1. Introduction
Amphotericin B (AmB) is a natural antifungal extracted from Streptomyces nodosus belonging to the macrolide family that interacts with the ergosterol of the fungal membrane. AmB is a broad-spectrum antifungal indicated for the treatment of severe invasive fungal infections (IFI) caused by
Cryptococcus neoformans,
Histoplasma spp,
Blastomyces spp,
Coccidiodes spp,
Mucor spp,
Aspergillus spp (except
A.terreus),
Candida spp (except
C.lusitaniae) [
1,
2,
3]. Despite its excellent spectrum of action, its use has been limited by the high incidence of nephrotoxicity when administered in the form of deoxycholate sodium salt [
4].
The use of liposomal AmB (L-AmB) represents a strategy to reduce renal damage and to increase the total effective dose administered. The administration of L-AmB maintains its mechanism of action and broad antifungal spectrum, and over the years, it has been shown to have a low risk of resistance with a better adverse effect profile [
5]. However, in critically ill patients, multiple confounding factors (i.e., sepsis, shock, vasoactive drugs and other nephrotoxic drugs) make it difficult to assess the impact of L-AmB administration on renal function. For this reason, observational studies with real-world data that include the greatest number of confounding factors can provide valuable clinical information on a subject that is still not well elucidated.
The aim of the current study was to evaluate renal function in adults (≥ 18 years of age) receiving L-AmB for more than 48 hours in the Intensive Care Unit (ICU) and to analyze the possible risk factors associated with renal impairment. Our hypothesis is that L-AmB administration does not significantly affect renal function and, therefore, we can consider its administration safe.
2. Results
2.1. Whole Population
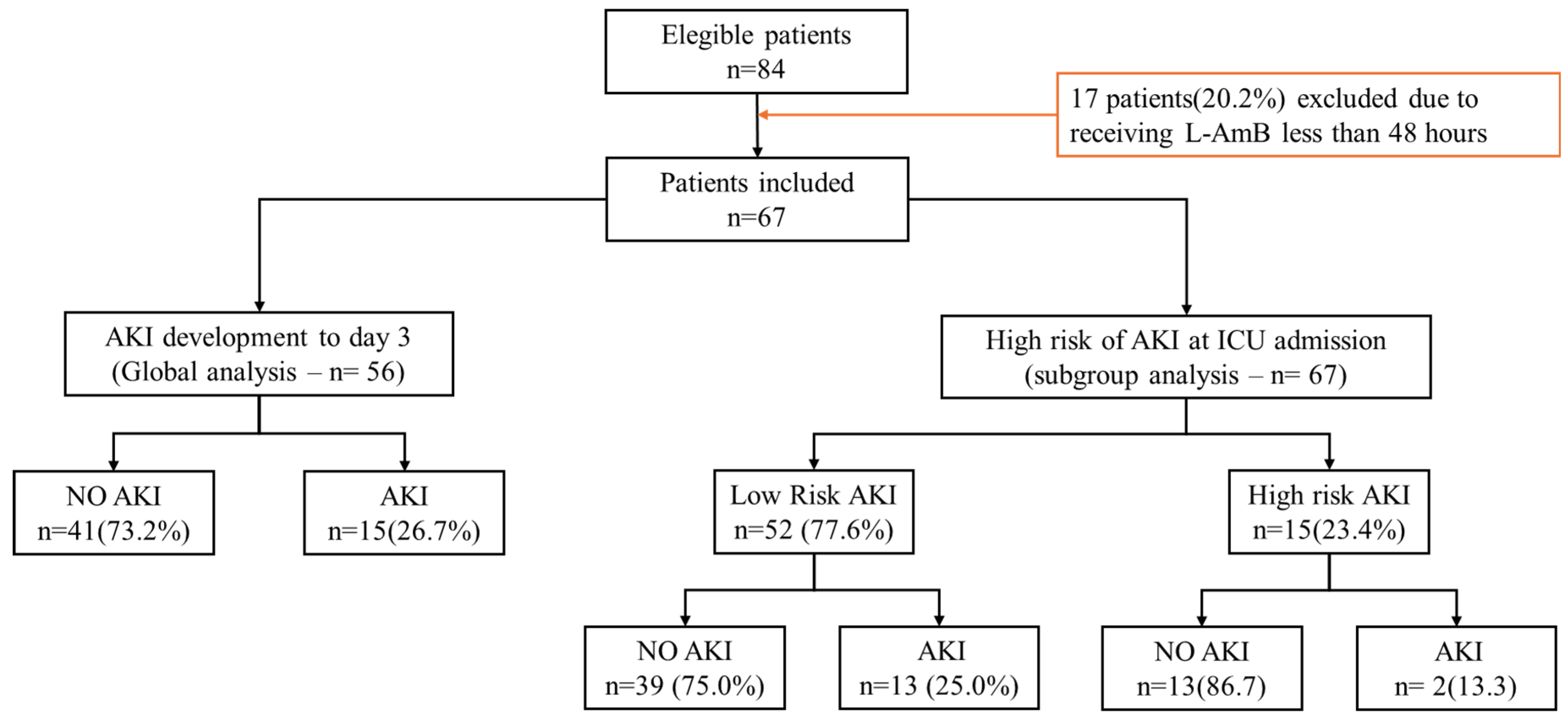
A total of 84 patients were eligible, 17 (20.2%) of whom received L-AmB less than 48 hours and were therefore excluded. Finally, 67 patients were included in the present analysis (
Figure 1). The median age was 61 years (53-71), 67% male with a median severity level according to the SOFA score of 4 points. The median ICU stay was 17 days, with a crude mortality of 34.3%. The median dose of L-AmB received was 3 mg/kg/day with a median total dose of 1.2 grams a day 7 (
