1. Introduction
Adipocyte-derived peptides or adipokines are versatile molecules whose activity has been thoroughly screened. Among the numerous adipokines identified, adiponectin (APN) stands out as a unique and promising peptide with relevance to various disorders. APN, a widely recognized peptide hormone released by white adipose tissue, has numerous physiological functions, including glucose and lipid homeostasis, anti-inflammation, metabolic modulations, and energy homeostasis [
1]. APN also appears to regulate ocular tissue, and studies have explored its relationship to age-related macular degeneration (AMD), one of the most common retinal disorders.
AMD is a leading cause of vision loss and blindness in older populations [
2]. It is characterized by the progressive degeneration of the macula, a part of the retina responsible for central vision [
3]. Advances in retinal imaging have been pivotal in deciphering biomarkers of progression to late AMD and have revealed that the deep retinal layers of the macula region are affected during this stage, with the adjoining vascular areas causing central vision loss. AMD is broadly divided into two types, dry AMD and wet AMD, based on the disease’s initial presentation and progression [
4]. Dry AMD is associated with yellow clumps of protein and other material known as drusen, macular thinning, lack of neovascular development, and slow progression. Wet AMD, which is more severe, is marked by the growth of abnormal blood vessels in the choroid, the layer present beneath the retina rich in blood vessels [
5,
6]. The progress of wet AMD involves the invasion of vessels from both the retinal pigment epithelium area and the space of the subretinal portion, eventually leading to central vision loss. Not all dry AMD progresses to wet AMD, but all wet AMD is preceded by some form of dry AMD, even if undiagnosed.
The relationship between APN and AMD has garnered attention because APN is known to have anti-inflammatory, vascular-protective properties and regulates metabolism, which could potentially play a role in the development and progression of AMD. Many in-depth investigations have suggested the contribution of the peptide APN in ophthalmic ailments, highlighting its ability to suppress retinal pigment epithelium cell proliferation and migration [
6,
7]. Furthermore, there is a positive correlation between APN levels and various ophthalmic parameters in the body, and therapies targeting APN show potential in preventing the vision loss associated with AMD. In the current review, we will discuss APN and its association with AMD and metabolic disorders, as well as provide thorough information about its therapeutic potential.
2. Adiponectin
2.1. Structure of Adiponectin
APN is a 30-kDa, 244-amino acid (247 in mice) peptide hormone that occurs at a much higher level than other adipokines in the circulation of rodents and humans (3 to 30 mg/mL) [
8]. APN is the product of the ADIPOQ gene and is primarily produced from white adipose tissue [
8]. APN is comprised of four distinct regions: a signal peptide (N-terminal), a short variable domain, a collagen-like fibrous region, and a C-terminal domain resembling a subcomponent of the C1 complex involved in complement activation. It has multifaceted roles in various physiological processes [
1], including regulating blood glucose levels, influencing fatty acid metabolism, maintaining lipid balance, controlling whole-body energy regulation, modulating immune responses and inflammation, and affecting aging and metabolic changes [
9,
10]. Furthermore, APN is involved in safeguarding ocular tissues [
11].
APN circulates in the blood in various forms—low-molecular-weight trimeric, high-molecular-weight hexameric and multimeric forms, and a globular fragment (gAPN)—each with distinct biological activities and target tissues [
12]. Animal and epidemiological studies strongly point to high-molecular-weight APN as the key structure for insulin-sensitizing effects. The gAPN fragment seems to have similar effects as the full-length molecule in certain contexts, particularly in studies using tumor cell lines. Interestingly, this gAPN fragment also shows strong metabolic effects in various tissues [
13].
2.2. Adiponectin Receptors and Signaling Pathway
AdipoR1 and AdipoR2 are two prominent receptors for APN. These receptors are members of the 7-transmembrane receptor family. They exhibit a structural orientation with an inward-facing amino terminus and an outward-facing carboxy terminus belonging to the progestin AdipoQ receptor protein group [
14]. This variation in structural alignment differentiates them from G-protein-coupled receptors [
1]. Their unique features include a site for zinc binding and a dedicated surface specifically designed to interact with APN, highlighting their remarkable individuality. These receptors play crucial roles in regulating reproductive functions in gonads by activating distinct signaling pathways. Previous research specified that AdipoR1 had a high affinity for the gAPN fragment and a low affinity for full-length peptide, whereas AdipoR2 displayed intermediate affinity for both forms.
When APN binds to AdipoR1 or AdipoR2, it activates intracellular signaling through the adaptor protein phosphotyrosine, interacting with the pleckstrin homology domain and leucine zipper 1 (APPL1) [
15]. APPL1 facilitates the activation of various pathways, including adenosine monophosphate-activated protein kinase (AMPK), p38 mitogen-activated kinase (MAPK), and extracellular signal-regulated kinase 1/2 MAPK, in response to APN. Moreover, APPL1 is involved in mediating the insulin-sensitizing effects of APN in peripheral tissues and modulating endothelial nitric oxide synthase function in endothelial cells. This emphasizes the intricate role of AdipoR1 and AdipoR2, along with APPL1, in orchestrating the physiological effects of APN throughout the body [
16].
