Submitted:
11 July 2024
Posted:
12 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Data Analysis
2.3. Calculating Antibiotic Resistance Index
2.4. Statistical Analysis
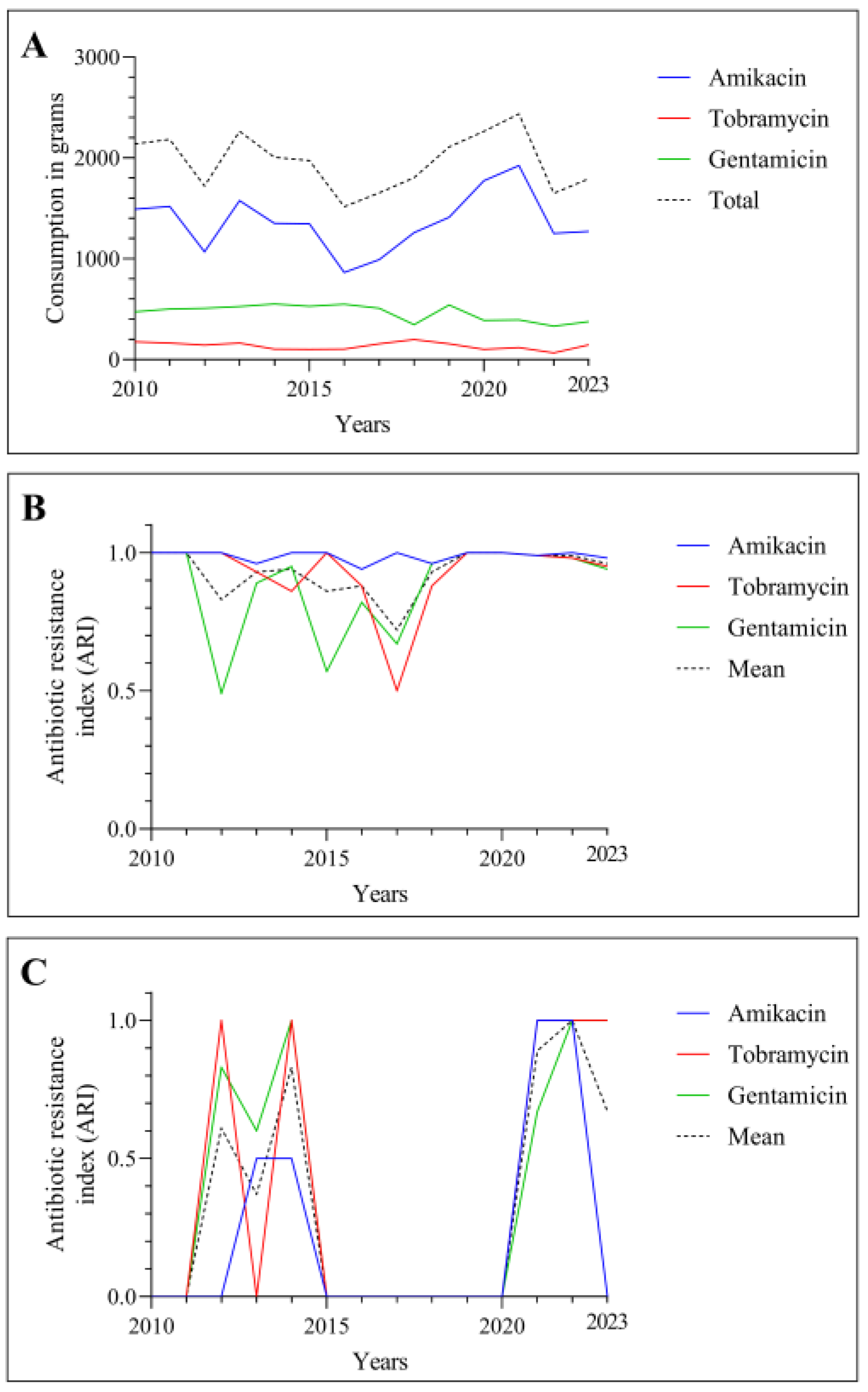
3. Results
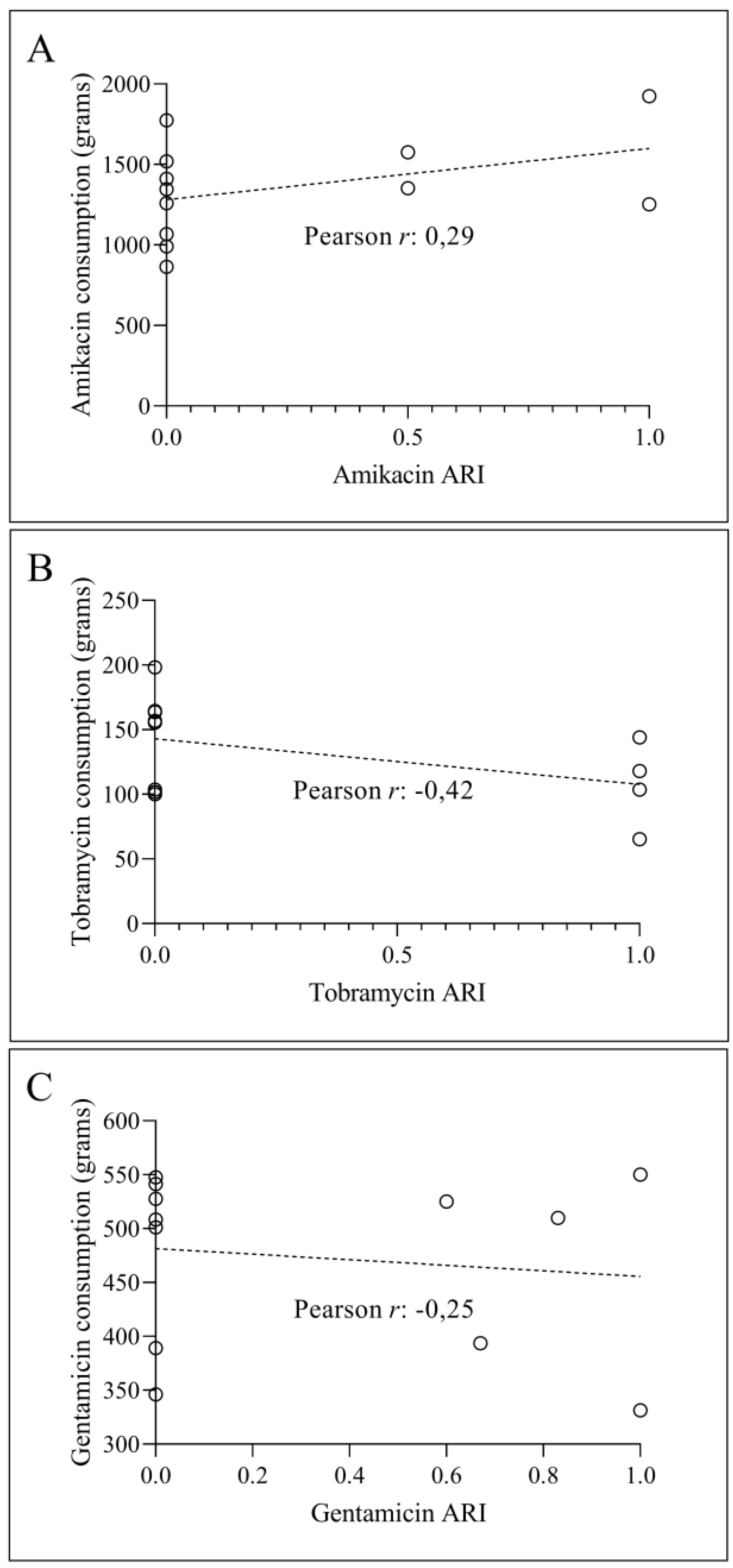
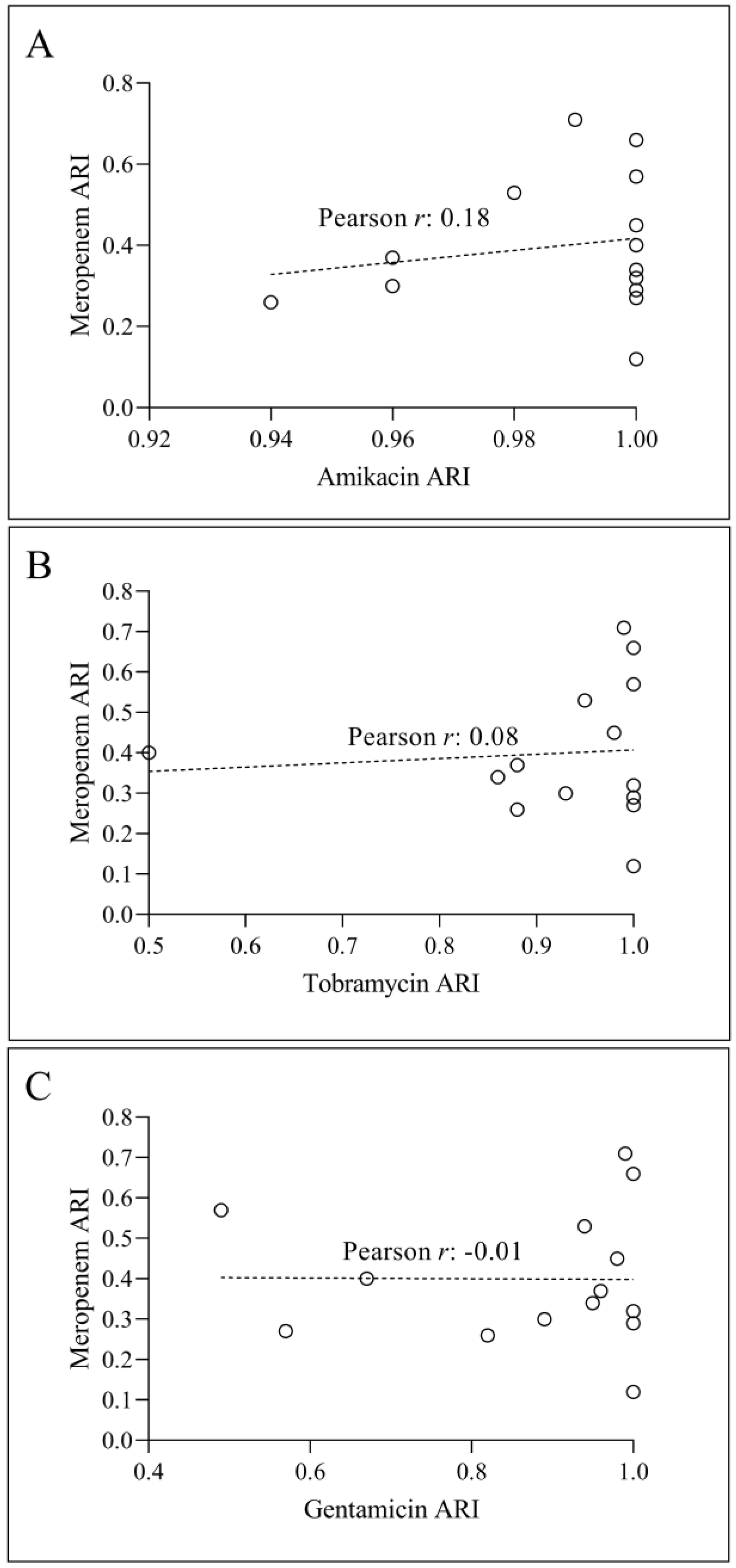