Table 1). During the observation period, there were no significant increases in serum creatinine and urea levels or daily urine output (
Figures S1–S3, Supplementary Materials). Only a significant increase in serum urea values at day 3 and 4 from baseline was observed (
Figure S2, Supplementary Materials). It should be noted that serum urea values were above the normal reference value at baseline (
Table 1).
2.2. Development of Renal Dysfunction
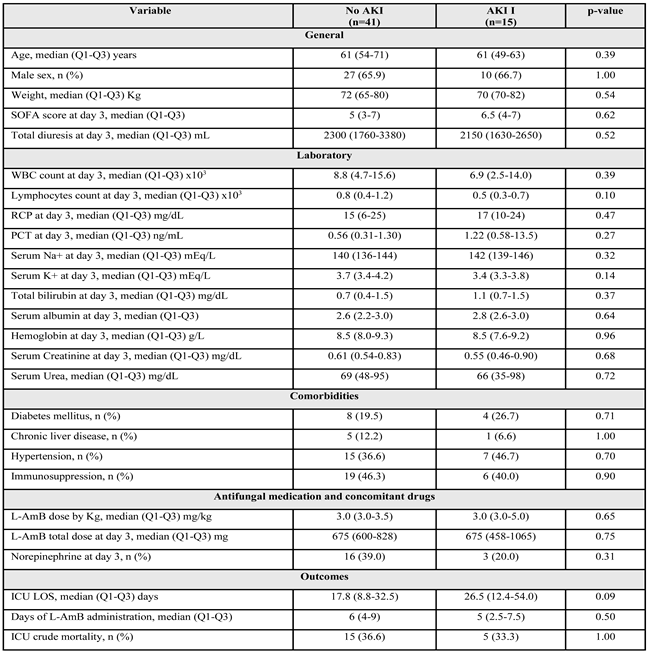
No patients met AKI III criteria. Fifty-six (83.6%) of the initial 67 patients were still admitted to the ICU on the third day. Of these patients, 15 (26.8%) met the AKI criteria with a median serum creatinine value of 0.55 (0.46-0.90) and a maximum value of 1.8 mg/dL. The characteristics of the patients on day 3 of ICU admission are shown in
Table 2. No significant differences were observed between the groups.
2.2. Development of Renal Dysfunction
No patients met AKI III criteria. Fifty-six (83.6%) of the initial 67 patients were still admitted to the ICU on the third day. Of these patients, 15 (26.8%) met the AKI criteria with a median serum creatinine value of 0.55 (0.46-0.90) and a maximum value of 1.8 mg/dL. The characteristics of the patients on day 3 of ICU admission are shown in
Table 2. No significant differences were observed between the groups.
2.3. Factors Associated with AKI Development
The association between AKI development and confounding variables was tested by developing multiple binary logistic regression models. The model was constructed with AKI development at day 3 as the dependent variable. Since no significant differences were observed between patients with and without AKI at day 3 (
Table 2), the independent variables were those considered clinically relevant and clinically plausible. The linear models were very unstable with no significance and very wide confidence intervals. The best model obtained (AIC 28.4) was the one that included as independent variables total L-AmB dose, SOFA score, age, sex, weight on admission, serum C-reactive protein and potassium level all at day 3 of treatment (
Figure S5, Supplementary Materials).
