Introduction
The skin is an important protection organ of the human body. It has a complex structure that is constantly renewed, acting as a barrier against various harmful agents [
1]. The skin has different layers: the outermost layer is the epidermis and, just below this, there is the dermis. There is also a layer composed mainly of adipose tissue called the hypodermis [
2].
Solar radiation can cause several types of damage to the organism depending on the duration and type of exposure [
3]. Individuals’ negligence regarding skin protection, coupled with excessive sun exposure, can contribute to the development of various skin pathologies. Inflammation, apoptosis and necrosis can occur following acute UV radiation exposure, which can lead to tissue damage. DNA damage can also occur, which leads to a higher probability of developing skin cancer [
4,
5].
The damage to cellular structures is mediated by oxidative stress that occurs with an exacerbated increase in reactive oxygen species (ROS), that is, very unstable and reactive molecules [
4,
6]. When this imbalance between oxidizing and antioxidant compounds occurs, there is oxidation of biomolecules and loss of their functions [
7]. To combat these damages, the skin has an enzymatic antioxidant system, which includes enzymes such as superoxide dismutase, glutathione peroxidase, and catalase. Antioxidants are important to counteract effects caused by ROS [
8,
9,
10,
11].
Therefore, some studies have sought to incorporate antioxidants in cosmetic formulations, constituting a viable and effective alternative for greater photoprotection [
12,
13,
14,
15]. Sunscreens are formulations designed to specifically attenuate the effect of UV radiation on the skin by means of physical or chemical action resulting in radiation absorption, dispersion, or reflection mechanisms. The quality control of a photoprotective formulation is essential, and this depends not only on its sun protection factor (SPF), but also on its physical-chemical properties, stability and solubility [
12,
16].
Rheology is an effective instrument for the physical and behavior analysis of formulation flow, and there are different methods available for the evaluation of the stability of cosmetic products [
17]. Furthermore, non-invasive in vivo methods have enabled a greater understanding of skin physiology [
18,
19] and these cutaneous biometrics can be used to assess the physicochemical properties of the skin and the behavior of a formulation within it. Such parameters that can be evaluated include skin hydration, pH, transepidermal water loss, melanin and erythema level, and temperature [
20].
The quality profile of a product also covers the sensory aspect and the degree of acceptance of the product by the target audience [
21,
22]. Some consumers are looking for products that meet the ethical requirements of their lifestyle. There is concern on the part of such individuals regarding the impacts that products generate on the environment, in themes such as cruelty-free, sustainability, and vegan formulations [
23,
24]. Vegan formulations are pharmaceutical preparations whose composition and raw materials are not of animal origin nor have been tested on animals [
25].
The present work evaluated the biophysical properties of two multifunctional formulations: Face Care Facial Moisturizing Cream® and the second formulation is a vegan test formulation. Additionally, a comparison was conducted between the two products regarding in vitro SPF, rheological profiles, and sensory analysis, encompassing male and female human volunteers.
Materials and Methods
The two formulations evaluated in this work were the commercialized Face Care Facial Moisturizing Cream (P1) with SPF 30 from the company PURIFIC PREMIUM
® and a vegan formulation (P2) provided by the company Naturelle
®. P1 and P2 are classified as multifunctional products and their compositions are shown in
Table 1:
Absorbance Scan
For the UV scan experiment, samples P1 and P2 were diluted in absolute ethanol at a concentration of 100 µg/mL. A scan was then carried out, measuring the absorbance from 250 to 400 nm in UV-Vis equipment (Shimadzu, UV-1700).
SPF Determination
The Mansur equation was used to calculate the SPF in vitro [
26]. The samples were diluted to 100 µg/mL in triplicate, and the absorbance of P1 and P2 was read between 290-320 nm at 5 nm intervals (Shimadzu, UV-1700). The SPF was calculated using the following equation:
where:
CF = correction factor (equal to 10);
EE (λ) = erythematogenic effect of radiation with wavelength λ;
Ι (λ) = intensity of sunlight at wavelength λ;
Abs (λ) = spectrophotometric reading of the absorbance of the sample solution at
wavelength (λ);
2 = Dilution factor
The EE (λ) x I (λ) values are given in the supplementary material (
Table S1).
Rheological Analysis
Continuous Flow Shear Rheometry
Rheograms were generated by means of a gradient rheometer and controlled shear stress MARS II® (Haake®), in continuous flow mode, at temperatures of 4, 25, 34, and 40 ± 0.1 °C, with parallel cone-plate geometry of 35 mm in diameter, separated by a fixed distance of 0.052 mm. It was found that the formulations did not break at up to 2000 s−1 of shear gradient. Therefore, the measurements of the flow curves were taken with a variation of the shear rates from 0 to 2000 s−1, in order to verify the behavior of the formulations submitted to such rates.
The upward and downward flow curves were calculated based on the Oswald de Waele equation (Power Law - Power Law), obtaining the
k and
n indices [
27]:τ =
k. γ
n, where τ is the shear stress (Pa),
k is the consistency index [(Pa·s)n], γ is the shear rate (s
−1), and
n is the flow behavior index (dimensionless).
In addition, the yield of each formulation was obtained using the Herschel-Buckley equation/model [
28]: τ = τ
0+ k. γ
n,
where τ is the shear stress (Pa), τ0 is the yield stress (Pa), k is the consistency index [(Pas)n], γ is the rate of shear (s−1), and n is the flow behavior index (dimensionless).
The hysteresis area was also obtained using the RheoWin 4.10.0000 program (Haake
®) and the thixotropy coefficient (Kt) was calculated using the equation [
29].
Oscillatory Rheometry
The samples were gently applied to the bottom plate, allowing a resting time of 1 min before each determination, and ensuring the minimum shear of the formulation [
30]. After determining the linear viscoelastic region, the frequency scan analysis from 0.1 to 10.0 Hz was performed. Viscosity (n ‘), tangent (tan), storage module (G’), and loss module (G”) were calculated using the RheoWin 4.10.0000 (Haake) software [
27]. Three repetitions were made for each sample.
Sensorial Analysis
A 9-point hedonic test was applied to perform the sensory analysis. The test was sample-blind, that is, the volunteers did not know which formulation was applied to their face. They also answered a test of intention to purchase the product, described by
Prudencio et al. [
37] and it is included in the supplementary material (Annex I).
Statistical Analysis
All data were analyzed using the ANOVA test, considering p <0.05 to be significant, followed by the Tukey test. Statistical analyzes were performed using the GraphPad Prism 5 software.
