1. Introduction
The global population is steadily increasing, and it is essential to adequately meet the demand for biomaterials and their usage in healthcare system. Biomaterials are used for the development of numerous medical devices leading to enhanced quality of life. Metal, polymer, ceramic and composites are the four main types of biomaterials that are used for the development of medical devices. The European Society for Biomaterials stated in 1987 that a biomaterial should be defined as a non-biological material that can be used in medical devices with the primary purpose of interacting with biological systems [
1,
2]. Its definition has evolved over time, and it is now defined as a material that interacts with biological systems to treat, evaluate, or replace any tissue/body function.
Every biomaterial is characterized by its biocompatibility [
3], meaning that it has ability to promote response from the host in certain circumstances. According to the Food and Drug Administration (FDA), biomaterial is only considered biocompatible if it does not harm the patient (U.S. Food & Drug Administration:
https://www.fda.gov/). Aside from biocompatibility, biomaterials must also meet the design criteria, as mechanical performance, geometry, and electric control [
4].
Over the last decade, discoveries in the various fields, in particular regenerative medicine, and tissue engineering, contributed to enormous increase in use of biological materials for medical applications. Although biomaterials offer great potential, the complexity of this field is currently considered as one of the major challenges for scientists, engineers and clinicians which are focused on the life quality improvement of people affected by various diseases.
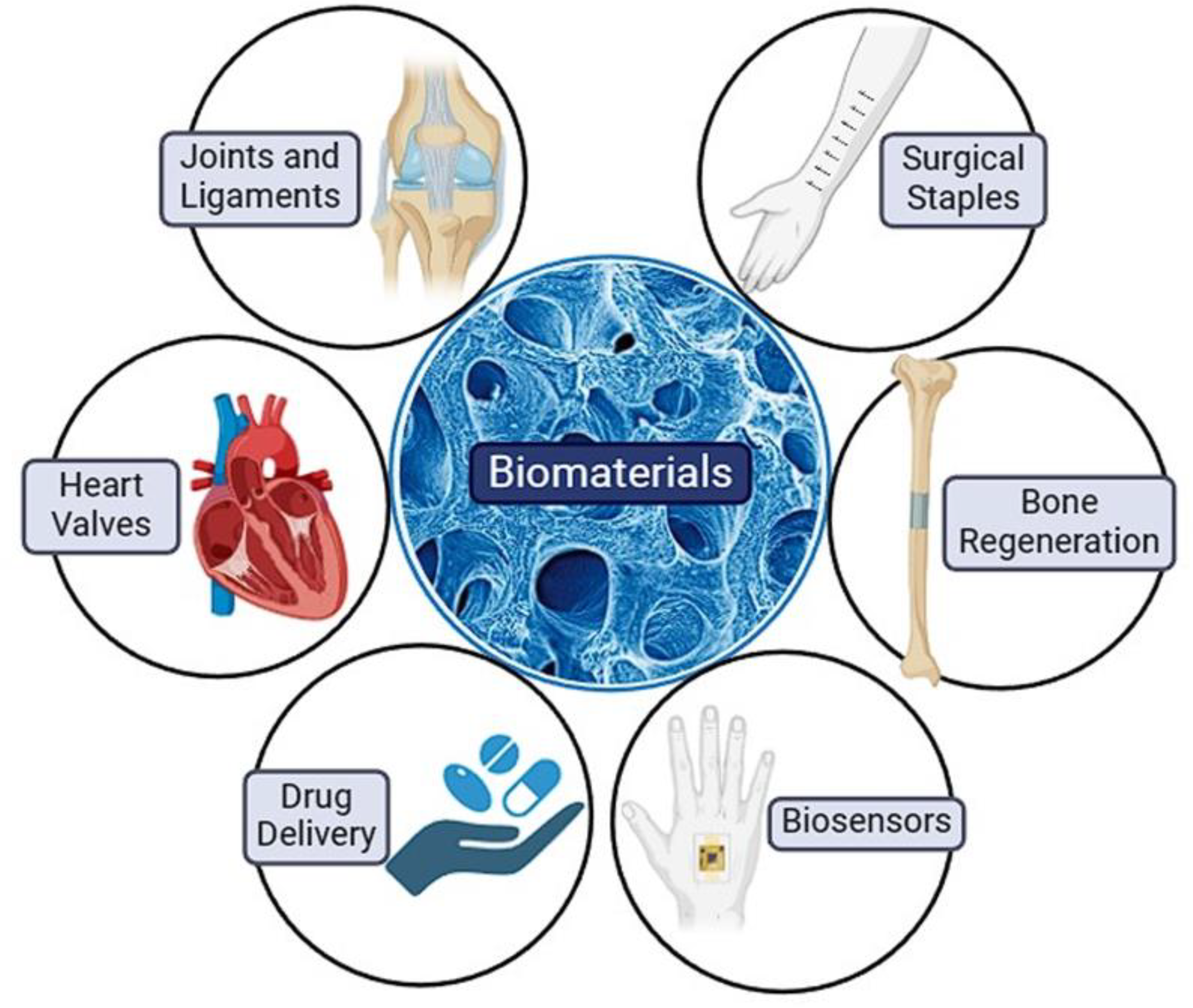
Due to numerous advantages of biomaterials including biodegradability, non-toxicity, and biocompatibility, they are considered to be highly effective in the replacement, support or enhancement of biological function in the damaged tissue and improvement in overall quality of life [
5,
6]. Tissue engineering implants, human tissue healing and regeneration, drug delivery management and systems, biosensors are among frequently used techniques and applications in which biomaterials are utilized [
7]. Tissue engineering implants include artificial ligaments or joints, heart valves and implants for hearing loss, human tissue healing and regeneration include surgical staples, bone regenerating hydrogels and dissolvable dressings, drug delivery management and systems include application or transfer of drugs to the target tissue or organ (e.g., drug-coated vascular stent), while biosensors include sensors with the ability to detect substance of interest (e.g., blood glucose levels monitoring sensors) (
Figure 1) [
8].
In this review, we provide insights including fundamental properties, case studies, and ethics related to the usage and application of biomaterials, further exploring their potential and role in healthcare and medicine.
2. Injectable Biomaterials, Bioactive Coatings, and Surface Modifications
Advancements in regenerative medicine and tissue engineering have led to a significant rise in the utilization of biological materials for medical applications in the past decade. These biological materials, also known as biomaterials, can be sourced from living organisms, or artificially created [
5]. They are classified as natural (e.g., cellulose, gelatine, alginate, chitosan) or synthetic (e.g., PLGA, PLA, PCL, fibronectin) based on their origin [
9].
Biomaterials offer various advantages such as biocompatibility, biodegradability, and non-toxicity, making them highly effective in enhancing biological functions, supporting damaged tissue, and ultimately improving quality of life. They are used in a wide range of applications including tissue engineering implants, human tissue healing, drug delivery systems, and biosensors.
Despite their potential, the complexity of biomaterials poses a significant challenge to researchers and clinicians aiming to enhance the quality of life of patients with diverse medical conditions. Interdisciplinary collaboration across fields like chemistry, biology, medicine, physics, engineering, materials science, and biotechnology is necessary for the development of these materials. Ensuring biomaterials meet biological requirements to prevent immune responses, as well as possessing suitable mechanical properties and corrosion resistance, are essential considerations.
2.1. Injectable Biomaterials
Evaluation of injectable biomaterials in regenerative medicine offers promising advancements, providing less invasive alternatives to conventional treatments [
5]. These biomaterials can be derived from natural sources, synthetic materials, or their composite combinations [
5,
9]. This approach is particularly beneficial for treating vertebral fractures, tumour resections, and craniofacial defects, facilitating accelerated repair processes [
6]. Injectable biomaterials are versatile for various clinical applications, enabling targeted delivery of bioactive molecules and growth factors to specific tissues [
9]. Meeting diverse treatment needs has led to the development of formulations and functionalities of injectable biomaterials. Commonly used materials in medical research for injectable biomaterials include alginate, collagen, gelatine, chitosan, fibrin in hydrogel or microsphere forms, as well as bioactive glasses, calcium phosphates, and polymethyl methacrylate (PMMA) in cement or paste formulations [
8,
10,
11].
2.2. Bioactive Coatings
Bioactive coatings play a critical role in medical device design by enhancing the bonding between implants and living tissues. These coatings facilitate stem cell differentiation into osteoblasts, bone ingrowth, and enhance implant integration [
12]. Furthermore, the recent development of biodegradable polymers as bioactive coatings has aimed to promote tissue formation, wound healing, and prevent infections [
13].
2.3. Surface Modifications
Surface modification of biomaterials is a vital procedure to address inadequate surface properties such as adhesion, adsorption, and biocompatibility prior to application [
14]. The body's immune response may lead to rejection of implanted biomaterials, while infections from microbial contamination can contribute to biomaterial dislocation and failure [
15]. Overcoming these challenges is essential, underscoring the importance of surface modification strategies [
14,
15]. Despite the rapid growth in biomaterial application and demand, challenges persist in production and safety, with biocompatibility and mechanical properties being crucial considerations [
16]. Failures related to biocompatibility present significant challenges, influenced by factors like shape, size, intended use, and duration of application [
17]. Corrosion and ionization of implants due to tissue reactions and the corrosive physiological environment are commonly observed biomaterial failures [
17].
3. Biomaterials for Drug Delivery
Drug delivery is a critical aspect of the pharmaceutical industry that aims to precisely administer therapeutic agents to specific target sites in the body [
18]. However, challenges such as drug release kinetics, poor tissue penetration, and overcoming physiological barriers like the blood-brain barrier limit the efficacy of current drug delivery systems [
19]. Developing drug carriers with properties like non-toxicity, biodegradability, and adjustable size and shape can enhance targeted drug delivery while minimizing side effects [
18]. The concept of a "magic bullet" introduced by Paul Ehrlich highlights the ideal drug delivery system that selectively targets diseased cells without affecting healthy ones [
19]. Biomedical engineers have made significant contributions to understanding drug delivery barriers and advancing novel drug delivery technologies [
19]. These technologies encompass various routes of drug administration and delivery vehicles, such as nanoparticles and lipid nanoparticles, to ensure effective drug delivery and protection of therapeutic agents from degradation [
20]. Engineering advancements like micro needle patches for painless vaccinations and innovative nanoparticle designs show promise for improving drug delivery methods and enhancing treatment outcomes [
20]. Additionally, optimized drug delivery vehicles, like nanoparticles loaded with anti-inflammatory agents for ailments such as acute respiratory distress syndrome (ARDS), hold potential for targeted and effective therapies [
20].
Nanoparticles revolutionize drug formulation and delivery by leveraging nanotechnology, a multidisciplinary field manipulating materials at the molecular level [
21]. With sizes ranging from 100 to 500 nm, nanoparticles can be tailored to deliver drugs to specific tissues, minimizing toxicity and enhancing treatment efficacy, notably in cancer therapy [
21]. Through injection, inhalation, or oral intake, nanoparticles interact with proteins in the body, facilitating drug distribution to organs via blood capillary absorption and lymphatic elimination [
21]. To evade immune activation and improve targeting, nanoparticle size and surface characteristics are crucial, with an optimal size of around 100 nm for efficient drug delivery and BBB traversal [
22]. Small nanoparticles exhibit faster drug release and reduced immune response, underscoring their potential in improving therapeutic outcomes [
22].
Effective drug release from nanoparticles hinges on various factors like pH, drug solubility, temperature, and diffusion processes within the nanoparticle matrix [
23]. To enhance biocompatibility and circulation time, hydrophilic surfaces, often coated with polymers like polyethylene glycol (PEG) or polyoxamer, are employed to prevent protein binding and premature drug loss [
23]. Erosion of the nanoparticle matrix facilitates controlled drug release, with the addition of auxiliary agents such as PEO-PPO mitigating early drug release due to polymer-drug interactions [
23]. Nanoparticles offer targeted drug delivery capabilities to damaged tissues through specific ligand coatings like peptides, antibodies, or proteins, minimizing off-target effects on healthy tissues [
24]. For instance, nanoparticle-based delivery systems can precisely deliver chemotherapeutic agents to tumour sites, reducing systemic toxicity and preserving normal tissues [
25]. Micelles and liposomes are other vehicles for localized chemotherapy delivery [
25].
Biomaterials play a critical role in interfacing with biological tissues by responding to specific biological signals. Hydrogels, a key biomaterial category, possess the ability to adapt their properties in response to biological recognition events, such as nutrient presence or enzyme activity [
26]. Comprised of up to 90-99% water, hydrogels form elastic networks through cross-linked polymers, offering biocompatibility and controllable swelling behaviour [
26]. These versatile materials can be designed using natural or synthetic components and have found diverse applications in medicine, including as adhesive cardiac patches for improved heart function restoration [
27].
Hydrogels are commonly used in soft contact lenses to allow gas diffusion while maintaining moisture on the eye's surface [
28]. In medical applications, hydrogel patches support tissue regeneration by creating a protective barrier for damaged tissues, promoting wound healing, and serving as depots for bioactive agent delivery [
28]. Microparticles, another significant drug delivery system, offer precise drug release, protection, and easy administration [
29]. These microparticles can be customized in size and morphology for various therapeutic needs, making them ideal for pulmonary drug delivery and the administration of insulin, corticosteroids, and chemotherapy agents [
30].
The interdisciplinary study of biomaterials by chemists, physicists, biologists, and pharmaceutical scientists is crucial for the development of innovative treatment and diagnostic techniques [
24]. The future of biomaterials in drug delivery holds promise for personalized and safer therapies through the manipulation of molecular sizes and surface properties to enhance drug delivery efficiency [
24].
4. Biomaterials in Cardiovascular Devices
The cardiovascular system is divided into pulmonary and systemic circulation loops, which work to ensure proper blood flow in the body. Pulmonary circulation facilitates oxygenation of blood, while systemic circulation distributes oxygen-rich blood to different body parts [
31].