AdipoR1 primarily stimulates the AMPK pathway, preventing hepatic glucose production and promoting fatty acid oxidation. AdipoR2 stands out by primarily activating the peroxisome proliferator-activated receptor (PPAR)-α pathway, leading to increased energy expenditure through enhanced fatty acid oxidation. Additionally, it possesses anti-inflammatory and antioxidant properties [
17].
3. Retinal Disorders and Oxidative Stress
The retina constitutes a segment of the central nervous system where light energy undergoes conversion into neuronal action potentials [
18]. The retina has the highest metabolic requirements of all tissues and is supported by a dense network of blood vessels [
8]. A healthy retinal vascular network is vital for the delivery of essential nutrients and oxygen. Disruption of this network leads to increased hypoxia and energy substrate deficiency, which is known to trigger retinal angiogenesis.
The retina faces a constant barrage of environmental threats, including light damage, oxidative stress, and genetic mutations that can cause protein misfolding. Retinal cells employ a sophisticated arsenal of protective mechanisms that combat these challenges and maintain cellular health, such as the heat shock response, the unfolded protein response, and autophagy [
19]. The retina is highly susceptible to oxidative stress damage because of exposure to visible light, ultraviolet radiation, and polyunsaturated fatty acids availability [
20]. Notably, the macula, with its high metabolic activity, is constantly exposed to high levels of reactive oxygen species, which play essential roles in various cellular functions. In addition to this, the macula’s high blood flow results in the abundant presence of exogenous reactive oxygen species in this area [
21]. Scientists are increasingly exploring the role of oxidative and nitrosative stress in macular diseases and other retinal conditions [
22]. While significant progress has been made in understanding the underlying mechanisms, effective treatments remain elusive. The future holds promise with ongoing research focused on developing personalized therapies based on individual genetics. This research suggests that oxidative and nitrosative stress are likely to be central to many future treatment strategies [
20].
AMD initiation and progression can be related to endogenous factors, like genetic predisposition, as well as exogenous factors. One such exogenous factor is an individual’s diet. In rabbits, a cholesterol-rich diet has been shown to cause AMD [
23]. In rats, 7-ketocholesterol, a product of cholesterol oxidation, and its esters have been associated with increased inflammation and angiogenesis [
24]. It is evident that prevalent conditions like hypertension and obesity are linked with AMD [
25,
26]. Besides variations in pathophysiological mechanisms in these retinal disorders, we find certain striking similarities in contributing factors such as genetic polymorphisms linked with high risk or severity of disease, oxidative stress, inflammatory response, metabolic perturbation, and cellular senescence [
27].
AMD has been closely associated with oxidative stress. It was observed that continued exposure of increased concentration of reactive oxygen species, lead to loss of superoxide dismutase (SOD) expression by AMD retinal pigment epithelium cells resulting in high oxidative stress [
21].
3.1. Age-Related Macular Degeneration
As the central area of the retina, the macula is essential for the formation of acute vision. Over time, damage to the macula results in macular degeneration. Macular degeneration is correlated with aging, progressing gradually over an extended period before the patient becomes aware of central vision loss [
28]. AMD is a multifactorial disorder with dysregulation in multiple pathways, such as the complement, lipid, angiogenic, inflammatory, and extracellular matrix pathways, resulting in pathogenesis [
29]. As discussed earlier there are two types (dry and wet) types of AMD [
4]. Dry AMD is more prevalent than wet AMD; however, wet AMD is more severe and can cause central blindness [
4] if treatment is delayed [
2]. Dry AMD is the chronic AMD form, which leads to some visual impairment or complete vision loss if untreated. Wet AMD, once established, progresses more rapidly than the dry form [
29].
AMD often goes unnoticed in its early stage as yellow deposits builds up under the retina termed as drusen [
30]. The latter stages of dry AMD are referred to as geographic atrophy (GA) [
31]. GA is characterized by scattered patches of degeneration in the light-sensitive retinal pigment epithelium and overlying photoreceptor cells. Untreated dry AMD may progress to wet AMD, known as choroidal neovascularization (CNV). In CNV, newly generated immature blood vessels spread from the underlying choroid towards the outer retina [
32]. It is well established that AMD affects the macula region of the retina completely. Understanding the molecular foundation of a disease offers the ability to apply that information in clinical applications. A notable illustration of therapy based on mechanisms is the use of anti-angiogenesis treatment for wet AMD [
29].
4. Adiponectin and Metabolic Disorders
Metabolic disorders are chiefly linked with visceral obesity, which increases cardiovascular mortality and morbidity. The relationship of APN with metabolic disorders has been explored extensively. APN can modulate the metabolism to combat various diseases related to metabolic dysregulation. Obesity and related comorbidities have been linked with decreased APN level. Decreased APN level has been linked to increased endocrine cancer risks [
33,
34,
35] and has been shown to increase the probability of developing obesity-related malignancies and increased occurrence of colon, gastric, prostate cancers and thus resulting in hard cancer diagnosis [
33]. This association of decreased APN level, obesity, and related comorbidities forms a vicious cycle.
