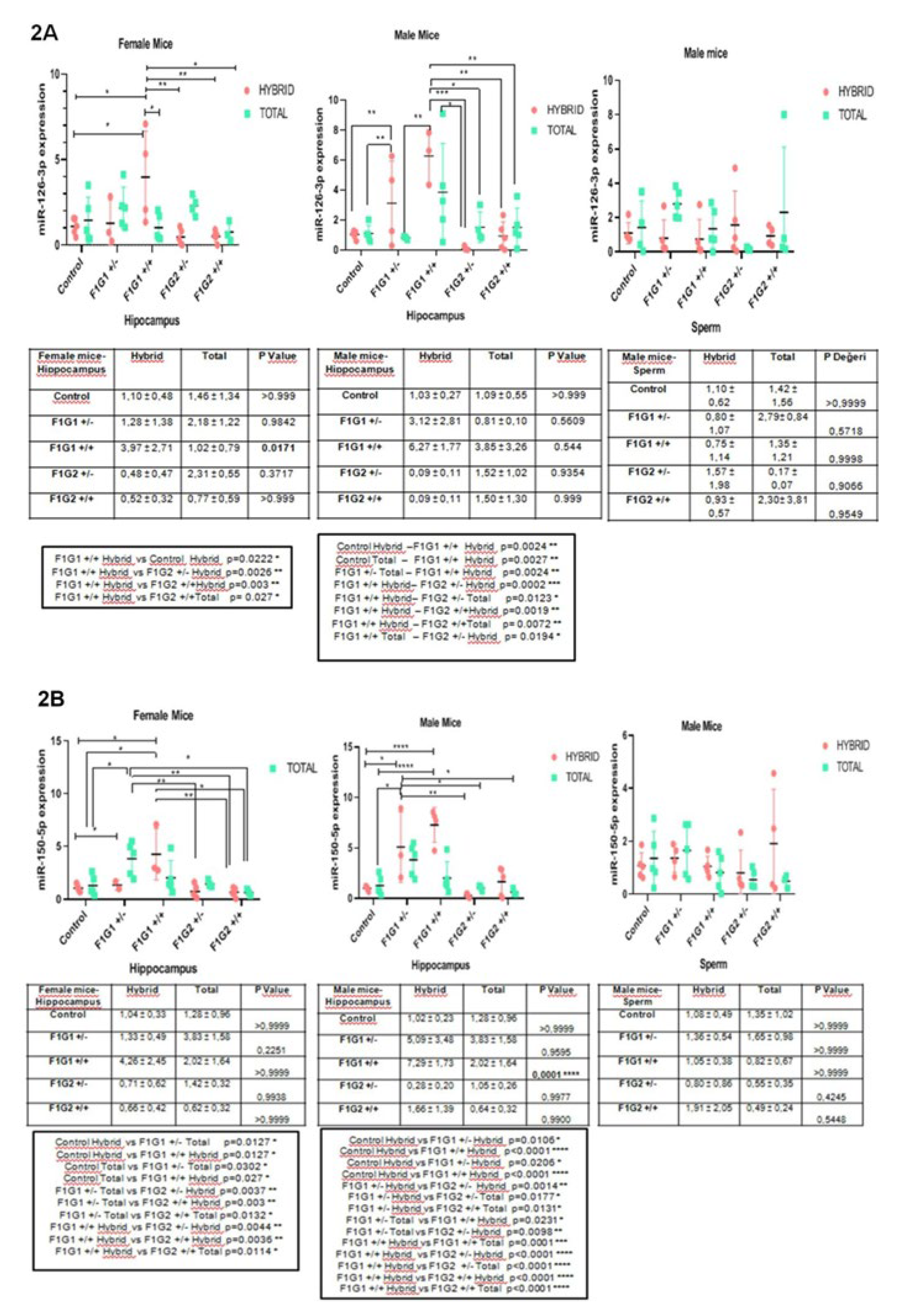
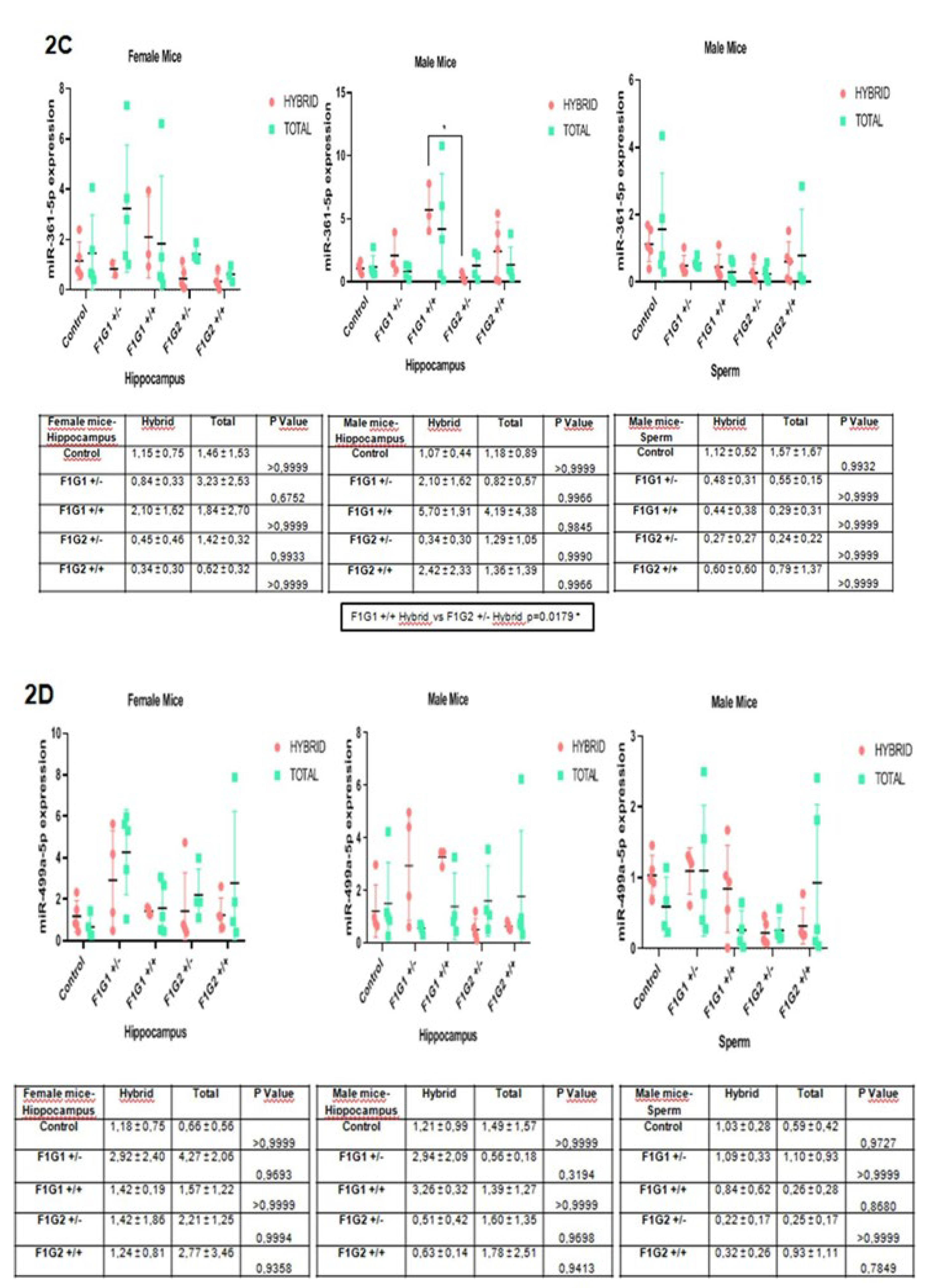
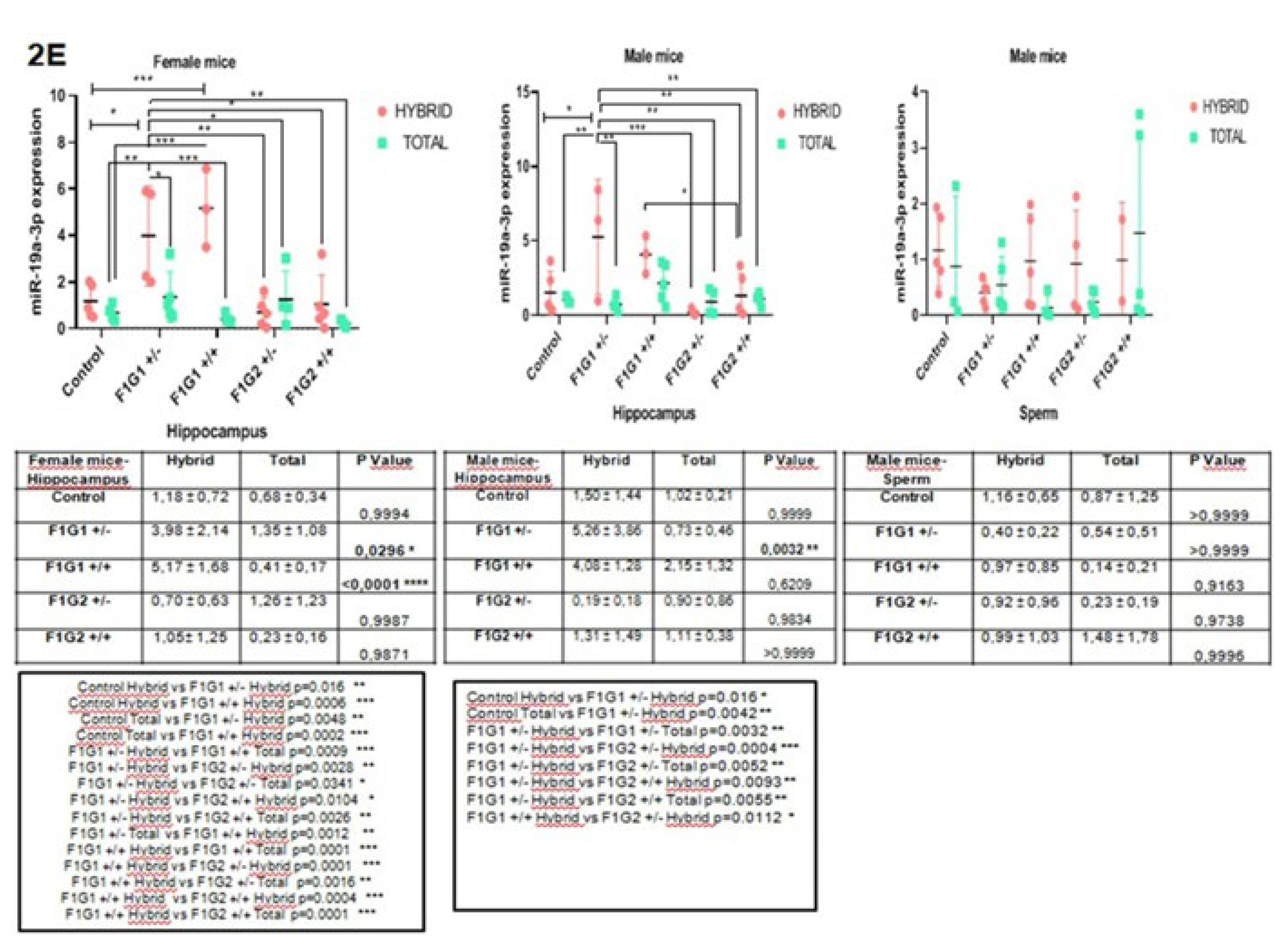
3.2. Sex-Dependent Distribution of Two Free or R-loop miRNA Fractions in Autism
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wormwood, K.L.; Charette, L.; Ryan, J.P.; Darie, C.C.; Woods, A.G. A Proteomics Investigation of Salivary Profiles as Potential Biomarkers for Autism Spectrum Disorder (ASD). Protein J 2023, 42(5), 607–620. [Google Scholar] [CrossRef] [PubMed]
- Dawson, G.; Rieder, A.D.; Johnson, MH. Prediction of autism in infants: progress and challenges. Lancet Neurol 2023, 22(3), 244–254. [Google Scholar] [CrossRef] [PubMed]
- Karaca, M.; Tahtasakal, R.; Dana, H.; Sahin, M.; Pirencioglu, S.N.; Tughan, E.; Dal, F.; Demirci, E.; Sener, E.F. Decreased levels of alpha synuclein in families with autism spectrum disorder and relationship between the disease severity. Brain Res 2023, 1814, 148410. [Google Scholar] [CrossRef] [PubMed]
- Sener, E.F.; Taheri, S.; Sahin, M.C.; Bayramov, K.K.; Marasli, M.K.; Zararsiz, G.; Mehmetbeyoglu, E.; Oztop, D.B.; Canpolat, M.; Canatan, H.; Ozkul, Y. Altered Global mRNA Expressions of Pain and Aggression Related Genes in the Blood of Children with Autism Spectrum Disorders. J Mol Neurosci 2019, 67(1), 89–96. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Pourtavakoli, A.; Hussen, B.M.; Taheri, M.; Ayatollahi, S.A. A Review on the Role of Genetic Mutations in the Autism Spectrum Disorder. Mol Neurobiol 2023, 60(9), 5256–5272. [Google Scholar] [CrossRef] [PubMed]