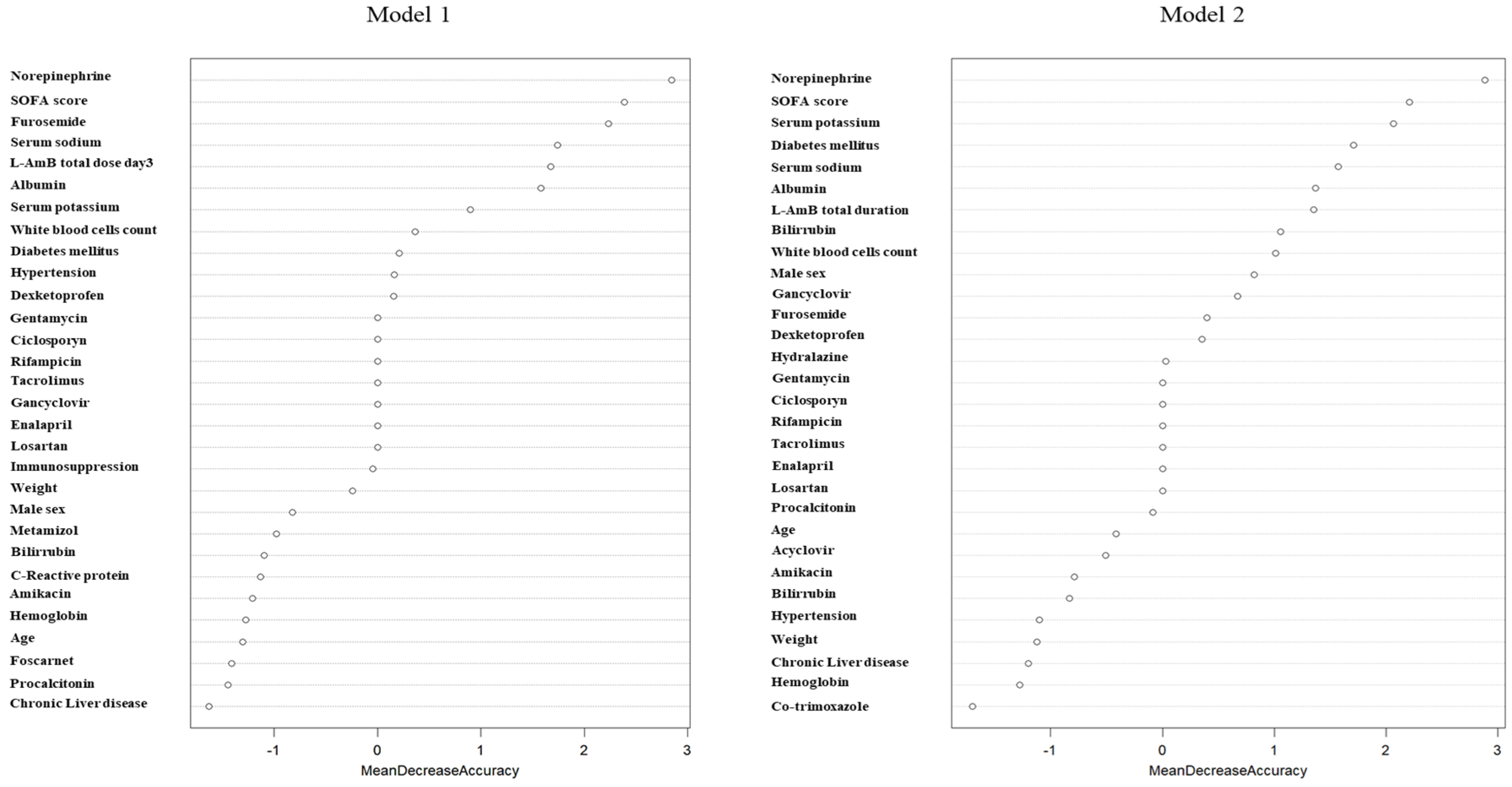
Due to the instability of linear models, a random forest model was developed to assess the impact of confounding variables on the development of AKI. Models developed with the inclusion of the variable total L-AmB dose at day 3 (Model 1; OOB= 31.3%) or total duration of L-AmB treatment (Model 2; OOB= 28.3%) showed that norepinephrine administration, severity measured by the SOFA score, furosemide, serum sodium and potassium concentrations were the variables with the highest contribution to the AKI development model (
Figure 2). Total L-AmB dose at day 3 (model 1) and duration of L-AmB treatment (model 2) were the fifth and seventh highest contributing variables, respectively.
2.4. Subgroups Analysis
Of the 67 patients included, 15 (21.7%) had a baseline (day 0) serum creatinine level above 1.0 mg/dL. This subgroup was considered to be at “high risk” of developing AKI. The general characteristics of patients are shown in
Table 1.