Many female reproductive disorders have been associated with metabolic disorders. Lower APN levels have been associated with higher incidences of diabetes [
36]. Altered adipocyte function occurs during visceral fat deposition, leading to reduced APN levels [
37]. Polycystic ovarian syndrome (PCOS) a common endocrine and metabolic disorder in women of childbearing age has also been scrutinized for its relationship with APN. APN exerts a positive response on metabolic parameters, resulting in the management of PCOS-related hyperandrogenism in female mice [
38,
39]. All the metabolic disorder discussed above are known to accelerate insulin resistance in individual further affecting the normal glucose pathway in common.
Research emphasizes the multifaceted benefits of APN, including its anti-atherogenic action that prevents the artery-clogging processes. The anti-inflammatory properties of APN highlight its ability to reduce inflammation within the body, offering promise for various inflammatory conditions [
40]. APN has been screened for its anti-inflammatory properties as it is known to trigger the secretion of the anti-inflammatory cytokine IL-10, which is derived from human adipose tissue [
41]. This crucial adipokine is known for its anti-diabetic effects by managing insulin resistance and glucose uptake and thus appears as a potential marker in managing and mitigating diabetes-related complications [
36].
5. Therapeutic Potential of Adiponectin
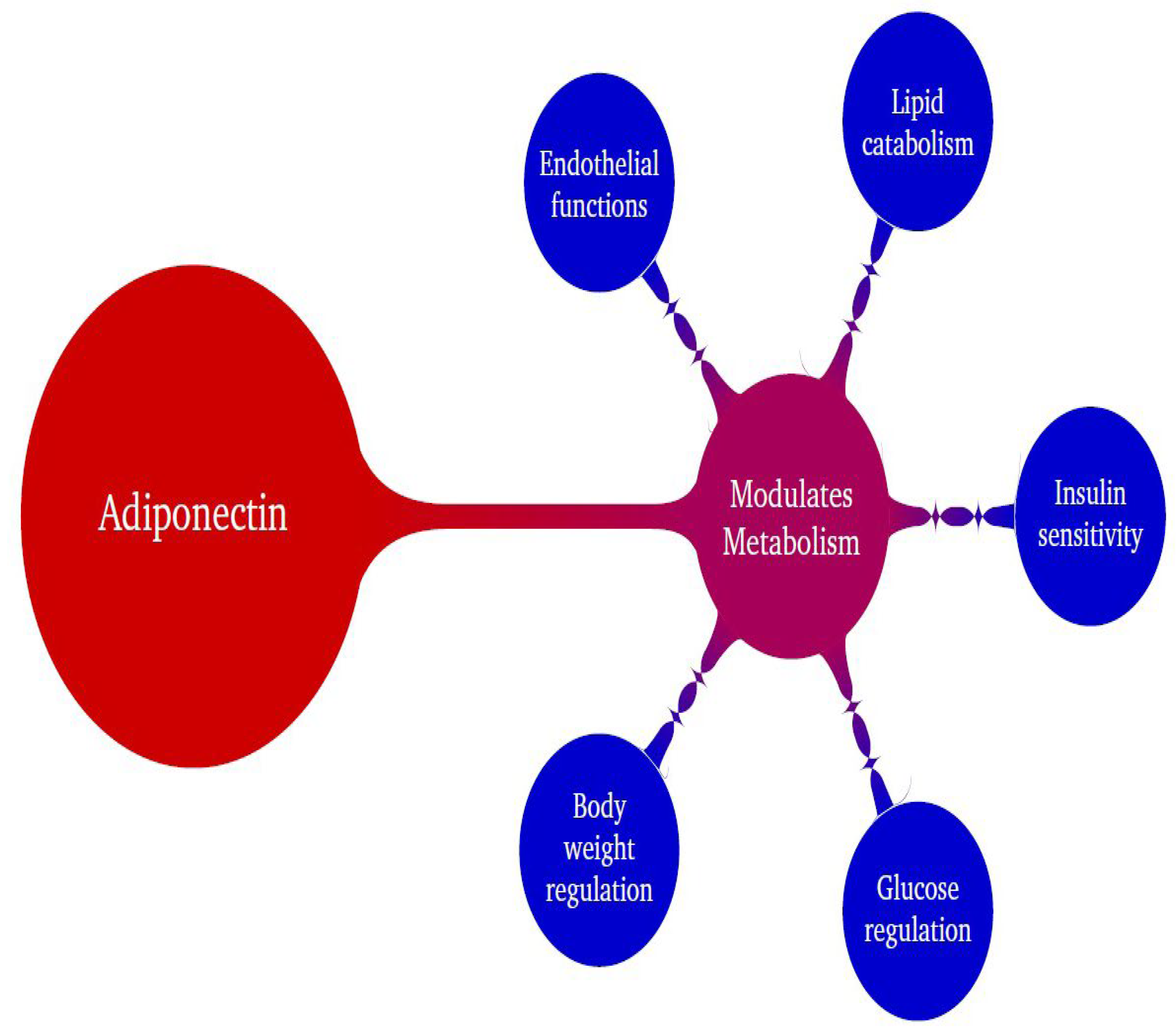
There is substantial research indicating that APN is a beneficial adipokine that regulates various physiological activities, and its modulation of metabolic processes has been well documented. APN participates in many physiological functions, such as glucose regulation, endothelial functions, and insulin sensitization (
Figure 1). It has tremendous capacity to combat most of the common disorders such as obesity, diabetes, cardiovascular diseases, cancers, neurological disorders, inflammatory disorders, and fertility-related issues [
1,
19,
39] and has also shown efficacy in treating PCOS in a mouse model [
40]. In addition to these common disorders, AMD also involves APN modulation.