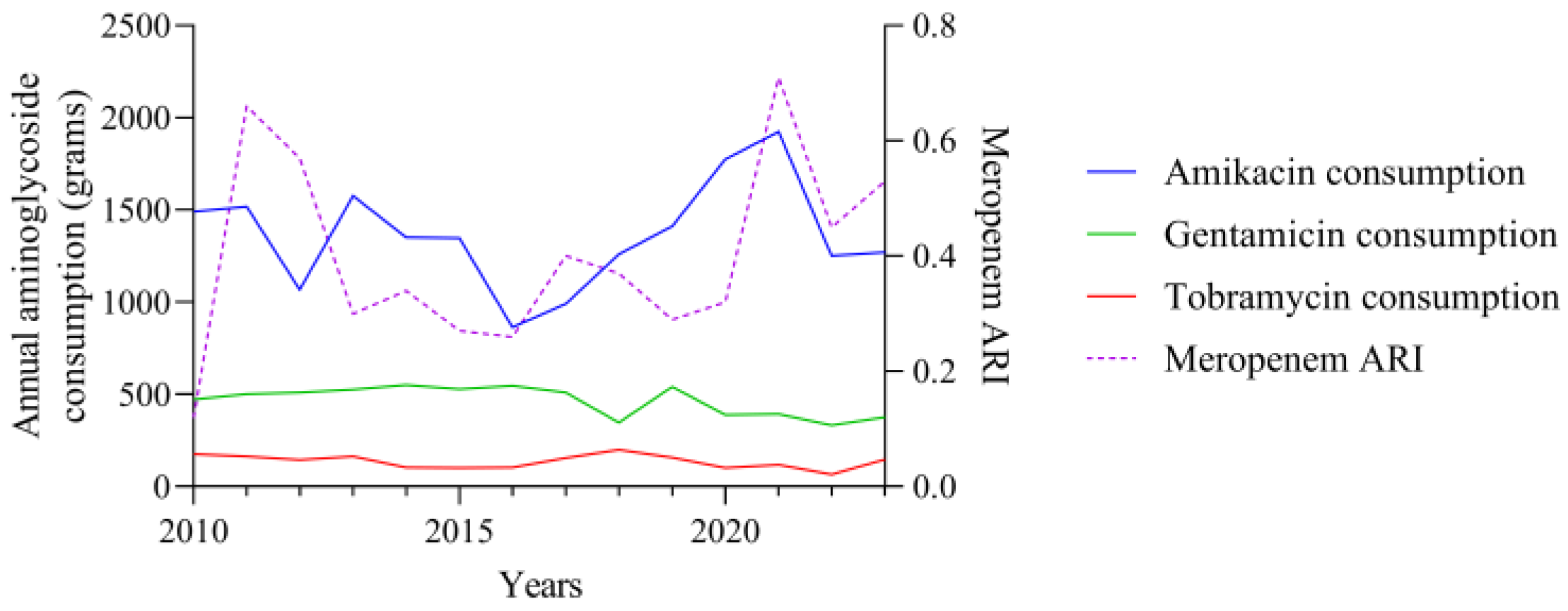
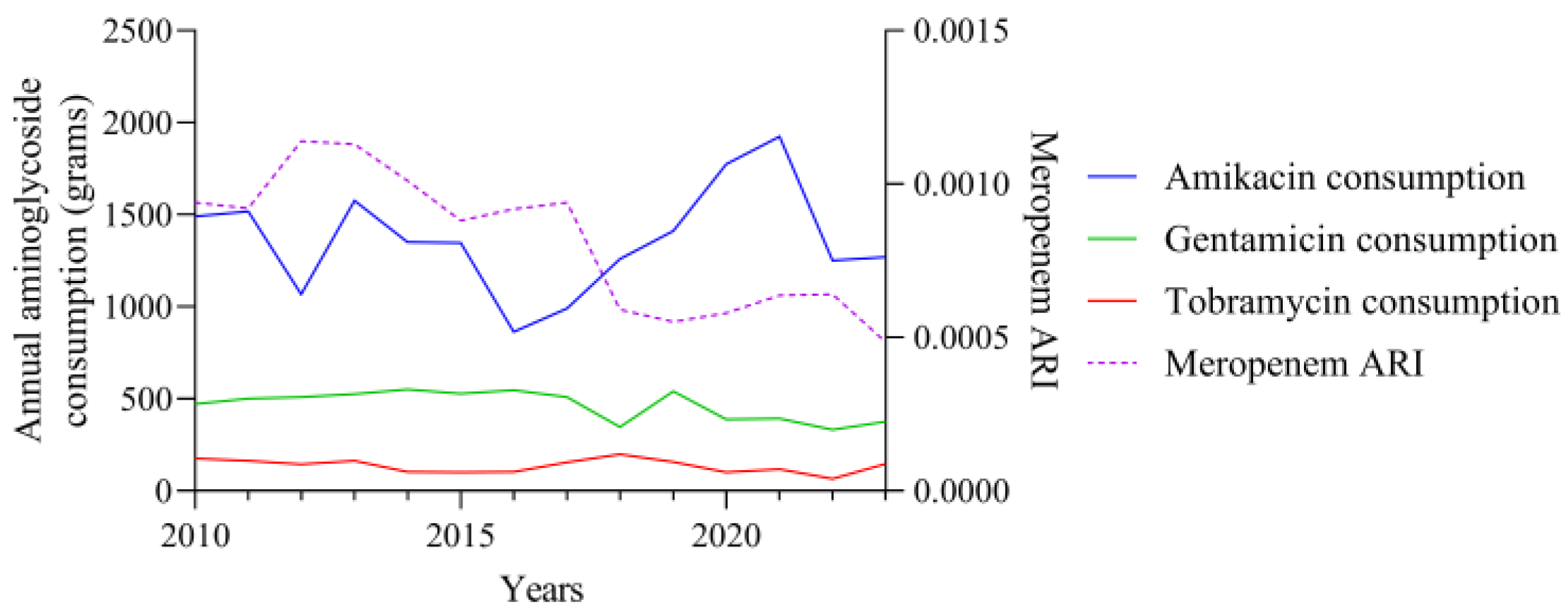
3.1. Consumption and ARI Statistics for the Three Aminoglycosides Tested