High risk of developing AKI subjects had a higher severity level as assessed by the SOFA score and as expected higher median serum creatinine and serum urea values, with no differences in urine output. In contrast, those patients had a lower body weight and fewer days of ICU stay and therefore received lower doses of L-AmB. However, although mortality was higher than in patients with a “lower risk” of AKI, it did not reach statistical significance.
2.5. AKI Development in Patients with “Lower Risk” of AKI at Baseline
No significant variations in serum creatinine levels and urine output were observed throughout the observation period (
Figures S6 and S7, Supplementary Materials). Although no significant variations in serum urea values were observed when considered overall, paired analysis showed significant increases on days 2, 3 and 4 compared to baseline (
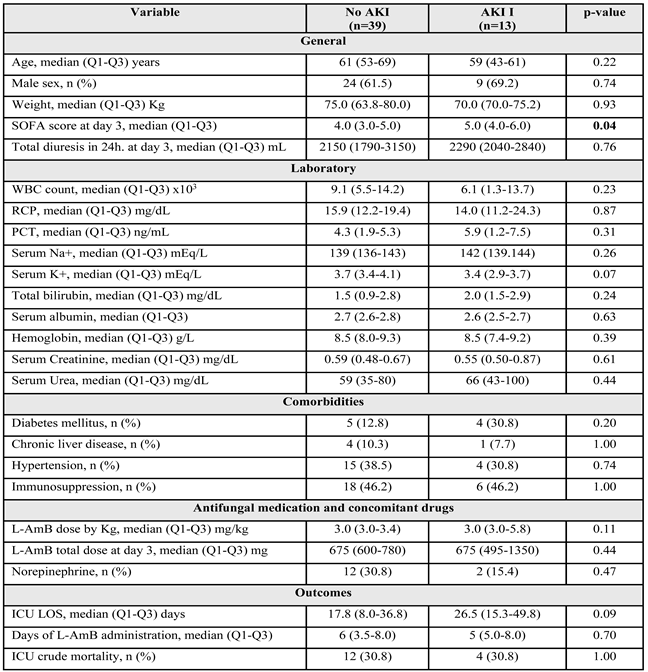
Figure S8). Thirteen (25.0%) of the 52 patients met the AKI criteria (
Table 3). No significant differences were observed between the groups, except in severity as measured by the SOFA score, which was slightly higher in the AKI group. The median serum creatinine values in the AKI and non-AKI groups were within the normal range. Only 1 patient met AKI II criteria who recorded a creatinine at day 3 of 1.22 mg/dL (within the value considered normal).
2.6. Factors Associated with AKI Development in Patients with Lower Risk of AKI
The random forest models performed using the variable total dose or total duration of L-AmB showed an OOB estimate of the error rate of 30% and 25% respectively. In model 1, serum potassium levels, procalcitonin (PCT), total L-AmB dose at day 3 and albumin were the variables with the highest contribution to the model. In model 2, serum potassium level, PCT, bilirubin and body weight were the major contributing variables. Interestingly, total L-AmB duration was not a variable recognized as being of interest in the model and was excluded (
Figure 3).
2.7. AKI Development in Patients with High Risk of AKI
3. Discussion
Our results using real-world data suggest that the administration of L-AmB in critically ill patients has a mild and clinically insignificant deleterious effect on renal function.
Different AKI criteria have been defined to identify hospitalized groups of patients at increased risk of mortality and/or need for renal replacement therapy. The RIFLE and AKIN criteria were specifically developed for average-sized adults. In 2012, the global organization KDIGO attempted to harmonize the AKI staging criteria due to the heterogeneity of definitions formerly used in medical literature [7,8].
Considering the aforementioned issues, we relied on the AKIN scale, which has been previously employed, particularly in research concerning renal recovery following the administration of L-AmB [9], thereby facilitating cross-comparison between studies.