5.1. Therapeutic Efforts of APN in Age-Related Macular Degeneration
Ocular disorders, such as AMD, diabetic retinopathy, and retinopathy of prematurity, are closely linked with changes in the circulating level and distribution of APN/AdipoRs or its variants. Neovascular AMD results in disturbance of the macula. Data from studies of a laser-induced mouse model of AMD suggest that APN participates in ameliorating neovascularization, [
42], counteracting retinal defects and CNV.
The APN 1 peptide (derived from gAPN) has shown efficacy in inhibiting CNV. Compared to a control peptide, subretinal administration of APN 1 resulted in 75% more inhibition of CNV; however, further rodent studies are needed to establish the effective doses of APN 1 for human administration. Presently we are devoid of scientific applications of APN 1 peptide for humans. It’s appropriate and profound understanding would illustrate the procedure and administration to the patients suffering from wet AMD evolving as a potential medication.
Two common and effective treatments to combat wet AMD are Avastin and Lucentis injected into the eye by ophthalmologist. These humanized antibodies against vascular endothelial growth factor (VEGF) were developed by Genentech [
4]. Avastin, a common cancer treatment, has emerged as an effective treatment for AMD [
43]. Lucentis demonstrates efficacy in treating wet AMD, but its cost (~20 times greater than Avastin) remains a significant concern. Another drug, Eylea, precisely developed for AMD, has a similar cost burden. These treatments are expensive and can produce potential adverse effects, including infections, hemorrhages, and ocular discomfort.
The protective role of APN in the retina has been established through several studies of AMD [
42]. APN levels showed a positive correlation with the retinal blood vessel diameter and blood velocity and flow, measured by Doppler velocimetry, in a population of Japanese males with Type 2 diabetes [
44].
Most research pertaining to an association between AMD and APN suggests a positive therapeutic relationship [
2]. Ocular disorders like AMD and many others are linked to altered circulating APN levels, and administration of APN decreases the pathophysiologic effects. APN is involved in modulating metabolic markers and establishes equilibrium among altered glucose and lipid levels [
15]. Treatment options for retinopathy mainly function to block overexpression of angiogenic factors. This can be achieved by intravitreal injections of antibodies that block angiogenic markers like VEGF, like those described previously [
8,
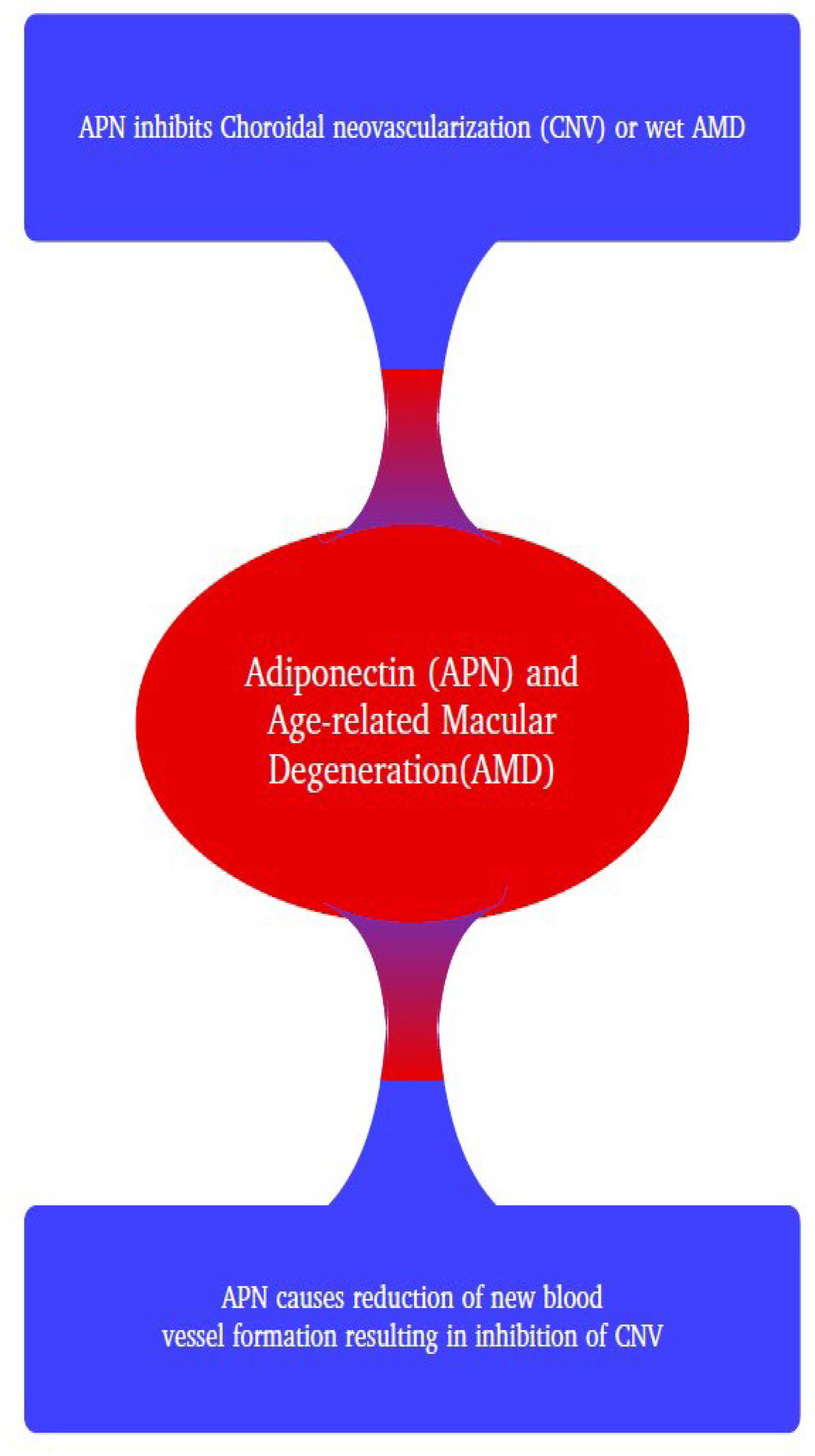
45]. Recent findings suggest that APN is an anti-angiogenic protein that has great potential in inhibiting the development of CNV [
42]. This research demonstrated that administration of APN in a laser-induced mouse model of AMD downregulated the expression of VEGF and subsequently inhibited CNV size (
Figure 2). Clinical studies have shown that anti-VEGF-A therapy may be a promising treatment option for CNV, indicating the potential to stabilize patients’ vision [