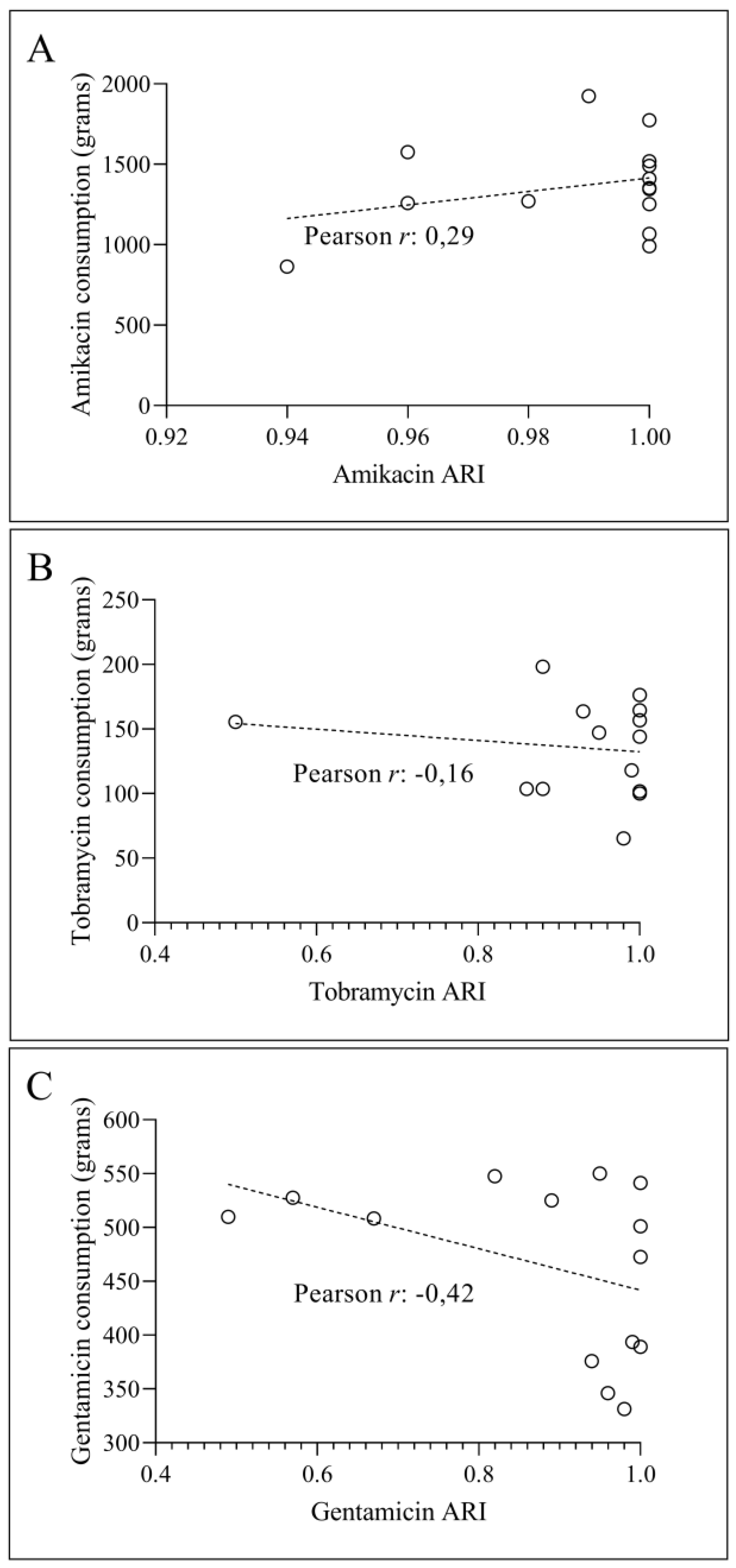
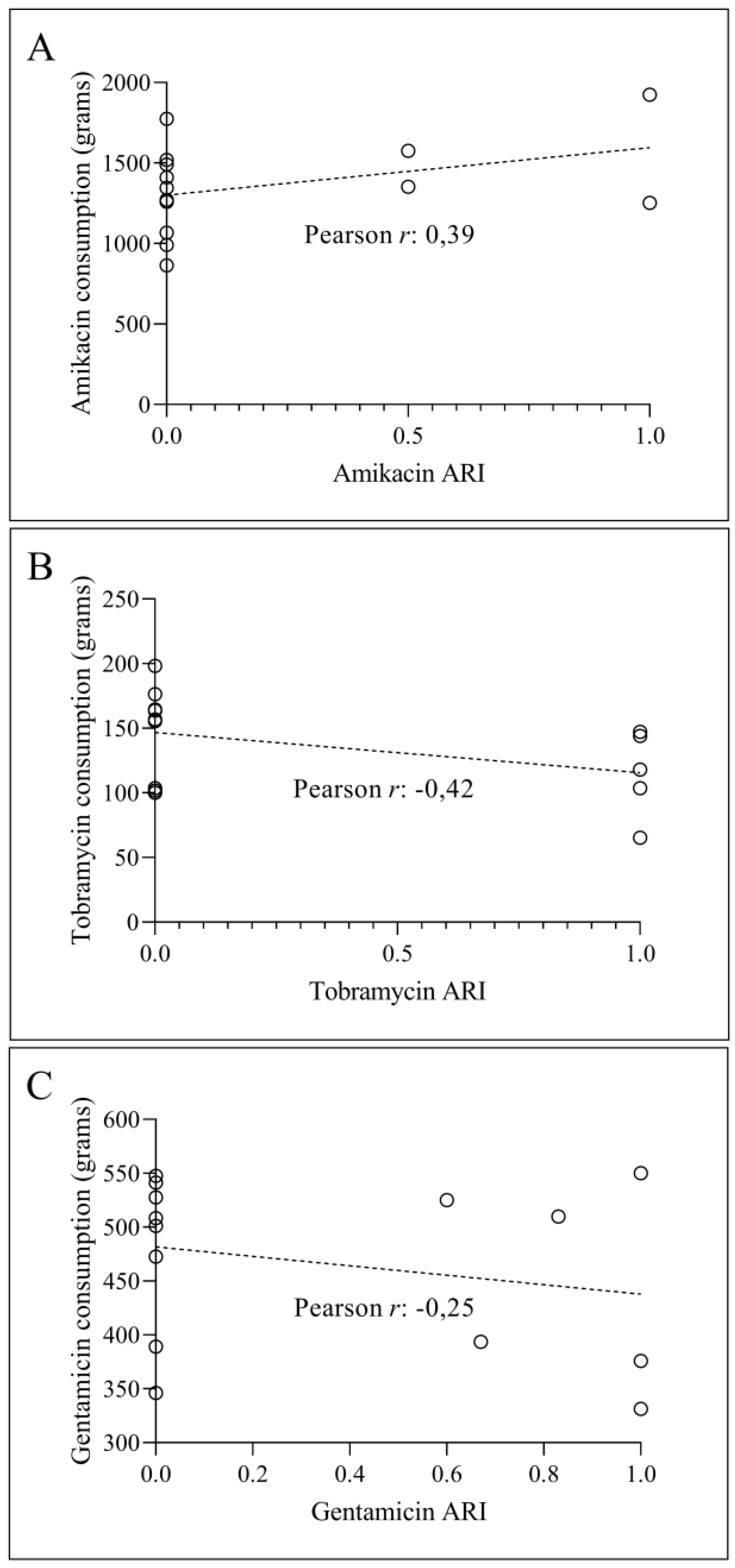
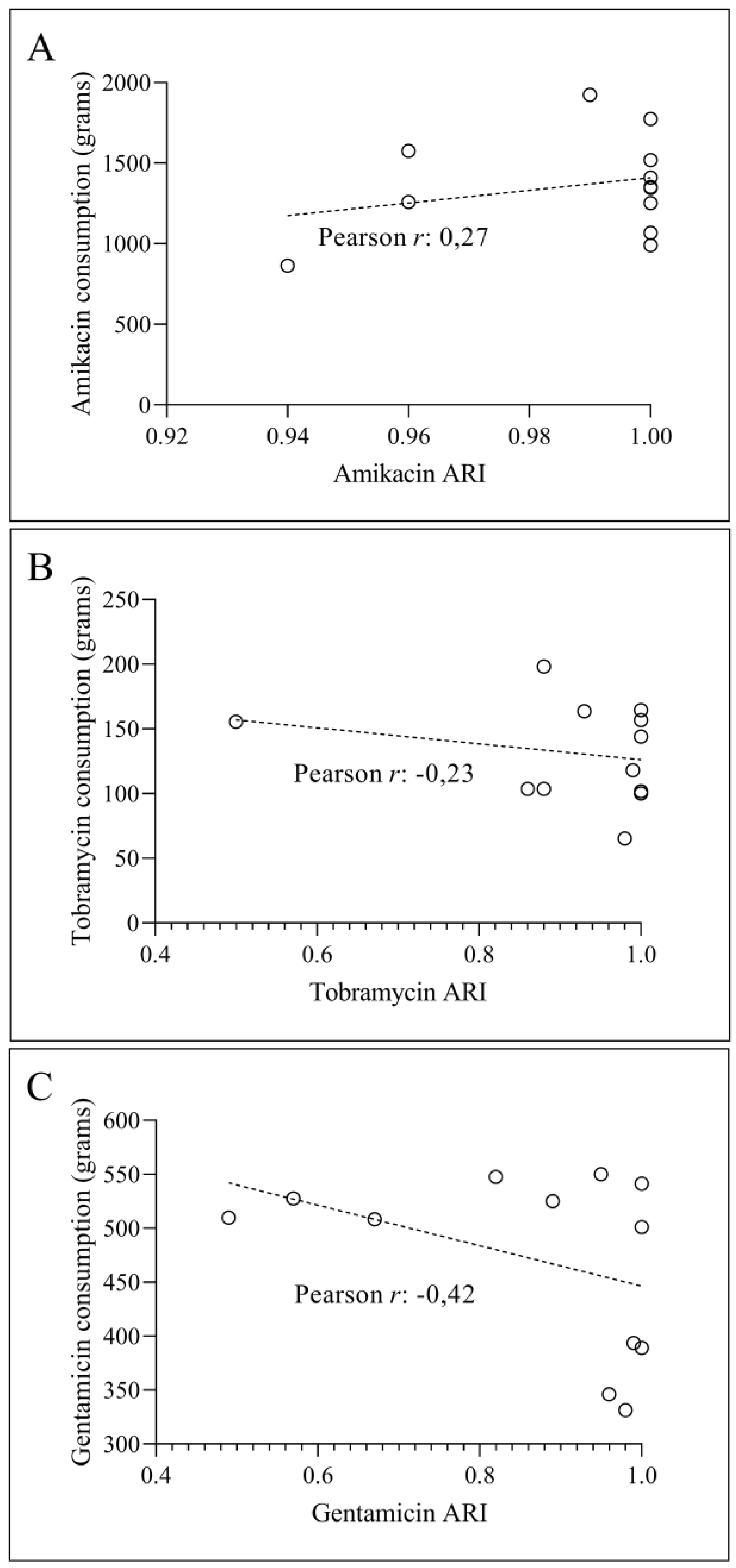
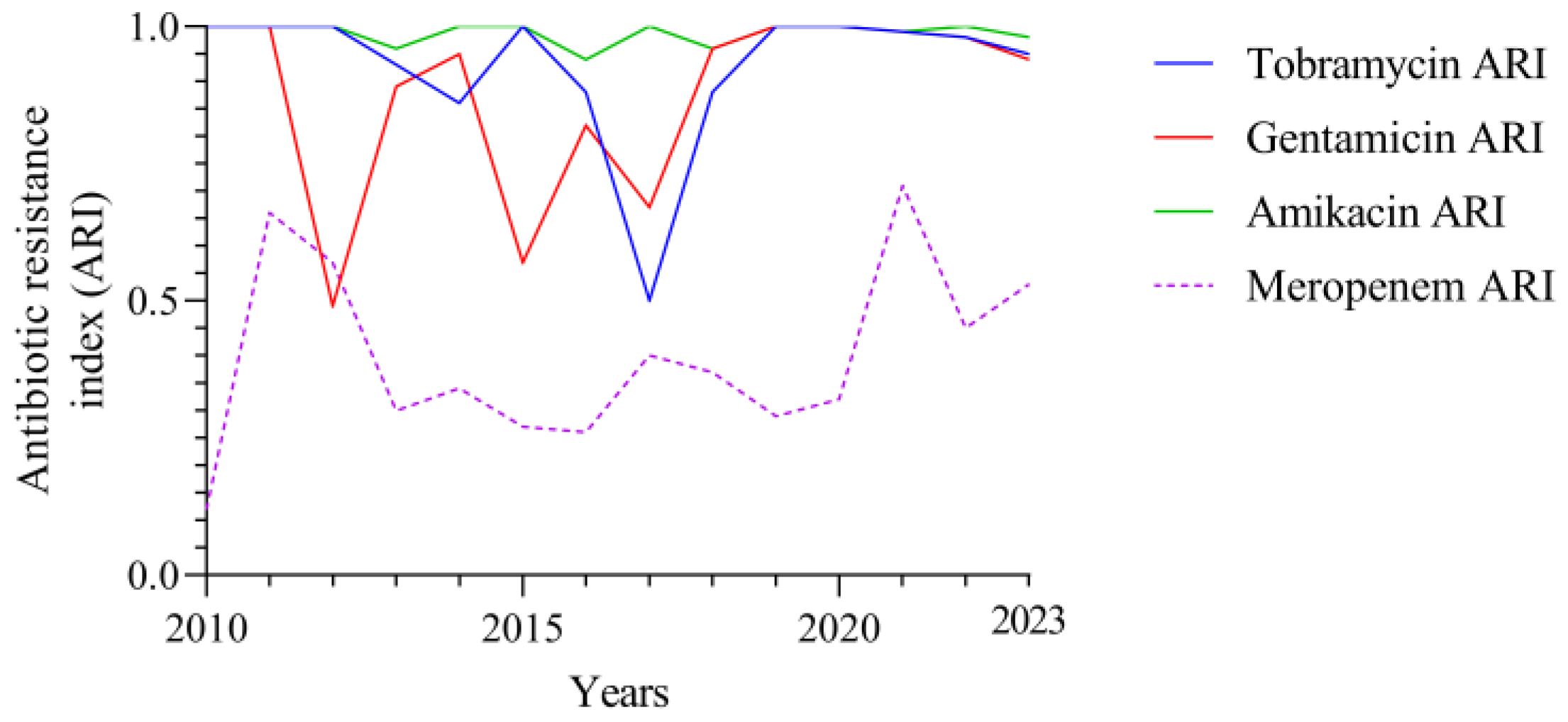