Several factors have been identified in the development of AKI. Takazono et al. [10] identified five AKI-associated factors in their Japanese study: prior treatment with ACE/ARBs inhibitors or carbapenems, concomitant administration of catecholamines (as a state of shock) or immunosuppressants and ≥ 3.52 mg/kg/day of L-AmB dosing. In addition, they found that hypokalemia (serum potassium < 3.5 mEq/L) before starting L-AmB therapy was associated with severe AKI (stage II-III). Our results are consistent with the harmful effect of concurrent treatment with catecholamines (specifically norepinephrine in our scenario) and the dosage of L-AmB in the development of AKI, although we did not establish a specific mg/kg/day cutoff. We have not studied the impact of hypokalemia on AKI severity; however, our random forest model indicates that serum potassium plays an important role in AKI development. Despite this, other factors such as previous exposure to ACE/ARB inhibitors or concomitant immunosuppressants did not significantly impact our AKI-development model. In contrast, data from Personett et al. [9] suggest that the L-AmB dose did not influence the probability of renal recovery, as renal worsening was not predicted by the daily dose of L-AmB at the time of AKI. They found no relationship between various factors (male sex, high weight, concomitant use of cyclosporine, vancomycin and ACE inhibitors) and the likelihood of recovering from a nephrotoxic event secondary to L-AmB exposure. In our setting, we did not assess the renal function recovery, but among our cohort, none of these factors influenced the development of AKI. A Spanish multicenter study [11] concluded that L-AmB treatment in ICU patients with impaired renal function had minimal impact on kidney function, as evidenced by serum creatinine values. These findings reaffirm L-AmB as a viable treatment option for IFI in critically ill patients, irrespective of their renal function at the initiation of therapy. Consistent with these data, our results demonstrate a similar trend as hypothesized by Álvarez-Lerma.
Due to the observed inconsistency of the linear regression model, we developed a non-linear tree-based technique using supervised machine learning tool (random forest) to assess the true impact of several covariates on AKI development. Although the RF model evidenced a significant association with the development of AKI as measured by creatinine increase, this rise had no clinical implications as most patients-maintained serum creatinine values within normal limits, requiring no therapeutic intervention. Additionally, in high-risk patients for developing AKI (identified on day 0), administration of L-AmB did not lead to elevated creatinine levels. Our random forest model for Low-risk AKI patients, demonstrated that the L-AmB dose contributes to the development of AKI, although other covariates (serum potassium and procalcitonin) showed greater impact, highlighting the multifactorial nature of AKI development in critically ill subjects. Interestingly, among patients categorized as high risk of developing AKI, serum creatinine values decreased significantly during the period of L-AmB administration. This observation probably reflects the impact of therapy on the resolution of sepsis and secondary organ dysfunction and should not be attributed to a direct protective action of L-AmB administration.
Multiple studies have assessed the nephrotoxic risks associated with L-AmB treatment. For example, in a multicenter study conducted in European hospitals, Ullmann AJ et al. [12] found a 28.6% global nephrotoxicity in patients treated with L-AmB. Along the same lines, the development of nephrotoxicity in immunocompromised patients with normal baseline kidney function reached 30.7%. Moreover, in patients with hematologic malignancies and probable invasive aspergillosis, Hachem et al. [12] demonstrated lower incidences of nephrotoxicity-related adverse events with L-AmB, both in primary therapy (2.8%) and salvage therapy (5.9%). Another trial performed by Wingard et al. [13] reported an incidence of nephrotoxicity in the L-AmB subgroup (dosage range: 3-5 mg/kg/day) ranging from 5.9% to 29.4%, depending on the magnitude by which the creatinine value exceeded the baseline (ranging from x1.5 to x3). Similarly, we observed nephrotoxicity rates ranging from 2.9% to 26.8%. Compared to our study, their population was notably younger than ours, as they recruited patients aged ≥ 2 years with suspected fungal infections and severe neutropenia. Their findings likely influenced the reported percentage of nephrotoxicity in their publication. In a phase I-II study on the safety and tolerance of L-AmB, Walsh et al. [
4] concluded that 50% of the patients exhibited laboratory features of nephrotoxicity, defined by a serum creatinine value ≥ 1.5 times greater than the baseline; 32% had a value ≥ 2 times greater than the baseline. Conversely, we observed lower nephrotoxicity rates according to AKIN criteria: 20.9% for AKI I patients and 1.9% for AKI II patients. In a double-blind trial conducted in severely immunocompromised patients, Cornely et al. [14] found no additional efficacy benefit with the 10 mg/kg/day dosing regimen compared to the standard 3 mg/kg/day regimen, despite observing increased rates of nephrotoxicity in the higher dosage group. Our findings are consistent with theirs, although our study included fewer patients receiving the high-loading dose regimen.
There are several limitations in this study. First, the reduced sample size and the single-center setting may lead to non-representative results. Although our study involved a small population, by limiting the study to a single center we aimed to reduce variability and ensure a more homogeneous sample. This approach provides clearer insights into the effects being studied. In addition, it should be taken into consideration that there are few L-AmB published studies addressing the occurrence of secondary nephrotoxicity.
Second, we only assessed the change in creatinine levels during the first week of treatment. Therefore, the impact of L-AmB on renal function beyond 7 days of observation cannot be ruled out. However, the median number of treatment days was 6 so the impact of prolonged treatment beyond 7 days should be limited.
