Submitted:
10 July 2024
Posted:
11 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
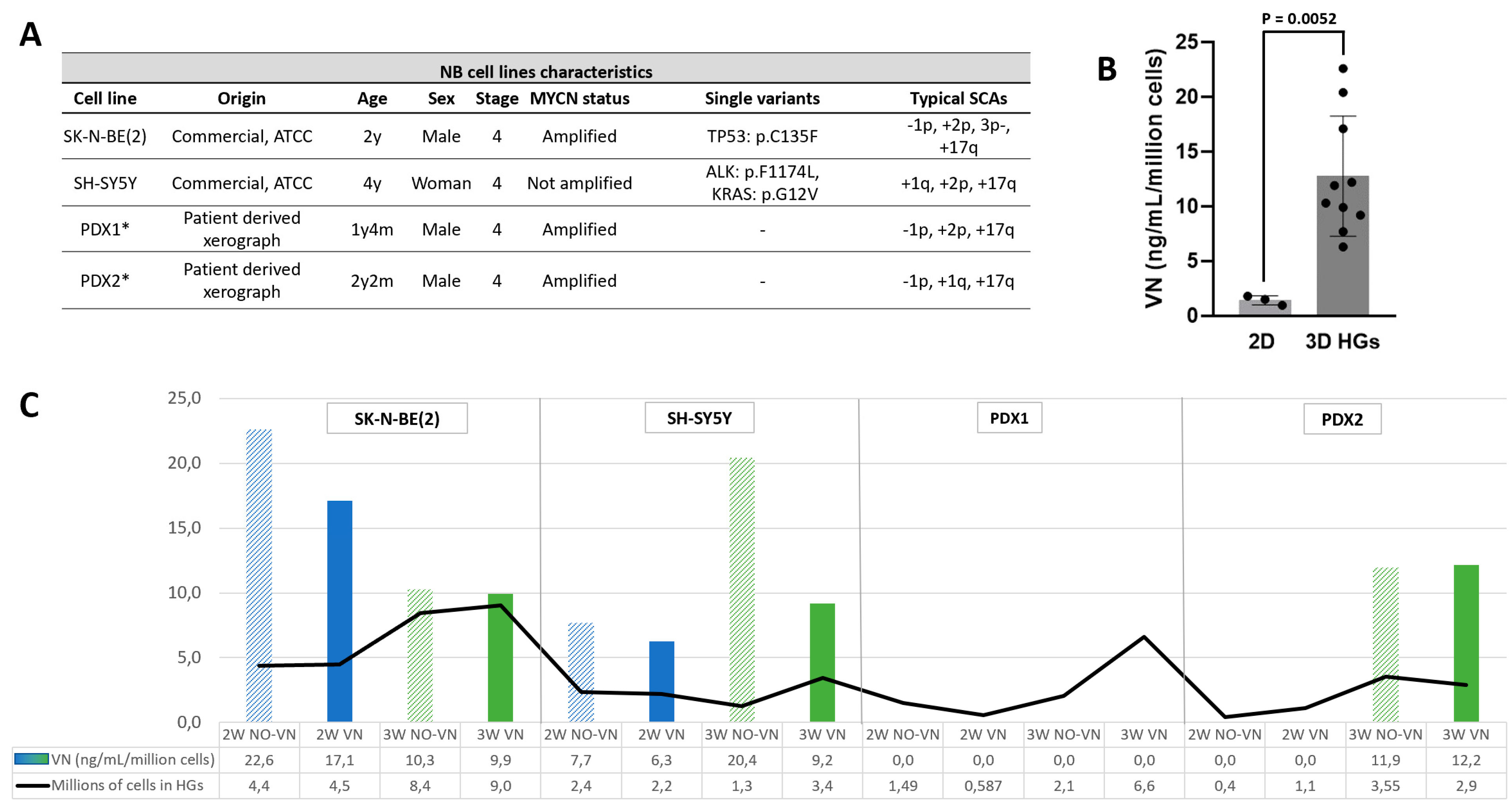
2.1. Higher VN Release in 3D Models
2.2. VN Secretion Independent of Cell Numbers
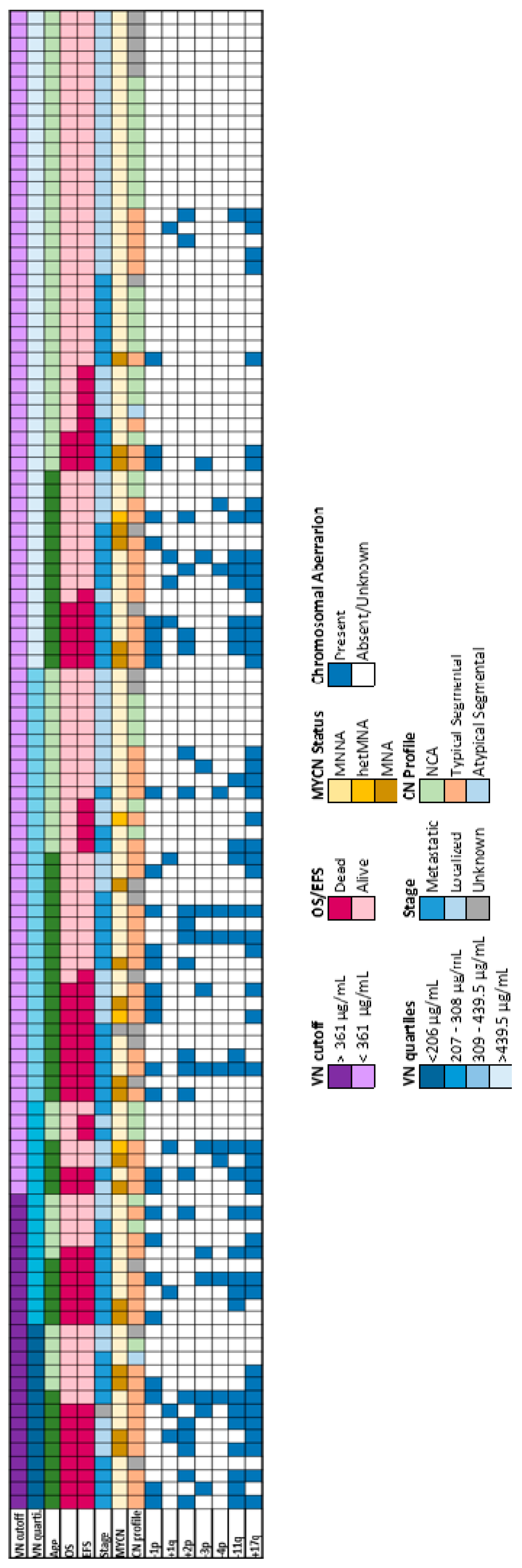
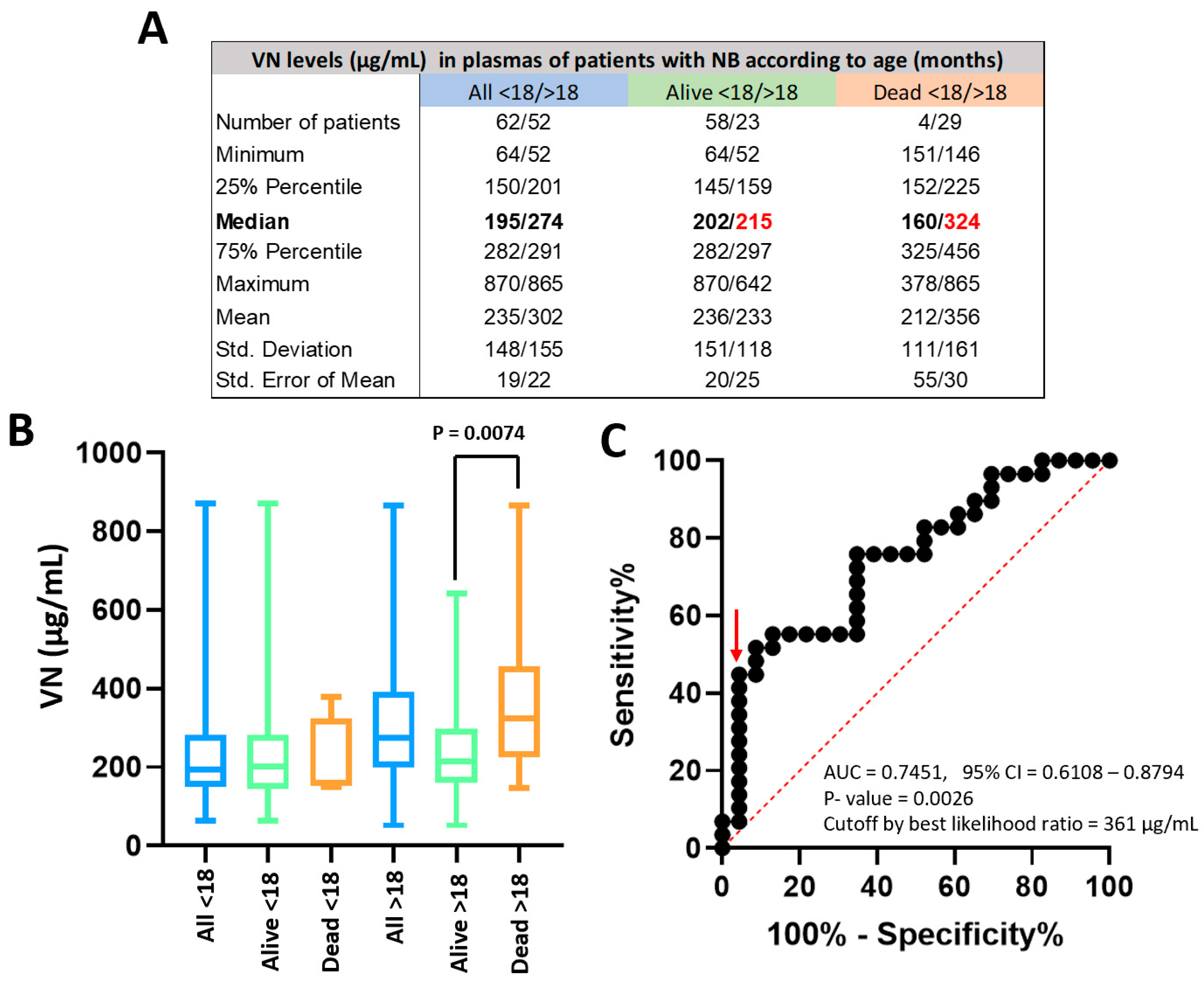
2.3. Clinical and Genetic Characteristics of NB Patients Included in the Study
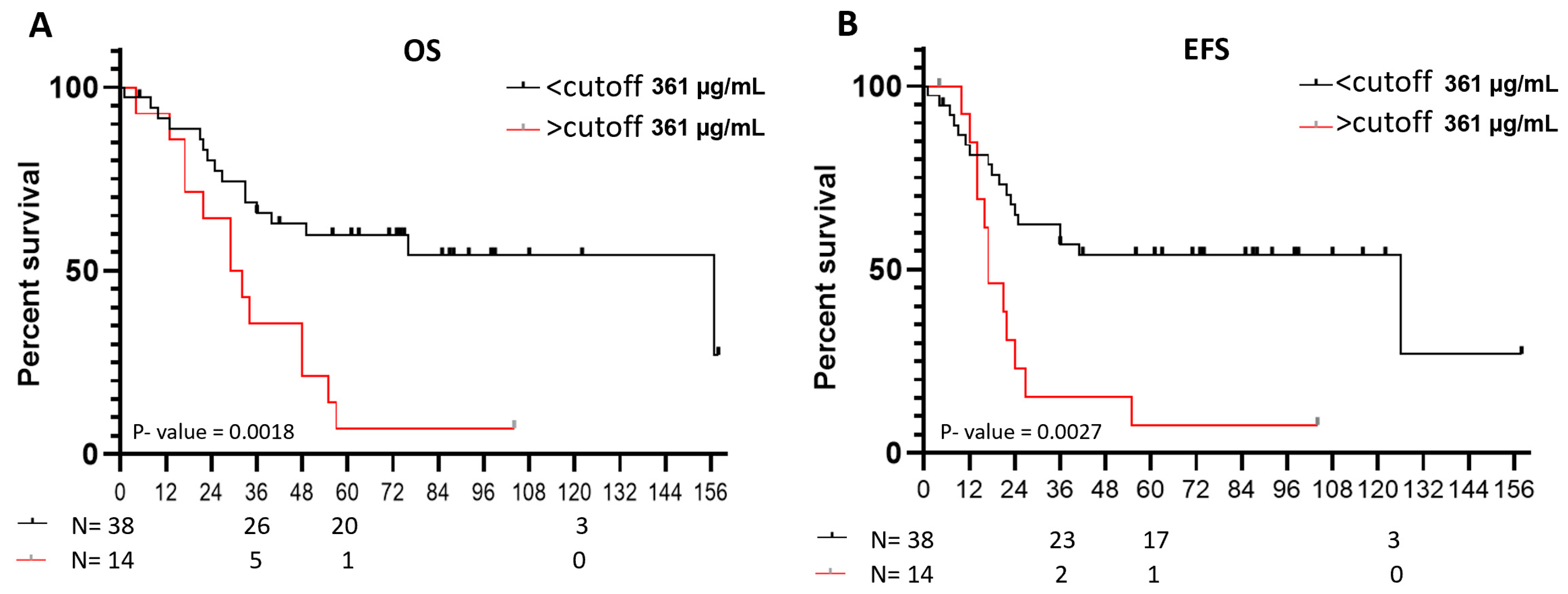
2.4. Discriminatory Power of Plasma VN Levels for NB Patient Outcomes
2.5. Plasma VN Level Association with Stage and Genetic Features of NB
3. Discussion
4. Materials and Methods
4.3. Patients and Samples
4.4. Plasma VN Determination
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schvartz, I.; Seger, D.; Shaltiel, S. Vitronectin. Int. J. Biochem. Cell Biol. 1999, 31, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Waltz, D.A.; Natkin, L.R.; Fujita, R.M.; Wei, Y.; Chapman, H.A. Plasmin and plasminogen activator inhibitor type 1 promote cellular motility by regulating the interaction between the urokinase receptor and vitronectin. J. Clin. Invest. 1997, 100, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.D.; Ferraris, G.M.S.; Andolfo, A.; Cunningham, O.; Sidenius, N. uPAR-induced cell adhesion and migration: Vitronectin provides the key. J. Cell Biol. 2007, 177, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Leavesley, D.I.; Kashyap, A.S.; Croll, T.; Sivaramakrishnan, M.; Shokoohmand, A.; Hollier, B.G.; Upton, Z. Vitronectin-Master controller or micromanager? IUBMB Life 2013, 65, n/a-n/a. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Panadero, R.; Noguera, I.; Cañete, A.; Navarro, S.; Noguera, R. Vitronectin as a molecular player of the tumor microenvironment in neuroblastoma. BMC Cancer 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Uhm, J.H.; Dooley, N.P.; Kyritsis, A.P.; Rao, J.S.; Gladson, C.L.; H U, N.-O.J. Vitronectin, a Glioma-derived Extracellular Matrix Protein, Protects Tumor Cells from Apoptotic Death 1. Clin Cancer Res. 1999, 5, 1587–1594. [Google Scholar] [PubMed]