- Moseley, R.L.; Hitchiner, R.; Kirkby, J.A. Self-reported sex diferences in high-functioning adults with autism: A meta-analysis. Molecular Autism 2018, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Apte, M.; Kumar, A. Correlation of mutated gene and signalling pathways in ASD. IBRO Neurosci Rep 2023, 14, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Sener, E.F.; Canatan, H.; Ozkul, Y. Recent Advances in Autism Spectrum Disorders: Applications of Whole Exome Sequencing Technology. Psyc Invest 2016, 13(3), 255–64. [Google Scholar] [CrossRef] [PubMed]
- Ozkul, Y.; Taheri, S.; Bayram, K.K.; Sener, E.F.; Mehmetbeyoğlu, E.; Oztop, D.B.; et al. A heritable profile of six miRNAs in autistic patients and mouse models. Scientific Reports 2020, 10(1), 9011. [Google Scholar] [CrossRef] [PubMed]
- Dana, H.; Bayram, K.K.; Delibaşı, N.; Tahtasakal, R.; Bayram, R.; Hamurcu, Z.; Sener, E.F. Disregulation of Autophagy in the Transgenerational Cc2d1a Mouse Model of Autism. Neuromolecular Med 2020, 22(2), 239–249. [Google Scholar] [CrossRef] [PubMed]
- Zamarbide, M.; Mossa, A.; Muñoz-Llancao, P.; Wilkinson, M.K.; Pond, H.L.; Oaks, A.W.; Manzini, M.C. Male-Specific cAMP Signaling in the Hippocampus Controls Spatial Memory Deficits in a Mouse Model of Autism and Intellectual Disability. Biol Psychiatry 2019, 85(9), 760–768. [Google Scholar] [CrossRef] [PubMed]