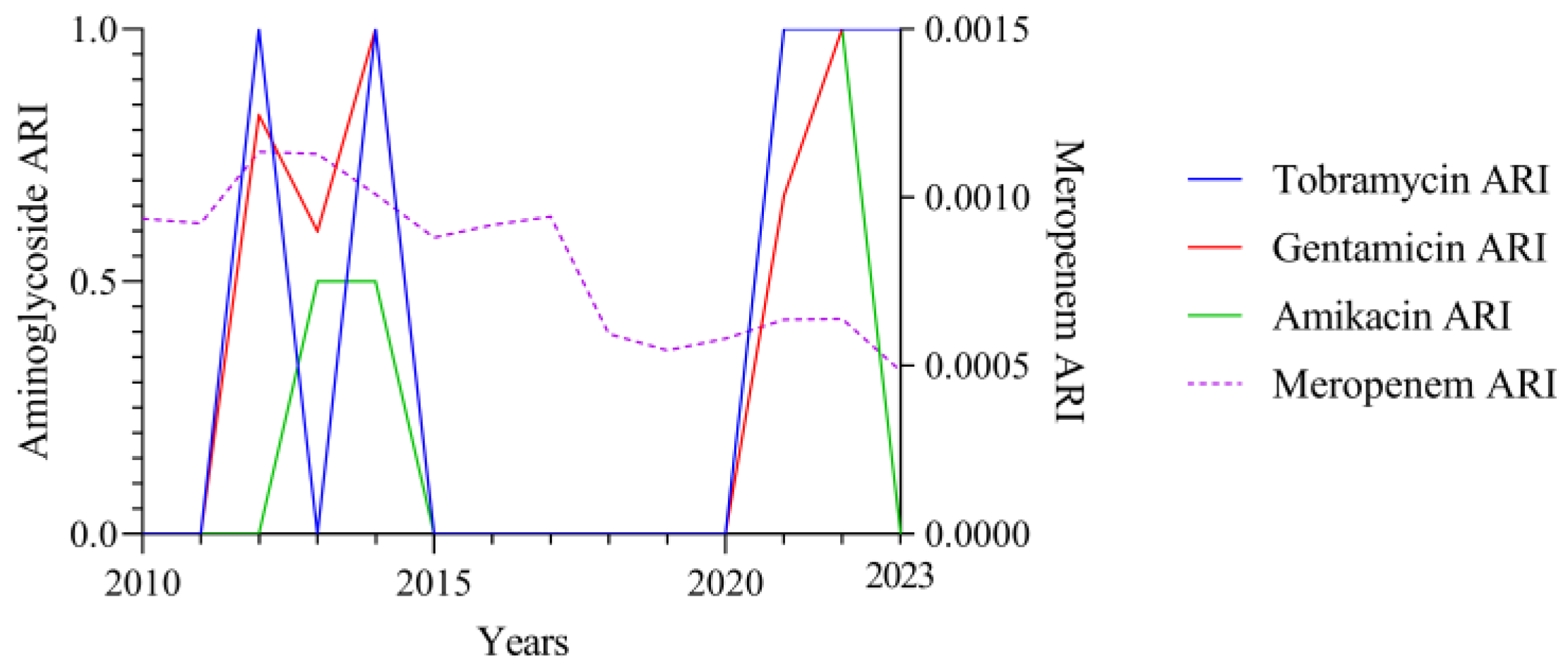
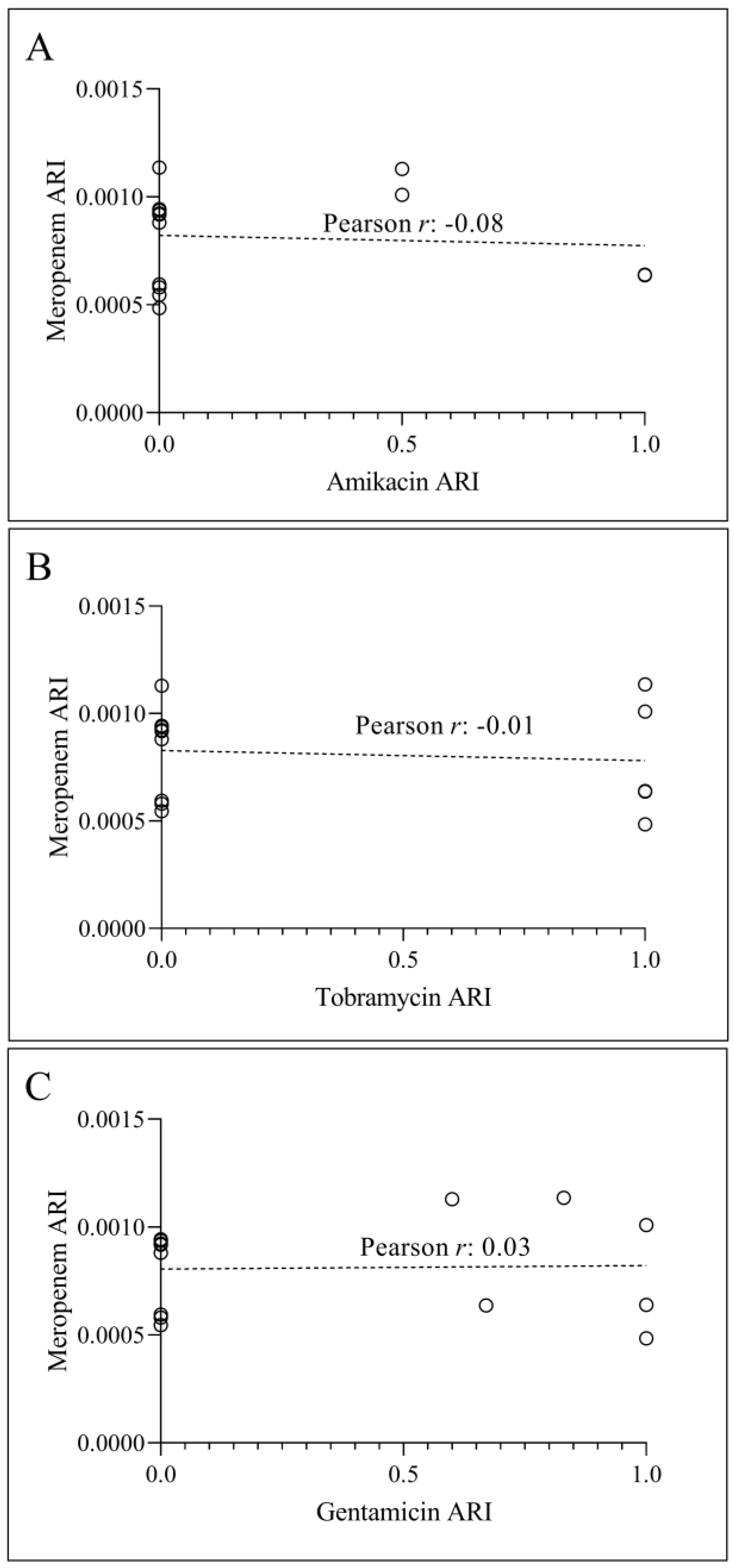
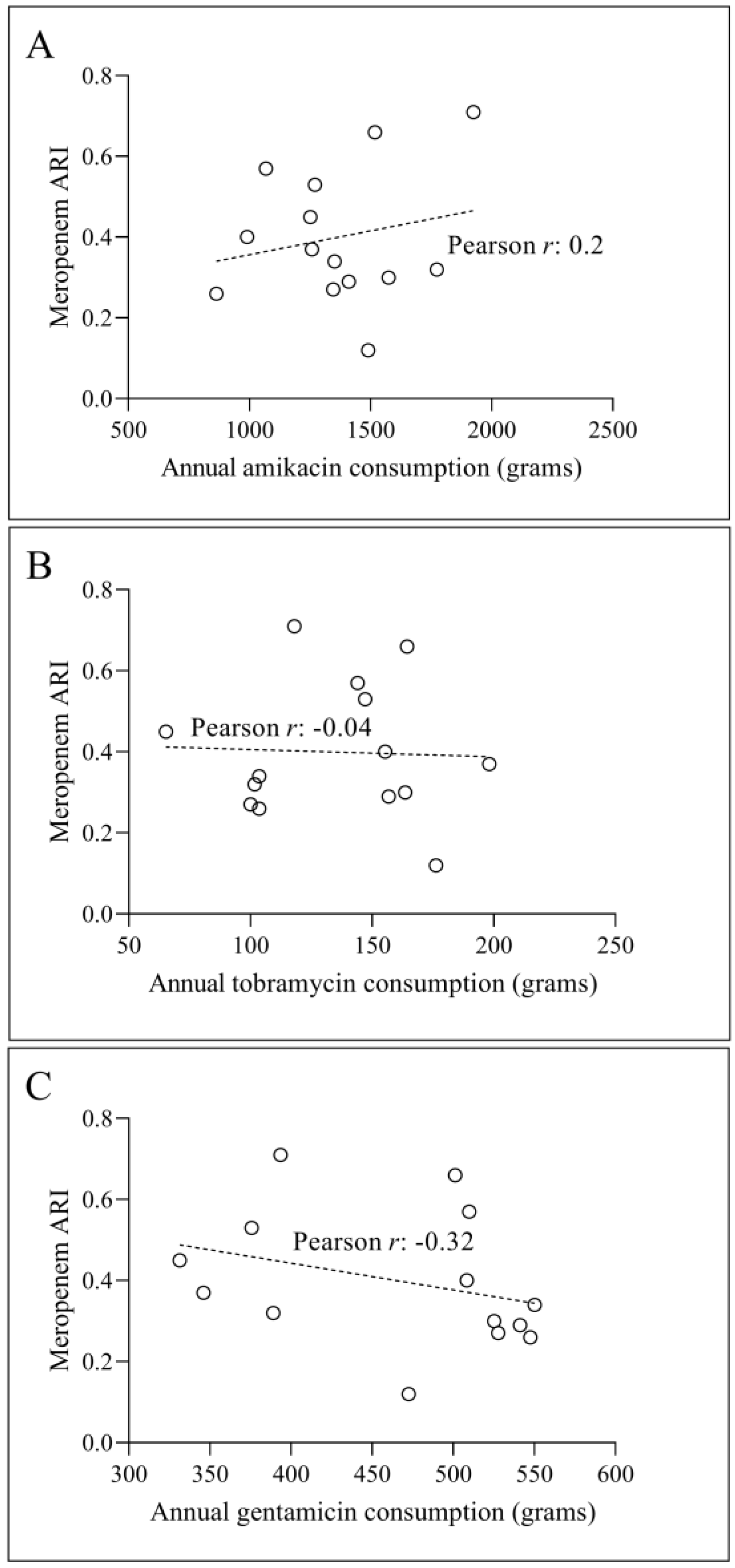
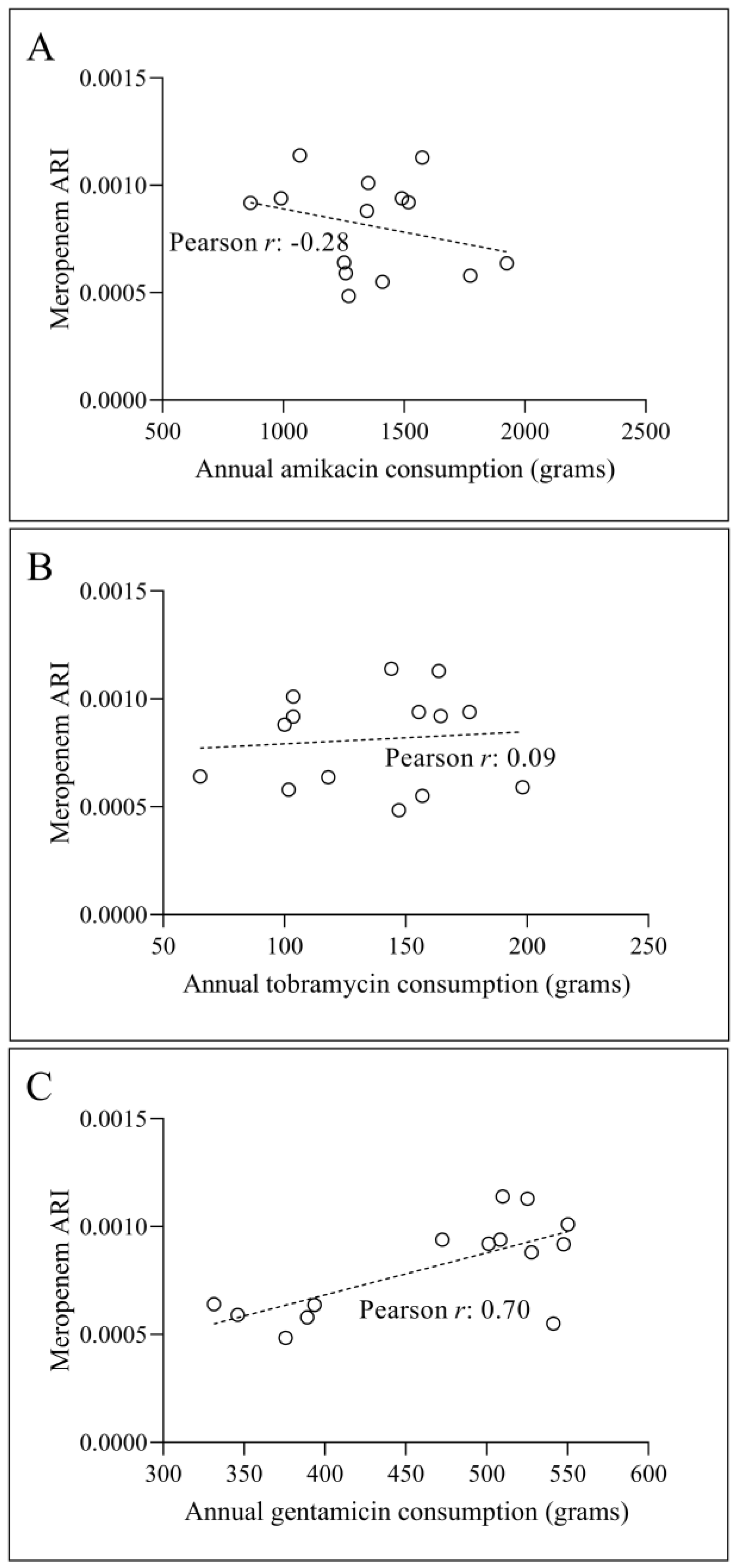
