Submitted:
11 July 2024
Posted:
12 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Echocardiography
2.3. Samples and Analytical Procedures
2.4. Isolation and Characterization of Human Plasma EVs
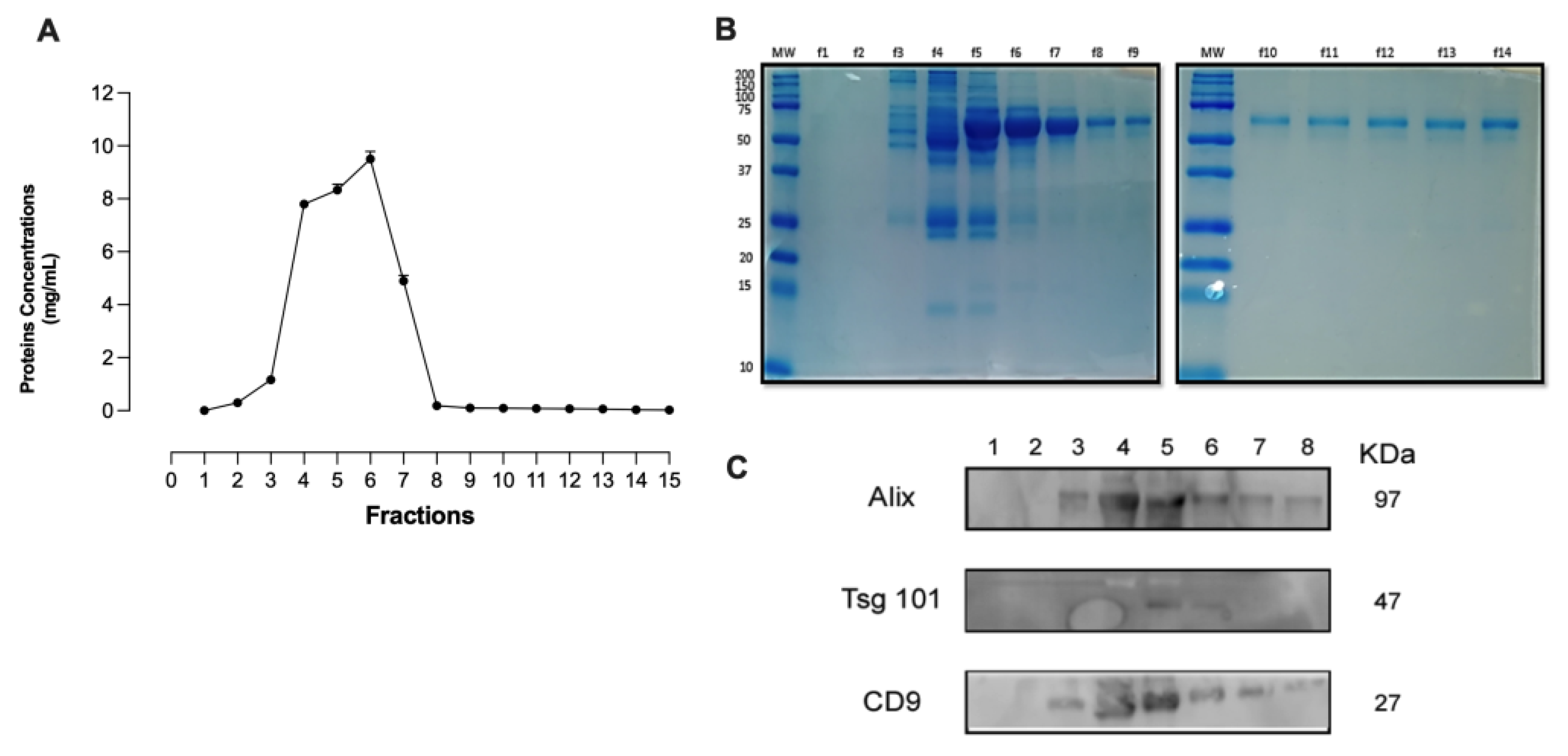
2.5. Size exclusion Chromatography (SEC)
2.6. Extracellular Vesicles Characterization
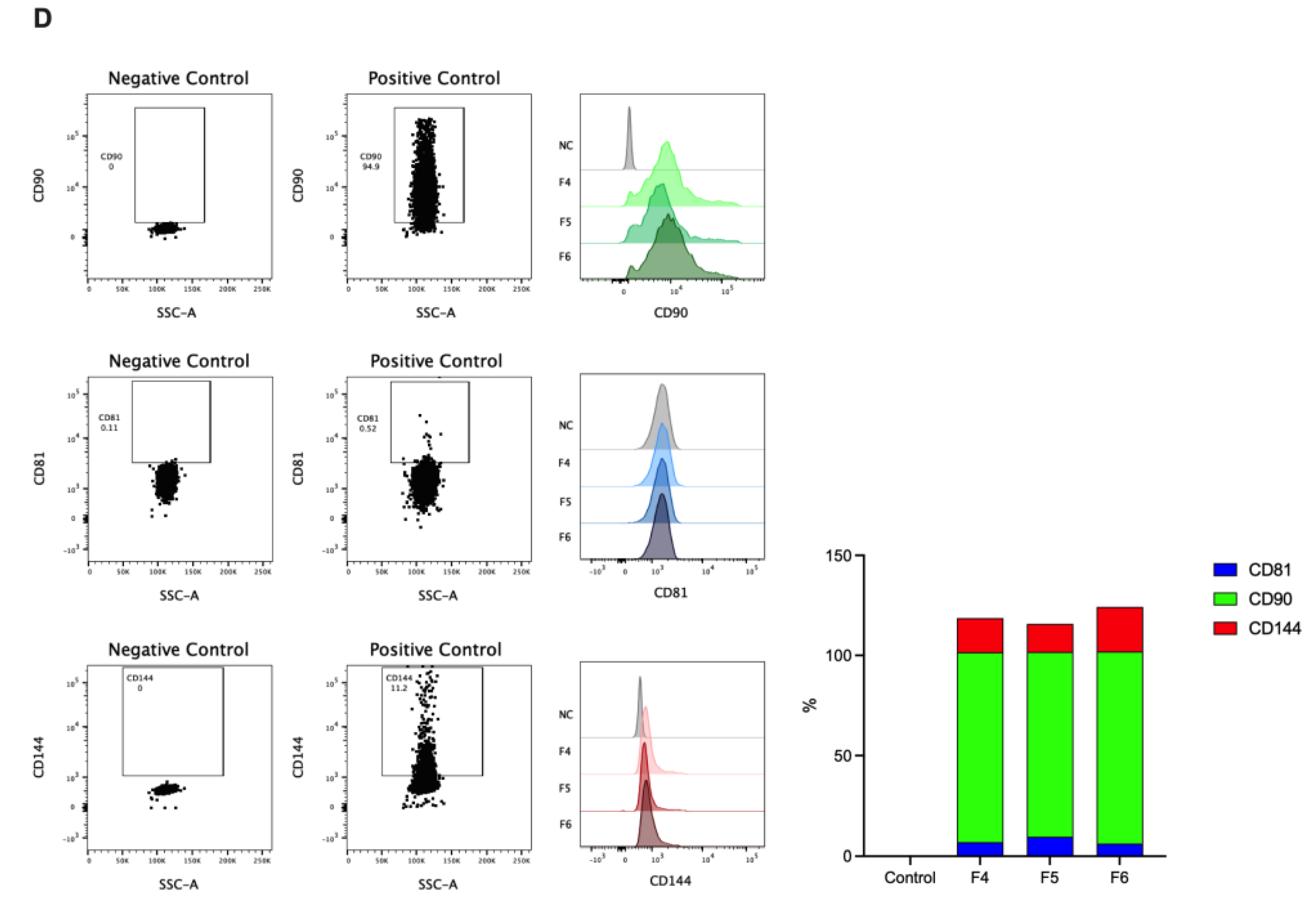
2.7. Flow cytometry of EVs