46].
Furthermore, comprehensive clinical studies in human subjects are imperative to ascertain the efficacy and safety of potential treatments before drawing definitive conclusions. Regarding conditions such as AMD and retinopathy of prematurity, there is an observed association with variations in circulating APN levels and the distribution of APN variants. Additional research has demonstrated the mitigation of retinal neovascularization and CNV through the administration of APN [
47].
5.2. Therapeutic Efforts Related to APN and Metabolic Disorders
APN has emerged as a potent and dynamic adipokine in addressing various metabolic disorders by regulating glucose and lipid levels. Studies have demonstrated that administering APN to both rodents and humans results in reduced body weight and brings about insulin-sensitizing, anti-atherogenic, and anti-inflammatory effects [
38,
48].
The role of APN in diabetes has been extensively explored. APN promotes insulin sensitivity, regulates glucose tolerance, and enhances fat metabolism [
49]. The insulin-sensitizing effects of APN are mediated through the activation of the PPAR-α receptor through phosphorylation and initiation of the AMPK cascade. Conversely, individuals with diabetes, insulin resistance, and hypertension, carrying mutations that exert a negative dominant effect on PPARγ, experience a significant reduction in APN levels. A newly identified insulin-sensitizing agent, Thiazolidinediones, has been recognized for its anti-diabetic properties. Thiazolidinediones acts as a PPARγ nuclear receptor agonist [
50] and induces an elevation in APN levels. Consequently, in the human context, low APN levels pose a risk factor for Type 2 diabetes [
51]. Studies have shown that certain medications, including statins, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers, may improve blood sugar levels and reduce insulin resistance by potentially increasing the production of APN [
52,
53].
Obesity and its related comorbidities are chiefly associated with altered adipokine levels [
54,
55]. Thus, the property of APN to act as an insulin sensitizer and reduce insulin resistance in obesity and its associated ailments demonstrates the effectiveness of this peptide. In a mouse model of PCOS, APN modulates various metabolic activities, resulting in the lowering of androgen and glucose and causing ovulation [
37,
38].
APN improves insulin sensitivity and glucose tolerance in obese animal models while also prolonging the normally reduced lifespan observed in diabetic obese mice [
55]. APN receptor agonists have also been categorized as therapeutic agents in combating obesity [
56,
57]. It has been well investigated and documented that APN peptide I has an inhibitory effect on CNV in rats [
58]. The two common receptors of APN, AdipoR1 and R2, are vital for glucose/lipid metabolism and insulin sensitivity [
51,
59]. Notably, the levels of circulating APN exhibit an inverse correlation with obesity, diabetes, and diseases associated with obesity [
60,
61,
62].