Results and Discussion
Absorbance Scan
Solar radiation can induce various types of damage to the skin, which varies depending on the duration and type of exposure [
3]. The Earth receives a constant stream of light photons from the sun, including infrared light (780–5000 nm), visible light (400–780 nm), and ultraviolet (UV) light (290–400 nm). Regarding to UV range, it can be categorized based on wavelength into UVC (200–280 nm), UVB (280–320 nm), and UVA (320–400 nm) [
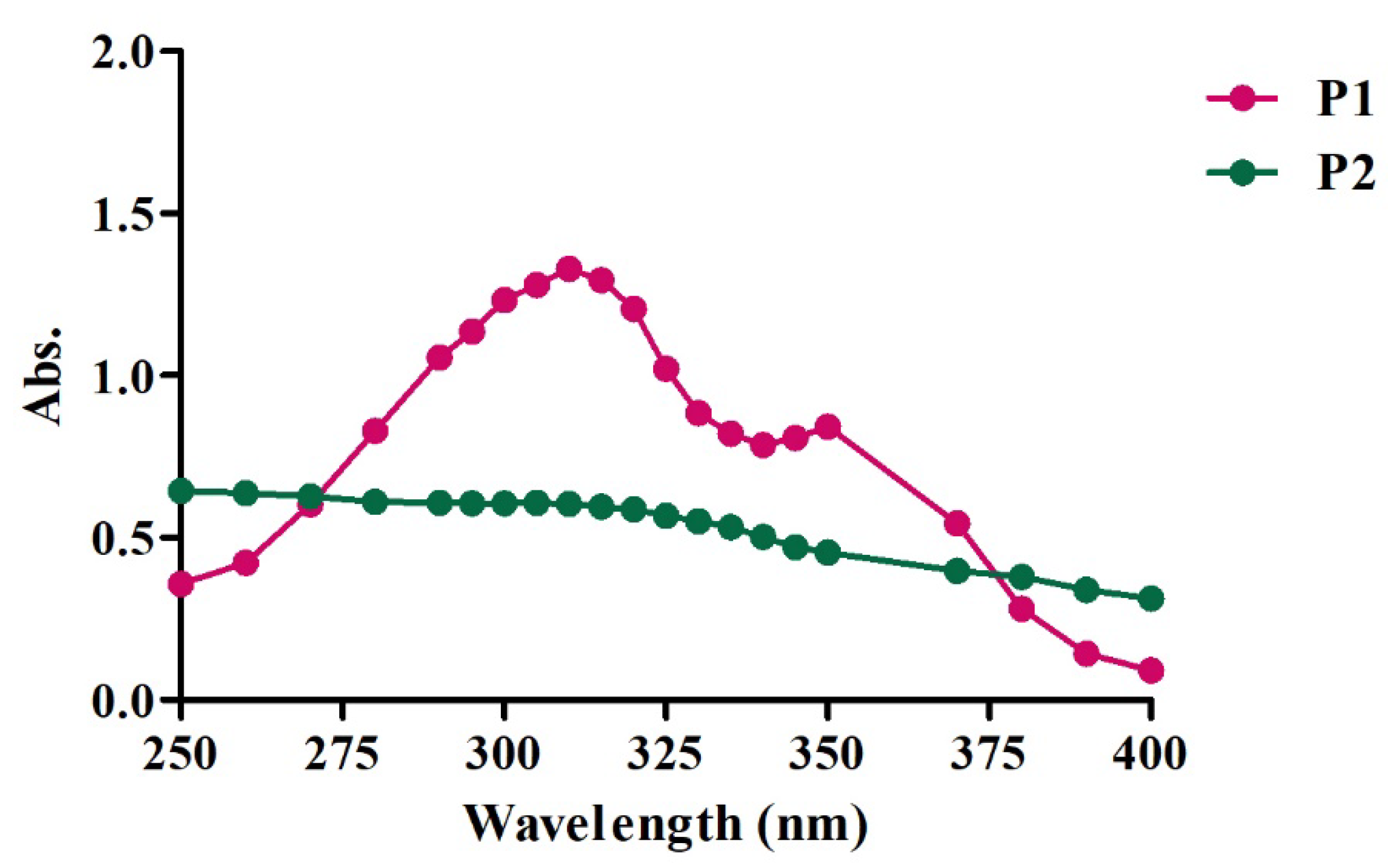
4]. The scanning results showed that the two products analyzed were able to absorb light in the 250 to 400 nm range. This encompasses almost all range of the electromagnetic spectrum of UV radiation, including UVA and UVB (
Figure 1).
P1 showed an absorption peak at 310 nm, which corresponds to UVB radiation. P2, on the other hand, did not show much variation in absorbance, which remained present throughout the range analyzed. It is well known that, in the event of an emission of UV light, sunscreens can protect the skin by absorbing the radiation, attenuating its effects on the skin [
12].
SPF Determination
SPF is an interesting tool for evaluating the effectiveness of multifunctional products. In vitro techniques for evaluating SPF have been developed and standardized, offering lower cost and labor than in vivo ones [
22]. The technique described by Mansur [
26] was used and the results were favorable for both products. P1 obtained an SPF of 25.21 ± 1.09, while P2’s result was 12.10 ± 0.43.
P1 has some chemical filters that can absorb UV radiation, such as
Diethylamino hydroxybenzoyl hexyl benzoate, an organic filter. The composition of P2 has more components and physical filters, such as zinc oxide and titanium dioxide, which act to reflect solar radiation. To complement UV protection, P2 contains antioxidant components (
Table 1) [
9].
Despite P2 achieving a lower SPF compared to P1, studies state that products with an SPF of 10 can absorb 90% of erythomatous radiation [
16]. P1 is marketed as SPF 30 (by in vivo tests), while P2 has not yet undergone in vivo testing. It is important to highlight that in vitro SPF results can differ from those obtained
in vivo. Studies show that the in vivo SPF for some formulations already on the market are higher than that achieved in vitro using the Mansur method [
61].
Rheological Analysis
Continuous Flow Shear Rheometry
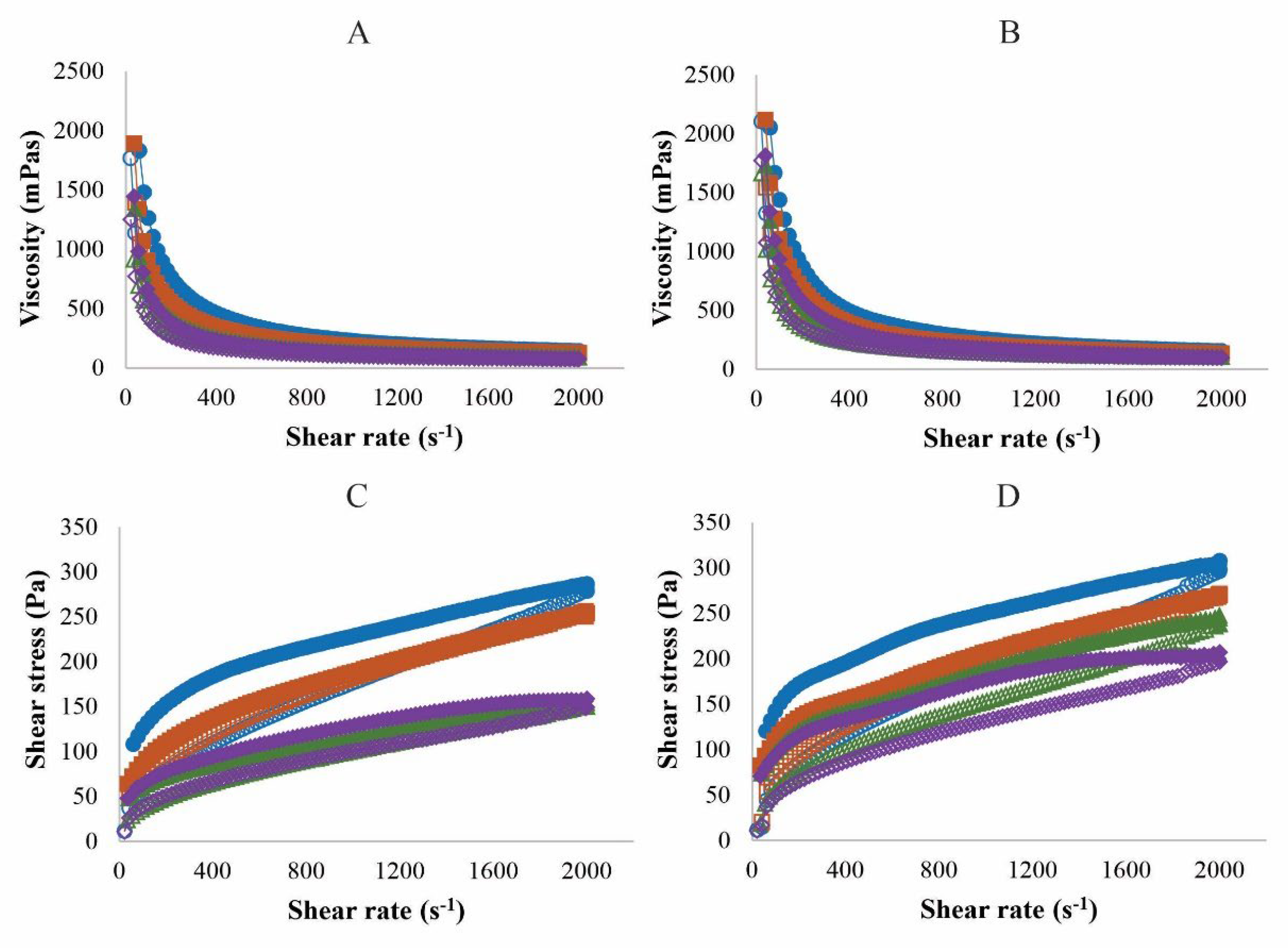
Rheological studies are tools to characterize cosmetic formulations and analyze their behavior under different conditions, obtaining characteristics such as spreadability on the skin and sensory aspects [
17]. Our results showed that both formulations are non-Newtonian fluids with pseudoplastic behavior (n < 1) (
Table 2); the viscosity decreased as the shear rate increased, a characteristic that can be observed in
Figure 2 (A and B). There was also a decrease in viscosity with increasing temperature, a characteristic that is reported in the literature for sunscreens [
38,
39].