Third, we do not calculate the glomerular filtration rate. This is not our usual practice when patients have serum creatinine levels within the normal range.
Fourth, the newer biomarkers of kidney damage were not used due to the retrospective nature of the study.
Fifth, we have not been able to obtain the baseline creatinine of the disease-free patient. However, the aim of the study was to evaluate the impact of L-AmB on creatinine after 72 hours of administration independently of the pre-administration creatinine value.
Finally, these results correspond to the population analyzed in our center and cannot be transferred to other populations or other centers without prior validation.
As a strength and to the best of our knowledge, this is the first study to incorporate supervised machine learning tools to relate the factors involved in L-AmB nephrotoxicity. However, further research is needed to shed light on the causes of L-AmB associated with AKI.
4. Materials and Methods
4.1. Study Design and Population
We carried out a retrospective, observational, single-center study over 6 years (2018 - 2024), which included critically ill patients (≥ 18 years of age) consecutively admitted to a 30-bed ICU. In the present analysis, we included all critically ill subjects who required administration of L-AmB for more than 48 consecutive hours due to a proven or probable fungal infection. We excluded patients with the following conditions:
4.2. Subgroup Analysis
Patients who had a serum creatinine value < 1.0 mg/dL obtained on the day before (day 0) the start of L-AmB infusion (day 1) were considered to have normal renal function (Normal reference laboratory value < 1.5 mg/dL). Patients who had a serum creatinine value above > 1.0 mg/dL on the day 0 were considered as high risk of developing AKI and they were analyzed as a particular subgroup with increased susceptibility to develop severe kidney dysfunction.
4.3. Variables
The variables studied are shown in
Table 1. These variables were obtained automatically from the clinical information system (CIS, Centricity Critical Care
®, GE) through the development of ETL (extract, transform and load) specifically created using SQL and Python.
The CIS automatically records all data from devices connected to the patient every two minutes, including hemodynamic variables, hourly diuresis, clinical parameters and laboratory values, as well as information on medication administered. In addition, healthcare professionals register all patient-related information throughout the ICU stay.
4.4. Definitions
Baseline Serum Creatinine Value
Because patients received L-AmB at different times during their ICU stay, the baseline creatinine value was considered to be that obtained on the day before (day 0) the start of L-AmB infusion (day 1).
High Risk of Developing-AKI Subgroup Patients
Patients with serum creatinine levels above 1.0 mg/dL on the day prior to the start of L-AmB infusion (day 0) were considered as a subgroup of patients at “high risk” of developing AKI with the start of L-AmB treatment (day 1). Conversely, those with serum creatinine levels below 1.0 mg/dL on day 0 were considered as a subgroup of patients without AKI and at lower risk of developing AKI. Both subgroups were analyzed separately.
Post-Treatment Renal Dysfunction Criteria
The diagnosis of renal dysfunction associated with L-AmB administration was made following the Acute Kidney Injury Network (AKIN) criteria for the diagnosis of acute renal failure described in the international KDIGO guidelines [
7]. For the diagnosis of AKI, the worst serum creatinine value 72 hours after L-AmB infusion was considered (day 3). This time window was selected because it is considered the minimum time necessary for the development of renal dysfunction.
AKIN grade I: defined as an absolute increase in serum creatinine value ≥ 0.3 mg/dL or an increase between 50% and 100% of the baseline value at 72h.
AKIN grade II: defined as an increase in serum creatinine > 100% (and up to 200%) over baseline at 72h.
AKIN grade III: defined as an increase in serum creatinine > 200% over baseline at 72h, or as an absolute increase in serum creatinine value ≥ 4.0 mg/dL (≥ 354 µmol/L) with an acute increase of at least 0.5 mg/dL (44 µmol/L) or in CRRT.
4.5. Ethical Considerations
Informed consent was not requested due to the retrospective (6 years), observational nature of the study and the collection of automatic anonymized data from the CIS. The study was conducted in accordance with the Declaration of Helsinki and the European Community Directive on Clinical Trials (2001/20/EC) of the European Parliament on Good Clinical Practice guidelines.
4.6. Statistical Analysis
Our analysis plan is based on the following five steps:
Step 1: The behavior of serum creatinine and urea levels as well as urinary volume during the study period (day 1 to day 7) was analyzed. Categorical variables are presented as number and percentage (%), and continuous variables as median and interquartile range (Q1-Q3). In order to analyze differences between groups, the Mann-Whitney U-test (continuous) and Chi-square (dichotomous) were used. Temporal differences between means were determined by Analysis Of Variance (ANOVA) and paired ANOVA with Bonferroni correction.