- Li, R.; Ren, M.; Chen, N.; Luo, M.; Zhang, Z.; Wu, J. Vitronectin Increases Vascular Permeability by Promoting VE-Cadherin Internalization at Cell Junctions. PLoS One 2012, 7, e37195. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.T.; Nash, G. Vitronectin (serum spreading factor): its localisation in normal and fibrotic tissue. J Clin Pathol 1988, 41, 1269–1272. [Google Scholar] [CrossRef] [PubMed]
- Heyman, L.; Leroy-Dudal, J.; Fernandes, J.; Seyer, D.; Dutoit, S.; Carreiras, F. Mesothelial vitronectin stimulates migration of ovarian cancer cells. Cell Biol. Int. 2010, 34, 493–502. [Google Scholar] [CrossRef]
- Radwan, A.F.; Ismael, O.E.; Fawzy, A.; El-Mesallamy, H.O. Evaluation of Serum Integrin αvβ3 & Vitronectin in the Early Diagnosis of Breast Cancer. Clin. Lab. 2019, 65, 1185–1193. [Google Scholar] [CrossRef]
- Edwards, S.; Lalor, P.F.; Tuncer, C.; Adams, D.H. Vitronectin in human hepatic tumours contributes to the recruitment of lymphocytes in an avb3-independent manner. Br. J. Cancer 2006, 95, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Ciereszko, A.; Dietrich, M.A.; Słowińska, M.; Nynca, J.; Ciborowski, M.; Kisluk, J.; Michalska-Falkowska, A.; Reszec, J.; Sierko, E.; Nikliński, J. Identification of protein changes in the blood plasma of lung cancer patients subjected to chemotherapy using a 2D-DIGE approach. PLoS One 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Subramanian, M.; Karaian, J.; Eklund, M.; Radhakrishnan, S.; Gana, N.; Rothwell, S.; Pollard, H.; Hu, H.; Shriver, C.D.; et al. Functional role of vitronectin in breast cancer. PLoS One 