- Sener, E.F.; Dana, H.; Tahtasakal, R.; Hamurcu, Z.; Taheri, S.; Delibasi, N.; Mehmetbeyoglu, E.; Sukranli, Z.Y.; Dal, F.; Tufan, E.; Oflamaz, A.O.; Doganyigit, Z.; Ozkul, Y.; Rassoulzadegan, M. Heterozygous Cc2d1a mice show sex-dependent changes in the Beclin-1/p62 ratio with impaired prefrontal cortex and hippocampal autophagy. Prog Neuropsychopharmacol Biol Psychiatry 2023, 125, 110764. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, L.B.; Li, B.; Zhao, Q.; Wei, W.; Leighton, L.J.; Bredy, T.W.; Pang, T.Y.; Hannan, A.J. Chronically high stress hormone levels dysregulate sperm long noncoding RNAs and their embryonic microinjection alters development and affective behaviours. Mol Psychiatry 2024, 29(3), 590–601. [Google Scholar] [CrossRef] [PubMed]
- Leir, S.H.; Paranjapye, A.; Harris, A. Functional genomics of the human epididymis: Further characterization of efferent ducts and model systems by single-cell RNA sequencing analysis. Andrology 2024, 12(5), 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Sendler, E.; Johnson, G.D.; Mao, S.; Goodrich, R.J.; Diamond, M.P. Stability, delivery and functions of human sperm RNAs at fertilization. Nucleic Acids Res 2013, 41, 4104–4117. [Google Scholar] [CrossRef] [PubMed]
- Rassoulzadegan, M.; Sharifi-Zarchi, A.; Kianmehr, L. DNA-RNA Hybrid (R-Loop): From a Unified Picture of the Mammalian Telomere to the Genome-Wide Profile. Cells 2021, 10(6), 1556. [Google Scholar] [CrossRef]
- Kianmehr, L.; Khazali, H.; Rajabi-Maham, H.; Sharifi-Zarchi, A.; Cuzin, F.; Rassoulzadegan, M. Genome-Wide Distribution of Nascent Transcripts in Sperm DNA, Products of a Late Wave of General Transcription. Cells 2019, 8(10), 1196. [Google Scholar] [CrossRef] [PubMed]