Step 2: The linear association between variables of interest (creatinine, urea and total L-AmB dose administered was determined by obtaining Spearman (Rho) correlation coefficient due to the non-parametric distribution of the data.
Step 3: The incidence of AKI on day 3 of L-AmB administration was determined in the general population according to the definition considered. A bivariate comparison was made between the groups with and without AKI on day 3 of observation.
Step 4: The impact of the different variables on the development of AKI was established by multivariate analysis (multiple logistic regression). The result is shown as Odds Ratio (OR) with a 95% confidence interval (95% CI). Values below 0.05 were considered significant. The assessment of model fit was performed using the Akaike’s information criterion (AIC).
Due to the instability of linear regression models a non-linear Random Forest (RF) model was developed to study the impact of covariates on the development of AKI. The Random Forest algorithm is a powerful non-linear tree-based learning technique in machine learning. It works by creating a series of decision trees during the training phase. Each tree is constructed using a random subset of the dataset to measure a different subset of features in each partition. This randomness introduces variability between individual trees, which reduces the risk of overfitting and improves overall prediction performance. In the final prediction, the algorithm aggregates the results of all trees, either by voting (for classification tasks) or by averaging (for regression tasks). This collaborative decision-making process, supported by multiple trees with their insights, provides an example of stable and accurate results.
The performance of the RF model was evaluated by the out-of-bag (OOB) error. This method allows measuring the prediction error of random forests, boosted decision trees and other machine learning models using bootstrap aggregation. Essentially, the OOB error measures how well the model predicts the OOB samples using only the trees that were not trained on those samples.
Step 5: The analysis was performed similarly in the subgroup of patients with “low risk of AKI” on day 0, and in the subgroup of patients with “high risk of AKI”, consisting of patients with a baseline (day 0) serum creatinine between 1.1 and 1.5 mg/dL. Although these values are within the normal range for the technique for our laboratory, levels above 1 mg/dL may express some degree of renal dysfunction, as the out-of-hospital basal creatinine is unknown.
All statistical analysis was performed using free software R (version 4.3.0).
5. Conclusions
Our results with real-world data suggest that L-AmB treatment in critically ill adults is safe and did not result in a significant increase in serum creatinine levels during the first week of treatment.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org. Figure S1–S11.
Author Contributions
Conceptualization, Ignasi Sacanella, Erika Esteve-Pitarch, Jessica Guevara-Chaux, Josep Gómez, Cecilia Casarino, Florencia Alés, Laura Canadell, Ignacio Martin-Loeches, Santiago Grau, Francisco Javier Candel, María Bodí and Alejandro Rodríguez; Formal analysis, Jessica Guevara-Chaux, Julen Berrueta, Alejandro García-Martínez, Josep Gómez and Alejandro Rodríguez; Investigation, Ignasi Sacanella, Erika Esteve-Pitarch, Jessica Guevara-Chaux, Alejandro García-Martínez, Cecilia Casarino, Florencia Alés, Francisco Javier Candel, María Bodí and Alejandro Rodríguez; Methodology, Julen Berrueta, Alejandro García-Martínez, Josep Gómez, Laura Canadell, Ignacio Martin-Loeches, Santiago Grau, Francisco Javier Candel, María Bodí and Alejandro Rodríguez; Resources, Erika Esteve-Pitarch and Julen Berrueta; Software, Julen Berrueta, Josep Gómez and Alejandro Rodríguez; Supervision, Erika Esteve-Pitarch; Validation, Alejandro García-Martínez, Laura Canadell, Ignacio Martin-Loeches and Alejandro Rodríguez; Writing—original draft, Ignasi Sacanella, Erika Esteve-Pitarch and Alejandro Rodríguez; Writing—review & editing, Ignasi Sacanella, Erika Esteve-Pitarch, Jessica Guevara-Chaux, Cecilia Casarino, Florencia Alés, Laura Canadell, Ignacio Martin-Loeches, Santiago Grau, Francisco Javier Candel, María Bodí and Alejandro Rodríguez. All authors will be informed about each step of manuscript processing including submission, revision, revision reminder, etc. via emails from our system or assigned Assistant Editor.
Funding
This research received no external funding.
Informed Consent Statement
Patient consent was waived due to the retrospective (6 years), observational nature of the study and the collection of automatic anonymized data from the CIS. The study was conducted in accordance with the Declaration of Helsinki and the European Community Directive on Clinical Trials (2001/20/EC) of the European Parliament on Good Clinical Practice guidelines.