2.8. Statistical Analysis
3. Results
3.1. Studied Group
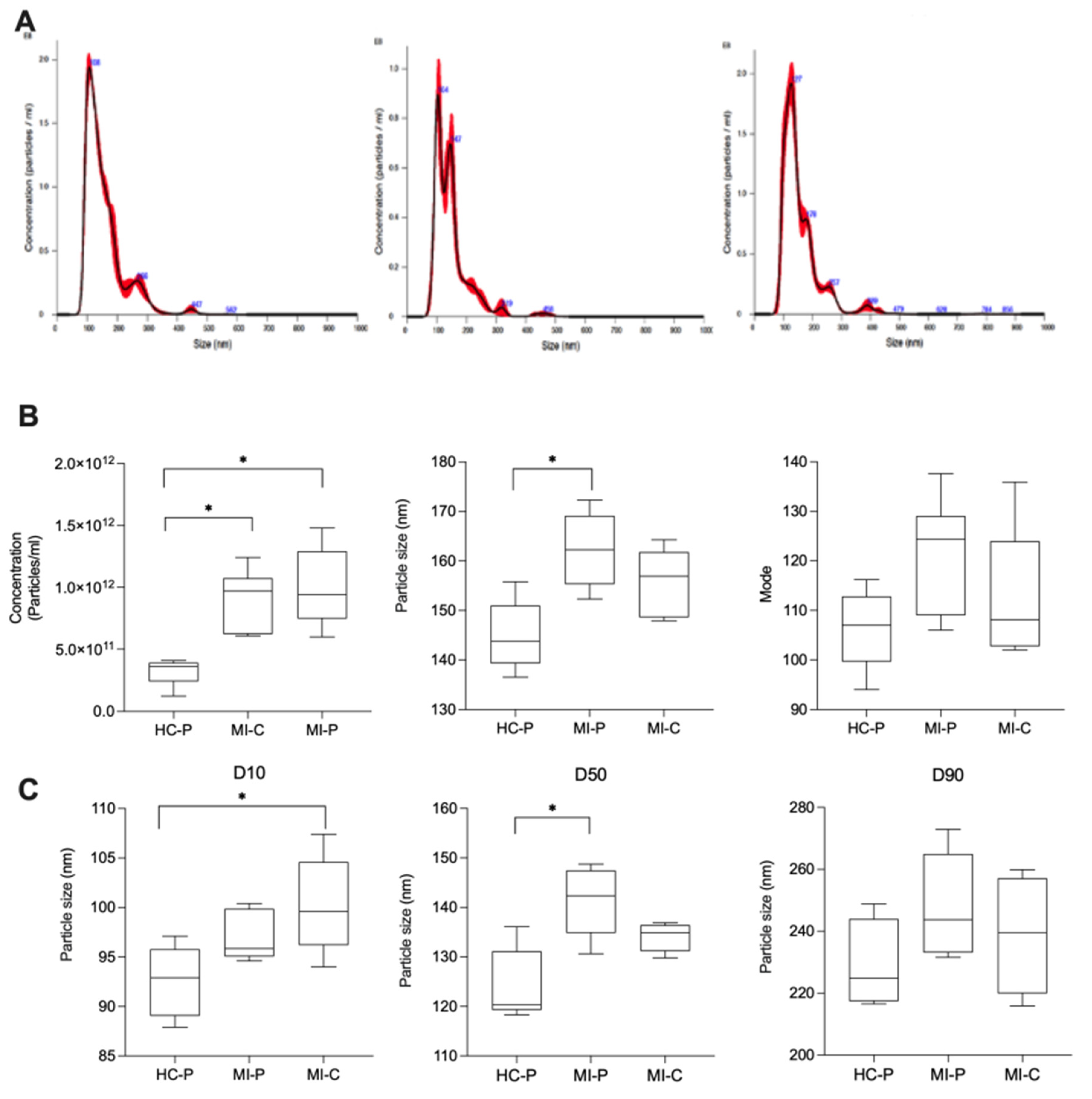
3.2. Characteristics of Peripheric and Coronary EVs from AMI (MI-P and MI-C) and Controls (HC-P)