The rheograms were better adjusted in the Herschel-Bulkley model, that is, the formulations started to flow after an initial shear stress (τ
0) and, later, they flowed with the increase in the shear rate. Thixotropy consists of a gradual reduction in viscosity under shear stress followed by a recovery of the structure when the stress is stopped [
17,
40]. It was observed that the shear gradient increased until reaching its maximum value (2000 s
−1) and, subsequently, the process was reversed by decreasing the gradient and generating the two curves [
40].
The rheological profiles of both formulations showed the presence of a hysteresis area, mainly at the extremes of temperature (4 °C and 40 °C) (
Figure 2). P2 had a significantly larger area of hysteresis at temperatures of 4 and 34 °C than P1 (
Table 2). The presence of a hysteresis area is an interesting finding, since it contributes to the release of the fragrance and the composition’s assets [
41]. The increase in the hysteresis area in photoprotective formulations may be related to the presence of emollients and emulsifiers, which alter the rheological behavior, causing a desirable effect for the formulation [
12,
42].
Regarding the consistency index values (
k),
Table 2 shows that there was a significant difference (p <0.05) comparing the values between P1 and P2 at each temperature. Since
k is related to the degree of resistance of the fluid to the flow [
17], it can be inferred that P2 is more consistent than P1 due its higher consistency index. The lowest temperature generated a higher
k value in both products, which can be explained by the fact that the temperature influences the consistency of the formulations [
43]. However, there was no significant variation in the
k value between the values at 25, 34, and 40 ºC. This fact demonstrates the possible stability with the gradual increase in temperature.
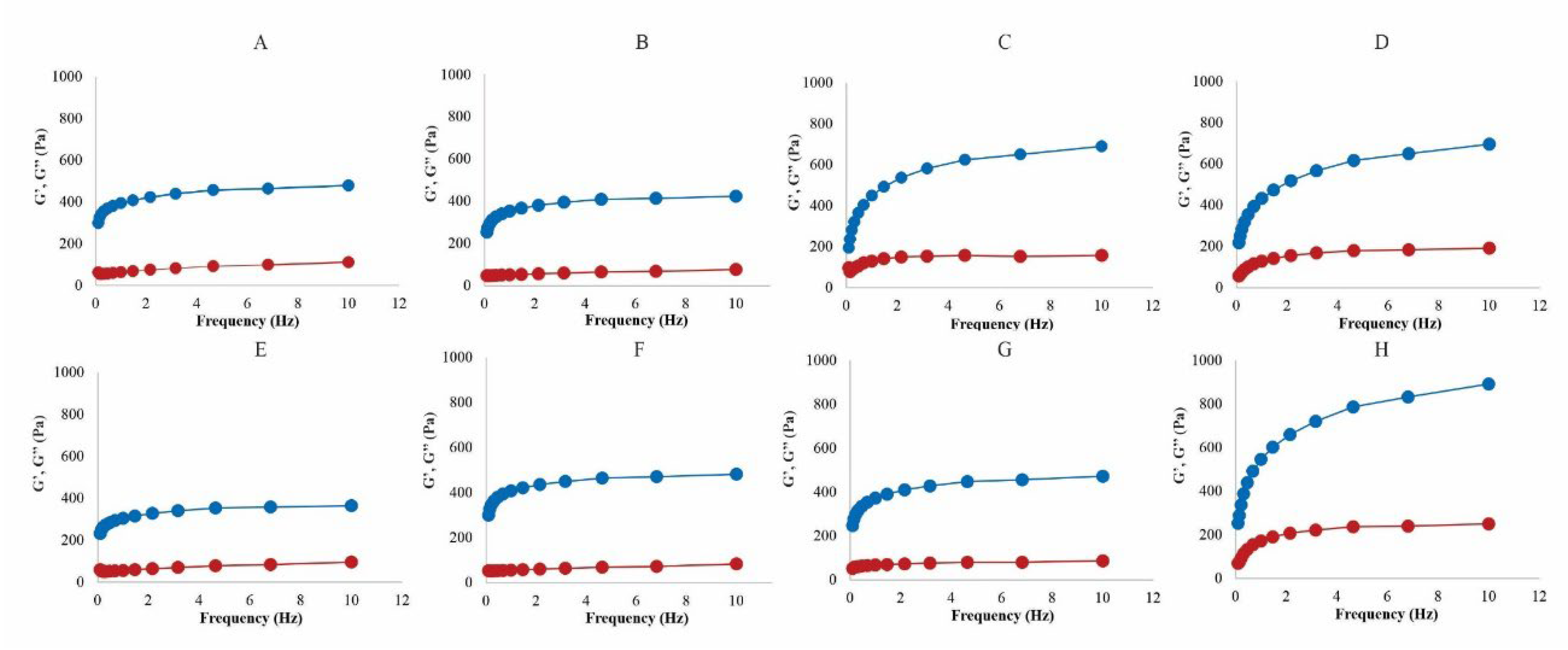
Oscillatory Rheometry
Many emulsions have viscoelastic properties that can be affected by oscillatory frequency and temperature [
44]. With P1 and P2, the increase in oscillatory frequency raised G’ mainly at the highest temperature (
Figure 3). The formulations presented a G’ (elastic modulus) greater than G” (loss modulus), confirming the characteristics of a viscoelastic system [
17,
45] (
Figure 3).
Studies with photoprotective formulations demonstrated a viscoelastic profile, that is, they had a predominant elastic behavior [
38,
46], with hysteresis or thixotropy area. These features are suitable for this kind of formulation since it facilitates the application, indicates the reversible variation of the viscosity with the time, and increases the stability [
12]. It is very important to analyze the rheological profiles of the formulations in order to modulate the desired sensory properties and also to analyze the quality and stability of products [
17].
