1. Introduction
The landscape of spine surgery has dramatically shifted with the adoption of navigation technologies, which have significantly enhanced the precision and safety of surgical procedures [
1]. In parallel, traditional free-hand techniques continued to develop, relying primarily on the surgeon’s expertise and anatomical familiarity. The integration of navigation systems into cervical spine surgery has been particularly transformative. The intricate anatomy of the cervical region demands high precision during surgery to avoid critical structures and minimize risks. Navigation technologies facilitate this by providing real-time, three-dimensional visualizations that enhance surgical accuracy.
These systems have significantly transformed cervical spine surgery, particularly due to the precision required to avoid critical structures [
2]. However, in anterior cervical spine surgery, traditional free-hand techniques remain prevalent, as direct visualization allows surgeons to leverage their expertise and familiarity with anatomy [
3]. Despite this, navigation systems offer substantial benefits, especially in complex cases where anatomy is distorted or pathology is near critical neurovascular structures [
4]. Intraoperative stereotactic navigation is increasingly used for accurate placement of spinal instrumentation and verification of anatomic landmarks under these challenging conditions [
5].
Anterior Cervical Discectomy and Fusion (ACDF), first performed in 1958, remains a cornerstone surgical procedure for addressing cervical spine conditions such as degenerative disc disease, infections, and tumors [
6]. Annually, this procedure is carried out more than 132,000 times across the United States [
7]. The rise of medical technology has seen a gradual shift towards navigation-guided ACDF, which leverages real-time imaging to enhance precision and improve surgical outcomes.
Given the evolution of ACDF techniques and the significant annual volume of procedures, a detailed comparative analysis is essential. This study aims to utilize the National Inpatient Sample (NIS) database to meticulously compare the outcomes of free-hand versus navigation-guided ACDF in terms of complications, costs, mortality, and length of stay. By analyzing these metrics, the study seeks to provide empirical evidence on the effectiveness and efficiency of navigation technologies in enhancing surgical precision and patient recovery. These insights can be crucial for informing clinical decision-making, optimizing surgical techniques, and ultimately leading to better patient outcomes.
2. Methods
This study utilized data from the Nationwide Inpatient Sample (NIS), a major public database on inpatient care managed by the Healthcare Cost and Utilization Project (HCUP). The NIS is a 20% sample of inpatient stays at HCUP-participating hospitals, translating to roughly 7 million admissions annually. This allows for generating national estimates using discharge weights included in the NIS data. The specific dataset employed in this study covered inpatient stays from January 1st, 2016 to December 31st, 2019, representing the most recent information available within the NIS at the time of the analysis.
Each dataset entry, referred to as a “case,” encapsulated a group of 5 patients, the analysis focused on 17,117 cases of one-level open Anterior Cervical Discectomy surgery, representing 85,085 patients. Within this cohort, 560 surgeries were guided by navigation technology, constituting 0.66% of the total. Patients meeting specific ICD-10 procedure codes for navigation-guided surgeries (8E09XBF, 8E09XBG, 8E09XBH) were included, while those with non-elective admissions, previous surgeries, or robotic interventions were excluded. Comorbidities and complications were identified via patient-specific ICD-10 codes.
We adopted propensity score matching to address disparities in variables such as ages and comorbidities between the navigation-guided and control groups. This statistical approach aimed to align the groups across various attributes, thereby fortifying the credibility of our analysis.
Due to the smaller size of the navigation group relative to the control group, we augmented the control group to be 20 times larger. This adjustment aimed to enhance the statistical robustness of our analysis while maintaining a balanced comparison between the groups. Through propensity score matching, we aligned the distribution of variables such as comorbidities, ages, gender composition, racial demographics, hospital sizes, and surgery years between the navigation-guided and control groups. This methodological refinement enabled a more thorough comparison and mitigated potential biases arising from initial group differences.
All analyses, including cross-tabulations and independent sample t-tests, were conducted, adopting a p-value threshold of less than 0.05 to establish statistical significance. A list of ICD-10 codes used in this study is included in the appendix for reference.
3. Results
Table 1 presents demographic and clinical features of single-level Anterior Cervical Discectomy and Fusion surgeries, contrasting between free-hand and navigation-guided techniques. Free-hand surgeries dominated 85020, while navigation-guided procedures accounted for 560. Both groups exhibited differences in age and gender distribution, with statistically significant variations noted. Navigation-guided surgeries showed higher Medicare usage and slightly lower reliance on private payers compared to the free-hand approach.