- Satir-Basaran, G.; Kianmehr, L.; Mehmetbeyoglu, E.; Korkmaz Bayram, K.; Memis, M.; Yilmaz, Z.; Tufan, E.; Taheri, S.; Kelestimur, F.; Rassoulzadegan, M. Mouse Paternal RNAs Initiate a Pattern of Metabolic Disorders in a Line-Dependent Manner. Front Genet 2022, 13, 839841. [Google Scholar] [CrossRef] [PubMed]
- Al-Hadid, Q.; Yang, Y. R-loop: an emerging regulator of chromatin dynamics. Acta Biochim Biophys Sin (Shanghai) 2016, 48(7), 623–31. [Google Scholar] [CrossRef] [PubMed]
- Scheuren, M.; Möhner, J; Zischler, H. R-loop landscape in mature human sperm: Regulatory and evolutionary implications. Front Genet 2023, 14, 1069871. [Google Scholar] [CrossRef] [PubMed]
- Patankar, A.; Gajbhiye, R.; Surve, S.; Parte, P. Epigenetic landscape of testis specific histone H2B variant and its influence on sperm function. Clin Epigenetics 2021, 13(1), 101. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; Schratt, G. miRNA regulation of social and anxiety-related behaviour. Cell Mol Life Sci 2020, 77(21), 4347–4364. [Google Scholar] [CrossRef] [PubMed]
- Stott, J.; Wright, T.; Holmes, J.; Wilson, J.; Griffiths-Jones, S.; Foster, D.; Wright, B. A systematic review of non-coding RNA genes with differential expression profiles associated with autism spectrum disorders. PLoS One 2023, 18(6), e0287131. [Google Scholar] [CrossRef] [PubMed]
- Vishnoi, A.; Rani, S. MiRNA Biogenesis and Regulation of Diseases: An Overview. Methods Mol Biol 2017, 1509, 1–10. [Google Scholar] [PubMed]