Various cardiovascular health issues can arise, including endocarditis, rheumatic heart disease, and conduction system irregularities. Cardiovascular disease encompasses coronary artery disease (CAD), coronary heart disease (CHD), peripheral artery disease (PAD), cerebrovascular disease, and aortic atherosclerosis. CAD, characterized by decreased myocardial perfusion, can lead to angina, myocardial infarction (MI), or heart failure, contributing to a significant proportion of heart conditions. Cerebrovascular disease involves strokes and transient ischemic attacks, while PAD primarily affects the extremities, often resulting in discomfort. Aortic atherosclerosis refers to thoracic and abdominal aneurysms associated with arterial disease. Atherosclerosis, caused by dyslipidaemia, immunological events, inflammation, and endothelial dysfunction, can lead to plaque formation and vessel stenosis, compromising blood flow [
32].
Significant progress has been made in the field of synthetic materials over centuries, enhancing our understanding of biomaterial interactions with human tissue. Biomaterials, used to modify natural tissue functions, constantly interact with bodily fluids to optimize physiological processes [
11]. Identified as substances capable of enhancing or replacing tissue, organs, or bodily functions, biomaterials exclude drugs and aim to improve the quality of life, according to the National Institutes of Health [
33]. Metals like stainless steel, cobalt, titanium, and shape memory alloys are prevalent in cardiovascular medicine for producing artificial heart valves, endovascular stents, and stent-graft combinations. Stainless steel, known for its high resistance to heat and ease of maintenance, is commonly used and sterilized due to its inert nature. 316L steel, characterized by high hardness, corrosion resistance, and blood compatibility, is frequently utilized in stents and artificial heart valves. Titanium, highly biocompatible with the human body, has a superior strength-to-weight ratio compared to stainless steel. It is inert, resistant to bodily fluids, and commonly employed in the production of defibrillators, pacemakers, stents, and artificial heart valves [
34]. Blood vascular prosthesis is a widely utilized technique for treating damaged blood vessels. Vascular bypass, also known as vascular graft technology, involves surgical intervention to alleviate obstruction by reconnecting blood vessels to facilitate easy blood flow. By bypassing damaged vessels, this procedure maintains normal circulation between healthy areas. The optimal resources for vascular bypass include the patient's own vessels or those from another individual.
Commonly used materials in this procedure include polyethylene terephthalate and polytetrafluoroethylene (PTFE). The objective is to develop multi-layered vascular grafts combining synthetic polymers (ePTFE, PET, PU, PLA, or PCL) with natural substances (collagen, elastin, gelatine, lecithin) and introducing biologically active compounds (peptides, vascular growth factors, antibodies, plasminogen activators) and specific cell types (HUVECs, HUASMCs, or fibroblasts) on each layer to mimic vascular system structure and cellular environment, enhancing biocompatibility and minimizing immune responses [
35]. Patients with valvular heart disease are often treated with a device or synthetic heart valve when one of the heart valves functions improperly. Prosthetic heart valves are categorized into two groups based on their functionality: biological and mechanical valves. Biological valves consist of biological or synthetic components like PTFE and Dacron. Elastomers, including polyurethanes and silicones, titanium, pyrolytic carbon, and metal alloys, are commonly used components in prosthetic heart valves (PHVs). Elastomers are valued for their ability to resist deformation under extreme conditions without breaking and returning to their original shape when stress is relieved. Polyurethanes are extensively studied for PHVs due to their biocompatibility and a range of formulations, but challenges include stability, susceptibility to calcification leading to early failure. Metals like titanium and stainless steel are frequently used in PHV construction due to their durability and compatibility. Pyrolytic carbon stands out for its exceptional properties such as toughness, clot prevention, and blood compatibility, making it an appealing material for PHVs. Bio-prosthetic heart valves, also known as tissue valves, demonstrate favourable hemodynamic properties without requiring continuous anticoagulant therapy as they are composed partially or entirely of biological materials sourced from humans or animals.
Natural biomaterials commonly used in bioprosthetic heart valves (BPHVs) include decellularized tissues such as small intestine submucosa, pericardium, heart valves, and arterial walls [
36]. Stents are devices utilized to open or bypass blood vessels carrying oxygenated blood to the heart. These stents, regardless of being metallic or plastic, are inserted into the blood vessel lumen [
11]. An exciting development in stent technology is the use of magnesium and its alloys known for their biodegradability, biosafety, structural qualities, and low restenosis risk. Biodegradable stents can also be made from zinc alloys and biodegradable polymers such as poly(lactic acid) (PLA) and polycaprolactone (PCL). These materials break down into elements metabolized by the patient, aiming to maintain blood vessel function and patency. Proper design and refinement of vascular stents are crucial to treat vessel obstructions, clear thrombi, and restore vascular function, ensuring secure placement and minimal restenosis risk [
37]. To ensure safe and effective use of medical devices in contact with blood, surface-induced adverse events like blood coagulation, thrombus formation, and complement activation must be prevented. Addressing these issues often involves surface modifications, coatings, or altering the physical and chemical properties of biomaterial surfaces. Blood clotting and cellular activation are triggered by protein adsorption, leading to device-related thrombus formation. Regulating the adsorption of clotting factors like immune globulins, fibrinogen, HMKW, and complement factors can help prevent activation of defence mechanisms and blood cells, ensuring the proper function of medical devices [
38].
5. Biomaterials in Orthopaedics
Biomaterials have played a crucial role in the evolution of orthopaedics, making significant contributions to the field. These substances have become increasingly vital with the advancement of modern medicine and material processing [
39]. They are tailored materials that can be used alone or in combination with complex systems to modulate living systems, guiding therapeutic interventions [
40]. Designed to interact with biological systems, biomaterials are particularly important for tissue, organ, and system reconstruction and replacement in cases of damage or dysfunction [
39]. Initially, biomaterial use in medicine featured inert materials mimicking the composition of hard tissues like cartilage and bones, but it has progressed with regenerative medicine playing a key role in tissue preservation, especially in the early stages of disease [
40]. This evolution has led to significant advancements in the field.
Biomaterials must exhibit biocompatibility, high bio-inertness, and biostability to ensure successful medical function without adverse reactions [
39]. These materials must also have suitable weight, elastic modulus, and practical applicability. Biomaterials, such as artificial joints and bone grafts, have transformed treatment approaches for musculoskeletal conditions, with ongoing advancements in orthopaedics applications for artificial joint modelling, bone grafting, and regeneration techniques [
39]. Recommendations are provided to improve healthcare practices in this field.
5.1. Biomaterials in Designing of Artificial Joints
Recent advancements in artificial joints, such as hip and knee replacements, have been significantly influenced by biomaterial innovations. Traditional metal choices like stainless steel and titanium, known for their strength and durability, have been long-standing favourites in orthopaedic treatments [
41]. However, a shift is occurring as orthopaedic practices increasingly adopt advanced biomaterials with a more nuanced selection approach. Modern artificial joint designs prioritize both structural integrity and biocompatibility, utilizing a range of biomaterials tailored to specific purposes. Various biomaterials and their classifications will be discussed in the subsequent sections.
5.2. Ceramics
Ceramics, prominent in everyday interactions, now offer a range of engineering designs tailored for high-temperature and high-strength applications [
42]. Through the fusion of ceramic powders like alumina and zirconia, orthopaedic ceramics have found a niche in joint replacements, particularly in total hip replacements where they account for over 40% of usage in the US and even higher in the UK [
43]. This trend is expected to continue with the expanding adoption of ceramics in various countries.
Orthopaedic ceramics' fracture resistance is influenced by internal flaws, such as poor fusing or large grains. To enhance fracture strength, experts work towards achieving nearly full density with alumina ceramics of smaller size. Despite their brittleness, these materials offer wear and scratch resistance due to their hard composition. Additionally, their increased hydrophilicity compared to metal surfaces reduces friction with bones, facilitating easier installation and smoother movement of patients [
43].
Alumina and zirconia are commonly used ceramic biomaterials in total joint replacement, with alumina historically favoured in hip replacements for its favourable qualities. Zirconia, which was popular between 1985 and 2001, saw a decline in global supply due to the failure of its primary manufacturing firm [
43]. Zirconia was preferred over alumina for its higher strength. Alumina matrix composites have been available since 2003, offering advantages over alumina in terms of fracture toughness, strength, and wear resistance [
43]. Oxidized zirconium, created by heating zirconium alloy devices in the presence of air, forms a black zirconium oxide ceramic intended for hard-on-soft bearings in total knee and hip arthroplasty. However, research and innovation may expand its use to hard-on-hard bearings in the future. Each ceramic component's unique characteristics determine its applications and preferences, contributing to positive patient outcomes through longer-lasting implants and reduced risks of complications [
43].
5.3. Polymeric Materials as Biomaterials
Polymers, both natural and synthetic, offer advantageous properties for orthopaedic procedures. They exhibit high intrinsic osteogenic and osteoimmune modulating potential, as well as the ability to withstand physiological stresses without fracturing or distortion [
39]. Natural polymers initially outperformed traditional synthetic materials in biological aspects, offering high chemical versatility and excellent biodegradability [
44]. Synthetic polymers were later developed to reduce host reactions to biomaterials. Non-biodegradable synthetic polymers display high biological inertness and customizable mechanical properties but may fail in orthopaedic implants due to issues such as stress shielding-induced bone resorption, infections, and poor tissue integration at the interface [
45]. Examples of such polymers include poly (methyl methacrylate), polyether ether ketone, and polyethylene. Biodegradable synthetic polymers, like polylactic acid, polyglycolic acid, and polylactic-co-glycolic acid, offer superior mechanical properties and thermal adaptability, making them suitable for regenerative medicine applications [
39]. The unique properties of polymers make them indispensable for potential advancements in orthopaedic care [
45].
5.4. Biomaterials as Bone Grafts
Biomaterials have significantly expanded the options for bone grafting, particularly in orthopaedic surgery. Bone grafts involve the transplantation of bone fragments within the body or from a donor to repair or replace damaged bones [
46]. Ideal biomaterial substitutes for bone repair should possess osteoinductive, osteogenic, and osteoconductive properties [
47]. Osteoinductive capabilities stimulate the recruitment and differentiation of host mesenchymal stem cells into bone and cartilage-forming cells, while osteogenic properties facilitate new bone formation using cells from the patient or a donor [
46]. Osteoconductive characteristics promote the growth of host capillaries, connective tissue, and mesenchymal cells within the implanted scaffold, creating a favourable environment for cell attachment, proliferation, and the formation of new bone tissue.
For bone replacements in orthopaedic surgery, materials must possess mechanical properties for support and stability to enable movement. They should also be hydrophilic to ensure good interface with human bone and minimize risks of complications such as systemic toxicity, teratogenic, carcinogenic, or antigenic reactions, which can lead to serious health issues [
46]. Ease of supply in large quantities at reduced costs, along with easy sterilization, is beneficial for healthcare practitioners to minimize material purchases [
48]. Further studies and innovations are necessary to develop the ideal bone reconstruction material. Substitutes can be natural or synthetic, each with distinct characteristics.
There are various classes of natural bone substitute materials, such as autografts, allografts, xenografts, photogenic material, and bone graft material from extracted teeth [
49]. Autografts have high osteogenic and osteoinductive properties, promoting bone formation and cell differentiation. However, their use may be limited due to donor site morbidity and limited tissue availability. Allografts, derived from the same species as the host, come in different forms and can be customized for specific patient needs. Xenografts, harvested from a species with a different genetic composition, present some challenges in usage. Photogenic materials, sourced from plant origins like coral and marine algae, offer alternative options [
50]. Bone graft material from extracted teeth is used by dentists to address dental deficiencies. The selection of natural graft materials depends on material characteristics and patient conditions, requiring collaboration among healthcare providers for optimal medical outcomes [
46].
5.5. Synthetic Bone Grafts
Synthetic bone substitute materials are categorized into three classes. Firstly, calcium phosphate ceramics like hydroxyapatite and tricalcium phosphate, and calcium phosphate cements, which are two or three-component systems consisting of materials such as hydroxyapatite and tricalcium phosphate [
51]. The second class incorporates composite bone substitute materials, composed of a matrix and reinforcements, which may be continuous or discontinuous [
46]. These synthetic substitutes are often utilized in conjunction with growth factors to enhance normal bone healing.
5.6. Biological Growth Factors
Growth factors (GFs) are molecules that can promote various cellular processes, including differentiation, proliferation, migration, and multicellular morphogenesis [
52]. In orthopaedics, experts have explored the localized administration of signalling modules as stimulators for tissue regeneration, aiming to avoid the possible risks associated with systemic exposure, such as increased cancer risk [
53]. However, the short effective half-life of growth factors at the surgical site, along with their limited interactions with the extracellular matrix and susceptibility to environmental changes, pose challenges for successful therapy [
54]. The development of recombinant technologies and drug delivery systems using various carriers is crucial to address the limitations in delivering growth factors effectively for therapeutic purposes.
5.7. Nanomaterials
Engineered nanomaterials, small substances utilized in medicine, technology, and engineering, replicate the intricate structure of natural bone matrix, fostering cell adhesion and proliferation, offering biocompatibility, and enhancing mechanical properties. In orthopaedics, nanotechnology focuses on leveraging scaffolds to enhance the interface between native bone and orthopaedics implants [
55]. Nanotechnology has the potential to revolutionize orthopaedics surgery by improving diagnosis and treatment. Understanding cell-biomaterial interactions at the nanoscale has propelled the increased use of nanomaterials in bone regeneration, surpassing the use of microscale materials [
56]. Ongoing research aims to further enhance the properties of these nanomaterials, ultimately leading to improved healthcare outcomes.
5.8. D Printing Technology
Three-dimensional printing, a key advancement in manufacturing and technology, facilitates the development of materials for customizable bone regeneration devices [
57]. Patient-specific instruments (PSIs) created through 3D printing, such as personalized surgical guides, enable surgeons to perform precise orthopaedics procedures [
58]. Compared to standard implants, 3D-printed implants offer advantages like reduced blood loss and lower readmission rates following surgery. The technology's adherence to regulatory standards has led to global approval [
57], and ongoing advancements are expected to significantly impact bone regeneration procedures in the near future.