Table 2 compares comorbidity rates between the control and navigation-guided groups in single-level Anterior Cervical Discectomy and Fusion procedures. Most comorbidities showed similar prevalence between groups, except for chronic anemia and congestive heart failure (CHF), which were significantly different.
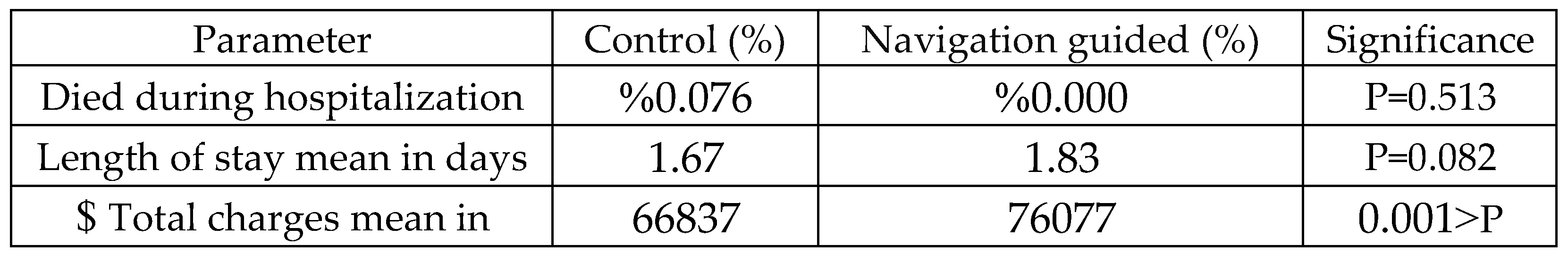
Table 3 compares clinical outcomes between the control and navigation-guided groups. Mortality rates were low and similar between groups. There was no statistically significant difference in length of stay (p=0.082). However, significant differences were observed in mean total charges (p<0.001), with navigation-guided procedures associated with higher costs.
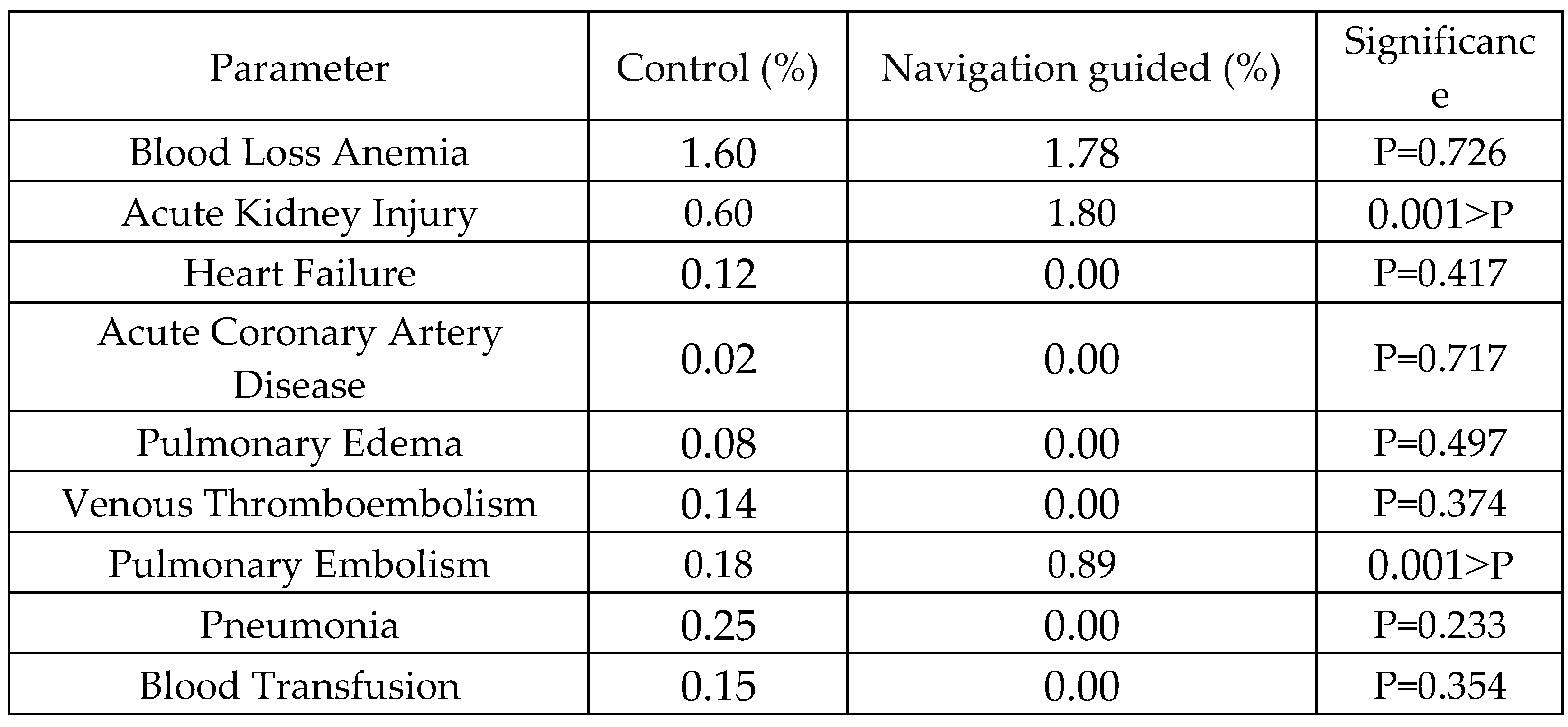
Table 4 compares postoperative complications between control and navigation-guided groups. While most complications showed no significant differences, acute kidney injury (P<0.001) and pulmonary embolism (P<0.001) were significantly different, with navigation-guided procedures associated with higher rates of acute kidney injury and pulmonary embolism compared to the control group.
Table 5 compares intraoperative complications between the control and navigation-guided groups in single-level Anterior Cervical Discectomy and Fusion procedures. There were no statistically significant differences in the prevalence of surgical site infection (P=0.631), dural tear (P=0.566), and injury to cervical spinal cord (P=0.092) between the two groups.
To ensure a fair comparison between the navigation-guided and control groups, we employed propensity score matching. This statistical technique effectively aligned variables such as ages, comorbidities, gender composition, and hospital sizes, enhancing the reliability of our analysis. Given the smaller size of the navigation group, we used a control group that is 20 times larger to maintain statistical accuracy. This adjustment facilitated a more comprehensive comparison, reducing potential biases and reinforcing the validity of our results.
Table 6 presents the comparison of demographic and clinical characteristics after propensity score matching (PSMA) between the control and navigation-guided groups in single-level Anterior Cervical Discectomy and Fusion procedures. The table includes parameters such as age, total surgeries, mortality rates, length of stay, and mean total charges, along with their respective significance levels. The data illustrate the effectiveness of PSMA in balancing the distribution of these variables between the two groups, enhancing the validity of our analysis. Notably, even after PSMA, navigation-guided procedures were associated with higher mean total charges compared to the control group by 7341
$.