- Schepici, G.; Cavalli, E.; Bramanti, P.; Mazzon, E. Autism Spectrum Disorder and miRNA: An Overview of Experimental Models. Brain Sci 2019, 9(10), 265. [Google Scholar] [CrossRef]
- Kondaurova, E.M.; Belokopytova, I.I.; Kulikova, E.A.; Khotskin, N.V.; Ilchibaeva, T.V.; Tsybko, A.S.; Popova, N.K.; Naumenko, V.S. On the role of serotonin 5-HT1A receptor in autistic-like behavior: сross talk of 5-HT and BDNF systems. Behav Brain Res 2023, 438, 114168. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Nardelli, J. Cellular and molecular introduction to brain development. Neurobiol Dis 2016, 92(Pt A), 3–17. [Google Scholar] [CrossRef]
- Yilmaz Sukranli, Z.; Korkmaz Bayram, K.; Mehmetbeyoglu, E.; Doganyigit, Z.; Beyaz, F.; Sener, E.F.; Taheri, S.; Ozkul, Y.; Rassoulzadegan, M. Trans Species RNA Activity: Sperm RNA of the Father of an Autistic Child Programs Glial Cells and Behavioral Disorders in Mice. Biomolecules 2024, 14(2), 201. [Google Scholar] [CrossRef] [PubMed]
- Tufan, E.; Taheri, S.; Karaca, Z.; Mehmetbeyoglu, E.; Yilmaz Sukranli, Z.; Korkmaz Bayram, K.; Ulutabanca, H.; Tanrıverdi, F.; Unluhizarci, K.; Rassoulzadegan, M.; Kelestimur, F. Alterations in Serum miR-126-3p Levels over Time: A Marker of Pituitary Insufficiency following Head Trauma. Neuroendocrinology 2024, 114(4), 315–330. [Google Scholar] [CrossRef] [PubMed]
- García-Muse, T.; Aguilera, A. R Loops: From Physiological to Pathological Roles. Cell 2019, 179(3), 604–618. [Google Scholar] [CrossRef] [PubMed]