6. Nanotechnology in Biomaterials
Nanotechnology, a rapidly growing field for the past 50 years, spans various disciplines like physics, biology, pharmacy, electronics, chemistry, and medicine, focusing on materials within the range of (1-100) nm [
59]. These nanomaterials possess unique properties that mimic extracellular matrix components, enabling direct delivery of active substances. Their high surface area to volume ratio grants them distinct characteristics, including enhanced mechanical properties and potential antiviral, antibacterial, and antifungal properties [
60]. Nanoparticles can penetrate cell membranes, aiding in protein absorption and making them valuable for drug delivery and tissue regeneration applications [
61]. Moreover, nanomaterials exhibit exceptional thermal and electrical conductivity, with the ability to transform non-magnetic materials into magnetic entities at the nanoscale level. Additionally, they offer biological advantages such as biocompatibility, low immunogenicity, and biodegradability [
61].
6.1. Applications of Nanomaterials
6.1.1. Drug Delivery Systems
Nanotechnology in drug delivery enhances therapeutic efficacy and minimizes side effects by utilizing nanomaterials as carriers for targeted delivery, reducing systemic distribution [
62]. These advancements are particularly crucial in cancer treatment, where precise drug delivery is vital. Common nanomaterial drug carriers include iron nanoparticles, dendrimers, liposomes, and polymeric micelles.
6.1.2. Liposomes
Liposomes, spherical vesicles with a phospholipid bilayer enclosing an aqueous space, fall within the size range of 0.01–5.0μm, positioning them as nano drug delivery systems. They were the first nanoparticle type to receive approval for medical applications, offering unique properties like the ability to encapsulate both hydrophilic and hydrophobic molecules, enhancing drug delivery versatility. Comprising biocompatible and biodegradable materials, liposomes safeguard drugs from degradation, improve bioavailability, and allow for efficient drug encapsulation [
63]. Moreover, their surface can be tailored to incorporate targeting ligands, antibodies, or functional groups for specific drug delivery purposes, making them ideal for targeted drug delivery, enhancing drug efficacy, and minimizing side effects.
6.1.3. Polymeric Micelles
Polymeric micelles, nanoscale structures resulting from the self-assembly of amphiphilic block copolymers in water, exhibit a core-shell architecture with dimensions ranging from 10 to 100 nm. The hydrophobic core enables the encapsulation of poorly soluble drugs, while the hydrophilic shell imparts stability and biocompatibility. These systems have garnered significant attention in targeted drug delivery owing to their efficacy in enhancing drug retention within tissues, protecting drugs from enzymatic degradation, and facilitating cellular uptake. Moreover, polymeric micelles possess biocompatibility and facile elimination pathways, thus minimizing toxicological risks. Particularly advantageous for highly toxic drug delivery, they are easily scalable and cost-effective to produce. In addition to oncology, polymeric micelles find application in various therapeutic domains including infectious diseases, cardiovascular disorders, inflammation, and neurological conditions [
64].
6.1.4. Iron Nanoparticles (FeNPs)
FeNPs, possessing magnetic traits, exhibit promise in targeted drug delivery. Leveraging a magnet enables the localization of these particles to specific treatment sites, thereby reducing the necessary dosage and minimizing systemic drug dispersion [
65].
6.1.5. Dendrimers
Dendrimers are polymers which consist of tendrils surrounding a hollow core. They can be used as drug carriers because of their properties. The drug can be loaded into them and as they contain polyethylene glycol (PEG) or the surface is PEGylated, the immunogenicity will be decreased, which leads to increasing drug residence time in the system, and the drug toxicity will be reduced [
66].
6.1.6. Tissue regeneration
Utilizing nanotechnology, biocompatible nanoscale scaffolds resembling normal extracellular matrices can be engineered for tissue regeneration, allowing for precise control over cellular behaviour and the development of implantable tissues. This approach finds applications in regenerating various tissues such as neural, cutaneous, osseous, cardiac, and cartilaginous tissues [
62,
67,
68].
6.1.7. Bone Regeneration
Nanomaterials like carbon nanotubes, graphene, and hydroxyapatite nanoparticles serve as scaffold materials for bone tissue regeneration by offering mechanical support and guiding natural regeneration processes. Their biodegradability allows for seamless integration within the body, eliminating the need for retrieval procedures. Additionally, these nanomaterials function as carriers for growth factors, bioactive compounds, and pharmaceuticals, augmenting bone repair and regeneration [
61].
6.1.8. Neural Tissue Regeneration
Electroactive, biodegradable, conductive nanomaterials were created by polymerizing poly(3,4-ethylenedioxythiophene) nanolayer onto the channel surface of chitosan/gelatine scaffolds. These scaffolds showed superior neural stem cell adhesion and proliferation. Neural stem cells cultured on these scaffolds successfully differentiated into mature neurons and astrocytes [
69].
6.1.9. Cardiac Tissue Regeneration
In cardiac tissue regeneration, nanomaterials offer diverse applications. They can be integrated into scaffolds to bolster mechanical support, foster cellular proliferation, and steer cell differentiation, facilitating the restoration of impaired cardiac tissue. Notably, carbon nanotubes feature prominently in constructing 3D scaffolds conducive to cardiomyocyte growth. Furthermore, nanomaterials like gold nanorods present opportunities for delivering bioactive agents such as microRNA and growth factors to enhance cellular activities [
70].
6.1.10. Cartilage Regeneration
Nanomaterials exhibit unique physicochemical characteristics at the nanoscale, exerting significant influence on cellular behaviour encompassing morphology, cytoskeletal organization, motility, and gene regulation. When harnessed in nanocomposite scaffolds mimicking human cartilage properties, these materials enhance cell growth, differentiation, adhesion, and extracellular matrix production. This emulation fosters improved tissue functionality and growth. Moreover, nanomaterial incorporation enhances the mechanical robustness of engineered cartilage constructs, rendering them more resilient to joint biomechanical stressors [
67].
6.1.11. Skin Regeneration
Nano-drug delivery systems enhance the efficacy of therapeutic agents like growth factors by prolonging drug release, shielding it from degradation, and augmenting skin retention, thereby fostering enhanced wound healing and skin regeneration. Nanohydrogels, characterized as three-dimensional polymeric networks, represent a promising approach for wound management, offering moisture retention, fluid absorption, and a conducive environment for wound recovery. In wound care, antimicrobial nanoparticles integrated into dressings play a pivotal role in infection prevention. Additionally, the integration of antimicrobial nanomaterials like hydroxyethyl cellulose-silver and nano-TiO2 hydrosol into scaffolds for skin regeneration underscores their potential to create a healing-oriented environment and promote tissue repair [
71].
7. Biodegradable and Bioresorbable Biomaterials, Biomaterials Regulation and Ethics, Biocompatibility and Immunomodulation
Biomaterials, essential in medical applications, must exhibit biocompatibility to avoid adverse host reactions. Compliance with FDA guidelines ensures patient safety (FDA). These materials must also fulfil diverse design criteria encompassing mechanical robustness, geometry, and electrical properties [
4]. Historical progression categorizes biomaterials into inert (1960-1970), bioactive (1980-1990), biodegradable (2000-2010), and smart biomaterials (2010-present). Inert biomaterials aimed at tissue replacement, while bioactive counterparts enhanced device efficacy through coatings. Biodegradables addressed infection risks prevalent in bioactives by promoting absorption within the body, obviating the need for replacement surgeries. The current era of biomaterials focuses on mimicking natural tissue structures to facilitate tissue repair. Despite advancements, first-generation biomaterials remain prevalent in clinical practice [
3,
4,
72].
7.1. Biodegradable and Bioresorbable Biomaterials
Biodegradable and bioresorbable biomaterials enable gradual degradation within the body, facilitating tissue regeneration without necessitating replacement. Of note, magnesium alloy emerges as a promising candidate for bioresorbable applications, although concerns regarding its use in implants exist [
73]. Scaffold engineering employs a range of biodegradable materials such as fossil-based polymers, poly (ε-caprolactone), poly(vinyl) alcohol, polyethylene glycol, polypropylene fumarate, polyurethane, modified polyurethanes, collagen, hyaluronic acid, chitosan, and fibrin [
74]. Biomaterials have revolutionized drug delivery systems, enhanced targeted therapy, and mitigated the adverse effects of conventional chemotherapy on healthy tissues. Notably, polyurethane drug delivery systems show promise in cancer treatment, offering a novel approach that instils optimism and novel prospects for cancer patients [
75].
Bone tissue engineering is a rapidly advancing field, emphasizing biodegradable biomaterials over nondegradable counterparts. Traditional biodegradable materials encompass metals, polymers, and ceramics, offering long-lasting treatments. Recent innovations include intelligent micro-nano materials and cell-based products, minimizing the need for repeat surgeries and reducing healthcare costs. Biodegradable ceramics, derived from natural clay and other components, play a crucial role in tissue repair, bone defect filling, and fracture healing. Hydroxyapatite, tricalcium phosphate, and dicalcium phosphate are commonly used ceramics, valued for their corrosion resistance, biocompatibility, and biological activity, aiding in gradual tissue regeneration [
76,
77].
7.2. Biomaterials Regulation and Ethics
Biomaterial development necessitates compliance with international and country-specific regulations and ethical guidelines. Following these standards ensures the efficacy, safety, and responsible utilization of biomaterials in various applications. Key guidelines include adherence to international standards and regulatory guidance such as global risk classification and access to medical device standards. Compliance with ethical considerations in preclinical animal and clinical human research is paramount. Addressing ethical challenges related to embryonic and foetal-derived tissues and gene therapy is essential. Ensuring informed consent, privacy protection, equitable access, affordability, environmental sustainability, and cultural sensitivity are critical aspects in the ethical development and application of biomaterials. Collaboration among diverse stakeholders is imperative for the establishment of universally recognized ethical regulations in this field [
77,
78,
79,
80] [
81].
7.3. Biocompatibility and Immunomodulation
In the context of immune response, maintaining immune homeostasis is crucial for defending against infections and managing tissue development, regeneration, and repair. A key focus in current research is on immunomodulation to enhance tissue regeneration and control immune responses. Immunomodulatory biologics, such as antibodies and drugs, have been developed to modulate immune activity in different conditions. However, challenges exist in their systemic administration, including short half-life, lack of targeting ability, and potential adverse reactions. Biocompatibility is a critical aspect in biomaterial development, ensuring that materials do not induce harmful effects in the body. Biocompatibility testing, required by regulatory bodies, is essential for various biomaterials used in medical applications, such as dental implants and prostheses. Enhancing resistance to bacterial infections is a key focus in biomaterial development to reduce hospital-acquired infections. Surface chemistry plays a significant role in biocompatibility, influencing cell adhesion, protein absorption, inflammatory response, antimicrobial properties, and biodegradation of biomaterials. Manipulating surface chemistry can regulate cell-material interactions and degradation rates, contributing to the overall success of biomaterials in medical applications [
82,
83,
84,
85,
86,
87,
88,
89].
8. Biomaterials for Tissue Engineering
8.1. Scaffold Design
Scaffold design plays a critical role in tissue engineering by providing a framework for cell attachment, proliferation, and differentiation, mimicking the extracellular matrix (ECM) of natural tissues. Various polymers, including natural, synthetic, and composite materials, are used in scaffold design to create biomaterials with specific properties conducive to tissue regeneration [
90]. Natural polymers such as collagen, fibrin, and hyaluronic acid promote cell adhesion and growth, resembling the microenvironment of natural tissues. Incorporating bioactive substances like peptides and growth factors enhances the regenerative capacity of these scaffolds [
91].
Synthetic polymers like poly(lactic-co-glycolic acid) (PLGA) and polyethylene glycol (PEG) offer controlled release of bioactive molecules, adjustable mechanical properties, and tailored degradation rates [
91]. Scaffold properties can be modified through polymer composition and structure manipulation to meet tissue engineering requirements. Techniques like electrospinning produce nanofibrous scaffolds that closely mimic natural tissue architecture [
92]. Composite materials, incorporating inorganic components or a blend of natural and synthetic polymers, provide versatility in scaffold design [
93]. For instance, incorporation of ceramic particles like tricalcium phosphate or hydroxyapatite improves bioactivity and mechanical strength, making them suitable for bone tissue engineering [
93]. Additionally, adding conductive materials such as graphene or carbon nanotubes facilitates electrical stimulation and enhances neural tissue regeneration. Scaffold design influences cell activity, nutrient transport, and tissue development, making it a fundamental aspect of tissue bioengineering [
94].
To ensure scaffold degradation aligns with tissue regeneration processes, factors like degradation mechanisms, structural design, and material selection must be considered [
74]. Biodegradable scaffolds made from collagen, PCL, or PLGA break down gradually into non-toxic byproducts, promoting tissue remodelling and growth [
74]. Bioactive degradation products released during scaffold breakdown can regulate cellular responses and tissue regeneration. Various techniques such as phase separation, 3D printing, and electrospinning are employed to control scaffold design and shape, optimizing properties like pore size, interconnectivity, and mechanical characteristics for tissue regeneration [
95]. Mechanical properties of scaffolds, including strength, elasticity, and stiffness, are tailored to match the characteristics of target tissues and withstand physiological stresses during tissue regeneration. Mechanical signals are crucial for controlling cell behaviour, differentiation, and tissue remodelling [
95].
8.2. Biocompatibility Assessment
Biomaterials in tissue culture serve most effective when they blend in into the biological environment without causing any negative side effects. This complex problem requires careful consideration of many important aspects, including material selection, immunogenicity assessment, degradation kinetics, evaluation of the cellular response, and creation of customized biomaterial formulations. Material Selection: The first step regarding biocompatibility is the careful selection of biomaterials, which combines the special benefits and challenges of synthetic polymers like polyethylene glycol (PEG), natural polymers like collagen, and composite materials. The material chosen needs to comply with mechanical and structural specifications and mimic the natural extracellular matrix [
91]. Immunogenicity Assessment: the examination of immunogenicity is closely related to biocompatibility. Thorough evaluation using in vitro tests indicates cytokine and cell responses, guiding mitigation efforts to reduce immunogenicity. This is essential for fostering a harmonious interaction between the biomaterial and the host tissue [
96]. Degradation Kinetics: Knowledge of degradation kinetics is necessary for temporal biocompatibility considerations. degradation rates that are ideal coincide with the timescales for tissue regeneration, avoiding damage to the scaffold and preventing the process of natural tissue remodelling. Optimizing the degradation properties guarantees sufficient assistance during crucial stages of recovery and renewal [
97]. Cellular Response Evaluation: the assessment of cellular response is fundamental to improving biocompatibility. Investigating cell adhesion, proliferation, and differentiation in the presence of the biomaterial is possible through in vitro research that makes use of cell culture models. Modifications to establish an environment conducive to tissue growth are guided by these investigations [
98]. Clinical Translation and Potential Implications: in addition to advancing science, successful biocompatibility enhancement in biomaterials has significant clinical implications. Better biocompatibility results in biomaterials that are safer and more effective for therapeutic applications, which include the regeneration of intricate tissues and organs as well as wound healing [
99]. To verify that biomaterials are safe and effective for use in regenerative medicine applications, biocompatibility assessment in biomaterial tissue engineering evaluates how biomaterials interact with biological systems. Introducing cultured cells to the biomaterials of interest is an essential part of this assessment process. Direct cell seeding into the biomaterial surface or exposure to soluble extracts or breakdown products of the biomaterial can accomplish this [
100].