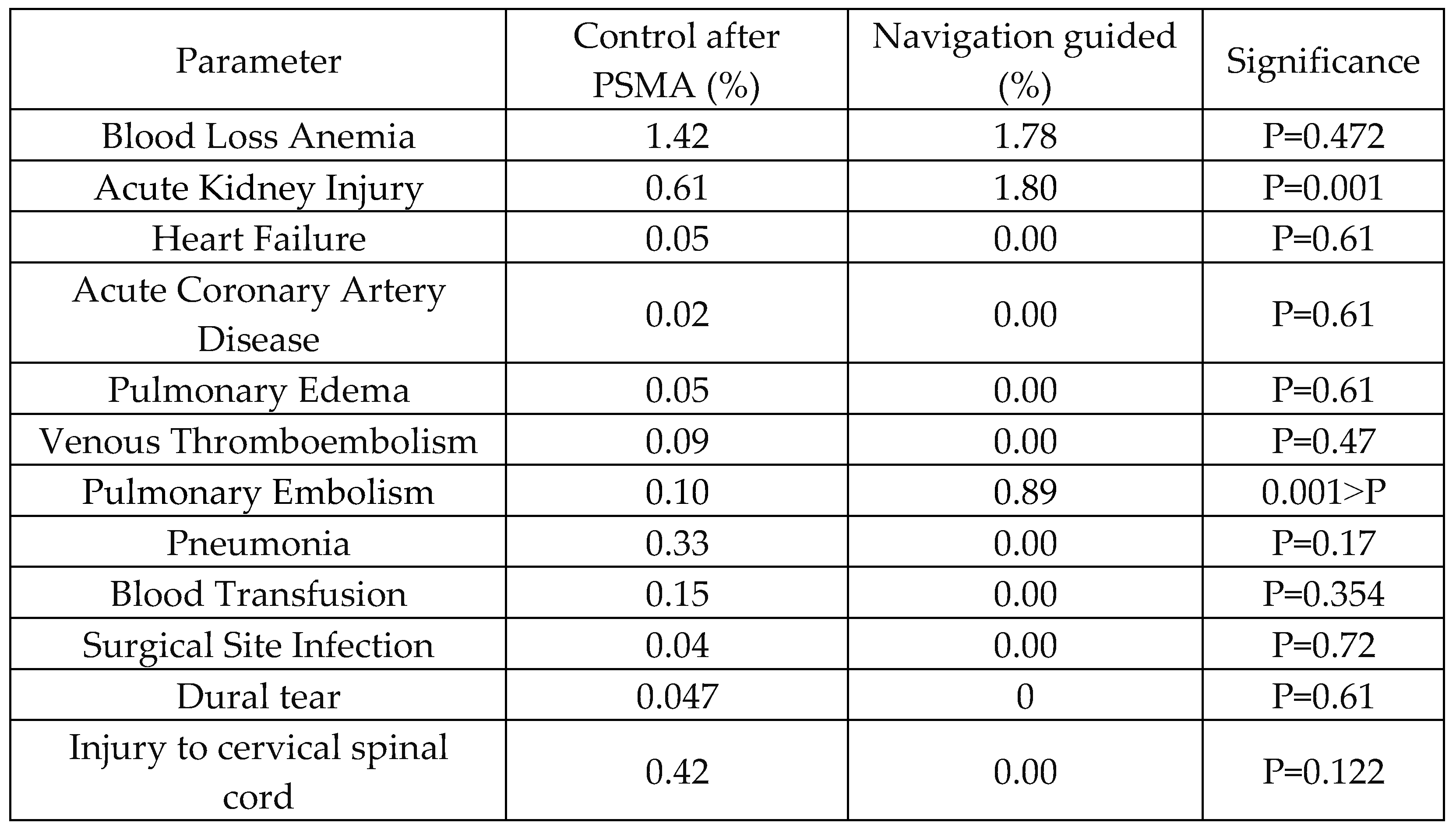
Table 7 provides insights into postoperative complications following single-level Anterior Cervical Discectomy and Fusion procedures, comparing outcomes between the control and navigation-guided groups after PSMA. The data reveal differences in complication rates, with acute kidney injury and pulmonary embolism, where navigation-guided procedures exhibited significantly higher rates compared to the control group (P=0.001 and P<0.001, respectively). However, no significant differences were observed in the rates of heart failure, acute coronary artery disease, pulmonary edema, venous thromboembolism, blood transfusion, surgical site infection, dural tear, or injury to the cervical spinal cord.
4. Discussion
Our investigation directly addresses the critical question of whether navigation surgery offers demonstrably superior outcomes compared to freehand techniques in the ACDF approach. By delving into this multifaceted issue, our study sheds light on the complexities inherent in this rapidly evolving surgical landscape.
From a financial perspective, we identified a statistically significant difference in mean total charges between navigation-assisted and freehand techniques. The analysis revealed a cost disparity of
$7,341, favoring the freehand approach. This finding aligns with existing literature, which suggests a trend of higher costs associated with spine navigation technology compared to traditional freehand surgery [
8,
9]. The underlying principle is that more advanced surgical technologies often incur greater implementation costs. As computer-assisted navigation becomes increasingly standardized and utilized in spinal surgeries, the economic burden on healthcare systems may rise due to the broader patient population undergoing these procedures [
10]. The advantages of using this technology, such as enhanced anatomical visualization and higher accuracy, should be carefully balanced against the disadvantages, such as longer operative times and higher financial expenses. Additional long-term studies are necessary to assess the cost-effectiveness of this navigation approach. Evaluating long-term outcomes and financial impacts will provide a more comprehensive understanding of the value of using this technology in surgical procedures. This includes analyzing potential cost savings from reduced complications and hospital readmissions and considering the initial investment and ongoing operational expenses associated with the technology. Our findings on cost differ significantly from earlier published studies, many of which predate the last decade. Studies on navigation technology in the ACDF approach do not address the financial aspect. These economic considerations raise pivotal questions about the cost-effectiveness of navigation surgery, prompting a nuanced evaluation of its benefits against financial investment [
11].