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- Tas, F.; Karabulut, S.; Bilgin, E.; Tastekin, D.; Duranyildiz, D. Clinical significance of serum fibronectin and vitronectin levels in melanoma patients. Melanoma Res. 2014, 24, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A.; Mueller, B.M. Melanoma cell migration on vitronectin: regulation by components of the plasminogen activation system. Int. J. Cancer 1997, 71, 116–122. [Google Scholar] [CrossRef]
- Cheung, N.-K. V; Dyer, M.A. Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat Rev Cancer 2013, 13, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Cohn, S.L.; Pearson, A.D.J.; London, W.B.; Monclair, T.; Ambros, P.F.; Brodeur, G.M.; Faldum, A.; Hero, B.; Iehara, T.; Machin, D.; et al. The International Neuroblastoma Risk Group (INRG) classification system: An INRG task force report. J. Clin. Oncol. 2009, 27, 289–297. [Google Scholar] [CrossRef]
- Von Stedingk, K.; De Preter, K.; Vandesompele, J.; Noguera, R.; Øra, I.; Koster, J.; Versteeg, R.; Påhlman, S.; Lindgren, D.; Axelson, H. Individual patient risk stratification of high-risk neuroblastomas using a two-gene score suited for clinical use. Int. J. Cancer 2015, 137, 868–877. [Google Scholar] [CrossRef]
- Vicente-Munuera, P.; Burgos-Panadero, R.; Noguera, I.; Navarro, S.; Noguera, R.; Escudero, L.M. The topology of vitronectin: A complementary feature for neuroblastoma risk classification based on computer-aided detection. Int. J. Cancer 2020, 146, 553–565. [Google Scholar] [CrossRef]
- López-Carrasco, A.; Martín-Vañó, S.; Burgos-Panadero, R.; Monferrer, E.; Berbegall, A.P.; Fernández-Blanco, B.; Navarro, S.; Noguera, R. Impact of extracellular matrix stiffness on genomic heterogeneity in MYCN-amplified neuroblastoma cell line. J. Exp. Clin. Cancer Res. 2020, 39. [Google Scholar] [CrossRef]