To test the compatibility and reactivity of the cells, cell seeding entails directly cultivating the cells onto the biomaterial's surface. Depending on the intended use and desirable cellular behaviour, cells can be seeded as monolayers or in three dimensional (3D) arrangements. To assess cytotoxicity and biological impacts, cells can be subjected to biomaterial extracts or degradation products. This entails observing the biological reactions of cells cultured in medium containing these extracts [
101]. Common assays used to measure cell viability and metabolic activity include MTT and live/dead staining. Evaluating gene expression, ECM formation, differentiation, proliferation, and behaviour of cells are all part of the biocompatibility assessment process. Biomaterials are designed with the use of techniques such as immunostaining, flow cytometry, and gene expression analysis, which helps in the characterization of cellular responses. The goal of biocompatibility assessment is to determine how biomaterials interact with constructions used in tissue engineering and facilitate tissue regeneration [
102]. In preclinical models or clinical trials, this entails evaluating tissue development, vascularization, and functional outcomes. Tissue morphology, vascular network formation, and functional tissue qualities are assessed by histological analysis, imaging modalities, and functional assays. These evaluations offer vital information regarding the safety and effectiveness of biomaterial-based treatments for in vivo tissue regeneration [
103].
8.3. Regenerative Medicine
Regenerative medicine-based biomaterials in tissue engineering are designed to promote tissue regeneration and repair, acting as a structural support for cell growth and differentiation to restore tissue function. Biomaterials utilized in regenerative medicine aim to be biocompatible, biodegradable, and capable of gradual replacement by newly formed tissue, integrating with surrounding tissues. These biomaterials are crucial for applications in bone, cartilage, skin, and neural tissue engineering, providing mechanical support and promoting cell differentiation and tissue regeneration [
104,
105,
106]. Biomaterial scaffolds mimic the natural extracellular matrix structure, promoting cell adhesion, proliferation, and differentiation through surface properties like chemistry and topography [
107].
These biomaterials play a key role in tissue-specific regeneration by enhancing cell viability and metabolic activity, enabling nutrient and bioactive chemical diffusion, and providing a temporary barrier to aid wound healing [
108,
109]. The controlled release of growth factors and signalling molecules from biomaterials promotes tissue repair, while shielding therapeutic cells like stem cells from immune rejection to create a conducive environment for tissue regeneration. Tailoring biomaterial characteristics ensures optimal tissue integration and functionality, reflecting natural tissue signals and regulating cell behaviour through bioactive compounds [
110]. By mimicking the extracellular matrix components and incorporating growth factors and cell-binding sites, biomaterials promote cell adhesion and trigger angiogenesis and tissue regeneration [
111]. The mechanical properties of biomaterial scaffolds, such as stiffness and elasticity, are matched to native tissue qualities to influence cellular responses and promote tissue repair [
112].
Regulatory control of the immune response is essential in tissue engineering for regenerative medicine. Biomaterials post-implantation modulate the host immune system's inflammatory and healing responses, impacting tissue integration, side effect reduction, and treatment effectiveness. Immunomodulatory biomaterials have shown promise in wound healing and tissue engineering applications but face challenges in regulatory approval and long-term safety for clinical translation. Personalized tissue engineering, enabled by customizable scaffold qualities tailored to individual patient needs through advanced imaging and 3D printing, facilitates tailored therapies that optimize tissue restoration while minimizing side effects [
113,
114]. Achieving precision medicine entails modifying surface chemistry, porosity, and architecture of biomaterials. The integration of biofunctionalization technologies, incorporating growth factors and signalling signals into scaffold architecture, further enhances personalized medicine and customized therapies for improved tissue regeneration outcomes [
115,
116]. Successful translation of individualized regenerative medicine from research to clinical practice requires collaboration among researchers, clinicians, and industry partners to ensure safety, effectiveness, and long-term success for a diverse range of tissue abnormalities and medical conditions [
114].
9. Biomaterials for Neural Interfaces
Biomaterials used in neural interfaces (NIs) for bidirectional communication with the nervous system in devices like neural implants, brain-machine interfaces (BMIs), and neuroprosthetics play a vital role in interacting with neural tissue effectively and safely. These devices stimulate neural tissue, record neural activities, and are essential in central nervous system (CNS) and peripheral nervous system (PNS) applications for neuro-modulatory and neuro-prosthetic purposes. Biocompatible biomaterials used in NIs need to prevent immune responses such as foreign body reaction and neural cell damage like glial scar to ensure seamless integration with neural tissue [
117,
118]. Notable biomaterials in NIs include medical-grade silicones, polyimides, and biocompatible metals like platinum, gold, and titanium. Additionally, functional biomaterials such as conducting polymers (e.g., polypyrrole, PEDOT), metals/alloys (platinum, iridium), and carbon-based materials (carbon nanotubes, graphene) with optimal electrical properties are crucial for effective neural signal recording and stimulation [
119,
120,
121,
122]. Furthermore, ensuring long-term stability in neural interface devices requires resistance to corrosion, oxidation, and degradation under physiological conditions. Strategies such as protective coatings, material selection, and surface modifications are employed to enhance device stability over time. Encapsulation, careful material selection, and surface modifications are key approaches to safeguard electronic components, enhance chemical and mechanical stability, and promote integration with surrounding tissue, ultimately improving the long-term performance and reliability of neural interfaces[
123,
124,
125].
9.1. Types of Interfaces
9.1.1. Neural implants
A variety of neural implant typologies, such as microelectrode arrays (MEAs), Utah electrode array (UEA), deep brain stimulation electrode leads (DBS), peripheral nerve stimulators, spinal cord stimulators (SCS), and intracranial electrodes have been developed and classified based on various discerning factors. Classification criteria include their anatomical location relative to nerves and neuronal cell bodies, delineating intraneural, epineural, perineural, intranuclear, or cortical positioning, and tissue geometry across species, distinguishing implants for the central nervous system (CNS) and peripheral nervous system (PNS) [
126]. This classification emphasizes the functional requirements and physiological considerations specific to different neural regions. Moreover, implants are categorized based on their functions, such as recording neural activity, stimulating neural tissues, or delivering pharmacological agents in a controlled manner [
127]. Designs are tailored to suit specific functional needs and the complex physiological environment in which the implants operate.
The electrode design of neural implants also plays a key role in their classification, with invasiveness level serving as a fundamental parameter. Electrode architecture can range from surface-level interfaces to deeply penetrating structures, resulting in varying levels of tissue interaction and physiological impact [
117]. Understanding these differences in invasiveness is crucial for comprehending the intricacies of electrode-tissue interfaces and their implications on neural function and integration. This comprehensive taxonomy, incorporating anatomical, functional, and invasiveness factors, offers a detailed framework for comprehending the diverse array of neural implants and their multifaceted purposes [
127].
9.1.2. BMIs
BMIs, also known as Brain-Computer Interfaces (BCIs), epitomize sophisticated closed-loop systems [
128,
129] meticulously engineered to construct a direct conduit between the human brain and extrinsic apparatuses or machineries, consisting of fNIRS and fNIRS-EEG devices [
130,
131]. These intricate interfaces provide a channel whereby patients can exert control or engage in interaction with external devices, connecting the covert communicative potential of their neural activity through signal amplification emanating directly from cerebral substrates [
132]. The overarching objective underpinning the design and implementation of BMIs is the realization of a symbiotic link between cerebral faculties and machine, thereby facilitating fluidic communication channels [
133,
134,
135] and intricate control paradigms.
9.1.3. Neuroprosthetics
Neuroprosthetics are advanced devices engineered to interface with neural substrates to restore compromised neurological functions following neurotrauma [
136,
137]. These interventions target a range of neurological disabilities, providing patients with enhanced sensory, motor, and cognitive autonomy. Examples include cochlear implants that restore auditory function in individuals with sensorineural hearing loss [
138] and prosthetic limbs controlled through neural interfaces, enabling precise motor commands for individuals with limb impairments or Tetraplegia [
139,
140].
Deep Brain Stimulation (DBS) stands out as another innovative neuroprosthetic approach for addressing movement disorders like Parkinson's disease and essential tremor by delivering targeted electrical stimuli to the basal ganglia, improving motor symptoms and quality of life [
141]. Visual prosthetics represent a cutting-edge frontier, aiming to restore vision in visually impaired individuals by stimulating the visual cortex with electrical signals to evoke light and visual perceptions [
142]. These neuroprosthetic advancements herald a transformative era in neurorehabilitation, offering individuals with neurological impairments renewed functional capabilities and increased quality of life.
9.2. Applications
Neural interfaces (NIs) have shown promising applications in the field of motor prosthetics, enabling the translation of neural signals encoding motor intentions into actionable commands for prosthetic limbs or assistive devices. This technology empowers individuals with motor impairments to perform volitional movements and interact with their environment [
131]. Furthermore, NIs calibrated for communication aids, such as language models, offer a means for individuals with speech impairments to express themselves through assistive communication devices, leveraging neural activity for expressive and receptive communication [
143]. In neurorehabilitation, NIs play a crucial role in facilitating neural plasticity and functional recuperation by incorporating closed-loop feedback mechanisms, such as augmented feedback modalities [
130,
139]. Additionally, NIs designed for neural prosthetic applications aim to restore lost sensory modalities or enhance perceptual capabilities by transducing neural signals into artificial sensory feedback, thereby ameliorating sensory deficits and reinstating sensory experiences [
144].
9.3. Biomaterials Used in Neural Implants, BMIs and Neuroprosthetics
There are several biomaterials used in the construction of neural implants, BMIs and neuroprosthetics to ensure compatibility and optimal performance. Considering biocompatibility, electrical properties, and long-term stability, some common biomaterials used in these devices include:
9.3.1. Silicon
Silicon-based materials, particularly Cubic silicon carbide (3C-SiC), are highly favored in brain-machine interfaces (BMIs) for their superior electrical properties and biocompatibility. 3C-SiC has emerged as a key material for constructing electrodes and integrated circuits in BMI frameworks, representing a significant advancement in neurotechnological applications [
119]. Its precise control over electrical conductivity and strong biocompatibility makes it an ideal candidate for interfacing with neural tissues with exceptional accuracy and effectiveness. This material plays a crucial role in enhancing the performance and reliability of neural interface systems by enabling high-fidelity neural signal acquisition and processing. Its versatility allows for the fabrication of both electrodes and integrated circuits, further highlighting its importance in advancing neural interface technology [
119,
145].
9.3.2. Electrode Coatings
Various coatings are applied to electrodes in neural interfaces to optimize their performance and biocompatibility. Examples of electrode coatings include platinum black, ruthenium oxide, platinum-iridium oxide, Amorphous Silicon carbide (a-SiC), PEDOT, and water-soluble poly-(styrenesulfonate) (PSS) [
119,
139,
145]. These coatings enhance charge transfer and reduce tissue damage, thereby improving the overall efficacy of neural recording applications. For instance, platinum black's porous microstructure increases its surface area, enhancing electrochemical activity and charge injection capabilities, leading to improved signal-to-noise ratios [
146,
147]. Ruthenium oxide coatings exhibit robust charge storage capacities and high charge injection limits, making them suitable for neural stimulation applications [
148,
149]. Additionally, polymer-based biomaterials such as polyimide and polyethylene glycol (PEG) serve as flexible substrates for electrodes, offering mechanical flexibility and biocompatibility. Various electrode coatings, such as platinum black, ruthenium oxide, platinum-iridium oxide, Amorphous Silicon carbide (a-SiC), PEDOT, and poly-(styrenesulfonate) (PSS), play a crucial role in enhancing the performance and biocompatibility of neural interfaces [
119,
139,
145]. These coatings improve charge transfer efficiency and reduce tissue damage, thereby optimizing neural recording applications. Furthermore, polymer-based biomaterials like polyimide and polyethylene glycol (PEG) serve as flexible substrates for electrodes, offering mechanical flexibility and biocompatibility [
11,
150]. These materials withstand mechanical stresses in neural tissues and provide enhanced flexibility for neural interface systems.
9.3.3. Biocompatible Metals
Metals such as gold, platinum, and titanium play vital roles in brain-machine interfaces (BMIs) due to their stability, biocompatibility, and electrical conductivity, making them ideal for use in electrode designs and neural tissue interconnects [
151]. Gold's inertness and corrosion resistance, along with platinum's exceptional conductivity, facilitate precise neural signal recording and stimulation, while titanium's mechanical robustness and biocompatibility ensure efficient integration within BMI frameworks [
152]. Biocompatible metals like iridium, tantalum, and stainless steel are also utilized in BMI architectures for their distinct mechanical properties, biocompatibility, and electrical conductivity [
153]. Iridium's charge injection capacity is valuable for precise neural stimulation, tantalum's corrosion resistance is essential for chronic implantation, and stainless steel's mechanical strength and cost-effectiveness make it suitable for structural support in BMI applications [
154,
155].