6. Conclusions
APN is a dynamic and multifaceted adipokine with diverse effects on various physiological processes, contributing to metabolic health and protection against various diseases. Modulation of metabolism, inflammation, and other physiological activities by this peptide showcases its versatile regulation and action. In addition, APN has been well-documented as a novel marker for combating ocular disorders.
The pathophysiology and mechanism of AMD, one of the most common causes of blindness in old age, has been extensively scrutinized. The association of APN with AMD in various experimental setups denotes the beneficial effect of this peptide. Thus, this adipokine can serve as a novel therapeutic target for future studies of treatments for combating visual disorders. Further exploration is vital for advancing our knowledge and potentially uncovering therapeutic avenues related to APN/AdipoRs in the context of AMD.
Author Contributions
Anusha Singh and Puran Bora conceived the idea. Anusha Singh wrote the manuscript. Puran Bora and Nalini Bora edited the manuscript.
Funding
This research received no external funding. But, this work was funded by grants from Jones Eye Institute, University of Arkansas for Medical Sciences, Little Rock, AR, 72205.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to acknowledge the Jones Eye Institute, University of Arkansas for Medical Sciences for all the funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Singh A, Choubey M, Bora P, Krishna A. Adiponectin and Chemerin: Contrary Adipokines in Regulating Reproduction and Metabolic Disorders. Reprod Sci. 2018, 25, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Bora, PS. New Discoveries in Retinal Cell Degeneration and Retinal Diseases. Biomolecules. 2023, 13, 1121. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A. The Diagnosis and Treatment of Age-Related Macular Degeneration. Dtsch Arztebl Int 2020, 117, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Logan C, Lyzogubov V, Bora N, Bora P. Role of Adiponectin Peptide I (APNp1) in Age-Related Macular Degeneration. Biomolecules. 2022, 12, 1232. [Google Scholar] [CrossRef]
- Girmens JF, Sahel JA, Marazova K. Dry age-related macular degeneration: A currently unmet clinical need. Intractable Rare Dis Res. 2012, 1, 103–114. [Google Scholar] [CrossRef]
- Deng Y, Qiao L, Du M, Qu C, Wan L, Li J, Huang L. Age-related macular degeneration: Epidemiology, genetics, pathophysiology, diagnosis, and targeted therapy. Genes & Diseases. 2022, 9, 62–79. [Google Scholar] [CrossRef]
- Mallardo M, Costagliola C, Nigro E, Daniele A. AdipoRon negatively regulates proliferation and migration of ARPE-19 human retinal pigment epithelial cells. Peptides. 2021, 146, 170676. [Google Scholar] [CrossRef]
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A Novel Serum Protein Similar to C1q, Produced Exclusively in Adipocytes (*). Journal of Biological Chemistry. 1995, 270, 26746–26749. [Google Scholar] [CrossRef] [PubMed]
- Fu Z, Gong Y, Löfqvist C, Hellström A, Smith LE. Review: Adiponectin in Retinopathy. Biochim Biophys Acta. 2016, 1862, 1392–1400. [Google Scholar] [CrossRef]
- Straub LG, Scherer PE. Metabolic Messengers: Adiponectin. Nat Metab. 2019, 1, 334–339. [Google Scholar] [CrossRef]
- Fantuzzi, G. Adiponectin in inflammatory and immune-mediated diseases. Cytokine. 2013, 64, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pacheco F, Martinez-Fuentes AJ, Tovar S, et al. Regulation of Pituitary Cell Function by Adiponectin. Endocrinology. 2007, 148, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Liu M, Liu F. Regulation of Adiponectin Multimerization, Signaling and Function. Best Pract Res Clin Endocrinol Metab. 2014, 28, 25–31. [Google Scholar] [CrossRef]
- Choubey M, Bora P. Emerging Role of Adiponectin/AdipoRs Signaling in Choroidal Neovascularization, Age-Related Macular Degeneration, and Diabetic Retinopathy. Biomolecules. 2023, 13, 982. [Google Scholar] [CrossRef]
- Kadowaki T, Yamauchi T. Adiponectin and Adiponectin Receptors. Endocr Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef]
- Thundyil J, Pavlovski D, Sobey CG, Arumugam TV. Adiponectin receptor signalling in the brain. Br J Pharmacol. 2012, 165, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Deepa SS, Dong LQ. APPL1: role in adiponectin signaling and beyond. Am J Physiol Endocrinol Metab. 2009, 296, E22–E36. [Google Scholar] [CrossRef] [PubMed]
- Matsunami T, Sato Y, Ariga S, Sato T, Kashimura H, Hasegawa Y, Yukawa M. Regulation of oxidative stress and inflammation by hepatic adiponectin receptor 2 in an animal model of nonalcoholic steatohepatitis. Int J Clin Exp Pathol. 2010, 3, 472–481. [Google Scholar]