- Petermann, E.; Lan, L.; Zou, L. Sources, resolution and physiological relevance of R-loops and RNA-DNA hybrids. Nat Rev Mol Cell Biol 2022, 23(8), 521–540. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.D.; Li, L.; Chan, W.Y. MicroRNAs: Key Regulators in the Central Nervous System and Their Implication in Neurological Diseases. Int J Mol Sci 2016, 17(6), 842. [Google Scholar] [CrossRef] [PubMed]
- Colak, D.; Zaninovic, N.; Cohen, M.S.; Rosenwaks, Z.; Yang, W.Y.; Gerhardt, J.; Disney, M.D.; Jaffrey, S.R. Promoter-bound trinucleotide repeat mRNA drives epigenetic silencing in fragile X syndrome. Science 2014, 343(6174), 1002–5. [Google Scholar] [CrossRef] [PubMed]
- Groh, M.; Gromak, N. Out of balance: R-loops in human disease. PLoS Genet 2014, 10(9), e1004630. [Google Scholar] [CrossRef] [PubMed]
- Skourti-Stathaki, K.; Kamieniarz-Gdula, K.; Proudfoot, N.J. R-loops induce repressive chromatin marks over mammalian gene terminators. Nature 2014, 516(7531), 436–9. [Google Scholar] [CrossRef] [PubMed]
- Azzalin, C.M. TERRA and the alternative lengthening of telomeres: a dangerous affair. FEBS Lett 2024, Mar 6. [CrossRef]
- Suster, I., Feng, Y. Multifaceted Regulation of MicroRNA Biogenesis: Essential Roles and Functional Integration in Neuronal and Glial Development. Int J Mol Sci 2021, 22(13), 6765.
- Komatsu, S.; Kitai, H.; Suzuki, H.I. Network Regulation of microRNA Biogenesis and Target Interaction. Cells 2023, 12(2), 306. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