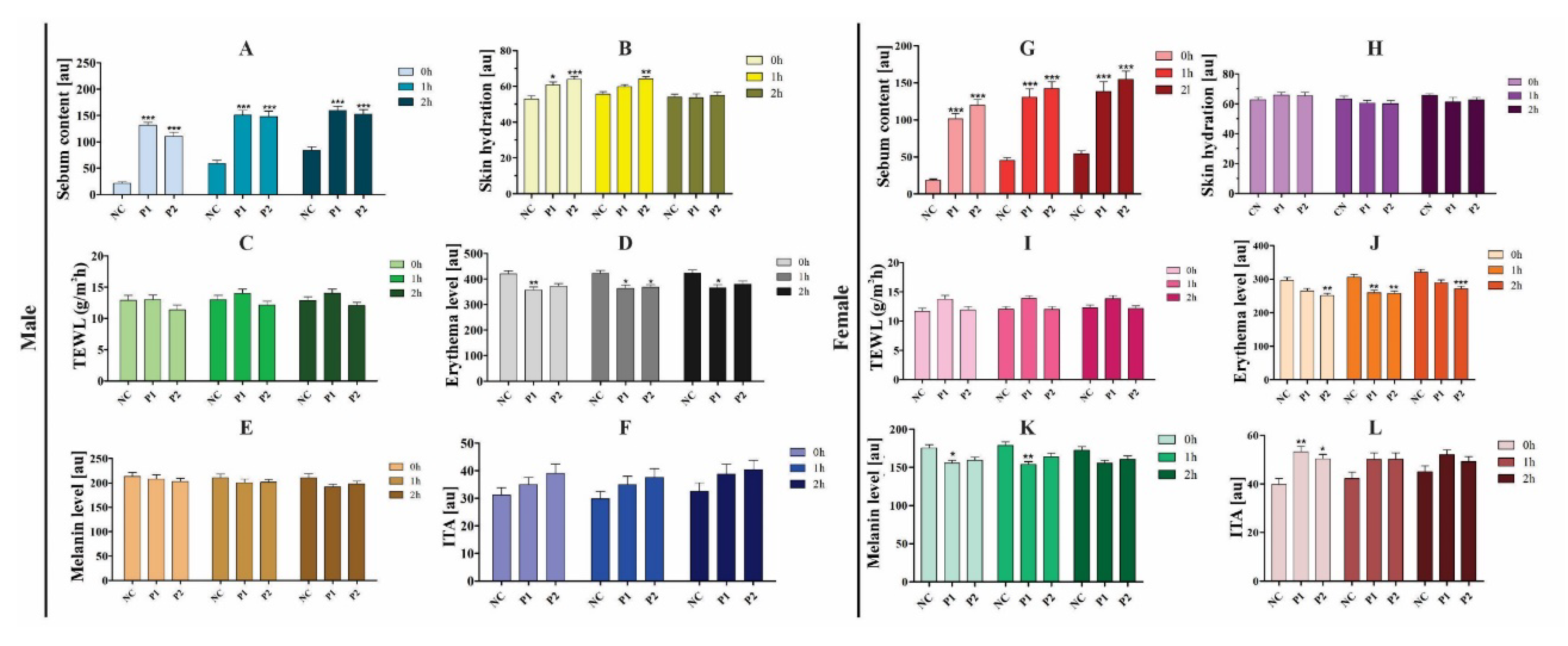
Assessment of Transepidermal Water Loss
Transepidermal water loss (TEWL), a biophysical parameter, refers to the stratum corneum’s ability to prevent uncontrolled water evaporation from the skin layers [
52]. According to the table specified by the manufacturer, values of 10-15 (AU) reveal a healthy skin condition of individuals. That result could be seen in both males (
Figure 4C) and females (
Figure 4I). In other words, in both sexes, with and without the presence of formulations, values below 15 were obtained, showing that the individuals were healthy in relation to this parameter.
A reduction in TEWL is observed when there is the application of occlusive components, which contribute to avoid water evaporation [
52]. In the formulation, it is possible to observe the presence of components such as polyglyceryl-10 pentaestearate, capric/caprylic triglyceride, and berrenyl alcohol, components already reported in multifunctional formulations that can fulfill the emollient and occlusive function. The use of multifunctional formulations for daily use becomes an excellent alternative for protection against solar radiation [
22], since exposure to solar radiation through UV rays generates several negative consequences on the skin [
4].
Assessment of Erythema Level and Melanin Content
Erythema, the redness caused by the vasodilation of cutaneous capillaries, is just one of the consequences of UV exposure in the skin [
53]. The level of skin erythema was analyzed when administering the two test products. These products were not irritating to the skin of the volunteers at the time analyzed. Furthermore, in both sexes, there was a significant decrease in the level of erythema following treatment (
Figure 4D and
Figure 4J).
It has been proven that plant extracts containing polyphenols have high antioxidant and anti-inflammatory activity, being able to also reduce erythema [
54,
55]. P1 has
Theobroma grandiflorum in its composition, a species known for its high content of phenolic compounds. In addition, P1 also contains seaweed and coumarin extract, known for their antioxidant and stimulating action [
56,
57]. The vegan formulation (P2) has in its constitution extracts of two red algae
Hypnea musciformis and
Gelidiela acerosa, and cucumber extract (
Cucumis sativus), active in the scavenging of free radicals, besides relieving the skin of cutaneous irritations [
58].
The skin phototype can be associated with the melanin content. Melanin determines the color of our hair and skin, and provides protection against UV radiation. The Fitzpatrick skin phototype describes different skin tones, photosensitivity, and response to tanning [
59]. The volunteers had phototypes I, II, and III, with melanin values as expected, since the melanin level did not exceed 250 (AU) in both sexes (
Figure 4E and
Figure 4K).
In the female volunteers, a significant decrease in the level of melanin was observed between zero and one hour with the application of P1 (
Figure 4K). Interestingly, according to the questionnaire in this study, most women reported using sunscreen daily. There are reports in the literature that the prolonged use of photoprotective formulations were able to reduce the melanin content in patients with hyperpigmentation [
32]. Another study proved that the administration of sunscreens containing antioxidants reduces the pigmentation of the skin and decreased the degradation of collagen in the dermis [
39].
Skin Color Assessment
The study also showed some results about the skin color of the volunteers. The men presented individual typology angles (ITAs) from 30º to 40º (AU), revealing an intermediate skin color. The women obtained ITAs from 40º to 55º (AU), featuring a white skin (
Figure 4F and
Figure 4L). Individuals with type I, II, and III may have lighter skin, and that claim was confirmed by analyzing the results of the ITAs of both sexes.
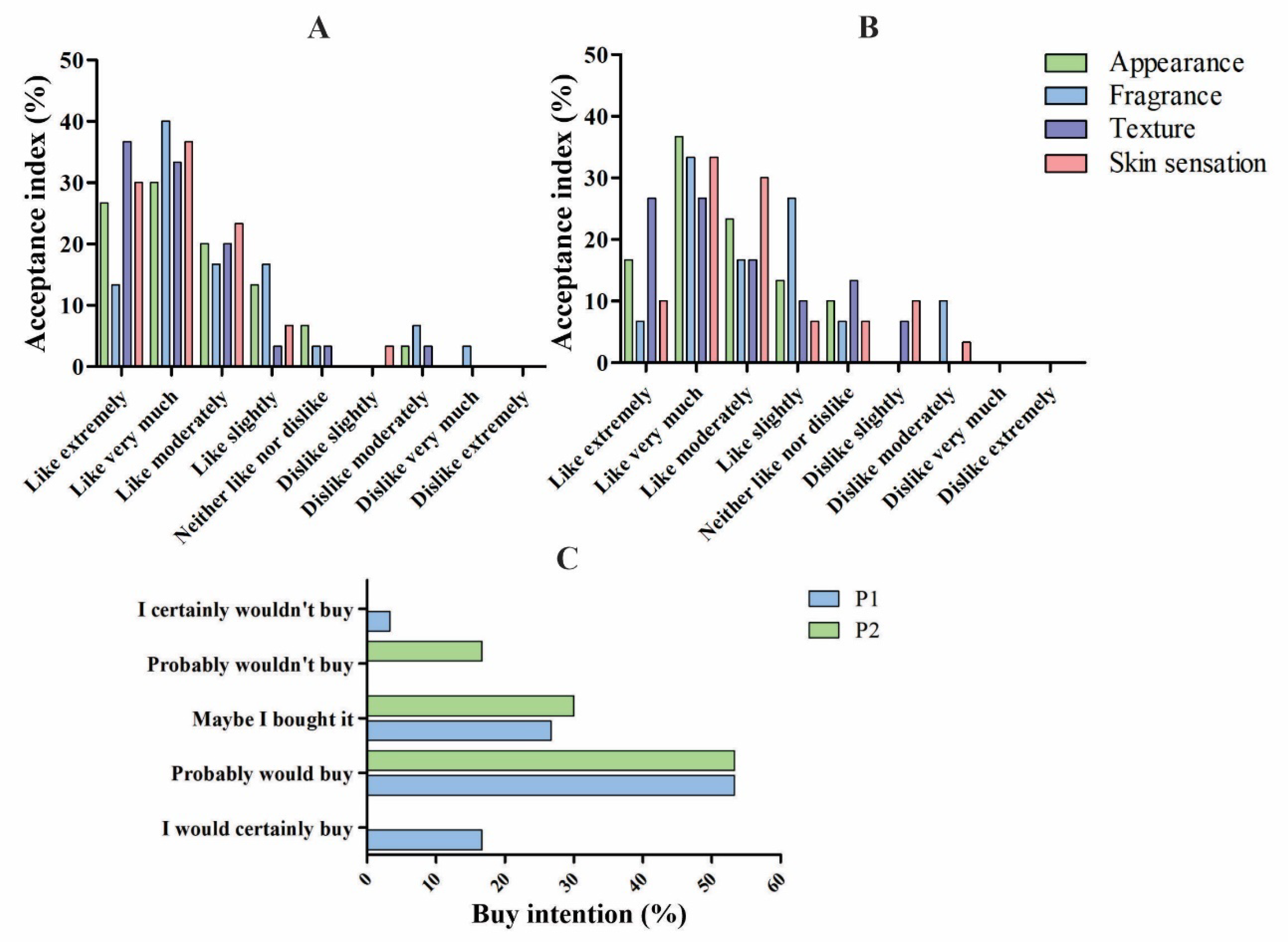
Sensorial Analysis
A sensory analysis was carried out on a 9-point hedonic scale, representing a scale with 9 categories, ranging from “I liked it very much” to “I disliked it very much”. Through this scale, opinions of the volunteers in relation to the products can be verified [
60]. For the hedonic test, the acceptance index was given in percentage, and the parameters investigated were: appearance, fragrance, texture, and sensation on the skin.