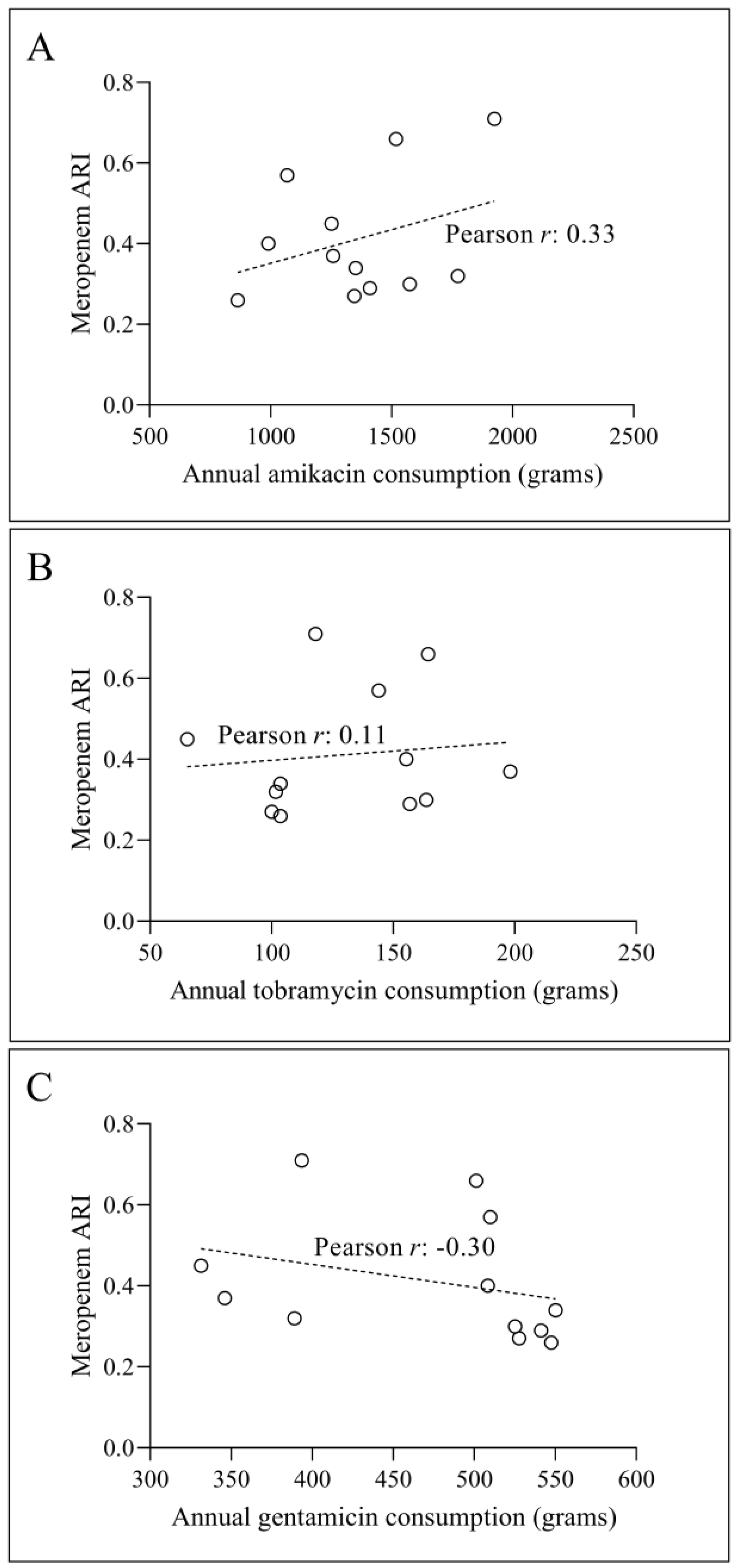
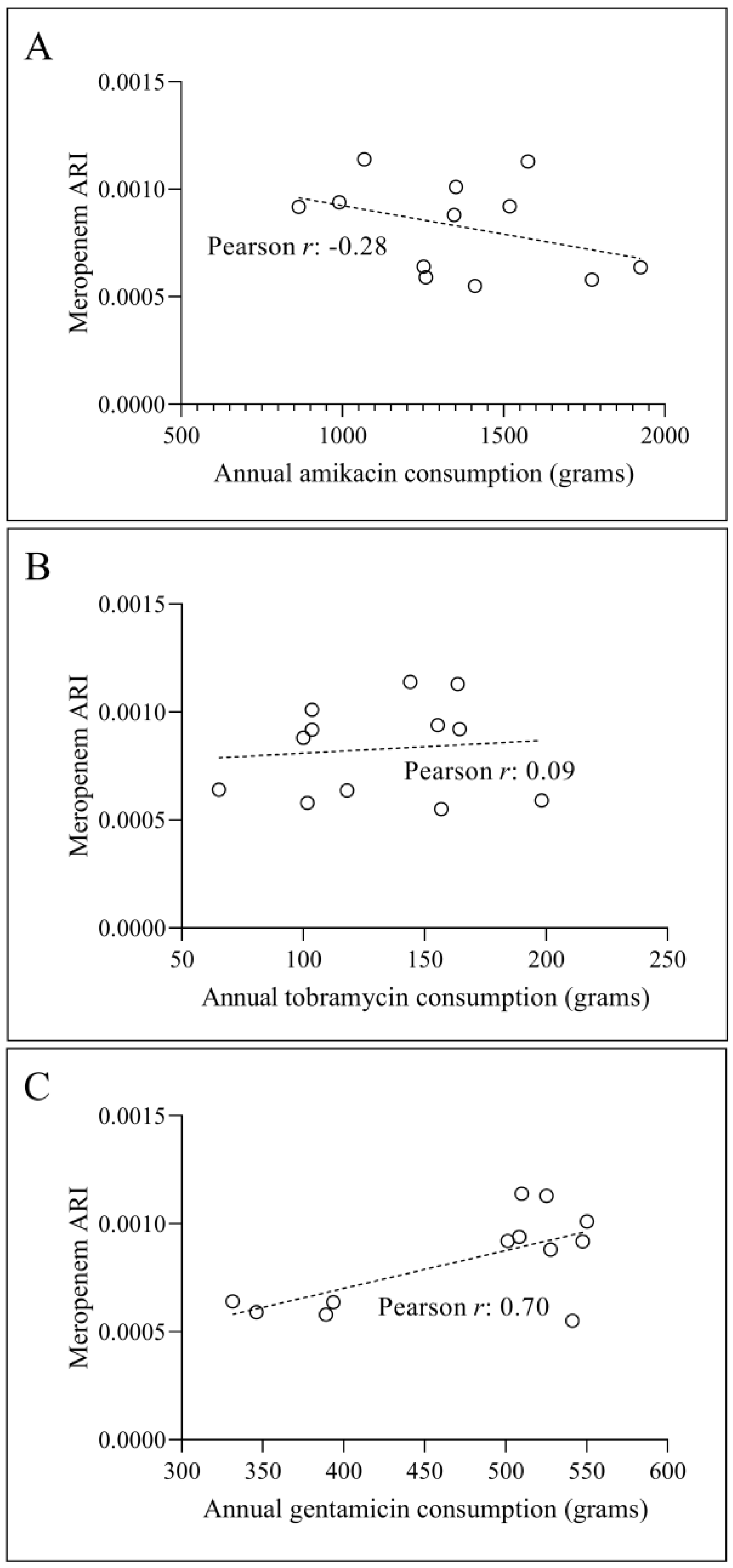
3.2. Temporal Correlation Dynamics Between Aminoglycoside Consumption and Resistance Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance. Available online: https://www.who.int/publications/i/item/9789240093461 (accessed on 6 July 2024).