Regarding the accuracy of the navigation technology, since our data lacks radiologic images, we assessed accuracy based on the absence of acute spinal injuries, as no such injuries occurred in any of the cases. This technology proved its accuracy of screw placement in the lumbar spine in comparison with the free-hand approach [
12]. Looking into the cervical approach, concerning the posterior and lateral mass approach, one study demonstrated that navigation percutaneous posterior pedicle screw fixation could be performed safely, finding these constructs biomechanically superior with lower neurovascular complication rates comparable to traditional lateral mass screw technique [
13]. Another study demonstrated that navigated surgery can lead to higher accuracy and even shorter operating times than standard navigated operations.
Moreover, it allows a reduction in radiation exposure for the medical staff [
9]. The superiority of screw placement accuracy in the lumbar spine achieved with navigation compared to freehand techniques has been well-established [
14,
15]. Extending this advantage to the cervical spine, particularly in the posterior and lateral mass approaches, a study demonstrated the safe implementation of navigated percutaneous posterior pedicle screw fixation, This technique offered biomechanical superiority and comparable, potentially lower, neurovascular complication rates compared to the traditional lateral mass screw technique [
16,
17]. Concerning the ACDF approach, a recent study showed navigation can safely and effectively restore cervical vertebral sequence, fully release spinal canal compression, and promote patients’ neurological recovery [
18]. A study including 193 anterior cervical discectomies and fusions (ACDFs) performed with 3D navigation and 728 performed with fluoroscopy found that, after adjusting for demographics and surgical characteristics, 3D navigation was associated with less lateral plate deviation and longer operative times per interspace; however, there were no significant associations with angular plate deviation, length of stay, perioperative complications [
19].
Mortality rates were low and comparable across both groups. While most complications showed no significant differences, navigation-assisted procedures were associated with a significantly increased risk of acute kidney injury and pulmonary embolism. Conversely, no significant differences were observed in surgical site infection, dural tear, or injury to the cervical spinal cord. The precise cause remains elusive within our extensive dataset; however, a plausible explanation may be the potential prolongation of surgeries associated with navigation assistance, which could lead to an increased risk of pulmonary embolism and subsequent renal implications. This finding necessitates a critical reevaluation of the purported benefits of robotic systems, particularly in light of adverse outcomes such as heightened postoperative complications [
20].
Given the smaller size of the navigation group, we used a 20-times larger control group to maintain statistical accuracy. This adjustment facilitated a more comprehensive comparison, reducing potential biases and reinforcing the validity of our results.
This study acknowledges certain methodological limitations, primarily the use of broad ICD-10 codes within an extensive dataset. Nevertheless, it provides a valuable large-scale analytical perspective. While granular details on individual patients are lacking, the considerable statistical power afforded by the vast sample size substantiates the credibility of our findings. It is crucial to recognize the inherent trade-off between the depth of patient-specific data [
21,
22,
23,
24] and the robust insights gained from analyzing a comprehensive dataset that includes thousands of freehand versus hundreds of navigational approaches in anterior cervical discectomy and fusion (ACDF) surgeries.
5. Conclusions
Using free-hand and navigation-guided techniques, we assessed the demographic, clinical, and outcome variables of single-level Anterior Cervical Discectomy and Fusion (ACDF) surgeries. Our findings indicate that while free-hand procedures were more prevalent, navigation-guided surgeries were associated with significantly higher usage of Medicare and increased total charges. Propensity score matching was employed to balance demographic and clinical characteristics, confirming the robustness of these findings. Notably, navigation-guided techniques showed a higher incidence of specific postoperative complications, such as acute kidney injury and pulmonary embolism, despite similar mortality rates and lengths of hospital stay compared to the free-hand approach. These results underscore the need for careful consideration of the costs and benefits associated with navigation-guided ACDF surgeries, particularly in light of the increased financial burden and risk of certain complications.