The results of P1 (
Figure 5A) showed that most volunteers answered “like very much” for the four parameters analyzed. For this formulation, “dislike extremely” was not selected by any individual for any of the parameters. For the fragrance, a small percentage answered “dislike very much”. There was also a small percentage of people who were indifferent to the parameters, answering “I neither like nor dislike”. The vast majority of the results were between “Like extremely and like slighty”.
For P2 (
Figure 5B), the result of the sensory analysis was similar to P1, in which most responses were positive. The appearance, texture, and sensation parameters on the skin obtained a greater number of “like very much” in P1 than in P2. P2 did not get any “dislike very much” and “dislike extremely” for the four parameters, a positive point to be taken into account. As in P1, most volunteers responded that they liked P2.
The purchase intention graph (
Figure 5C) revealed that most volunteers would probably buy both formulations (more than 50%). P2 did not obtain any votes for “Certainly would buy” and “Certainly would not buy”. Approximately 17% of volunteers would probably not buy P2, and approximately 4% would certainly not buy P1. Finally, almost 20% of volunteers would certainly buy P1.
Studies have already been reported using this type of affective test to verify the acceptance of photoprotective formulations, in addition to applying a 7-point purchase intention test, and there was a good acceptance profile [
37]. Determining the tactile characteristics of cosmetic products through sensory analysis is of great importance, as it generates additional improvements that could be made to achieve consumer acceptance [
38]. In addition, there are few reports in the literature of the sensory analysis of vegan photoprotective multifunctional formulations, making this study relevant to expanding knowledge among different audiences in the cosmetic market.
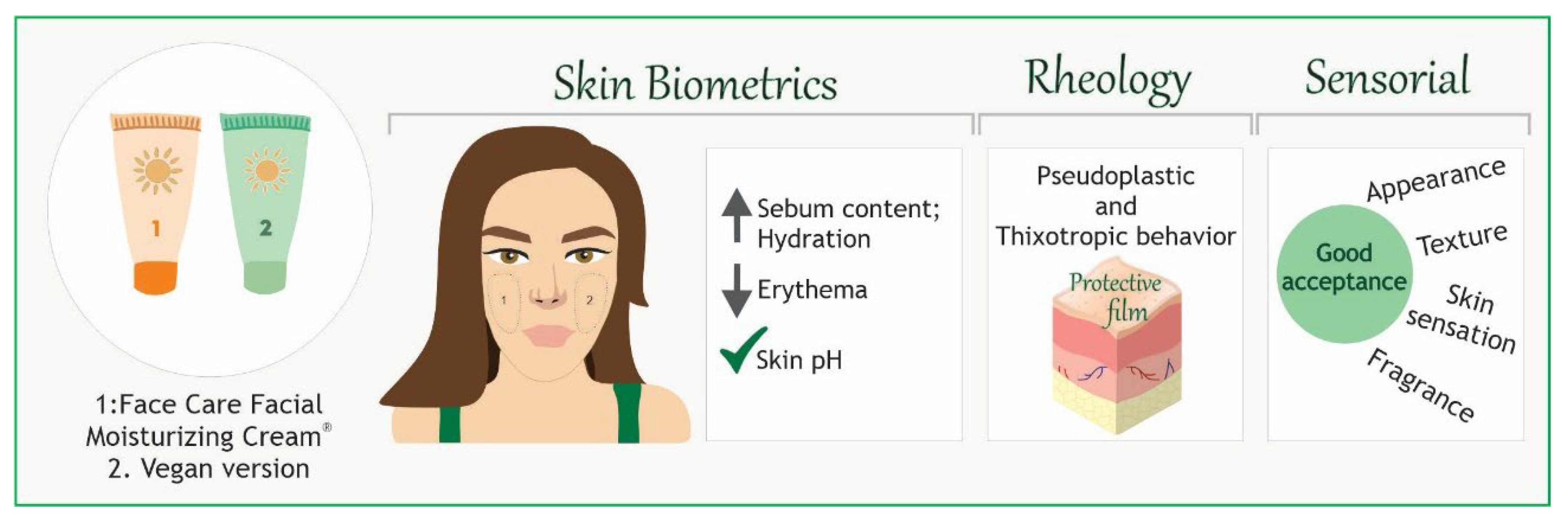
Conclusion
The study evaluated biophysical, rheological and sensorial parameters of Face Care Facial Moisturizing Cream
® (P1) and a vegan formulation (P2) by in vitro and in vivo tests. The formulations are photoprotective, presenting a SPF in vitro higher than 10. They increased the cutaneous sebum content, which can form an emulsion with water, playing a role in maintaining the hydration of the skin surface. There was an increase in hydration, maintenance of cutaneous pH, and reduction of erythema. In addition, the formulations had very similar rheological profiles, exhibiting pseudoplastic and thixotropic behavior, important for the dispersion of the present assets and to form a protective film. The sensory analysis showed a promising result for both products, which obtained great purchase intention scores by the participating volunteers (
Figure 6). The vegan formulation presents itself as a viable alternative to access a distinct market.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
K.C.N. and C.V.N. conceptualized the project. K.C.N. and B.L.A. conducted the experimental work. M.L.B. and R.S.S. contributed to result interpretation. Data curation and graphics were made by K.C.N. and L.A.A. The initial draft was written by K.C.N. Funding acquisition was managed by R.B., S.O.S.L., T.U.N., and C.V.N. Project administration and review were conducted by C.V.N. All authors collectively analyzed the findings and contributed to shaping the final manuscript.
Acknowledgment
The authors acknowledge the funding provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior -Brasil (CAPES).
Conflict of Interest
None.
References
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive compounds for skin health: A review. Nutrients. 2021, 13, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Tortora, G.J.; Derrickson, B.; Tortora, G.J. Principles of anatomy and physiology. 12th ed. Wiley, editor. Hoboken, N.J; 2009. 1288 p.