9.3.4. Nanoparticles
Carbon nanofibers (CNF) and carbon nanotubes (CNT) are recognized for their exceptional mechanical and electrical properties, making them versatile biomaterials for various biomedical applications [
156]. The incorporation of minocycline into poly (lactic-co-glycolic acid) (PLGA) nanoparticles enables controlled and sustained release of this antibiotic and anti-inflammatory agent at targeted sites, enhancing localized therapeutic efficacy [
157]. Silver nanowires (AgNW), one-dimensional nanostructures with high electrical conductivity, flexibility, and optical transparency, are essential materials in flexible electronics, transparent conductive films, and biomedical devices [
158]. Their unique properties, including high aspect ratio and large surface area, offer advantages in electrical and thermal conductivity, as well as surface-enhanced plasmon resonance effects, positioning them as promising candidates for advanced electronic and optoelectronic technologies [
159,
160].
9.3.5. Ceramic Materials
Ceramic materials, such as alumina and glass, are essential in the construction of neural interfaces (NIs) [
161]. These ceramics provide insulation to protect electronic components from environmental factors and serve as protective packaging against mechanical stress and moisture ingress. In addition, ceramics offer inherent biocompatibility, creating a barrier between neural tissues and electronic components in NIs, reducing the risk of adverse tissue reactions and ensuring long-term functionality of the implanted system [
162].
9.3.6. Fluorescent Materials
DAPI, or 4’,6-diamidino-2-phenylindole, is a widely utilized fluorescent molecule in neural biocompatibility studies for precise identification of cell nuclei in immunohistochemistry [
163]. By binding to DNA and emitting blue fluorescence under ultraviolet excitation, DAPI enables accurate quantification of cell nuclei, facilitating analyses of cellular distribution, proliferation, and viability within neural tissues [
164]. Other fluorescent materials, such as fluorescent proteins (e.g., green fluorescent protein, GFP) and organic dyes (e.g., fluorescein isothiocyanate, FITC), are also valuable in neural biocompatibility studies, offering distinct advantages in visualizing specific cellular structures or processes [
165]. Fluorescent proteins, genetically integrated into cells, allow longitudinal tracking of cellular dynamics in live imaging, enabling real-time monitoring of cellular behaviors in neural tissues. Organic dyes, known for their versatility and spectral properties, serve as crucial tools for multiplexed labeling of various cellular components, supporting comprehensive analyses of neural tissue morphology and function.
Quantum dots, a promising class of fluorescent materials in neural biocompatibility studies, are characterized by narrow emission spectra and exceptional photostability [
166]. Comprising semiconductor nanocrystals, quantum dots exhibit high brightness and resistance to photobleaching, making them suitable for long-term imaging and high-resolution visualization of cellular structures in neural tissues. Their tunable emission spectra enable multiplexed labeling and simultaneous imaging of multiple cellular components, facilitating comprehensive analyses of neural tissue architecture and function [
167].
10. Biomaterials: Clinical Applications and Case Studies
10.1. Hydrogel Sheets in Burn Wound Treatment
Burns and open wounds exhibit distinct pathophysiological characteristics, with burns causing significant fluid loss necessitating effective treatment modalities such as hydrogel sheets. These sheets, composed of crosslinked polymer chains forming three-dimensional networks, possess high fluid absorption capacities due to numerous hydrophilic groups (-COOH, -OH, -CONH
2, -SO
3H), rendering them pliable and gentle. Typically derived from natural biopolymers like alginate, collagen, and chitosan, hydrogel sheets can also be synthesized from materials like polyvinyl alcohol and polyethylene glycol [
168]. Each hydrogel sheet comprises a hydrophilic polymer insoluble in water and water itself, with a notable trait of causing no immunological reactions and transparency facilitating wound healing assessment. Demonstrating efficacy across varying burn severities, hydrogel sheets are commonly employed for initial burn treatment, offering cooling, hydration, exudate absorption, and wound contamination prevention [
169]. Moreover, as demonstrated in a study comparing hydrogel sheets and silver sulfadiazine cream (SSD) in treating second-degree burns in 50 patients, hydrogel-treated patients exhibited faster recovery in 15 days compared to 21 days with SSD, with fewer reapplications (5 vs. 25) and enhanced usability, minimizing patient discomfort [
169].
10.2. Biodegradable Vascular Scaffold (BVS)
Cardiovascular diseases, predominantly coronary artery disease (CAD), are a leading global cause of mortality, characterized by arterial plaque accumulation hindering sufficient supply of blood, oxygen, and nutrients to the heart [
170]. Percutaneous coronary interventions with stents have traditionally been the primary approach for acute coronary conditions, yet present drawbacks, notably impeding positive vessel remodelling and future revascularization, along with risks of delayed endothelialisation and localized inflammation [
171]. Addressing these limitations, bioresorbable scaffolds (BVSs) have emerged as a promising alternative, requiring considerations such as flexibility, biocompatibility, and sufficient mechanical support for at least 6 months post-implantation to facilitate arterial healing. Bioresorbable polymers (e.g., salicylic acid, poly-l-lactide) and metals (e.g., magnesium alloy, iron alloy) are commonly employed materials due to their temporary support capabilities and concurrent vessel healing, contrasting solid metal stents. Notably, BVSs are advantageous for their complete bio-resorption and dynamic arterial diametric modulation, particularly appealing for younger patient cohorts [
172].
10.3. Dental implants
Patients experiencing multiple tooth loss commonly opt for dental implants, crucial for both aesthetics and overall health. Dental implants are surgically placed in the jawbone to serve as artificial tooth roots, supporting prosthetic teeth. Through osseointegration, natural bone fuses with the implants, providing a durable solution that has made dental implants the most prevalent biomaterials in recent years [
173]. Predominantly composed of metals like titanium, tantalum, and alloys, such as titanium-zirconium combinations for enhanced bone response [
174], dental implants have garnered popularity due to their strength, biocompatibility, and corrosion resistance [
175]. Research involving 10,871 implants placed over a 24-year span revealed a 3.3% implant failure rate, commonly associated with low bone density, prior sinus lift surgery, and smoking habits. Despite these challenges, success rates exceed 97% over 10 years, highlighting the high efficacy of dental implants [
173,
176].
10.4. Contact Lenses
Contact lenses (CLs) are crucial ocular prosthetic devices worn directly on the eye, serving as alternatives to spectacles, with a global user base exceeding 150 million people [
177]. Primarily utilized for vision correction, ocular rehabilitation, and with additional indications for prevention, diagnosis, and aesthetics, key considerations when selecting contact lenses include comfort, durability, practicality, and visual stability. Contact lenses are broadly classified into rigid gas-permeable hard lenses and flexible, high-water-content soft lenses, primarily composed of polymeric materials such as PMMA and HEMA-derived hydrogels [
178,
179]. Hydrogel-based lenses, offering oxygen permeability and high-water content for enhanced comfort, currently dominate the market, representing 65% of contact lens usage [
180]. Comparative studies demonstrating no significant differences between spectacles and contact lenses have led to a preference for contact lenses by patients, with long-term contact lens wear shown to be more effective for vision correction in adults and children, provided eye discomfort is mitigated [
181,
182].
11. Conclusions
An enormous surge in the utilization of biological materials for medical purposes has been observed over the past decade, attributable to advancements in regenerative medicine and tissue engineering. These materials have demonstrated their significance across a spectrum of applications, including tissue engineering implants, facilitation of human tissue healing and regeneration, management of drug delivery systems, and utilization in biosensors. Generally, biomaterials are hailed for their remarkable efficacy in restoring, reinforcing, or augmenting biological function within damaged tissues, thereby enhancing overall quality of life. The summary of the applications can be obtained from the
Table 1.
The requisites for biomaterials within the domain of NIs are clearly delineated by their essential attributes of biocompatibility, favourable electrical properties, and sustained long-term stability. This triad underscores the pivotal role of biomaterials in the intricate domain of neural interface engineering. Making informed choices and conducting comprehensive assessments of biomaterial properties emerge as decisive factors in the successful development and deployment of neural implants, brain-machine interfaces (BMIs), and neuroprosthetics. The delicate interplay between biomaterial characteristics and neural environments necessitates a meticulous equilibrium, ensuring that chosen materials not only elicit minimal host tissue responses but also seamlessly integrate with the neural milieu, thereby fostering enduring and efficient communication between the neural interface and the intricate biological context.
Evidence gleaned from contemporary trends points towards healthcare persisting as one of the most rapidly evolving sectors in the foreseeable future. Hence, it is imperative to remain abreast of these advancements and devise innovative strategies to attain favourable health outcomes. Orthopaedic surgery stands to reap immense benefits from these innovations, with the utilization of biomaterials such as ceramics and polymers in the design of vital artificial joints like those for the hip and knee. The assessment of biocompatibility ensures effective interaction between biomaterials and host tissues, mitigating adverse effects and promoting integration.
Furthermore, the amalgamation of biomaterials with regenerative medicine holds tremendous potential for resolving an array of clinical challenges, encompassing organ failure and tissue abnormalities. Biomaterials facilitate the creation of functional tissue constructs capable of reinstating organ function and expediting healing through pioneering methodologies such as bioactive chemical inclusion, cell encapsulation, and 3D printing technology.
It is paramount for biomaterial development to adhere to international, and sometimes country-specific, regulations and guidelines. Ethics stands as a cornerstone in the development and application of all biomaterials. It is worth noting that adherence to these guidelines ensures the efficacy, safety, and responsible utilization of biomaterials across diverse fields. However, further research is imperative to enhance their biodegradability, non-toxicity, and biocompatibility profiles, thus paving the way for even more impactful applications in the future.
Author Contributions
Conceptualization, A.H.-J. and V.N.U.; methodology, N.I., A.G., A.H., S.K., A.T., B.K., M.H., A.A.B., H.L., A.S.; validation, A.A.A., M.M.T., formal analysis, N.I.; investigation, A.H.-J.; writing—original draft preparation, N.I., A.G., A.H., S.K., A.T., B.K., M.H., A.A.B., H.L., A.S., A.A.A., M.M.T., A.H.-J. and V.N.U. writing—review and editing, V.N.U., and A.H.-J.; visualization, A.S.; supervision, A.H.-J and V.N.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article has no new data was created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- The Williams Dictionary of Biomaterials; Liverpool University Press, 1999; ISBN 978-0-85323-734-1.
- ESB European Society for Biomaterials. Available online: https://esbiomaterials.eu/Cms/Content/past-esb (accessed on 11 May 2024).
- Marin, E.; Boschetto, F.; Pezzotti, G. Biomaterials and Biocompatibility: An Historical Overview. J. Biomed. Mater. Res. A 2020, 108, 1617–1633. [Google Scholar] [CrossRef] [PubMed]
- Zadpoor, A. Meta-Biomaterials. Biomater. Sci. 2020, 8, 18–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Biomaterials in Medical Applications. Polymers 2023, 15, 847. [Google Scholar] [CrossRef] [PubMed]
- Oleksy, M.; Dynarowicz, K.; Aebisher, D. Advances in Biodegradable Polymers and Biomaterials for Medical Applications—A Review. Molecules 2023, 28, 6213. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Kumar, A. Biomaterials and Bioengineering Tomorrow’s Healthcare. Biomatter 2013, 3, e24717. [Google Scholar] [CrossRef] [PubMed]
- Socci, M.C.; Rodríguez, G.; Oliva, E.; Fushimi, S.; Takabatake, K.; Nagatsuka, H.; Felice, C.J.; Rodríguez, A.P. Polymeric Materials, Advances and Applications in Tissue Engineering: A Review. Bioengineering 2023, 10, 218. [Google Scholar] [CrossRef]
- Eldeeb, A.E.; Salah, S.; Elkasabgy, N.A. Biomaterials for Tissue Engineering Applications and Current Updates in the Field: A Comprehensive Review. AAPS PharmSciTech 2022, 23, 267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ma, B.; Hu, K.; Yuan, B.; Sun, X.; Song, X.; Tang, Z.; Lin, H.; Zhu, X.; Zheng, Y.; et al. Evidence-Based Biomaterials Research. Bioact. Mater. 2022, 15, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, A. An Overview on Biomaterials and Its Applications in Medical Science. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1116, 012178. [Google Scholar] [CrossRef]
- Ercan, H.; Durkut, S.; Koc-Demir, A.; Elçin, A.E.; Elçin, Y.M. Clinical Applications of Injectable Biomaterials. Adv. Exp. Med. Biol. 2018, 1077, 163–182. [Google Scholar] [CrossRef]
- Raucci, M.G.; D’Amora, U.; Ronca, A.; Ambrosio, L. Injectable Functional Biomaterials for Minimally Invasive Surgery. Adv. Healthc. Mater. 2020, 9, e2000349. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liang, C.; Wei, Z.; Bai, Y.; Bhaduri, S.B.; Webster, T.J.; Bian, L.; Yang, L. Injectable Biomaterials for Translational Medicine. Mater. Today 2019, 28, 81–97. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Ansari, S.; Chen, C.; Xu, X.; Akiyama, K.; Snead, M.L.; Zadeh, H.H.; Shi, S. Co-Encapsulation of Anti-BMP2 Monoclonal Antibody and Mesenchymal Stem Cells in Alginate Microspheres for Bone Tissue Engineering. Biomaterials 2013, 34, 6572–6579. [Google Scholar] [CrossRef] [PubMed]
- Bidarra, S.J.; Barrias, C.C.; Granja, P.L. Injectable Alginate Hydrogels for Cell Delivery in Tissue Engineering. Acta Biomater. 2014, 10, 1646–1662. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Liu, K.; Jiao, T.; Zhang, N.; Ma, K.; Zhang, R.; Zou, Q.; Ma, G.; Yan, X. An Injectable Self-Assembling Collagen-Gold Hybrid Hydrogel for Combinatorial Antitumor Photothermal/Photodynamic Therapy. Adv. Mater. Deerfield Beach Fla 2016, 28, 3669–3676. [Google Scholar] [CrossRef] [PubMed]
- Adeosun, S.; Ilomuanya, M.; Gbenebor, O.; Dada, M.; Odili, C. Biomaterials for Drug Delivery: Sources, Classification, Synthesis, Processing, and Applications. In; 2020 ISBN 978-1-83962-479-7.
- Biomaterials for Targeted Drug Delivery Applications | Frontiers Research Topic. Available online: https://www.frontiersin.org/research-topics/59135/biomaterials-for-targeted-drug-delivery-applications (accessed on 12 May 2024).