- Monferrer, E.; Sanegre, S.; Martín-Vañó, S.; García-Lizarribar, A.; Burgos-Panadero, R.; López-Carrasco, A.; Navarro, S.; Samitier, J.; Noguera, R. Digital image analysis applied to tumor cell proliferation, aggressiveness, and migration-related protein synthesis in neuroblastoma 3d models. Int. J. Mol. Sci. 2020, 21, 1–17. [Google Scholar] [CrossRef]
- Monferrer, E.; Martín-Vañó, S.; Carretero, A.; García-Lizarribar, A.; Burgos-Panadero, R.; Navarro, S.; Samitier, J.; Noguera, R. A three-dimensional bioprinted model to evaluate the effect of stiffness on neuroblastoma cell cluster dynamics and behavior. Sci. Rep. 2020, 10, 6370. [Google Scholar] [CrossRef]
- Monferrer, E.; Dobre, O.; Trujillo, S.; González Oliva, M.A.; Trubert-Paneli, A.; Acevedo-León, D.; Noguera, R.; Salmeron-Sanchez, M. Vitronectin-based hydrogels recapitulate neuroblastoma growth conditions. Front. cell Dev. Biol. 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Repetto, O.; Caggiari, L.; De Zorzi, M.; Elia, C.; Mussolin, L.; Buffardi, S.; Pillon, M.; Muggeo, P.; Casini, T.; Steffan, A.; et al. Quantitative Plasma Proteomics to Identify Candidate Biomarkers of Relapse in Pediatric/Adolescent Hodgkin Lymphoma. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Braoudaki, M.; Lambrou, G.I.; Vougas, K.; Karamolegou, K.; Tsangaris, G.T.; Tzortzatou-Stathopoulou, F. Protein biomarkers distinguish between high- and low-risk pediatric acute lymphoblastic leukemia in a tissue specific manner. J. Hematol. Oncol. 2013, 6, 1–20. [Google Scholar] [CrossRef]
- Kadowaki, M.; Sangai, T.; Nagashima, T.; Sakakibara, M.; Yoshitomi, H.; Takano, S.; Sogawa, K.; Umemura, H.; Fushimi, K.; Nakatani, Y.; et al. Identification of vitronectin as a novel serum marker for early breast cancer detection using a new proteomic approach. J. Cancer Res. Clin. Oncol. 