- Ansary TM, Hossain MR, Kamiya K, Komine M, Ohtsuki M. Inflammatory molecules associated with ultraviolet radiation-mediated skin aging. Int J Mol Sci. 2021, 22. [Google Scholar] [CrossRef]
- Afaq, F. Natural agents: Cellular and molecular mechanisms of photoprotection. Arch Biochem Biophys. 2011, 508, 144–51. [Google Scholar] [CrossRef] [PubMed]
- Sgarbi, F.C.; Carmo EDDo Rosa, L.E.B. Radiação ultravioleta e carcinogênese. Rev Ciencias Médicas. 2007, 16, 245–50. [Google Scholar]
- Scandalios, J.G. Oxidative stress: Molecular perception and transduction of signals triggering antioxidant gene defenses. Brazilian J Med Biol Res. 2005, 38, 995–1014. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Antioxidant constituents of three selected red and green color Amaranthus leafy vegetable. Sci Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rehana, D.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. Evaluation of antioxidant and anticancer activity of copper oxide nanoparticles synthesized using medicinally important plant extracts. Biomed Pharmacother. 2017, 89, 1067–77. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.M.; Volpato, H.; Lazarin-Bidóia, D.; Desoti, V.C.; de Souza, R.O.; Fonseca, M.J.V.; et al. The extended production of UV-induced reactive oxygen species in L929 fibroblasts is attenuated by posttreatment with Arrabidaea chica through scavenging mechanisms. J Photochem Photobiol B Biol et]. 2018, 178, 175–81. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J Dermatol Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef]
- Limón-Pacheco, J.; Gonsebatt, M.E. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat Res - Genet Toxicol Environ Mutagen. 2009, 674, 137–47. [Google Scholar] [CrossRef]
- Felippim, E.C.; Marcato, P.D.; Maria, P.; Gonçalves, B.; Campos, M. Development of Photoprotective Formulations Containing Nanostructured Lipid Carriers : Sun Protection Factor, Physical-Mechanical and Sensorial Properties. AAPS PharmSciTech. 2020, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Piccinino, D.; Capecchi, E.; Tomaino, E.; Gabellone, S.; Gigli, V.; Avitabile, D.; et al. Nano-structured lignin as green antioxidant and uv shielding ingredient for sunscreen applications. Antioxidants. 2021, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Shetty, P.K.; Venuvanka, V.; Jagani, H.V.; Chethan, G.H.; Ligade, V.S.; Musmade, P.B.; et al. Development and evaluation of sunscreen creams containing morin-encapsulated nanoparticles for enhanced UV radiation protection and antioxidant activity. Int J Nanomedicine. 2015, 10, 6477–91. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.; Hegde, A.R.; Shetty, P.K.; Gollavilli, H.; Managuli, R.S.; Kalthur, G.; et al. Sunscreen creams containing naringenin nanoparticles: Formulation development and in vitro and in vivo evaluations. Photodermatol Photoimmunol Photomed. 2018, 34, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Tofetti MH de, F.C.; de Oliveira, V.R. A importância do uso do filtro solar na prevenção do fo. Rev Científica da Univ Fr. 2006, 6, 59–66. [Google Scholar] [CrossRef]
- Huang, N. Rheological Characterization of Pharmaceutical and Cosmetic Formulations for Cutaneous Applications. Curr Pharm Des. 2019, 25, 2349–63. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, M.; Nowak, I. Noninvasive evaluation of the influence of aucubin-containing cosmetic macroemulsion on selected skin parameters. J Cosmet Dermatol. 2021, 20, 1022–30. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, U.; Koop, U.; Leneveu-Duchemin, M.C.; Osterrieder, K.; Bielfeldt, S.; Chkarnat, C.; et al. Multicentre comparison of skin hydration in terms of physical-, physiological- and product-dependent parameters by the capacitive method (Corneometer CM 825). Int J Cosmet Sci. 2003, 25, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Malina, Y.; Duarte, B.; Pessoa, U.F. Métodos biofísicos não invasivos para avaliação da eficácia de cosméticos. 2013. https://bdigital.ufp.pt/handle/10284/4097. Acessed 07 Dez 2022.
- Mara, G.; Gonçalves, S.; Maria, P.; Gonçalves, B.; Campos, M. Aplicação de métodos de biofísica no estudo da eficácia de produtos dermocosméticos. 2009, 45, 1–10. [CrossRef]
- Morocho-Jácome, A.L.; Freire, T.B.; de Oliveira, A.C.; de Almeida, T.S.; Rosado, C.; Velasco, M.V.R.; et al. In vivo SPF from multifunctional sunscreen systems developed with natural compounds—A review. J Cosmet Dermatol. 2021, 20, 729–37. [Google Scholar] [CrossRef]
- Flor, J.; Mazin, M.R.; Ferreira, L.A. Cosméticos Naturais, Orgânicos e Veganos. Cosmet Toilet. 2019, 31, 30–6. [Google Scholar]
- Lee, J.; Kwon, K.H. Good ingredients from foods to vegan cosmetics after COVID-19 pandemic. J Cosmet Dermatol. 2022, 21, 3190–9. [Google Scholar] [CrossRef] [PubMed]
- Miguel, I.; Coelho, A.; Bairrada, C.M. Modelling attitude towards consumption of vegan products. Sustain. 2021, 13, 1–17. [Google Scholar] [CrossRef]
- Mansur, J.D.S.; Breder, M.N.R.; Mansur, M.C.D.A.; Azulay, R.D. Determinação do fator de proteção solar por espectrofotometria. An. Bras. Dermatol. 1986, 121–124. [Google Scholar]
- Bruschi, M.L.; Jones, D.S.; Panzeri, H.; Gremião, M.P.D.; Freitas, O.; Lara, E.H.G. Semisolid Systems Containing Propolis for the Treatment of Periodontal Disease: In Vitro Release Kinetics, Syringeability, Rheological, Textural, and Mucoadhesive Properties. Semisolid Syst Treat Periodontal Dis. 2007, 96, 2074–89. [Google Scholar] [CrossRef]
- Hemphill, T.; Campos, W.; Pilehvari, A. Yield-power law model more accurately predicts mud rheology. Oil Gas J. 1993, 91, 45–50. [Google Scholar]
- Sovilj, V.; Milanovic, J.; Petrovic, L. Influence of gelatin-sodium stearoyl lactylate interaction on the rheological properties of gelatin gels. Colloids Surfaces A Physicochem Eng Asp. 2013, 417, 211–6. [Google Scholar] [CrossRef]
- Said Dos Santos, R.; Bassi da Silva, J.; Rosseto, H.C.; Vecchi, C.F.; Campanholi K da, S.S.; Caetano, W.; et al. Emulgels containing propolis and curcumin: The effect of type of vegetable oil, poly(acrylic acid) and bioactive agent on physicochemical stability, mechanical and rheological properties. Gels. 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.B.; Pathak, M.; Parrish, J.A. Protection of human skin against the effects of the sunburn ultraviolet (290-320nm), in sunlight and man, normal and abnormal photobiological responses. Univ Tokio. 1974, 751. [Google Scholar]
- Martini, A.P.M.; Maia Campos, P.M.B.G. Influence of visible light on cutaneous hyperchromias: Clinical efficacy of broad-spectrum sunscreens. Photodermatol Photoimmunol Photomed. 2018, 34, 241–8. [Google Scholar] [CrossRef]
- Courage-Khazaka-Scientific-Devices. Multi Probe Adapter 9. 2022. https://www.courage-khazaka.de/de/wissenschaftliche-produkte/alle/sondensysteme/16-wissenschaftliche-produkte/alle-produkte/75-mpa-d. Acessed 07 Dez 2022.