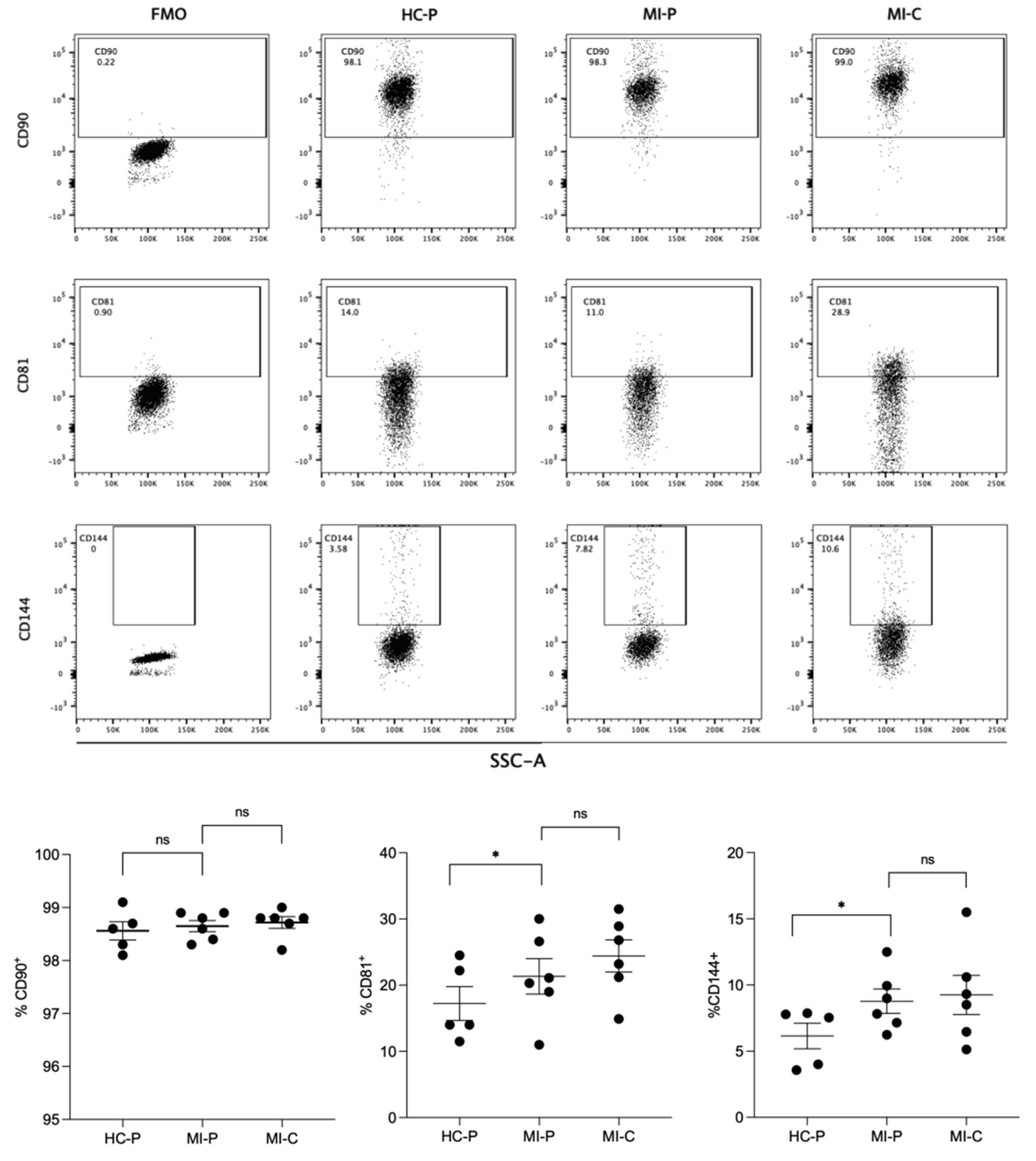
3.3. Immunophenotype Characterization of Extracellular Vesicles by Flow Cytometry
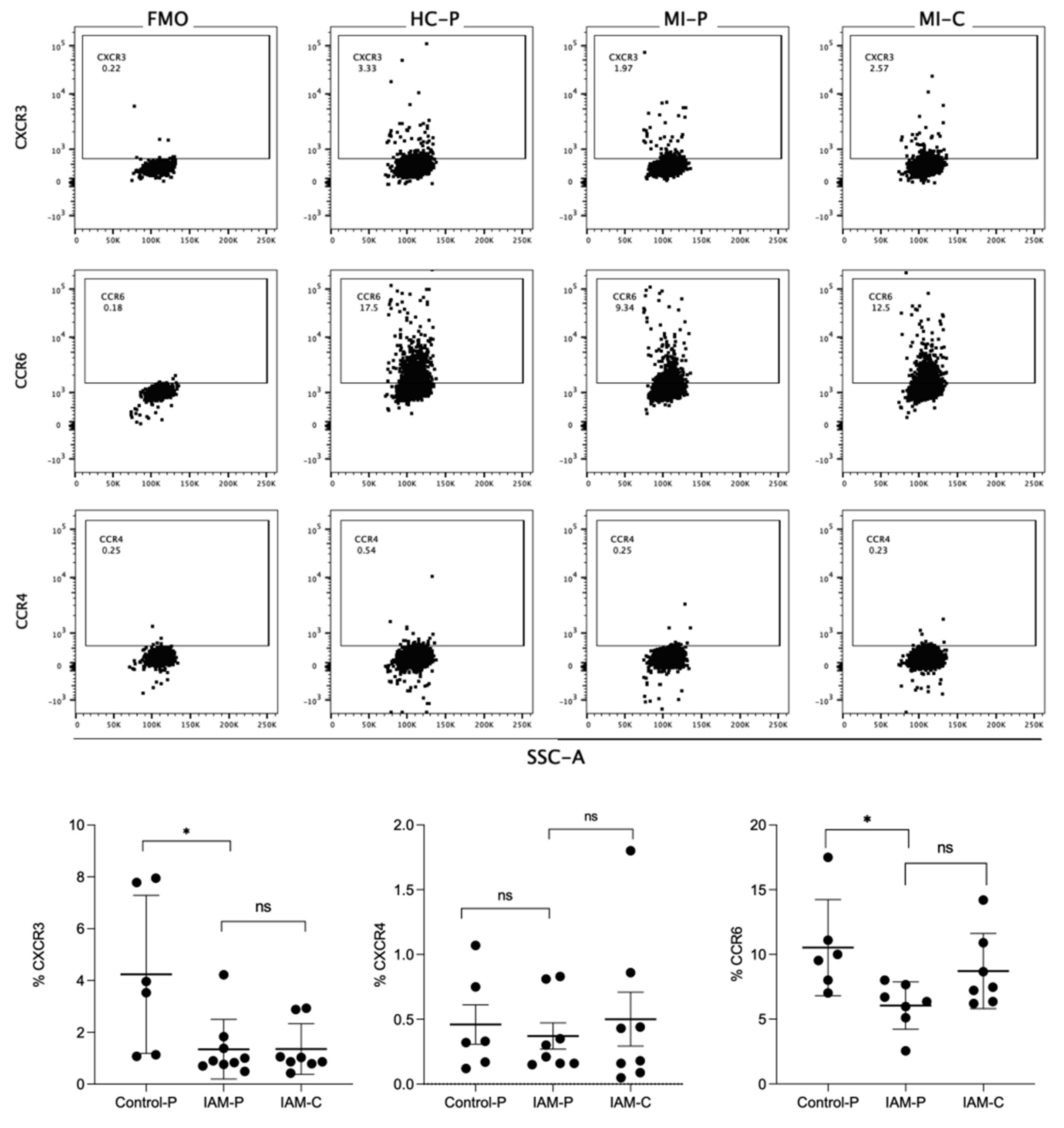
3.3. Characterization of the Chemokine Receptor Profile of Extracellular Vesicles by Flow Cytometry
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Saleh M, Ambrose JA. Understanding myocardial infarction. F1000Res. 2018;7.