2011, 137, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Turan, T.; Torun, M.; Atalay, F.; Gönenç, A.; Turk, J. Endometrial ve Ovaryum Kanserinde Tanısal Değer Yönünden Serum Biyobelirteci Olarak Vitronektin, Solubl Epitel-Kaderin ve TGF-β1’in Değerlendirilmesi Assessment of Vitronectin, Soluble Epithelial-Cadherin and TGF-β1 as a Serum Biomarker with Predictive Value for Endometrial and Ovarian Cancers. Pharm Sci 2017, 14, 141–147. [Google Scholar] [CrossRef]
- Ortega-Martínez, I.; Gardeazabal, J.; Erramuzpe, A.; Sanchez-Diez, A.; Cortés, J.; García-Vázquez, M.D.; Pérez-Yarza, G.; Izu, R.; Luís Díaz-Ramón, J.; de la Fuente, I.M.; et al. Vitronectin and dermcidin serum levels predict the metastatic progression of AJCC I–II early-stage melanoma. Int. J. Cancer 2016, 139, 1598–1607. [Google Scholar] [CrossRef]
- Fukushima, Y.; Tamura, M.; Nakagawa, H.; Itoh, K. Induction of glioma cell migration by vitronectin in human serum and cerebrospinal fluid. J. Neurosurg. 2007, 107, 578–585. [Google Scholar] [CrossRef]
- Schneider, G.; Suszynska, M.; Kakar, S.; Ratajczak, M.Z. Vitronectin in the ascites of human ovarian carcinoma acts as a potent chemoattractant for ovarian carcinoma: Implication for metastasis by cancer stem cells. J. cancer stem cell Res. 2016, 4, 1. [Google Scholar] [CrossRef]
- Tugcu, D.; Devecioglu, O.; Unuvar, A.; Ekmekci, H.; Ekmekci, O.B.; Anak, S.; Ozturk, G.; Akcay, A.; Aydogan, G. Plasma Levels of Plasminogen Activator Inhibitor Type 1 and Vitronectin in Children With Cancer. Clin. Appl. Thromb. Hemost. 2016, 22, 28–33. [Google Scholar] [CrossRef] [PubMed]
- López-Carrasco, A.; Berbegall, A.P.; Martín-Vañó, S.; Blanquer-Maceiras, M.; Castel, V.; Navarro, S.; Noguera, R. Intra-Tumour Genetic Heterogeneity and Prognosis in High-Risk Neuroblastoma. Cancers (Basel). 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Braekeveldt, N.; Wigerup, C.; Gisselsson, D.; Mohlin, S.; Merselius, M.; Beckman, S.; Jonson, T.; Börjesson, A.; Backman, T.; Tadeo, I.; et al. Neuroblastoma patient-derived orthotopic xenografts retain metastatic patterns and geno- and phenotypes of patient tumours. Int. J. Cancer 2015, 136, E252–E261. [Google Scholar] [CrossRef] [PubMed]