- Dalgleish, T.; Williams, J.M.G.; Golden, A.-M.J.; Perkins, N.; Barrett, L.F.; Barnard, P.J.; et al. Scientific Measurements of Skin and Hair. J Exp Psychol Gen. 2007, 136, 23–42. [Google Scholar] [CrossRef]
- Courage-Khazaka-Scientific-Devices. Information and Operating Instructions for the Multi Probe Adapter MPA and its probes. Koln, Germany; 2007.
- Lode, M.; Buraczewska, I.; Brostro, U. Artificial reduction in transepidermal water loss improves skin barrier function. Br. J. Dermatol. 2007, 82–6. [Google Scholar] [CrossRef]
- Prudencio, S.; Prude, H.; Ceratti, V.S. ; Ceratti, V.S. Avaliação sensorial de formulações fotoprotetoras em diferentes bases cosméticas. Rev Saúde e Pesqui. 2012, 5, 487–94. [Google Scholar]
- Yarovaya, L. Correlation between sensory and instrumental characterization of developed sunscreens containing grape seed extract and a commercial product. Int J Cosmet Sci. 2022, 44, 569–87. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.; de Freitas, L.A.P.; Maia Campos, P.M.B.G. Topical Formulation Containing Beeswax-Based Nanoparticles Improved In Vivo Skin Barrier Function. AAPS PharmSciTech. 2017, 18, 2505–16, http://link.springer.com/10.1208/s12249-017-0737-x. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Moturi, V.; Lee, Y. Thixotropic property in pharmaceutical formulations. J Control Release. 2009, 136, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Arancibia, C.; Castro, C.; Jublot, L.; Costell, E.; Bayarri, S. Colour, rheology, flavour release and sensory perception of dairy desserts. Influence of thickener and fat content. Lwt - Food Sci Technol. 2015, 62, 408–16. [Google Scholar] [CrossRef]
- Osterwalder, U.; Sohn, M.; Herzog, B. Global state of sunscreens. Photodermatol Photoimmunol Photomed. 2014, 30, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.; de Freitas, L.A.P.; Maia Campos, P.M.B.G. Topical Formulation Containing Beeswax-Based Nanoparticles Improved In Vivo Skin Barrier Function. AAPS PharmSciTech. 2017, 18, 2505–16. [Google Scholar] [CrossRef] [PubMed]
- Said, R.; Cássia, H.; Bassi, J.; Félix, C.; Caetano, W.; Luciano, M. The effect of carbomer 934P and different vegetable oils on physical stability, mechanical and rheological properties of emulsion-based systems containing propolis. 2020, 307. [CrossRef]
- Huang, Z.; Delparastan, P.; Burch, P.; Cheng, J.; Cao, Y.; Messersmith, P.B. Injectable dynamic covalent hydrogels of boronic acid polymers cross-linked by bioactive plant-derived polyphenols. Trans Annu Meet Soc Biomater Annu Int Biomater Symp. 2019, 40, 61. [Google Scholar] [CrossRef]
- Tadros, T.F. Correlation of viscoelastic properties of stable and flocculated suspensions with their interparticle interactions. Adv Colloid Interface Sci. 1996, 68, 97–200. [Google Scholar] [CrossRef]
- Khavkin, J.; Ellis, D.A.F. Aging Skin: Histology, Physiology, and Pathology. Facial Plast Surg Clin North Am. 2011, 19, 229–34. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, K.P.; Elsner, P.; Berardesca, E.; Maibach, H.I. Bioengineering of the Skin: Skin Imaging and Analysis, Informa Healthcare USA. Inc, New York. 2007.
- Man, M.Q.; Xin, S.J.; Song, S.P.; Cho, S.Y.; Zhang, X.J.; Tu, C.X.; et al. Variation of skin surface pH, sebum content and stratum corneum hydration with age and gender in a large chinese population. Skin Pharmacol Physiol. 2009, 22, 190–9. [Google Scholar] [CrossRef]
- Maan, A.A.; Nazir, A.; Khan, M.K.I.; Ahmad, T.; Zia, R.; Murid, M.; et al. The therapeutic properties and applications of Aloe vera: A review. J Herb Med. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Melo, M.O.; Campos, P.M.B.G. G. Técnicas para Avaliar a Hidratação e a Oleosidade da Pele. Cosmet Toilet. 2016, 28, 30–4. [Google Scholar]
- Melo, M.O; Campos, P.M.B.G. Função de Barreira da Pele e pH Cutâneo. Bioengenharia Cutânea. 2016, 28, 34–8. [Google Scholar]
- Matsumura, Y.; Ananthaswamy, H.N. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004, 195, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J. New Developments in Photoprotection of Human Skin. Skin Pharmacol Appl Skin Physiol. 2001, 14, 401–7. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhang, X.; Chen, F.; Wang, M. Dietary polyphenols as photoprotective agents against UV radiation. J Funct Foods. 2017, 30, 108–18. [Google Scholar] [CrossRef]
- Balboa, E.M.; Conde, E.; Moure, A.; Falqué, E.; Domínguez, H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013, 138, 1764–85. [Google Scholar] [CrossRef]
- Pugliese, A.G.; Tomas-barberan, F.A.; Truchado, P.; Genovese, M.I.; Prestes, L. Flavonoids, Proanthocyanidins, Vitamin C, and Antioxidant Activity of Theobroma grandif lorum (Cupuassu) Pulp and Seeds. J. Agric. Food Chem. 2013, 61, 2720–28. [Google Scholar] [CrossRef]
- Chakraborty, K.; Joseph, D.; Joy, M.; Raola, V.K. Characterization of substituted aryl meroterpenoids from red seaweed Hypnea musciformis as potential antioxidants. Food Chem. 2016, 212, 778–88. [Google Scholar] [CrossRef] [PubMed]
- Dinish, X.L.U.S.; Aguirre, J.; Bi, R.; Dev, K.; Binte, A.; Attia, E.; et al. Optoacoustic mesoscopy analysis and quantitative estimation of specific imaging metrics in Fitzpatrick skin phototypes II to V. J. Biophotonics. 2019, 12, 1–9. [Google Scholar] [CrossRef]
- Lim, J. Hedonic scaling: A review of methods and theory. Food Qual Prefer. 2011, 22, 733–47. [Google Scholar] [CrossRef]
- Mbanga, L.; Mulenga, M.; Mpiana, P.T.; Bokolo, K.; Mumbwa, M.; Mvingu, K. Determination of sun protection factor (SPF) of some body creams and lotions marketed in Kinshasa by ultraviolet spectrophotometry. Int. J. Adv. Res. Chem. Sci. 2014, 1, 7–13. [Google Scholar]
Figure 1.
UV radiation scan covering 250 to 400 nm with P1 and P2 at a concentration of 100 µg/mL.
Figure 1.
UV radiation scan covering 250 to 400 nm with P1 and P2 at a concentration of 100 µg/mL.
Figure 2.
Rheological behavior of P1 and P2. Viscosity as a function of the shear rate, flow diagram of P1 (A) and P2 (B). Shear stress as a function of the shear rate, flow diagram of P1 (C) and P2 (D). The analyses were performed at temperatures of 4 °C (⬤), 25 °C (∎), 34 °C (▲), and 40 °C (♦). The closed symbol represents the forward curve and the open symbol represents the return curve. Each rheogram is the average of at least 3 replicates with a variation coefficient of less than 10%.
Figure 2.
Rheological behavior of P1 and P2. Viscosity as a function of the shear rate, flow diagram of P1 (A) and P2 (B). Shear stress as a function of the shear rate, flow diagram of P1 (C) and P2 (D). The analyses were performed at temperatures of 4 °C (⬤), 25 °C (∎), 34 °C (▲), and 40 °C (♦). The closed symbol represents the forward curve and the open symbol represents the return curve. Each rheogram is the average of at least 3 replicates with a variation coefficient of less than 10%.
Figure 3.