- Reinecke H, Minami E, Zhu WZ, Laflamme MA. Cardiogenic differentiation and transdifferentiation of progenitor cells. Circ Res. 2008;103(10):1058-71.
- Moreno Ruiz N. Modificación de los criterios de Sgarbossa para el diagnóstico de infarto agudo de miocardio en presencia de bloqueo de rama izquierda. Revista de la Facultad de Medicina 2015;63(1):151-4.
- Martinez-Greene, J.A.; Hernandez-Ortega, K.; Quiroz-Baez, R.; Resendis-Antonio, O.; Pichardo-Casas, I.; Sinclair, D.A.; Budnik, B.; Hidalgo-Miranda, A.; Uribe-Querol, E.; Ramos-Godinez, M.D.P.; et al. Quantitative proteomic analysis of extracellular vesicle subgroups isolated by an optimized method combining polymer-based precipitation and size exclusion chromatography. J Extracell Vesicles 2021, 10, e12087. [CrossRef]
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer 2019, 18, 75. [CrossRef]
- Zhu, J.; Lu, K.; Zhang, N.; Zhao, Y.; Ma, Q.; Shen, J.; Lin, Y.; Xiang, P.; Tang, Y.; Hu, X.; et al. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif Cells Nanomed Biotechnol 2018, 46, 1659-1670. [CrossRef]
- Giricz, Z.; Varga, Z.V.; Baranyai, T.; Sipos, P.; Paloczi, K.; Kittel, A.; Buzas, E.I.; Ferdinandy, P. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J Mol Cell Cardiol 2014, 68, 75-78. [CrossRef]
- Mackie, A.R.; Klyachko, E.; Thorne, T.; Schultz, K.M.; Millay, M.; Ito, A.; Kamide, C.E.; Liu, T.; Gupta, R.; Sahoo, S.; et al. Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circ Res 2012, 111, 312-321. [CrossRef]
- de Couto, G.; Gallet, R.; Cambier, L.; Jaghatspanyan, E.; Makkar, N.; Dawkins, J.F.; Berman, B.P.; Marban, E. Exosomal MicroRNA Transfer Into Macrophages Mediates Cellular Postconditioning. Circulation 2017, 136, 200-214. [CrossRef]
- Sahoo, S.; Losordo, D.W. Exosomes and cardiac repair after myocardial infarction. Circ Res 2014, 114, 333-344. [CrossRef]
- Lazar, E.; Benedek, T.; Korodi, S.; Rat, N.; Lo, J.; Benedek, I. Stem cell-derived exosomes - an emerging tool for myocardial regeneration. World J Stem Cells 2018, 10, 106-115. [CrossRef]
- Ju, C.; Shen, Y.; Ma, G.; Liu, Y.; Cai, J.; Kim, I.M.; Weintraub, N.L.; Liu, N.; Tang, Y. Transplantation of Cardiac Mesenchymal Stem Cell-Derived Exosomes Promotes Repair in Ischemic Myocardium. J Cardiovasc Transl Res 2018, 11, 420-428. [CrossRef]
- Yao, J.; Huang, K.; Zhu, D.; Chen, T.; Jiang, Y.; Zhang, J.; Mi, L.; Xuan, H.; Hu, S.; Li, J.; et al. A Minimally Invasive Exosome Spray Repairs Heart after Myocardial Infarction. ACS Nano 2021, 15, 11099-11111. [CrossRef]
- Huang, L.; Yang, L.; Ding, Y.; Jiang, X.; Xia, Z.; You, Z. Human umbilical cord mesenchymal stem cells-derived exosomes transfers microRNA-19a to protect cardiomyocytes from acute myocardial infarction by targeting SOX6. Cell Cycle 2020, 19, 339-353. [CrossRef]