- Newall, F.; Johnston, L.; Ignjatovic, V.; Summerhayes, R.; Monagle, P. Age-related plasma reference ranges for two heparin-binding proteins – vitronectin and platelet factor 4. Int. J. Lab. Hematol. 2009, 31, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Trigg, R.M.; Shaw, J.A.; Turner, S.D. Opportunities and challenges of circulating biomarkers in neuroblastoma. Open Biol. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Zogchel, L.M.J. van; Wezel, E.M. van; Wijk, J. van; Stutterheim, J.; Bruins, W.S.C.; Zappeij-Kannegieter, L.; Slager, T.J.E.; Schumacher-Kuckelkorn, R.; Verly, I.R.N.; Schoot, C.E. van der; et al. Hypermethylated RASSF1A as Circulating Tumor DNA Marker for Disease Monitoring in Neuroblastoma. JCO Precis Oncol. 2020, 4, PO.19.00261. [CrossRef]
- Filipe, E.C.; Chitty, J.L.; Cox, T.R. Charting the unexplored extracellular matrix in cancer. Int. J. Exp. Pathol. 2018, 99, 58–76. [Google Scholar] [CrossRef] [PubMed]
- Hahn, W.C.; Bader, J.S.; Braun, T.P.; Califano, A.; Clemons, P.A.; Druker, B.J.; Ewald, A.J.; Fu, H.; Jagu, S.; Kemp, C.J.; et al. An expanded universe of cancer targets. Cell 2021, 184, 1142–1155. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.; Bryndza, E.; Poniewierska-Baran, A.; Serwin, K.; Suszynska, M.; Sellers, Z.P.; Merchant, M.L.; Kaliappan, A.; Ratajczak, J.; Kucia, M.; et al. Evidence that vitronectin is a potent migration-enhancing factor for cancer cells chaperoned by fibrinogen: a novel view of the metastasis of cancer cells to low-fibrinogen lymphatics and body cavities. 7. Oncotarget, 2016; 7, 69829–69843. [Google Scholar] [CrossRef]