- Drug Delivery Systems. Available online: https://www.nibib.nih.gov/science-education/science-topics/drug-delivery-systems-getting-drugs-their-targets-controlled-manner (accessed on 12 May 2024).
- Park, H.S.; Nam, S.H.; Kim, J.; Shin, H.S.; Suh, Y.D.; Hong, K.S. Clear-Cut Observation of Clearance of Sustainable Upconverting Nanoparticles from Lymphatic System of Small Living Mice. Sci. Rep. 2016, 6, 27407. [Google Scholar] [CrossRef] [PubMed]
- Sykes, E.A.; Dai, Q.; Sarsons, C.D.; Chen, J.; Rocheleau, J.V.; Hwang, D.M.; Zheng, G.; Cramb, D.T.; Rinker, K.D.; Chan, W.C.W. Tailoring Nanoparticle Designs to Target Cancer Based on Tumor Pathophysiology. Proc. Natl. Acad. Sci. U. S. A. 2016, 113, E1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yeo, Y. Controlled Drug Release from Pharmaceutical Nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.A.; Saleh, A.M. Applications of Nanoparticle Systems in Drug Delivery Technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Ma, Y.; Yu, S.; Ji, C. Smart Multifunctional Magnetic Nanoparticle-Based Drug Delivery System for Cancer Thermo-Chemotherapy and Intracellular Imaging. ACS Appl. Mater. Interfaces 2016, 8, 24502–24508. [Google Scholar] [CrossRef] [PubMed]
- Ulijn, R.V.; Bibi, N.; Jayawarna, V.; Thornton, P.D.; Todd, S.J.; Mart, R.J.; Smith, A.M.; Gough, J.E. Bioresponsive Hydrogels. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Advanced Drug Delivery Micro- and Nanosystems for Cardiovascular Diseases. Mol. Basel Switz. 2022, 27, 5843. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Clegg, J.R.; Anselmo, A.C.; Mitragotri, S. Hydrogels in the Clinic. Bioeng. Transl. Med. 2020, 5, e10158. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F. Microparticles Used as Drug Delivery Systems. In Proceedings of the Smart Colloidal Materials; Richtering, W., Ed.; Springer: Berlin, Heidelberg, 2006; pp. 15–21. [Google Scholar]
- El-Sherbiny, I.M.; El-Baz, N.M.; Yacoub, M.H. Inhaled Nano- and Microparticles for Drug Delivery. Glob. Cardiol. Sci. Pract. 2015, 2015, 2. [Google Scholar] [CrossRef]
- Chaudhry, R.; Miao, J.H.; Rehman, A. Physiology, Cardiovascular. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2024. [Google Scholar]
- Olvera Lopez, E.; Ballard, B.D.; Jan, A. Cardiovascular Disease. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2024. [Google Scholar]
- Nii, T.; Katayama, Y. Biomaterial-Assisted Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 8657. [Google Scholar] [CrossRef] [PubMed]
- Milazzi Kovačević, R. Biomaterijali u kardiovaskularnoj medicini. info:eu-repo/semantics/bachelorThesis, University of Zagreb. Faculty of Mechanical Engineering and Naval Architecture, 2022.
- Padera, R.F.; Schoen, F.J. 2.5.2a - Cardiovascular Medical Devices: Heart Valves, Pacemakers and Defibrillators, Mechanical Circulatory Support, and Other Intracardiac Devices. In Biomaterials Science (Fourth Edition); Wagner, W.R., Sakiyama-Elbert, S.E., Zhang, G., Yaszemski, M.J., Eds.; Academic Press, 2020; pp. 999–1032 ISBN 978-0-12-816137-1.
- Taghizadeh, B.; Ghavami, L.; Derakhshankhah, H.; Zangene, E.; Razmi, M.; Jaymand, M.; Zarrintaj, P.; Zarghami, N.; Jaafari, M.R.; Moallem Shahri, M.; et al. Biomaterials in Valvular Heart Diseases. Front. Bioeng. Biotechnol. 2020, 8, 529244. [Google Scholar] [CrossRef] [PubMed]
- Malisz, K.; Świeczko-Żurek, B. VASCULAR STENTS -MATERIALS AND MANUFACTURING TECHNOLOGIES. 2022, 166, 22–28. [CrossRef]
- Kuchinka, J.; Willems, C.; Telyshev, D.V.; Groth, T. Control of Blood Coagulation by Hemocompatible Material Surfaces-A Review. Bioeng. Basel Switz. 2021, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Al-Shalawi, F.D.; Mohamed Ariff, A.H.; Jung, D.-W.; Mohd Ariffin, M.K.A.; Seng Kim, C.L.; Brabazon, D.; Al-Osaimi, M.O. Biomaterials as Implants in the Orthopedic Field for Regenerative Medicine: Metal versus Synthetic Polymers. Polymers 2023, 15, 2601. [Google Scholar] [CrossRef] [PubMed]
- Im, G.-I. Biomaterials in Orthopaedics: The Past and Future with Immune Modulation. Biomater. Res. 2020, 24, 7. [Google Scholar] [CrossRef] [PubMed]
- Amirtharaj Mosas, K.K.; Chandrasekar, A.R.; Dasan, A.; Pakseresht, A.; Galusek, D. Recent Advancements in Materials and Coatings for Biomedical Implants. Gels 2022, 8, 323. [Google Scholar] [CrossRef] [PubMed]
- Dermeik, B.; Travitzky, N. Laminated Object Manufacturing of Ceramic-Based Materials. Adv. Eng. Mater. 2020, 22, 2000256. [Google Scholar] [CrossRef]
- Kurtz, S.M. Chapter 1: Primer on Ceramic Biomaterials in Orthopedics. Available online: https://biomed.drexel.edu/orthoceramics/Encyclopedia/chapter1.html (accessed on 12 May 2024).
- Reichert, C.L.; Bugnicourt, E.; Coltelli, M.-B.; Cinelli, P.; Lazzeri, A.; Canesi, I.; Braca, F.; Martínez, B.M.; Alonso, R.; Agostinis, L.; et al. Bio-Based Packaging: Materials, Modifications, Industrial Applications and Sustainability. Polymers 2020, 12, 1558. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Bhatnagar, D.; Bansal, D.; Batra, H.; Goyal, N. Recent Advancements in Nanomaterials for Biomedical Implants. Biomed. Eng. Adv. 2022, 3, 100029. [Google Scholar] [CrossRef]
- Georgeanu, V.A.; Gingu, O.; Antoniac, I.V.; Manolea, H.O. Current Options and Future Perspectives on Bone Graft and Biomaterials Substitutes for Bone Repair, from Clinical Needs to Advanced Biomaterials Research. Appl. Sci. 2023, 13, 8471. [Google Scholar] [CrossRef]
- Pinto, P.O.; Branquinho, M.V.; Caseiro, A.R.; Sousa, A.C.; Brandão, A.; Pedrosa, S.S.; Alvites, R.D.; Campos, J.M.; Santos, F.L.; Santos, J.D.; et al. The Application of Bonelike® Poro as a Synthetic Bone Substitute for the Management of Critical-Sized Bone Defects - A Comparative Approach to the Autograft Technique - A Preliminary Study. Bone Rep. 2021, 14, 101064. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.; Sheppard, M.; Gorasia, G.; Arora, P.; Cooper, M.; Mulligan, S. Drivers, Opportunities and Best Practice for Sustainability in Dentistry: A Scoping Review. J. Dent. 2021, 112, 103737. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.P. Bone Grafts in Dental Medicine: An Overview of Autografts, Allografts and Synthetic Materials. Materials 2023, 16, 4117. [Google Scholar] [CrossRef]
- Kim, J.; Koo, B.-K.; Knoblich, J.A. Human Organoids: Model Systems for Human Biology and Medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhao, M.; Lash, B.; Martino, M.M.; Julier, Z. Growth Factor Engineering Strategies for Regenerative Medicine Applications. Front. Bioeng. Biotechnol. 2020, 7, 469. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Garcia, A.; Rodriguez-Merchan, E.C. Orthobiologics: Current Role in Orthopedic Surgery and Traumatology. Arch. Bone Jt. Surg. 2022, 10, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Kawai, T.; Goto, K.; Matsuda, S. Clinical Application of Injectable Growth Factor for Bone Regeneration: A Systematic Review. Inflamm. Regen. 2019, 39, 20. [Google Scholar] [CrossRef] [PubMed]
- Pokkalath, A.; Nadar, D.; Ravikumar, P.; Sawarkar, S.P. Chapter 11 - Nanomaterials for Orthopaedic Implants and Applications. In Handbook on Nanobiomaterials for Therapeutics and Diagnostic Applications; Anand, K., Saravanan, M., Chandrasekaran, B., Kanchi, S., Jeeva Panchu, S., Chen, Q., Eds.; Elsevier, 2021; pp. 229–270 ISBN 978-0-12-821013-0.
- Hajiali, H.; Ouyang, L.; Llopis-Hernandez, V.; Dobre, O.; Rose, F.R.A.J. Review of Emerging Nanotechnology in Bone Regeneration: Progress, Challenges, and Perspectives. Nanoscale 2021, 13, 10266–10280. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Mitra, I.; Bose, S. 3D Printing for Bone Regeneration. Curr. Osteoporos. Rep. 2020, 18, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Wixted, C.M.; Peterson, J.R.; Kadakia, R.J.; Adams, S.B. Three-Dimensional Printing in Orthopaedic Surgery: Current Applications and Future Developments. JAAOS Glob. Res. Rev. 2021, 5, e20–00230. [Google Scholar] [CrossRef] [PubMed]
- Gowda, B.H.J.; Ahmed, M.G.; Chinnam, S.; Paul, K.; Ashrafuzzaman, M.; Chavali, M.; Gahtori, R.; Pandit, S.; Kesari, K.K.; Gupta, P.K. Current Trends in Bio-Waste Mediated Metal/Metal Oxide Nanoparticles for Drug Delivery. J. Drug Deliv. Sci. Technol. 2022, 71, 103305. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, P.; Fu, Z.; Meng, S.; Dai, L.; Yang, H. Applications of Nanomaterials in Tissue Engineering. RSC Adv. 2021, 11, 19041–19058. [Google Scholar] [CrossRef]
- Triantafyllopoulos, I.K.; Papaioannou, N.A. Application of Nanotechnology in Medicine. Smart Biomaterials and Biosensors. Acta Orthop. Traumatol. Hell. 2022, 73. [Google Scholar]
- Farooque, F.; Wasi, M.; Mughees, M.M. Liposomes as Drug Delivery System: An Updated Review. J. Drug Deliv. Ther. 2021, 11, 149–158. [Google Scholar] [CrossRef]
- Vinchurkar, R.H.; Kuchekar, A.B. Polymeric Micelles: A Novel Approach towards Nano-Drug Delivery System. Biosci. Biotechnol. Res. Asia 2021, 18, 629–649. [Google Scholar] [CrossRef]
- Panda, M.K.; Panda, S.K.; Singh, Y.D.; Jit, B.P.; Behara, R.K.; Dhal, N.K. Role of Nanoparticles and Nanomaterials in Drug Delivery: An Overview. In Advances in Pharmaceutical Biotechnology: Recent Progress and Future Applications; Patra, J.K., Shukla, A.C., Das, G., Eds.; Springer: Singapore, 2020; ISBN 9789811521959. [Google Scholar]
- Patel, V.; Rajani, C.; Paul, D.; Borisa, P.; Rajpoot, K.; Youngren-Ortiz, S.R.; Tekade, R.K. Chapter 8 - Dendrimers as Novel Drug-Delivery System and Its Applications. In Drug Delivery Systems; Tekade, R.K., Ed.; Advances in Pharmaceutical Product Development and Research; Academic Press, 2020; pp. 333–392 ISBN 978-0-12-814487-9.