Institutional Review Board Statement
The study was conducted under exempt status granted by the institutional review board, and the requirement for informed consent was waived due to the de-identified nature of the NIS dataset.
Informed Consent Statement
Irrelevant.
Acknowledgments
Irrelevant.
List of Abbreviations (A-Z):
HCUP: Healthcare Cost and Utilization Project
ICD-10: International Classification of Diseases, 10th Revision
LOS: Length of Stay
NIS: Nationwide Inpatient Sample
SPSS: Statistical Package for the Social Sciences
TKA: Total Hip Arthroplasty
UKA: Unicompartmental Knee Arthroplasty
Conflicts of Interest
None.
References
- Overley, S. C., Cho, S. K., Mehta, A. I. & Arnold, P. M. Navigation and Robotics in Spinal Surgery: Where Are We Now? Neurosurgery 80, S86–S99 (2017). [PubMed]
- Wallace, N. et al. Computer-assisted navigation in complex cervical spine surgery: tips and tricks. J. Spine Surg. 6, 136–144 (2020). [CrossRef]
- Butt, B. B., Piche, J., Gagnet, P., Patel, R. & Aleem, I. Stereotactic navigation in anterior cervical spine surgery: surgical setup and technique. J. Spine Surg. 6, 598–605 (2020). [PubMed]
- Pirris, S. M. & Nottmeier, E. W. A case series on the technical use of three-dimensional image guidance in subaxial anterior cervical surgery: Three-dimensional image guidance in subaxial anterior cervical surgery. Int. J. Med. Robot. 11, 44–51 (2015). [PubMed]
- Kim, J.-S., Eun, S. S., Prada, N., Choi, G. & Lee, S.-H. Modified transcorporeal anterior cervical microforaminotomy assisted by O-arm-based navigation: a technical case report. Eur. Spine J. 20, 147–152 (2011).
- Kirnaz, S. et al. Intraoperative image guidance for cervical spine surgery. Ann. Transl. Med. 9, 93–93 (2021). [CrossRef]
- Gould, H., Sohail, O. A. & Haines, C. M. Anterior cervical discectomy and fusion: Techniques, complications, and future directives. Semin. Spine Surg. 32, 100772 (2020).
- Maman D, Mahamid A, Finkel B, Gan-Or H, Fournier L, Berkovich Y, Behrbalk E. Comparative evaluation of postoperative outcomes and expenditure between robotic and conventional single-level lumbar fusion surgery: a comprehensive analysis of nationwide inpatient sample data. Eur Spine J. 2024 May 7.
- Carl B, Bopp M, Pojskic M, Voellger B, Nimsky C. Standard navigation versus intraoperative computed tomography navigation in upper cervical spine trauma. Int J Comput Assist Radiol Surg. 2019 Jan;14(1):169-182. [CrossRef]
- Dominy CL, Tang JE, Arvind V, Cho BH, Selverian S, Shah KC, Kim JS, Cho SK. Trends in the Charges and Utilization of Computer-Assisted Navigation in Cervical and Thoracolumbar Spinal Surgery. Asian Spine J. 2022 Oct;16(5):625-633. [CrossRef]
- Dea N, Fisher CG, Batke J, Strelzow J, Mendelsohn D, Paquette SJ, Kwon BK, Boyd MD, Dvorak MF, Street JT. Economic evaluation comparing intraoperative cone beam CT-based navigation and conventional fluoroscopy for the placement of spinal pedicle screws: a patient-level data cost-effectiveness analysis. Spine J. 2016 Jan 1;16(1):23-31. [CrossRef]
- La Rocca G, Mazzucchi E, Pignotti F, Nasto LA, Galieri G, Rinaldi P, De Santis V, Pola E, Sabatino G. Navigated, percutaneous, three-step technique for lumbar and sacral screw placement: a novel, minimally invasive, and maximally safe strategy. J Orthop Traumatol. 2023 Jun 29;24(1):32. [CrossRef]
- Coric D, Rossi V. Percutaneous Posterior Cervical Pedicle Instrumentation (C1 to C7) With Navigation Guidance: Early Series of 27 Cases. Global Spine J. 2022 Apr;12(2_suppl):27S-33S.