- Lee, H.-J.; Cha, H.-J.; Lim, J.-S.; Lee, S.H.; Song, S.Y.; Kim, H.; Hancock, W.S.; Yoo, J.S.; Paik, Y.-K. Abundance-Ratio-Based Semiquantitative Analysis of Site-Specific N-Linked Glycopeptides Present in the Plasma of Hepatocellular Carcinoma Patients. Proteome Res. 2014, 13, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Benachour, H. ; Leroy-Dudal, J; Agniel, R; Wilson, J; Briand, M; Carreiras, F; Gallet, O. Vitronectin (Vn) glycosylation patterned by lectin affinity assays-A potent glycoproteomic tool to discriminate plasma Vn from cancer ascites Vn. J Mol Recognit, 2018; 31, e2690. [Google Scholar] [CrossRef]
- Hao, W.; Zhang, X.; Xiu, B.; Yang, X.; Hu, S.; Liu, Z.; Duan, C.; Jin, S.; Ying, X.; Zhao, Y.; et al. Vitronectin: a promising breast cancer serum biomarker for early diagnosis of breast cancer in patients. Tumor Biol. 2016, 37, 8909–8916. [Google Scholar] [CrossRef]
- Chen, M.H.; Lu, C.; Sun, J.; Chen, X.D.; Dai, J.X.; Cai, J.Y.; Chen, X.L. Diagnostic and prognostic value of serum vitronectin levels in human glioma. J. Neurol. Sci. 2016, 371, 54–59. [Google Scholar] [CrossRef]
- Gladson, C.L.; Cheresht, D.A. Glioblastoma Expression of Vitronectin and the av/83 Integrin. Adhesion Mechanism for Transformed Glial Cells Key words: glioblastoma * invasion * vitronectin * avβ3 integrin. J. Clin. Invest 1991, 88, 1924–1932. [Google Scholar] [CrossRef] [PubMed]
- Mikuła-Pietrasik, J.; Uruski, P.; Tykarski, A. ; Książek, · Krzysztof The peritoneal “soil” for a cancerous “seed”: a comprehensive review of the pathogenesis of intraperitoneal cancer metastases. Cell. Mol. Life Sci 2018, 75, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Yamada, S.; Kawasaki, H. Distribution of vitronectin in plasma and liver tissue: Relationship to chronic liver disease. Hepatology 1994, 20, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Stoks, M.; Vieco-Martí, I.; Noguera, I.; Sánchez-Sánchez, M.; Burgos-Panadero, R.; Navarro, S.; Noguera, R. Digital image analysis workflows for evaluation of cell behavior and tumor microenvironment to aid therapeutic assessment in high-risk neuroblastoma. Comput. Biol. Med. 2023, 164, 107364. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Panadero, R.; El Moukhtari, S.H.; Noguera, I.; Rodríguez-Nogales, C.; Martín-Vañó, S.; Vicente-Munuera, P.; Cañete, A.; Navarro, S.; Blanco-Prieto, M.J.; Noguera, R. Unraveling the extracellular matrix-tumor cell interactions to aid better targeted therapies for neuroblastoma. Int. J. Pharm. 2021, 608, 121058. [Google Scholar] [CrossRef] [PubMed]
- Kurozumi, K.; Ichikawa, T.; Onishi, M.; Fujii, K.; Date, I. Cilengitide treatment for malignant glioma: current status and future direction. Neurol. Med. Chir. (Tokyo). 2012, 52, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Nabors, L.B.; Stupp, R.; Mikkelsen, T. Cilengitide: an integrin-targeting arginine-glycine-aspartic acid peptide with promising activity for glioblastoma multiforme. Expert Opin. Investig. Drugs 2008, 17, 1225–1235. [Google Scholar] [CrossRef]
- Hasturk, O.; Jordan, K.E.; Choi, J.; Kaplan, D.L. Enzymatically crosslinked silk and silk-gelatin hydrogels with tunable gelation kinetics, mechanical properties and bioactivity for cell culture and encapsulation. 2019. [CrossRef]
- Villamón, E.; Piqueras, M.; Meseguer, J.; Blanquer, I.; Berbegall, A.P.; Tadeo, I.; Hernández, V.; Navarro, S.; Noguera, R. NeuPAT: An intranet database supporting translational research in neuroblastic tumors. Comput. Biol. Med. 2013, 43, 219–228. [Google Scholar] [CrossRef]
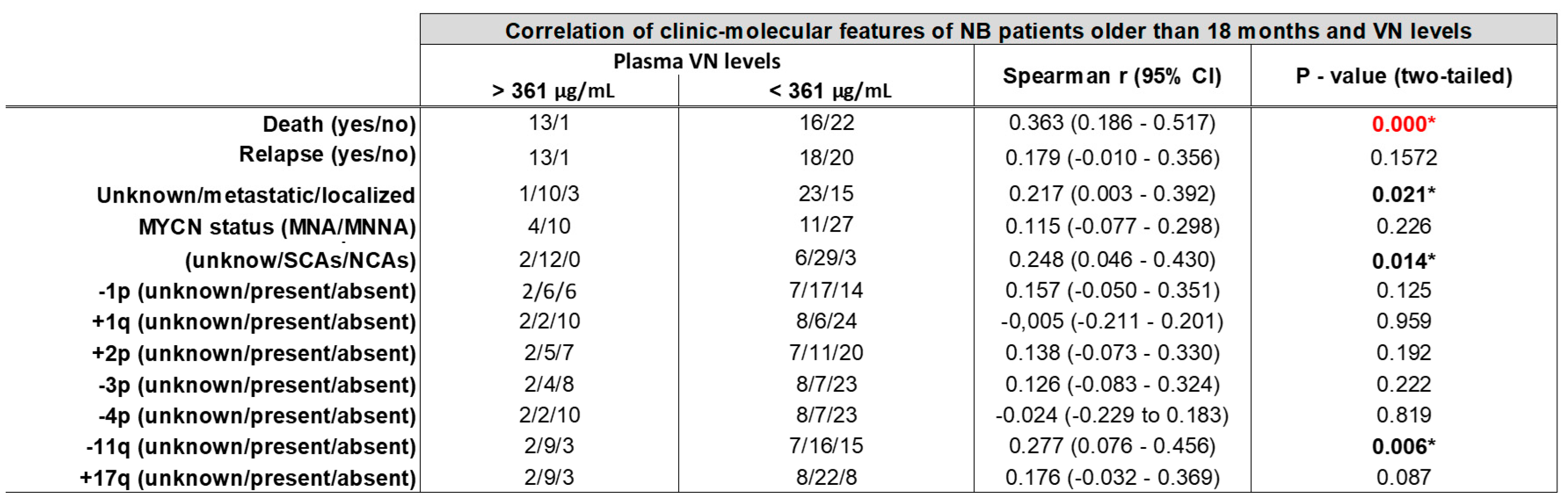 |
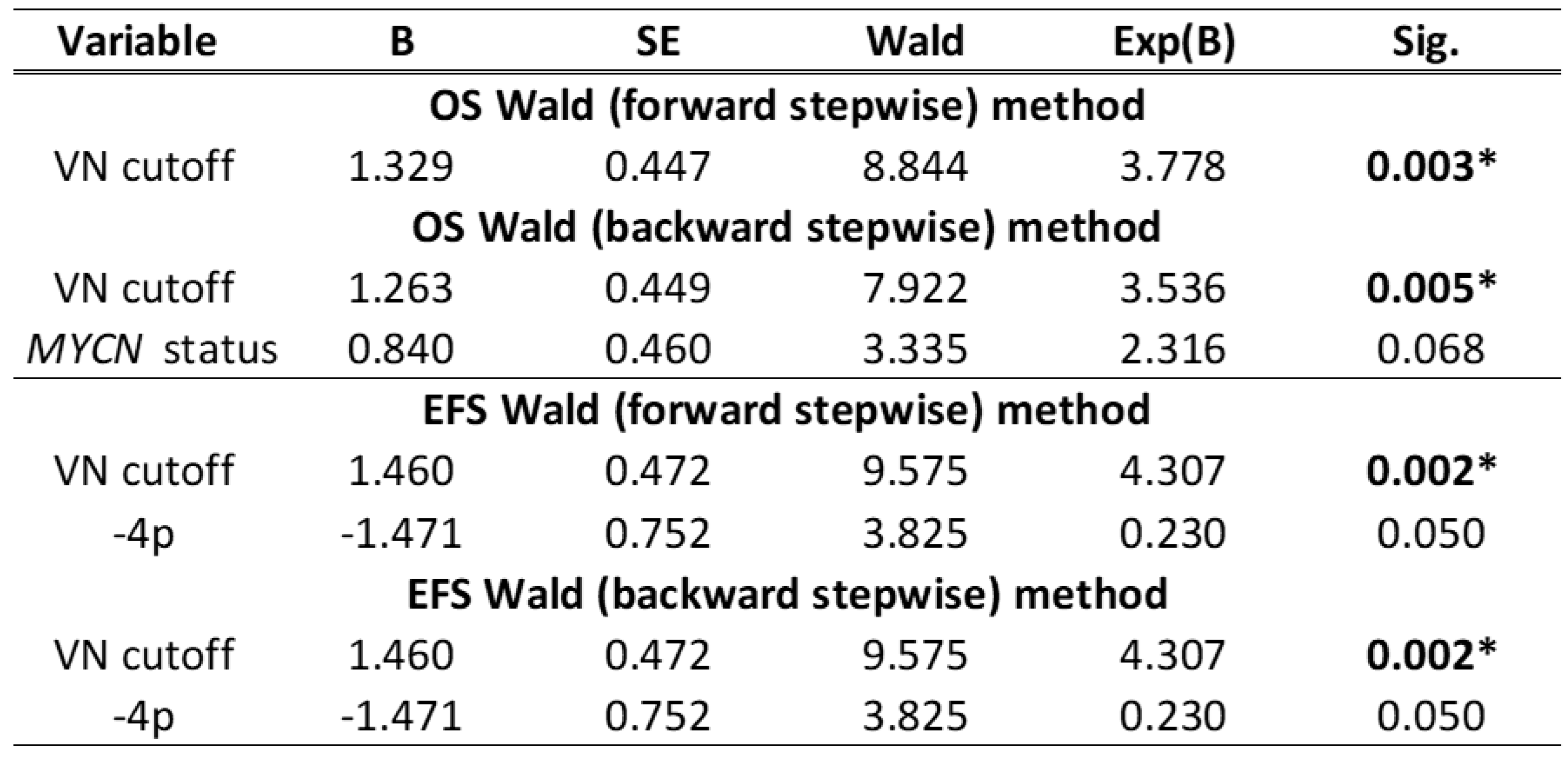 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