- Eftekhari, A.; Maleki Dizaj, S.; Sharifi, S.; Salatin, S.; Rahbar Saadat, Y.; Zununi Vahed, S.; Samiei, M.; Ardalan, M.; Rameshrad, M.; Ahmadian, E.; et al. The Use of Nanomaterials in Tissue Engineering for Cartilage Regeneration; Current Approaches and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 536. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Hong, X.; Wang, J.; Feng, L.; Fan, T.; Guo, R.; Zhang, H. 2D Nanomaterials for Tissue Engineering and Regenerative Nanomedicines: Recent Advances and Future Challenges. Adv. Healthc. Mater. 2021, 10, 2001743. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Gherasim, O.; Gherasim, T.G.; Grumezescu, V.; Grumezescu, A.M.; Teleanu, D.M. Nanomaterial-Based Approaches for Neural Regeneration. Pharmaceutics 2019, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- R. Amin, D.; Sink, E.; Narayan, S.P.; Abdel-Hafiz, M.; Mestroni, L.; Peña, B. Nanomaterials for Cardiac Tissue Engineering. Molecules 2020, 25, 5189. [Google Scholar] [CrossRef]
- Wang, W.; Lu, K.; Yu, C.; Huang, Q.; Du, Y.-Z. Nano-Drug Delivery Systems in Wound Treatment and Skin Regeneration. J. Nanobiotechnology 2019, 17, 82. [Google Scholar] [CrossRef]
- Todros, S.; Todesco, M.; Bagno, A. Biomaterials and Their Biomedical Applications: From Replacement to Regeneration. Processes 2021, 9, 1949. [Google Scholar] [CrossRef]
- Gv, J.; Setti, S.G. Bio-Engineering and Bio-Design of New Generation Bioresorbable Implants. Indian J. Biochem. Biophys. IJBB 2021, 58, 118–126. [Google Scholar] [CrossRef]
- Vach Agocsova, S.; Culenova, M.; Birova, I.; Omanikova, L.; Moncmanova, B.; Danisovic, L.; Ziaran, S.; Bakos, D.; Alexy, P. Resorbable Biomaterials Used for 3D Scaffolds in Tissue Engineering: A Review. Mater. Basel Switz. 2023, 16, 4267. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, M.; Kędra, K. Biomedical Polyurethanes for Anti-Cancer Drug Delivery Systems: A Brief, Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 8181. [Google Scholar] [CrossRef]
- Wei, S.; Ma, J.-X.; Xu, L.; Gu, X.-S.; Ma, X.-L. Biodegradable Materials for Bone Defect Repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Fathi, M.; Savabi, O.; Tayebi, L.; Vashaee, D. Biodegradable Magnesium Bone Implants Coated with a Novel Bioceramic Nanocomposite. Mater. Basel Switz. 2020, 13, 1315. [Google Scholar] [CrossRef] [PubMed]
- Schuh, J.C.L.; Funk, K.A. Compilation of International Standards and Regulatory Guidance Documents for Evaluation of Biomaterials, Medical Devices, and 3-D Printed and Regenerative Medicine Products. Toxicol. Pathol. 2019, 47, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Hunckler, M.D.; Levine, A.D. Navigating Ethical Challenges in the Development and Translation of Biomaterials Research. Front. Bioeng. Biotechnol. 2022, 10, 949280. [Google Scholar] [CrossRef] [PubMed]
- Hyun, I.; Scharf-Deering, J.C.; Lunshof, J.E. Ethical Issues Related to Brain Organoid Research. Brain Res. 2020, 1732, 146653. [Google Scholar] [CrossRef] [PubMed]
- Sekar, M.P.; Budharaju, H.; Zennifer, A.; Sethuraman, S.; Vermeulen, N.; Sundaramurthi, D.; Kalaskar, D.M. Current Standards and Ethical Landscape of Engineered Tissues-3D Bioprinting Perspective. J. Tissue Eng. 2021, 12, 20417314211027677. [Google Scholar] [CrossRef] [PubMed]
- Bu, W.; Wu, Y.; Ghaemmaghami, A.M.; Sun, H.; Mata, A. Rational Design of Hydrogels for Immunomodulation. Regen. Biomater. 2022, 9, rbac009. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Xiong, Y.; Knoedler, S.; Lu, L.; Panayi, A.C.; Alfertshofer, M.; Jiang, D.; Rinkevich, Y.; Lin, Z.; Zhao, Z.; et al. Immunomodulatory Nanosystems: Advanced Delivery Tools for Treating Chronic Wounds. Res. Wash. DC 2023, 6, 0198. [Google Scholar] [CrossRef] [PubMed]
- Raut, H.K.; Das, R.; Liu, Z.; Liu, X.; Ramakrishna, S. Biocompatibility of Biomaterials for Tissue Regeneration or Replacement. Biotechnol. J. 2020, 15, e2000160. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Kolahreez, D.; Ramakrishna, S.; Williams, D. Key Terminology in Biomaterials and Biocompatibility. Curr. Opin. Biomed. Eng. 2019, 10, 45–50. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, H.; Wang, M.; Mantovani, D.; Yang, K.; Witte, F.; Tan, L.; Yue, B.; Qu, X. Immunomodulatory Biomaterials against Bacterial Infections: Progress, Challenges, and Future Perspectives. Innov. Camb. Mass 2023, 4, 100503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mou, L.; Jiang, X. Surface Chemistry of Gold Nanoparticles for Health-Related Applications. Chem. Sci. 2020, 11, 923–936. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Mitra, I.; Goodman, S.B.; Kumar, M.; Bose, S. Improving Biocompatibility for Next Generation of Metallic Implants. Prog. Mater. Sci. 2023, 133, 101053. [Google Scholar] [CrossRef] [PubMed]
- Jurak, M.; Wiącek, A.E.; Ładniak, A.; Przykaza, K.; Szafran, K. What Affects the Biocompatibility of Polymers? Adv. Colloid Interface Sci. 2021, 294, 102451. [Google Scholar] [CrossRef] [PubMed]
- Naziya sayeed, R.K. Scaffolds In Tissue Engineering. Ann. Romanian Soc. Cell Biol. 2020, 142–152. [Google Scholar]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Chen, Y.; Wang, X.; Xu, X.; Shen, Y.; Khan, A.U.R.; Aldalbahi, A.; Fetz, A.E.; Bowlin, G.L.; El-Newehy, M.; et al. Electrospinning Nanofiber Scaffolds for Soft and Hard Tissue Regeneration. J. Mater. Sci. Technol. 2020, 59, 243–261. [Google Scholar] [CrossRef]
- Shahi, S.; Karbasi, S.; Ahmadi, T.; Naeimi, F.; Goodarzi, V.; Ebrahimi-Barough, S. Evaluation of Physical, Mechanical and Biological Properties of β-Tri-Calcium Phosphate/Poly-3-Hydroxybutyrate Nano Composite Scaffold for Bone Tissue Engineering Application. Mater. Technol. 2021, 36, 237–249. [Google Scholar] [CrossRef]
- Luo, Y. Chapter 19 - Three-Dimensional Scaffolds. In Principles of Tissue Engineering (Fifth Edition); Lanza, R., Langer, R., Vacanti, J.P., Atala, A., Eds.; Academic Press, 2020; pp. 343–360 ISBN 978-0-12-818422-6.
- Zhang, Y.; Zhang, M.; Cheng, D.; Xu, S.; Du, C.; Xie, L.; Zhao, W. Applications of Electrospun Scaffolds with Enlarged Pores in Tissue Engineering. Biomater. Sci. 2022, 10, 1423–1447. [Google Scholar] [CrossRef] [PubMed]
- Lock, A.; Cornish, J.; Musson, D.S. The Role of In Vitro Immune Response Assessment for Biomaterials. J. Funct. Biomater. 2019, 10, 31. [Google Scholar] [CrossRef]
- Kirillova, A.; Yeazel, T.R.; Asheghali, D.; Petersen, S.R.; Dort, S.; Gall, K.; Becker, M.L. Fabrication of Biomedical Scaffolds Using Biodegradable Polymers. Chem. Rev. 2021, 121, 11238–11304. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A. The Summary of the Most Important Cell-Biomaterial Interactions That Need to Be Considered during in Vitro Biocompatibility Testing of Bone Scaffolds for Tissue Engineering Applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 1036–1051. [Google Scholar] [CrossRef]
- Yu, J.R.; Navarro, J.; Coburn, J.C.; Mahadik, B.; Molnar, J.; Holmes, J.H.; Nam, A.J.; Fisher, J.P. Current and Future Perspectives on Skin Tissue Engineering: Key Features of Biomedical Research, Translational Assessment, and Clinical Application. Adv. Healthc. Mater. 2019, 8, e1801471. [Google Scholar] [CrossRef] [PubMed]
- Othman, Z.; Cillero Pastor, B.; van Rijt, S.; Habibovic, P. Understanding Interactions between Biomaterials and Biological Systems Using Proteomics. Biomaterials 2018, 167, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Basu, B. Probing Toxicity of Biomaterials and Biocompatibility Assessment. In Biomaterials for Musculoskeletal Regeneration: Concepts; Basu, B., Ed.; Springer: Singapore, 2017; ISBN 978-981-10-3059-8. [Google Scholar]
- Natarajan, A.B.Mt.; Sivadas, V.P.D.; Nair, P.D.P.D. 3D-Printed Biphasic Scaffolds for the Simultaneous Regeneration of Osteochondral Tissues. Biomed. Mater. 2021, 16, 054102. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.B.; Englund, E.K.; Chen, S.; Frank, L.R.; Ward, S.R. Medical Imaging of Tissue Engineering and Regenerative Medicine Constructs. Biomater. Sci. 2021, 9, 301–314. [Google Scholar] [CrossRef]
- Wasyłeczko, M.; Sikorska, W.; Chwojnowski, A. Review of Synthetic and Hybrid Scaffolds in Cartilage Tissue Engineering. Membranes 2020, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, D.; Dey, N.; Bhardwaj, N.; Mandal, B.B. Emerging and Innovative Approaches for Wound Healing and Skin Regeneration: Current Status and Advances. Biomaterials 2019, 216, 119267. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.; Qu, K.-Y.; Zhang, X.-F.; Huang, N.-P. Recent Advances in the Application of Two-Dimensional Nanomaterials for Neural Tissue Engineering and Regeneration. ACS Biomater. Sci. Eng. 2021, 7, 3503–3529. [Google Scholar] [CrossRef] [PubMed]
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular Matrix-Based Materials for Regenerative Medicine. Nat. Rev. Mater. 2018, 3, 159–173. [Google Scholar] [CrossRef]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Mater. Basel Switz. 2015, 8, 5744–5794. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Ali, O.; Han, S.-B.; Kim, D.-H. Mechanobiological Strategies to Enhance Stem Cell Functionality for Regenerative Medicine and Tissue Engineering. Front. Cell Dev. Biol. 2021, 9, 747398. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Handy, R.D.; Upton, M.; Besinis, A. Review of Antimicrobial Nanocoatings in Medicine and Dentistry: Mechanisms of Action, Biocompatibility Performance, Safety, and Benefits Compared to Antibiotics. ACS Nano 2023, 17, 7064–7092. [Google Scholar] [CrossRef] [PubMed]
- Bason, C.; Gallorini, M.; Berardi, A.C. The Extracellular Matrix, Growth Factors and Morphogens in Biomaterial Design and Tissue Engineering. In Extracellular Matrix for Tissue Engineering and Biomaterials; Berardi, A.C., Ed.; Springer International Publishing: Cham, 2018; ISBN 978-3-319-77023-9. [Google Scholar]
- Mahla, R.S. Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int. J. Cell Biol. 2016, 2016, 6940283. [Google Scholar] [CrossRef] [PubMed]
- Savoji, H.; Godau, B.; Hassani, M.S.; Akbari, M. Skin Tissue Substitutes and Biomaterial Risk Assessment and Testing. Front. Bioeng. Biotechnol. 2018, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Fang, H.; Wang, C.; Wang, Y.; Qi, C.; Zhang, Y.; Zhou, Q.; Huang, M.; Wang, M.; Wu, M. 3D Printing of Customized Functional Devices for Smart Biomedical Systems. SmartMat n/a. [CrossRef]
- Julier, Z.; Park, A.J.; Briquez, P.S.; Martino, M.M. Promoting Tissue Regeneration by Modulating the Immune System. Acta Biomater. 2017, 53, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Stace, E.T.; Dakin, S.G.; Mouthuy, P.-A.; Carr, A.J. Translating Regenerative Biomaterials Into Clinical Practice. J. Cell. Physiol. 2016, 231, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, V.; Cui, X.T. Carbohydrate Based Biomaterials for Neural Interfacing. J. Mater. Chem. B 2022, 10, 4714–4740. [Google Scholar] [CrossRef] [PubMed]
- Hatsopoulos, N.G.; Donoghue, J.P. The Science of Neural Interface Systems. Annu. Rev. Neurosci. 2009, 32, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Beygi, M.; Bentley, J.T.; Frewin, C.L.; Kuliasha, C.A.; Takshi, A.; Bernardin, E.K.; La Via, F.; Saddow, S.E. Fabrication of a Monolithic Implantable Neural Interface from Cubic Silicon Carbide. Micromachines 2019, 10, 430. [Google Scholar] [CrossRef]
- Pisciotta, A.; Lunghi, A.; Bertani, G.; Di Tinco, R.; Bertoni, L.; Orlandi, G.; Biscarini, F.; Bianchi, M.; Carnevale, G. PEDOT: PSS Promotes Neurogenic Commitment of Neural Crest-Derived Stem Cells. Front. Physiol. 2022, 13, 930804. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Pajorova, J.; Tomkova, M.; Matejka, R.; Broz, A.; Stepanovska, J.; Prazak, S.; Skogberg, A.; Siljander, S.; Kallio, P. Applications of Nanocellulose/Nanocarbon Composites: Focus on Biotechnology and Medicine. Nanomaterials 2020, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Barrese, J.C.; Rao, N.; Paroo, K.; Triebwasser, C.; Vargas-Irwin, C.; Franquemont, L.; Donoghue, J.P. Failure Mode Analysis of Silicon-Based Intracortical Microelectrode Arrays in Non-Human Primates. J. Neural Eng. 2013, 10, 066014. [Google Scholar] [CrossRef] [PubMed]
- Knaack, G.L.; McHail, D.G.; Borda, G.; Koo, B.; Peixoto, N.; Cogan, S.F.; Dumas, T.C.; Pancrazio, J.J. In Vivo Characterization of Amorphous Silicon Carbide As a Biomaterial for Chronic Neural Interfaces. Front. Neurosci. 2016, 10, 301. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.J.; Kolarcik, C.L.; Kozai, T.D.Y.; Luebben, S.D.; Sapp, S.A.; Zheng, X.S.; Nabity, J.A.; Cui, X.T. Ultrasoft Microwire Neural Electrodes Improve Chronic Tissue Integration. Acta Biomater. 2017, 53, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.C.; Sy, J.C.; Falcón-Banchs, R.; Cima, M.J. Three Dimensional In Vitro Glial Scar Model to Investigate Local Strain Effects from Micromotion around Neural Implants. Lab. Chip 2017, 17, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Sharif, N.A. Electrical, Electromagnetic, Ultrasound Wave Therapies, and Electronic Implants for Neuronal Rejuvenation, Neuroprotection, Axonal Regeneration, and IOP Reduction. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2023, 39, 477–498. [Google Scholar] [CrossRef]