- Kanno H, Handa K, Murotani M, Ozawa H. A Novel Intraoperative CT Navigation System for Spinal Fusion Surgery in Lumbar Degenerative Disease: Accuracy and Safety of Pedicle Screw Placement. J Clin Med. 2024 Apr 4;13(7):2105. [CrossRef]
- Shahi P, Subramanian T, Araghi K, Singh S, Asada T, Maayan O, Korsun M, Singh N, Tuma O, Dowdell J, Sheha E, Qureshi S, Iyer S. Comparison of Robotics and Navigation for Clinical Outcomes After Minimally Invasive Lumbar Fusion. Spine (Phila Pa 1976). 2023 Oct 1;48(19):1342-1347.
- Tanaka M, Zygogiannnis K, Sake N, Arataki S, Fujiwara Y, Taoka T, de Moraes Modesto TH, Chatzikomninos I. A C-Arm-Free Minimally Invasive Technique for Spinal Surgery: Cervical and Thoracic Spine. Medicina (Kaunas). 2023 Oct 6;59(10):1779. [CrossRef]
- Coric D, Rossi V. Navigated, Percutaneous Posterior Cervical Minimally Invasive Surgery Fixation: Technique and Nuances. Int J Spine Surg. 2022 Oct;16(S2):S8-S13. [CrossRef]
- Tang Y, Li H, Zhang S, Liu H, Zhang J, Yang H, Zhang K, Wang G, Chen K. Comparison of Anterior Cervical Discectomy Fusion Combined with Lateral Mass Screw and with Cervical Pedicle Screw Fixation Surgery under O-Arm Navigation for Single-Stage Management of Severe Lower Cervical Fracture Dislocation. Orthop Surg. 2023 Oct;15(10):2647-2655. [CrossRef]
- Nie JZ, Weber MW, Revelt NJ, Nordmann NJ, Watson VL, Nie JW, Menezes SA, Delfino K, Cozzens JW, Espinosa JA, Amin D, Acakpo-Satchivi L. Comparison of Using Intraoperative Computed Tomography-Based 3-Dimensional Navigation and Fluoroscopy in Anterior Cervical Diskectomy and Fusion for Cervical Spondylosis. World Neurosurg. 2022 May;161:e740-e747.
- Joseph JR, Smith BW, Liu X, Park P (2017) Current applications of robotics in spine surgery: a systematic review of the literature. Neurosurg Focus.
- Maman, D., Mahamid, A., Finkel, B. et al. Comparative evaluation of postoperative outcomes and expenditure between robotic and conventional single-level lumbar fusion surgery: a comprehensive analysis of nationwide inpatient sample data. Eur Spine J (2024). [CrossRef]
- Maman, D., Laver, L., Becker, R., Mahamid, A. & Berkovich, Y. (2024) Robotic-assisted total knee arthroplasty reduces postoperative complications and length of stay without increased cost compared to navigation-guided techniques: a national analysis. Knee Surgery, Sports Traumatology, Arthroscopy, 1–7. [CrossRef]
- Maman, D., Laver, L., Becker, R., Takrori, L., Mahamid, A., Finkel, B., Gan-Or, H.,Yonai, Y. & Berkovich, Y. (2024) Trends and Epidemiology in Robotic-Assisted Total Knee Arthroplasty: Reduced Complications and Shorter Hospital Stays. Knee Surgery, Sports Traumatology, Arthroscopy. [CrossRef]
- Maman, D.; Mahamid, A.; Yonai, Y.; Berkovich, Y. Comparing Complication Rates, Costs, and Length of Stay between Unicompartmental and Total Knee Arthroplasty: Insights from a Big Data Analysis Using the National Inpatient Sample Dataset. J. Clin. Med. 2024, 13, 3888. [CrossRef]
Table 1.
Demographic and Clinical Characteristics of Single-Level Anterior Cervical Discectomy and Fusion Procedures: Control vs Navigation-Guided Approaches.
Table 1.