Data Availability Statement
The data supporting the conclusions of this study are available from the Hospital Joan XXIII de Tarragona (Spain), but restrictions are placed on the free availability of these data by the health authorities of Catalonia, so they are not publicly available. However, the data can be obtained from the corresponding author (AR) upon reasonable request and with the permission of the Technical Secretary and the person responsible for data management at Hospital Joan XXIII de Tarragona (Spain).
Acknowledgments
We express our gratitude to the dedicated staff members of the Pharmacy, Intensive Care, Infectious Disease, Microbiology, and Nursing Departments at Hospital Universitari Joan XXIII. This project would not have been possible without their collaborative efforts.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Martinez, R. An Update on the Use of Antifungal Agents. J Bras Pneumol 2006, 32, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Klepser, M. The Value of Amphotericin B in the Treatment of Invasive Fungal Infections. J Crit Care 2011, 26, 225.e1–225e10. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Toledo, E.; Jiménez-Delgadillo, A.U.; Manzano-Gayosso, P. Antifúngicos Poliénicos. Mecanismo de Acción y Aplicaciones. Revista de la Facultad de Medicina 2020, 63, 7–17. [Google Scholar] [CrossRef]
- TJ Walsh; RW Finberg; C Arndt; J Hiemenz; C Schwartz; D Bodensteiner; P Pappas; N Seibel; RN Greenberg; S Dummer; et al. Liposomal Amphotericin B for Empirical Therapy in Patients with Persistent Fever and Neutropenia. N Engl J Med 1999, 764–771.
- Deray, G. Amphotericin B Nephrotoxicity. J Antimicrob Chemother 2002, 49, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.J. Acute Kidney Injury. Nat Rev Dis Primers 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- KDIGO International Section 2: AKI Definition. Kidney Int Suppl (2011) 2012, 2, 19–36. [CrossRef] [PubMed]
- Rocha, P.N.; Kobayashi, C.D.; De Carvalho Almeida, L.; De Oliveira Dos Reis, C.; Santos, B.M.; Glesby, M.J. Incidence, Predictors, and Impact on Hospital Mortality of Amphotericin B Nephrotoxicity Defined Using Newer Acute Kidney Injury Diagnostic Criteria. Antimicrob Agents Chemother 2015, 59, 4759–4769. [Google Scholar] [CrossRef] [PubMed]
- Personett, H.A.; Kayhart, B.M.; Barreto, E.F.; Tosh, P.; Dierkhising, R.; Mara, K.; Leung, N. Renal Recovery Following Liposomal Amphotericin B-Induced Nephrotoxicity. Int J Nephrol 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Takazono, T.; Tashiro, M.; Ota, Y.; Obata, Y.; Wakamura, T.; Miyazaki, T.; Nishino, T.; Izumikawa, K. Factor Analysis of Acute Kidney Injury in Patients Administered Liposomal Amphotericin B in a Real-World Clinical Setting in Japan. Sci Rep 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Lerma, F.; Cruz Soriano, M.; Rodríguez, M.; Catalán, M.; María Llorente, A.; Vidart, N.; Garitacelaya, M.; Maraví, E.; Fernández, E.; Alvarado, F.; et al. Impact of Liposomal Amphotericin B on Renal Function in Critically Ill Patients with Renal Function Impairment. Revista española de Quimioterapia 2012, 25, 206–215. [Google Scholar] [PubMed]
- Ullmann, A.J.; Sanz, M.A.; Tramarin, A.; Barnes, R.A.; Wu, W.; Gerlach, B.A.; Krobot, K.J.; Gerth, W.C. Prospective Study of Amphotericin B Formulations in Immunocompromised Patients in 4 European Countries. Clinical infectious diseases 2006, 43, e29–e38. [Google Scholar] [CrossRef] [PubMed]
- Wingard, J.R.; White, M.H.; Anaissie, E.; Raffalli, J.; Goodman, J.; Arrieta, A. A Randomized, Double-Blind Comparative Trial Evaluating the Safety of Liposomal Amphotericin B versus Amphotericin B Lipid Complex in the Empirical Treatment of Febrile Neutropenia. Clinical Infectious Disease 2000, 31, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Maertens, J.; Bresnik, M.; Ebrahimi, R.; Ullmann, A.J.; Bouza, E.; Heussel, C.P.; Lorttiolary, O.; Rieger, C.; Boehrne, A.; et al. Liposomal Amphotericin B as Initial Therapy for Invasive Mold Infection: A Randomized Trial Comparing a High-Loading Dose Regimen with Standard Dosing (AmBiLoad Trial). Clinical Infectious Diseases 2007, 44, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).