- Mea, H.J.; Yong, P.V.C.; Wong, E.H. An Overview of Acinetobacter Baumannii Pathogenesis: Motility, Adherence and Biofilm Formation. Microbiological Research 2021, 247, 126722. [Google Scholar] [CrossRef] [PubMed]
- Ayoub Moubareck, C.; Hammoudi Halat, D. Insights into Acinetobacter Baumannii: A Review of Microbiological, Virulence, and Resistance Traits in a Threatening Nosocomial Pathogen. Antibiotics 2020, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.-J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter Baumannii: Pathogenesis, Antibiotic Resistance Mechanisms, and Prospective Treatment Options. Front. Cell. Infect. Microbiol. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Gordon, N.C.; Wareham, D.W. Multidrug-Resistant Acinetobacter Baumannii: Mechanisms of Virulence and Resistance. International Journal of Antimicrobial Agents 2010, 35, 219–226. [Google Scholar] [CrossRef]
- Orosz, L.; Burián, K. The “COVID Effect” in Culture-Based Clinical Microbiology: Changes Induced by COVID-19 Pandemic in a Hungarian Tertiary Care Center. Journal of Infection and Public Health 2024, 17, 102453. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Cuzon, G.; Naas, T. The Real Threat of Klebsiella Pneumoniae Carbapenemase-Producing Bacteria. The Lancet Infectious Diseases 2009, 9, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Podschun, R.; Ullmann, U. Klebsiella Spp. as Nosocomial Pathogens: Epidemiology, Taxonomy, Typing Methods, and Pathogenicity Factors. Clin Microbiol Rev 1998, 11, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Paczosa, M.K.; Mecsas, J. Klebsiella Pneumoniae: Going on the Offense with a Strong Defense. Microbiol Mol Biol Rev 2016, 80, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.M.; Farshadzadeh, Z.; Janabadi, S.; Musavi, M.; Shahi, F.; Moradi, M.; Khoshnood, S. Evaluating the Frequency of Carbapenem and Aminoglycoside Resistance Genes among Clinical Isolates of Acinetobacter Baumannii from Ahvaz, South-West Iran. New Microbes New Infect 2020, 38, 100779. [Google Scholar] [CrossRef] [PubMed]
- EUCAST: Disk Diffusion Methodology. Available online: https://www.eucast.org/ast_of_bacteria/disk_diffusion_methodology/ (accessed on 2 June 2022).
- De Socio, G.V.; Rubbioni, P.; Botta, D.; Cenci, E.; Belati, A.; Paggi, R.; Pasticci, M.B.; Mencacci, A. Measurement and Prediction of Antimicrobial Resistance in Bloodstream Infections by ESKAPE Pathogens and Escherichia Coli. Journal of Global Antimicrobial Resistance 2019, 19, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.; Rafei, R.; Gunawan, C.; Harmer, C.J.; Hamidian, M. Variants of Tn6924, a Novel Tn7 Family Transposon Carrying the blaNDM Metallo-β-Lactamase and 14 Copies of the aphA6 Amikacin Resistance Genes Found in Acinetobacter Baumannii. Microbiol Spectr 2022, 10, e0174521. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Xu, L.; Chen, Y. Drug Resistance and Susceptibility of Amikacin in Children with Extended-Spectrum Beta-Lactamase-Producing Enterobacterales: A Systematic Review with Meta-Analysis. Diagnostic Microbiology and Infectious Disease 2023, 106, 115956. [Google Scholar] [CrossRef] [PubMed]
- Sedláková, M.H.; Urbánek, K.; Vojtová, V.; Suchánková, H.; Imwensi, P.; Kolář, M. Antibiotic Consumption and Its Influence on the Resistance in Enterobacteriaceae. BMC Res Notes 2014, 7, 454. [Google Scholar] [CrossRef] [PubMed]
- Alawi, M.M.; Tashkandi, W.A.; Basheikh, M.A.; Warshan, F.M.; Ghobara, H.A.; Ramos, R.B.; Guiriba, M.L.; Ayob, O.; Janah, S.S.; Sindi, A.A.; et al. Effectiveness of Antimicrobial Stewardship Program in Long-Term Care: A Five-Year Prospective Single-Center Study. Interdiscip Perspect Infect Dis 2022, 2022, 8140429. [Google Scholar] [CrossRef] [PubMed]
- Balázs, B.; Tóth, Z.; Nagy, F.; Kovács, R.; Tóth, H.; Nagy, J.B.; Tóth, Á.; Szarka, K.; Majoros, L.; Kardos, G. The Role of Uniform Meropenem Usage in Acinetobacter Baumannii Clone Replacement. Antibiotics (Basel) 2021, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter Baumannii Antibiotic Resistance Mechanisms. Pathogens 2021, 10, 373. [Google Scholar] [CrossRef]
- Ahmed, O.B.; Asghar, A.H.; Bamaga, M.; Bahwerth, F.S.; Ibrahim, M.E. Characterization of Aminoglycoside Resistance Genes in Multidrug-Resistant Klebsiella Pneumoniae Collected from Tertiary Hospitals during the COVID-19 Pandemic. PLOS ONE 2023, 18, e0289359. [Google Scholar] [CrossRef] [PubMed]
- Almaghrabi, R.; Clancy, C.J.; Doi, Y.; Hao, B.; Chen, L.; Shields, R.K.; Press, E.G.; Iovine, N.M.; Townsend, B.M.; Wagener, M.M.; et al. Carbapenem-Resistant Klebsiella Pneumoniae Strains Exhibit Diversity in Aminoglycoside-Modifying Enzymes, Which Exert Differing Effects on Plazomicin and Other Agents. Antimicrob Agents Chemother 2014, 58, 4443–4451. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