Demographic and Clinical Characteristics of Single-Level Anterior Cervical Discectomy and Fusion Procedures: Control vs Navigation-Guided Approaches.
| Parameter |
Control (%) |
Navigation guided (%) |
Significance |
| Total Surgeries |
85020 |
560 |
- |
| Average Age (y) |
55.55 |
58.21 |
P<0.0001 |
| Female (%) |
51.6 |
58 |
P=0.002 |
| Payer - Medicare (%) |
33.9 |
41.1 |
P<0.0001 |
| Payer - Medicaid (%) |
10.7 |
10.7 |
| Payer - Private (%) |
44.5 |
42 |
| Payer - Other (including self-pay) (%) |
10.9 |
6.2 |
| Median Income - 0-25 percentile (%) |
25.1 |
20.9 |
P=0.128 |
| Median Income - 26-50 percentile (%) |
27.2 |
28.2 |
| Median Income - 51-75 percentile (%) |
26.3 |
29.1 |
| Median Income - 76-100 percentile (%) |
21.3 |
21.8 |
Table 2.
Comparison of Comorbidities Between Control and Navigation-Guided Groups in Single-Level Anterior Cervical Discectomy and Fusion Procedures.
Table 2.
Comparison of Comorbidities Between Control and Navigation-Guided Groups in Single-Level Anterior Cervical Discectomy and Fusion Procedures.
| Parameter |
Control (%) |
Navigation guided (%) |
Significance |
| Hypertension Diagnosis |
43.7 |
43.8 |
P=0.985 |
| Dyslipidemia Diagnosis |
30 |
30.4 |
P=0.846 |
| Sleep Apnea Diagnosis |
9.5 |
10.7 |
P=0.345 |
| Chronic Anemia |
2.3 |
0.9 |
P=0.024 |
| Mental Disorders |
40.4 |
39.3 |
P=0.602 |
| Alzheimer Disease |
0.1 |
0.3 |
P=0.985 |
| Parkinson Disease |
0.5 |
0.9 |
P=0.123 |
| Type 2 Diabetes |
19 |
21.4 |
P=0.141 |
| Renal Disease |
3.8 |
3.6 |
P=0.812 |
| CHF |
0.9 |
1.8 |
P=0.040 |
| Chronic Lung Disease |
8.0 |
7.1 |
P=0.457 |
| Osteoporosis Diagnosis |
2.3 |
2.7 |
P=0.591 |
Table 3.
Comparison of Hospitalization Outcomes and Costs Between Control and Navigation-Guided Groups in Single-Level Anterior Cervical Discectomy and Fusion Procedures.
Table 3.
Comparison of Hospitalization Outcomes and Costs Between Control and Navigation-Guided Groups in Single-Level Anterior Cervical Discectomy and Fusion Procedures.
Table 4.
Incidence of Postoperative Complications Between Control and Navigation-Guided Groups in Single-Level Anterior Cervical Discectomy and Fusion Procedures.
Table 4.
Incidence of Postoperative Complications Between Control and Navigation-Guided Groups in Single-Level Anterior Cervical Discectomy and Fusion Procedures.
Table 5.
Comparison of Intraoperative Complications Between Control and Navigation-Guided Groups in Single-Level Anterior Cervical Discectomy and Fusion Procedures.
Table 5.
Comparison of Intraoperative Complications Between Control and Navigation-Guided Groups in Single-Level Anterior Cervical Discectomy and Fusion Procedures.
| Parameter |
Control (%) |
Navigation guided (%) |
Significance |
| Surgical Site Infection |
0.04 |
0 |
P=0.631 |
| Dural tear |
0.059 |
0 |
P=0.566 |
| Injury to cervical spinal cord |
0.51 |
0 |
P=0.092 |
Table 6.
Comparison of Demographic and Clinical Characteristics After Propensity Score Matching Between Control and Navigation-Guided Groups.
Table 6.
Comparison of Demographic and Clinical Characteristics After Propensity Score Matching Between Control and Navigation-Guided Groups.
| Parameter |
Control after PSMA |
Navigation guided |
Significance |
| AGE in years |
58.3 |
58.21 |
P=0.564 |
| Total Surgeries |
11200 |
560 |
- |
| Died during hospitalization (%) |
0.047 |
0 |
P=0.61 |
| Length of stay mean in days |
1.69 |
1.83 |
P=0.136 |
| Total charges mean in $
|
68736 |
76077 |
P=0.002 |
Table 7.
Comparison of Demographic and Clinical Characteristics After Propensity Score Matching Between Control and Navigation-Guided Groups.
Table 7.
Comparison of Demographic and Clinical Characteristics After Propensity Score Matching Between Control and Navigation-Guided Groups.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).