Storage module G ‘(
⬤) and loss module G’’(
⬤) depending on the frequency of P1 at temperatures of 4 °C (A), 25 °C (B), 34 °C (C), and 40 °C (D), and P2 at temperatures of 4 °C (E), 25 °C (F), 34 °C (G), and 40 °C (H). Each rheogram is the average of at least 3 replicates with a variation coefficient of less than 10%. Tangent δ, on the other hand, remained relatively constant with an increase in frequency, but P1 had the tangent values higher for higher temperatures (34 and 40 °C). Both products showed tangent δ values and less than 1, at all temperatures studied, indicating that the viscoelasticity of the formulations was also maintained (supplementary material,
Figure S1).
Figure 3.
Storage module G ‘(
⬤) and loss module G’’(
⬤) depending on the frequency of P1 at temperatures of 4 °C (A), 25 °C (B), 34 °C (C), and 40 °C (D), and P2 at temperatures of 4 °C (E), 25 °C (F), 34 °C (G), and 40 °C (H). Each rheogram is the average of at least 3 replicates with a variation coefficient of less than 10%. Tangent δ, on the other hand, remained relatively constant with an increase in frequency, but P1 had the tangent values higher for higher temperatures (34 and 40 °C). Both products showed tangent δ values and less than 1, at all temperatures studied, indicating that the viscoelasticity of the formulations was also maintained (supplementary material,
Figure S1).
Figure 4.
Skin sebum content assessment in male (A) and female (G) individuals, skin hydration for male (B) and female (H), transepidermal water loss for male (C) and female (I), skin erythema for male (D) and female (J), melanin level for male (E) and female (K), and skin color in male (F) and female (L) (MPA 9, Courage-Khazaka) at time 0, time 1 (one hour after application), and time 2 (two hours after application). (NC) negative control, (P1) product 1, (P2) product 2. * p <0.05, ** p <0.01, and *** p <0.001 indicate a significant difference compared with NC, according to one-way ANOVA with Tukey test.
Figure 4.
Skin sebum content assessment in male (A) and female (G) individuals, skin hydration for male (B) and female (H), transepidermal water loss for male (C) and female (I), skin erythema for male (D) and female (J), melanin level for male (E) and female (K), and skin color in male (F) and female (L) (MPA 9, Courage-Khazaka) at time 0, time 1 (one hour after application), and time 2 (two hours after application). (NC) negative control, (P1) product 1, (P2) product 2. * p <0.05, ** p <0.01, and *** p <0.001 indicate a significant difference compared with NC, according to one-way ANOVA with Tukey test.
Figure 5.
Acceptance index (%) using the adopted parameters: appearance, fragrance, texture, and skin sensation for (A) P1 and (B) P2. 5-point purchase intention graph (%) for P1 and P2 (C).
Figure 5.
Acceptance index (%) using the adopted parameters: appearance, fragrance, texture, and skin sensation for (A) P1 and (B) P2. 5-point purchase intention graph (%) for P1 and P2 (C).
Figure 6.
Graphical abstract demonstrating a multifunctional photoprotective formulation (P1) and its vegan version (P2) on the human skin. They had an SPF higher than 10, by in vitro tests. They also proved to be beneficial to the skin, with an increase in hydration, cutaneous sebum, and a reduction of erythema. Moreover, the sensory analysis showed a promising result for both products, great purchase intention scores by the participating volunteers.
Figure 6.
Graphical abstract demonstrating a multifunctional photoprotective formulation (P1) and its vegan version (P2) on the human skin. They had an SPF higher than 10, by in vitro tests. They also proved to be beneficial to the skin, with an increase in hydration, cutaneous sebum, and a reduction of erythema. Moreover, the sensory analysis showed a promising result for both products, great purchase intention scores by the participating volunteers.
Table 1.
Components of Face Care Facial Moisturizing Cream® (P1) and a vegan test formulation (P2) (INCI - International Nomenclature Cosmetic Ingredient).
Table 1.
Components of Face Care Facial Moisturizing Cream® (P1) and a vegan test formulation (P2) (INCI - International Nomenclature Cosmetic Ingredient).
| P1 |
P2 |
| Aqua (water) |
Aqua (water) |
| Tribehenin PEG-20 esters |
Caprylic/capric triglyceride |
| Theobroma Grandiflorum seed butter |
Titanium dioxide |
| Tocopheryl acetate |
Hydrated silica |
| C12-15 Alkyl benzoate |
Hydrogen dimethicone |
| Diethylamino hidroxybenzoyl hexyl benzoate |
Aluminium hydroxide |
| Ethylhexyl triazone |
Zinc oxide |
| Algae extract |
Triethoxycaprylylsilane |
| Bis-ethylhexyloxyphenol methoxyphenyl triazine |
Cetearyl olivate/sorbitan olivate |
| Ethylhexyl methoxycinnamate |
Propanediol |
| Titanium dioxide |
Coco-caprylate/caprate, |
| Hydrated silica |
Polyglyceryl-10 pentastearate |
| Hydrogen dimethicone |
Behenyl alcohol |
| Aluminium hydroxide |
Sodium stearoyl lactylate |
| Dimethicone |
Squalene, |
| Panthenol |
Hypnea musciformis extract |
| Glycerin |
Gellidiela acerosa extract |
| Disodium EDTA |
Cucumis sativus (cucumber) seed extract |
| Acrylates/C10-30 alkyl acrylate crosspolymer |
Ammonium acryloyldimethyltaurate/vp copolymer |
| Triethanolamine |
Phenoxyethanol |
| Biosaccharide gum 4 |
Ethylhexylglycerin |
| Methylisothiazolinone |
Tocopheryl acetate |
| Phenoxyethanol |
Sodium stearoyl glutamate |
| Cyclomethicone |
Disodium EDTA |
| Parfum (Fragrance) |
Parfum (fragrance) |
| Xanthan gum |
|
| Cyclopentasiloxane |
|
| Dimethicone crosspolymer |
|
| Glass butylphenyl methylpropional |
|
| Alpha-isomethyl ionone |
|
| Coumarin |
|
| Hexyl cinnamal |
|
| Linalool |
|
Table 2.
– Results of the hysteresis areas, k, n, and τ0, at temperatures of 4, 25, 34, and 40 °C for P1 and P2. Means with the same letter are significantly different comparing P1 with P2 (p < 0.05) according to the one-way ANOVA with Tukey test.
Table 2.
– Results of the hysteresis areas, k, n, and τ0, at temperatures of 4, 25, 34, and 40 °C for P1 and P2. Means with the same letter are significantly different comparing P1 with P2 (p < 0.05) according to the one-way ANOVA with Tukey test.
| Table . |
k (Pa.s) |
n (dimensionless) |
τ0 (Pa) |
Hysteresis area (Pa/s) |
| P1 |
| 4 |
c35.08 ± 1.41 |
0.27 ± 0.01 |
g36.50 ± 2.57 |
a89097.50 ± 9034.23 |
| 25 |
d14.66 ± 0.95 |
0.37 ± 0.00 |
17.37 ± 2.47 |
12934.00 ± 832.60 |
| 34 |
e12.29 ± 0.46 |
0.35 ± 0.02 |
17.80 ± 2.82 |
b30046.67 ± 2832.85 |
| 40 |
f13.88 ± 1.48 |
0.30 ± 0.02 |
h11.82 ± 3.04 |
57483.33 ± 6890.91 |
| |
P2 |
| 4 |
c41.25 ± 2.68 |
0.26 ± 0.00 |
g15.45 ± 4.56 |
a105525.00 ± 1951.71 |
| 25 |
d22.67 ± 0.47 |
0.32 ± 0.00 |
22.73 ± 0.81 |
26993.33 ± 12.79 |
| 34 |
e20.95 ± 1.31 |
0.33 ± 0.00 |
18.07 ± 3.31 |
b61085.00 ± 2990.85 |
| 40 |
f24.38 ± 5.13 |
0.28 ± 0.01 |
h2.93 ± 0.85 |
66178.00 ± 13671.29 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).