- Luan, L.; Robinson, J.T.; Aazhang, B.; Chi, T.; Yang, K.; Li, X.; Rathore, H.; Singer, A.; Yellapantula, S.; Fan, Y.; et al. Recent Advances in Electrical Neural Interface Engineering: Minimal Invasiveness, Longevity and Scalability. Neuron 2020, 108, 302. [Google Scholar] [CrossRef] [PubMed]
- Mridha, M.F.; Das, S.C.; Kabir, M.M.; Lima, A.A.; Islam, Md.R.; Watanobe, Y. Brain-Computer Interface: Advancement and Challenges. Sensors 2021, 21, 5746. [Google Scholar] [CrossRef]
- Lee, M.B.; Kramer, D.R.; Peng, T.; Barbaro, M.F.; Liu, C.Y.; Kellis, S.; Lee, B. Clinical Neuroprosthetics: Today and Tomorrow. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2019, 68, 13–19. [Google Scholar] [CrossRef]
- Cantillo-Negrete, J.; Carino-Escobar, R.I.; Ortega-Robles, E.; Arias-Carrión, O. A Comprehensive Guide to BCI-Based Stroke Neurorehabilitation Interventions. MethodsX 2023, 11, 102452. [Google Scholar] [CrossRef]
- Chen, J.; Xia, Y.; Zhou, X.; Vidal Rosas, E.; Thomas, A.; Loureiro, R.; Cooper, R.J.; Carlson, T.; Zhao, H. fNIRS-EEG BCIs for Motor Rehabilitation: A Review. Bioengineering 2023, 10, 1393. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, S.; Yang, Y.; Zhang, K.; He, E.; Liang, W.; Luo, J.; Wu, Y.; Cai, X. Nanomaterial-Based Microelectrode Arrays for in Vitro Bidirectional Brain–Computer Interfaces: A Review. Microsyst. Nanoeng. 2023, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.N.; Chou, N.; Jang, J.-W.; Choe, H.K.; Kim, S. A 3D Flexible Neural Interface Based on a Microfluidic Interconnection Cable Capable of Chemical Delivery. Microsyst. Nanoeng. 2021, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Mukae, N.; Arata, J.; Iwata, H.; Iramina, K.; Iihara, K.; Hashizume, M. A Multichannel-near-Infrared-Spectroscopy-Triggered Robotic Hand Rehabilitation System for Stroke Patients. IEEE Int. Conf. Rehabil. Robot. Proc. 2017, 2017, 158–163. [Google Scholar] [CrossRef]
- Rapoport, B.I.; Kedzierski, J.T.; Sarpeshkar, R. A Glucose Fuel Cell for Implantable Brain–Machine Interfaces. PLOS ONE 2012, 7, e38436. [Google Scholar] [CrossRef] [PubMed]
- Bonizzato, M. Neuroprosthetics: An Outlook on Active Challenges toward Clinical Adoption. J. Neurophysiol. 2021, 125, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Vardalakis, N.; Wagner, F.B. Neuroprosthetics: From Sensorimotor to Cognitive Disorders. Commun. Biol. 2023, 6, 14. [Google Scholar] [CrossRef]
- Rahman, M.T.; Chari, D.; Ishiyama, G.; Lopez, I.; Quesnel, A.M.; Ishiyama, A.; Nadol, J.B.; Hansen, M.R. Cochlear Implants: Causes, Effects and Mitigation Strategies for the Foreign Body Response and Inflammation. Hear. Res. 2022, 422, 108536. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Das, R.; Iversen, H.K.; Puthusserypady, S. Review on Motor Imagery Based BCI Systems for Upper Limb Post-Stroke Neurorehabilitation: From Designing to Application. Comput. Biol. Med. 2020, 123, 103843. [Google Scholar] [CrossRef]
- Flesher, S.N.; Downey, J.E.; Weiss, J.M.; Hughes, C.L.; Herrera, A.J.; Tyler-Kabara, E.C.; Boninger, M.L.; Collinger, J.L.; Gaunt, R.A. A Brain-Computer Interface That Evokes Tactile Sensations Improves Robotic Arm Control. Science 2021, 372, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Malek, N. Deep Brain Stimulation in Parkinson’s Disease. Neurol. India 2019, 67, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Niketeghad, S.; Pouratian, N. Brain Machine Interfaces for Vision Restoration: The Current State of Cortical Visual Prosthetics. Neurotherapeutics 2019, 16, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Speier, W.; Arnold, C.; Pouratian, N. Integrating Language Models into Classifiers for BCI Communication: A Review. J. Neural Eng. 2016, 13, 031002. [Google Scholar] [CrossRef] [PubMed]
- Wolpaw, J.R.; Thompson, A.K. Enhancing Neurorehabilitation by Targeting Beneficial Plasticity. Front. Rehabil. Sci. 2023, 4, 1198679. [Google Scholar] [CrossRef] [PubMed]
- Golabchi, A.; Woeppel, K.M.; Li, X.; Lagenaur, C.F.; Cui, X.T. Neuroadhesive Protein Coating Improves the Chronic Performance of Neuroelectronics in Mouse Brain. Biosens. Bioelectron. 2020, 155, 112096. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Perez, A.; Villa, R.; Sanchez-Vives, M.; Guimerà-Brunet, A.; Hernández-Ferrer, J. Quantification of Signal-to-Noise Ratio in Cerebral Cortex Recordings Using Flexible MEAs With Co-Localized Platinum Black, Carbon Nanotubes, and Gold Electrodes. Front. Neurosci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-C.; Wang, M.-H.; Ge, C.-F.; Ji, B.-W.; Guo, Z.-J.; Wang, X.-L.; Yang, B.; Li, C.-Y.; Liu, J.-Q. The Use of a Double-Layer Platinum Black-Conducting Polymer Coating for Improvement of Neural Recording and Mitigation of Photoelectric Artifact. Biosens. Bioelectron. 2019, 145, 111661. [Google Scholar] [CrossRef] [PubMed]
- Atmaramani, R.; Chakraborty, B.; Rihani, R.T.; Usoro, J.; Hammack, A.; Abbott, J.; Nnoromele, P.; Black, B.J.; Pancrazio, J.J.; Cogan, S.F. Ruthenium Oxide Based Microelectrode Arrays for in Vitro and in Vivo Neural Recording and Stimulation. Acta Biomater. 2020, 101, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Veerapandian, M.; Avti, P.K.; Ravichandiran, V. Ruthenium Bipyridine Sensitized MoO3 Multifunctional Nanostructures: Study of Opto-Electrochemical Properties, Biocompatibility and Bioimaging. Colloids Surf. B Biointerfaces 2017, 154, 315–320. [Google Scholar] [CrossRef]
- Rao, S.S.; Han, N.; Winter, J.O. Polylysine-Modified PEG-Based Hydrogels to Enhance the Neuro–Electrode Interface. J. Biomater. Sci. Polym. Ed. 2011, 22, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Giliopoulos, D.; Zamboulis, A.; Giannakoudakis, D.; Bikiaris, D.; Triantafyllidis, K. Polymer/Metal Organic Framework (MOF) Nanocomposites for Biomedical Applications. Molecules 2020, 25, 185. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Liu, Y.; Xiao, L.; Zhang, C. Advanced Metallic and Polymeric Coatings for Neural Interfacing: Structures, Properties and Tissue Responses. Polymers 2021, 13, 2834. [Google Scholar] [CrossRef] [PubMed]
- Merrill, D.R.; Stecker, M.M. Chapter 7 - The Electrode—Principles of the Neural Interface and Materials. In Essential Neuromodulation (Second Edition); Arle, J.E., Shils, J.L., Eds.; Academic Press, 2022; pp. 131–174 ISBN 978-0-12-817000-7.
- Zhang, Y.; Le, S.; Li, H.; Ji, B.; Wang, M.-H.; Tao, J.; Liang, J.-Q.; Zhang, X.-Y.; Kang, X.-Y. MRI Magnetic Compatible Electrical Neural Interface: From Materials to Application. Biosens. Bioelectron. 2021, 194, 113592. [Google Scholar] [CrossRef]
- Sung, C.; Jeon, W.; Nam, K.S.; Kim, Y.; Butt, H.; Park, S. Multimaterial and Multifunctional Neural Interfaces: From Surface-Type and Implantable Electrodes to Fiber-Based Devices. J. Mater. Chem. B 2020, 8, 6624–6666. [Google Scholar] [CrossRef] [PubMed]
- Vajrala, V.S.; Saunier, V.; Nowak, L.G.; Flahaut, E.; Bergaud, C.; Maziz, A. Nanofibrous PEDOT-Carbon Composite on Flexible Probes for Soft Neural Interfacing. Front. Bioeng. Biotechnol. 2021, 9, 780197. [Google Scholar] [CrossRef] [PubMed]
- Holmkvist, A.D.; Agorelius, J.; Forni, M.; Nilsson, U.J.; Linsmeier, C.E.; Schouenborg, J. Local Delivery of Minocycline-Loaded PLGA Nanoparticles from Gelatin-Coated Neural Implants Attenuates Acute Brain Tissue Responses in Mice. J. Nanobiotechnology 2020, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Krukiewicz, K.; Britton, J.; Więcławska, D.; Skorupa, M.; Fernandez, J.; Sarasua, J.-R.; Biggs, M.J.P. Electrical Percolation in Extrinsically Conducting, Poly(ε-Decalactone) Composite Neural Interface Materials. Sci. Rep. 2021, 11, 1295. [Google Scholar] [CrossRef] [PubMed]
- Mallavarapu, A.; Ajay, P.; Sreenivasan, S.V. Enabling Ultrahigh-Aspect-Ratio Silicon Nanowires Using Precise Experiments for Detecting the Onset of Collapse. Nano Lett. 2020, 20, 7896–7905. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Liu, B.; Fan, G.; Glamazda, A.; Ludwig, F.; Wulferding, D.; Schilling, M.; Gao, R.; Lemmens, P. Tailoring the Surface Plasmon Resonance Energy of Au Nanowire Arrays by Defect Management and Thermal Treatment. Phys. E Low-Dimens. Syst. Nanostructures 2020, 121, 114092. [Google Scholar] [CrossRef]
- Park, Y.; Chung, T.S.; Lee, G.; Rogers, J.A. Materials Chemistry of Neural Interface Technologies and Recent Advances in Three-Dimensional Systems. Chem. Rev. 2022, 122, 5277–5316. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, C.J.; Ecker, M.; Kozai, T.D.Y.; Malliaras, G.G.; Meng, E.; Voit, W. Recent Advances in Neural Interfaces—Materials Chemistry to Clinical Translation. MRS Bull. 2020, 45, 655–668. [Google Scholar] [CrossRef]
- Aktas, B.; Ozgun, A.; Kilickap, B.D.; Garipcan, B. Cell Adhesion Molecule Immobilized Gold Surfaces for Enhanced Neuron-Electrode Interfaces. J. Biomed. Mater. Res. B Appl. Biomater. 2024, 112, e35310. [Google Scholar] [CrossRef]
- Domínguez-Bajo, A.; Rosa, J.M.; González-Mayorga, A.; Rodilla, B.L.; Arché-Núñez, A.; Benayas, E.; Ocón, P.; Pérez, L.; Camarero, J.; Miranda, R.; et al. Nanostructured Gold Electrodes Promote Neural Maturation and Network Connectivity. Biomaterials 2021, 279, 121186. [Google Scholar] [CrossRef]
- Pan, T.; Lu, D.; Xin, H.; Li, B. Biophotonic Probes for Bio-Detection and Imaging. Light Sci. Appl. 2021, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Karatum, O.; Nizamoglu, S. Optoelectronic Neural Interfaces Based on Quantum Dots. ACS Appl. Mater. Interfaces 2022, 14, 20468–20490. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Hoseini, S.J.; Milan, P.B.; Hooshmand, S.; Kim, H.-W.; Mozafari, M. Quantum Dots: A Review from Concept to Clinic. Biotechnol. J. 2020, 15, 2000117. [Google Scholar] [CrossRef]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Mol. Basel Switz. 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Cook, K.A.; Martinez-Lozano, E.; Sheridan, R.; Rodriguez, E.K.; Nazarian, A.; Grinstaff, M.W. Hydrogels for the Management of Second-Degree Burns: Currently Available Options and Future Promise. Burns Trauma 2022, 10, tkac047. [Google Scholar] [CrossRef] [PubMed]
- Shahjehan, R.D.; Bhutta, B.S. Coronary Artery Disease. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2024. [Google Scholar]
- Im, S.H.; Im, D.H.; Park, S.J.; Jung, Y.; Kim, D.-H.; Kim, S.H. Current Status and Future Direction of Metallic and Polymeric Materials for Advanced Vascular Stents. Prog. Mater. Sci. 2022, 126, 100922. [Google Scholar] [CrossRef]
- Peng, X.; Qu, W.; Jia, Y.; Wang, Y.; Yu, B.; Tian, J. Bioresorbable Scaffolds: Contemporary Status and Future Directions. Front. Cardiovasc. Med. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Gupta, N.; Weber, D.D.S. Dental Implants. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2024. [Google Scholar]
- Alghamdi, H.S.; Jansen, J.A. The Development and Future of Dental Implants. Dent. Mater. J. 2020, 39, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.C.S.; Agrelli, A.; Andrade, A.N.; Mendes-Marques, C.L.; Arruda, I.R.S.; Santos, L.R.L.; Vasconcelos, N.F.; Machado, G. Titanium Dental Implants: An Overview of Applied Nanobiotechnology to Improve Biocompatibility and Prevent Infections. Mater. Basel Switz. 2022, 15, 3150. [Google Scholar] [CrossRef] [PubMed]
- French, D.; Ofec, R.; Levin, L. Long Term Clinical Performance of 10 871 Dental Implants with up to 22 Years of Follow-up: A Cohort Study in 4247 Patients. Clin. Implant Dent. Relat. Res. 2021, 23, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Moreddu, R.; Vigolo, D.; Yetisen, A.K. Contact Lens Technology: From Fundamentals to Applications. Adv. Healthc. Mater. 2019, 8, e1900368. [Google Scholar] [CrossRef] [PubMed]
- Gurnani, B.; Kaur, K. Contact Lenses. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2024. [Google Scholar]
- Musgrave, C.S.A.; Fang, F. Contact Lens Materials: A Materials Science Perspective. Mater. Basel Switz. 2019, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Contact Lenses 101, Part 1: Contact Lens Materials. Available online: https://coopervision.com/practitioner/ecp-viewpoints/technicians-and-staff/contact-lenses-101-part-1-contact-lens-materials (accessed on 26 May 2024).
- Fogt, J.S.; Weisenberger, K.; Fogt, N. Visual Performance with Multifocal Contact Lenses and Progressive Addition Spectacles. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2022, 45, 101472. [Google Scholar] [CrossRef] [PubMed]
- Kandel, H. Quality-of-Life Outcomes of Long-Term Contact Lens Wear: A Systematic Review. Contact Lens Anterior Eye 2022, 45, 101521. [Google Scholar] [CrossRef]
- Beygi, M.; Dominguez-Viqueira, W.; Feng, C.; Mumcu, G.; Frewin, C.L.; La Via, F.; Saddow, S.E. Silicon Carbide and MRI: Towards Developing a MRI Safe Neural Interface. Micromachines 2021, 12, 126. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).