- Lee KS, Lin S, Copland DA, Dick AD, Liu J. Cellular senescence in the aging retina and developments of senotherapies for age-related macular degeneration. J Neuroinflammation. 2021, 18, 32. [Google Scholar] [CrossRef]
- Athanasiou D, Aguilà M, Bevilacqua D, Novoselov SS, Parfitt DA, Cheetham ME. The cell stress machinery and retinal degeneration. FEBS Letters. 2013, 587, 2008–2017. [Google Scholar] [CrossRef]
- Nebbioso M, Franzone F, Lambiase A, Bonfiglio V, Limoli PG, Artico M, Taurone S, Vingolo EM, Greco A, Polimeni A. Oxidative Stress Implication in Retinal Diseases—A Review. Antioxidants. 2022, 11, 1790. [Google Scholar] [CrossRef]
- andau K, Kurz-levin M. Chapter 4 - Retinal disorders. In: Kennard C, Leigh RJ, eds. Handbook of Clinical Neurology. Vol 102. Neuro-ophthalmology. Elsevier; 2011, 97-116. [CrossRef]
- Dasari B, Prasanthi JR, Marwarha G, Singh BB, Ghribi O. Cholesterol-enriched diet causes age-related macular degeneration-like pathology in rabbit retina. BMC Ophthalmol. 2011, 11, 22. [Google Scholar] [CrossRef]
- Amaral J, Lee JW, Chou J, Campos MM, Rodríguez IR. 7-Ketocholesterol Induces Inflammation and Angiogenesis In Vivo: A Novel Rat Model. Apte RS, ed. PLoS ONE. 2013, 8, e56099. [Google Scholar] [CrossRef]
- Cho YK, Park DH, Jeon IC. Medication trends for Age-related macular degeneration. Int J Mol Sci. 2021, 22, 118377. [Google Scholar] [CrossRef]
- Wong T, Mitchell P. The eye in hypertension. The Lancet. 2007, 369, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Copland DA, Theodoropoulou S, Liu J, Dick AD. A Perspective of AMD Through the Eyes of Immunology. Invest Ophthal Vis Sci. 2018, 59, AMD83–AMD92. [Google Scholar] [CrossRef] [PubMed]
- Handa, JT. How does the macula protect itself from oxidative stress? Mol Asp Med. 2012, 33, 418–435. [Google Scholar] [CrossRef] [PubMed]
- Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. The Lancet. 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Ambati J, Fowler BJ. Mechanisms of Age-Related Macular Degeneration. Neuron. 2012, 75, 26–39. [Google Scholar] [CrossRef]
- Thomas CJ, Mirza RG, Gill MK. Age-Related Macular Degeneration. Med Clin North Am. 2021, 105, 473–491. [Google Scholar] [CrossRef]
- Zweifel SA, Imamura Y, Spaide TC, Fujiwara T, Spaide RF. Prevalence and significance of subretinal drusenoid deposits (reticular pseudodrusen) in age-related macular degeneration. Ophthalmology. 2010, 117, 1775–1781. [Google Scholar] [CrossRef]
- Bird, AC. Therapeutic targets in age-related macular disease. J Clin Invest. 2010, 120, : 3033–3041. [Google Scholar] [CrossRef]
- Tumminia A, Vinciguerra F, Parisi M, et al. Adipose Tissue, Obesity and Adiponectin: Role in Endocrine Cancer Risk. Int J Mol Sci. 2019, 20, 2863. [Google Scholar] [CrossRef]
- Hebbard L, Ranscht B. Multifaceted roles of Adiponectin in cancer. Best Pract Res Clin Endocrinol Metab. 2014, 28, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Spranger J, Kroke A, Möhlig M, et al. Adiponectin and protection against type 2 diabetes mellitus. The Lancet. 2003, 361, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y. The metabolic syndrome and adipocytokines. FEBS Letters. 2006, 580, 2917–2921. [Google Scholar] [CrossRef] [PubMed]
- Singh A, Bora P, Krishna A. Direct action of adiponectin ameliorates increased androgen synthesis and reduces insulin receptor expression in the polycystic ovary. Biochem Biophys Res Commun. 2017, 488, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Singh A, Bora P, Krishna A, Singh A, Bora P, Krishna A. Systemic adiponectin treatment reverses polycystic ovary syndrome-like features in an animal model. Reprod Fertil Dev. 2018, 30, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P. Adiponectin: a key adipocytokine in metabolic syndrome. Clin Sci. 2006, 110, 267–278. [Google Scholar] [CrossRef]
- Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, Maeda K, Nagaretani H, Kishida K, Maeda N, Nagasawa A, Funahashi T, Matsuzawa Y. Adiponectin Specifically Increased Tissue Inhibitor of Metalloproteinase-1 Through Interleukin-10 Expression in Human Macrophages. Circulation. 2004, 109, 2046–2049. [Google Scholar] [CrossRef] [PubMed]
- Bora PS, Kaliappan S, Lyzogubov VV, Tytarenko RG, Thotakura S, Viswanathan T, Bora NS. Expression of adiponectin in choroidal tissue and inhibition of laser induced choroidal neovascularization by adiponectin. FEBS Letters. 2007, 581, 1977–1982. [Google Scholar] [CrossRef] [PubMed]
- Nancy M. Holekamp MD. Review of Neovascular Age-Related Macular Degeneration Treatment Options. 2019, 25. Accessed November 9, 2023. https://www.ajmc.com/view/review-of-neovascular-agerelated-macular-degeneration-treatment-options.
- Omae T, Nagaoka T, Yoshida A. Relationship Between Retinal Blood Flow and Serum Adiponectin Concentrations in Patients With Type 2 Diabetes Mellitus. Invest Ophthal Vis Sci. 2015, 56, 4143–4149. [Google Scholar] [CrossRef]
- Hellström A, Smith LE, Dammann O. Retinopathy of prematurity. The Lancet. 2013, 382, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Ozveren, M. Results of Intravitreal Anti-VEGF Injection in Choroidal Neovascularization Caused by Pathologies Other Than Age-Related Macular Degeneration. Beyoglu Eye J. 2020, 5, 129–134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ranibizumab and Bevacizumab for Neovascular Age-Related Macular Degeneration. N Engl J Med. 2011, 364, 1897–1908. [CrossRef] [PubMed]
- Khoramipour K, Chamari K, Hekmatikar AA, Ziyaiyan A, Taherkhani S, Elguindy NM, Bragazzi NL. Adiponectin: Structure, Physiological Functions, Role in Diseases, and Effects of Nutrition. Nutrients. 2021, 13, 1180. [Google Scholar] [CrossRef]
- Siitonen N, Pulkkinen L, Lindström J, Kolehmainen M, Eriksson JG, Venojärvi M, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Tuomilehto J, Uusitupa M. Association of ADIPOQ gene variants with body weight, type 2 diabetes and serum adiponectin concentrations: the Finnish Diabetes Prevention Study. BMC Med Genet. 2011, 12, 5. [Google Scholar] [CrossRef]
- Chiarelli F, Marzio DD. Peroxisome proliferator-activated receptor-γ agonists and diabetes: Current evidence and future perspectives. Vasc Health Risk Manag. 2008, 4, 297–304. [Google Scholar] [CrossRef]
- Nguyen TMD. Adiponectin: Role in Physiology and Pathophysiology. Int J Prev Med 2020, 11, 136. [CrossRef]
- Kitamura N, Takahashi Y, Yamadate S, Asai S. Angiotensin II receptor blockers decreased blood glucose levels: a longitudinal survey using data from electronic medical records. Cardiovasc Diabetol 2007, 6, 26. [CrossRef]
- Furuhashi M, Ura N, Higashiura K, Murakami H, Tanaka M, Moniwa N, Yoshida D, Shimamoto K. Blockade of the Renin-Angiotensin System Increases Adiponectin Concentrations in Patients With Essential Hypertension. Hypertension. 2003, 42, 76–81. [Google Scholar] [CrossRef]
- Pereira S, Alvarez-Leite JI. Adipokines: biological functions and metabolically healthy obese profile. J Receptor, Ligand Channel Res. 2014, 7, 15–25. [Google Scholar] [CrossRef]
- Katsareli EA, Dedoussis GV. Biomarkers in the field of obesity and its related comorbidities. Expert Opin Ther Tar. 2014, 18, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Greenhill, C. Adiponectin receptor agonists—possible therapeutic approach? Nat Rev Endocrinol. 2014, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Lee CH, Hung YJ. Possible new therapeutic approach for obesity-related diseases: Role of adiponectin receptor agonists. J Diab Invest. 2015, 6, 264–266. [Google Scholar] [CrossRef]
- Lyzogubov VV, Tytarenko RG, Bora NS, Bora PS. Inhibitory role of adiponectin peptide I on rat choroidal neovascularization. Biochimica et Biophysica Acta. 2 0121, 823, 1264–1272. [Google Scholar] [CrossRef]
- Greenhill, C. Adiponectin receptor agonists—possible therapeutic approach? Nat Rev Endocrinol. 2014, 10, 4–4. [Google Scholar] [CrossRef] [PubMed]
- Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and Insulin Resistance. Mol Med. 2008, 14, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto S, Matsushita Y, Nakagawa T, Hayashi T, Noda M, Mizoue T. Circulating adiponectin levels and risk of type 2 diabetes in the Japanese. Nutr Diabetes. 2014, 4, e130. [Google Scholar] [CrossRef] [PubMed]
- Di Zazzo E, Polito R, Bartollino S, Nigro E, Porcile C, Bianco A, Daniele A, Moncharmont B. Adiponectin as Link Factor between Adipose Tissue and Cancer. Int J Mol Sci. 2019, 20, 839. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).