- Vicencio, J.M.; Yellon, D.M.; Sivaraman, V.; Das, D.; Boi-Doku, C.; Arjun, S.; Zheng, Y.; Riquelme, J.A.; Kearney, J.; Sharma, V.; et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J Am Coll Cardiol 2015, 65, 1525-1536. [CrossRef]
- Jansen, F.; Yang, X.; Proebsting, S.; Hoelscher, M.; Przybilla, D.; Baumann, K.; Schmitz, T.; Dolf, A.; Endl, E.; Franklin, B.S.; et al. MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J Am Heart Assoc 2014, 3, e001249. [CrossRef]
- Sinning, J.M.; Losch, J.; Walenta, K.; Bohm, M.; Nickenig, G.; Werner, N. Circulating CD31+/Annexin V+ microparticles correlate with cardiovascular outcomes. Eur Heart J 2011, 32, 2034-2041. [CrossRef]
- Nozaki, T.; Sugiyama, S.; Koga, H.; Sugamura, K.; Ohba, K.; Matsuzawa, Y.; Sumida, H.; Matsui, K.; Jinnouchi, H.; Ogawa, H. Significance of a multiple biomarkers strategy including endothelial dysfunction to improve risk stratification for cardiovascular events in patients at high risk for coronary heart disease. J Am Coll Cardiol 2009, 54, 601-608. [CrossRef]
- Jimenez, J.J.; Jy, W.; Mauro, L.M.; Soderland, C.; Horstman, L.L.; Ahn, Y.S. Endothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosis. Thromb Res 2003, 109, 175-180. [CrossRef]
- World Health Organization. Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovasculardiseases#tab=tab_1 (accessed on 15 March 2020).
- Otero-Ortega, L.; Alonso-Lopez, E.; Perez-Mato, M.; Laso-Garcia, F.; Gomez-de Frutos, M.C.; Diekhorst, L.; Garcia-Bermejo, M.L.; Conde-Moreno, E.; Fuentes, B.; Alonso de Lecinana, M.; et al. Similarities and Differences in Extracellular Vesicle Profiles between Ischaemic Stroke and Myocardial Infarction. Biomedicines 2020, 9. [CrossRef]
- Barile, L.; Lionetti, V.; Cervio, E.; Matteucci, M.; Gherghiceanu, M.; Popescu, L.M.; Torre, T.; Siclari, F.; Moccetti, T.; Vassalli, G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res 2014, 103, 530-541. [CrossRef]
- Oporto, K.; Radojkovic, C.; Mellisho, E.A.; Zuniga, F.; Ormazabal, V.; Guzman-Gutierrez, E.; Nova-Lamperti, E.; Rodriguez-Alvarez, L.; Aranda, M.; Escudero, C.; et al. Adenosine promoted angiogenesis mediated by the release of small extracellular vesicles from human endothelial progenitor cells. Microvasc Res 2023, 148, 104498. [CrossRef]
- Contreras, H.; Alarcón-Zapata, A.; Nova-Lamperti, E.; Ormazabal, V.; Varas-Godoy, M.; Salomon, C.; Zuniga, F. Comparative study of size exclusion chromatography for isolation of small extracellular vesicle from cell-conditioned media, plasma, urine, and saliva. Front. Nanotechnol 2023, 5, 1146772. [CrossRef]
- Vion, A.C.; Ramkhelawon, B.; Loyer, X.; Chironi, G.; Devue, C.; Loirand, G.; Tedgui, A.; Lehoux, S.; Boulanger, C.M. Shear stress regulates endothelial microparticle release. Circ Res 2013, 112, 1323-1333. [CrossRef]
- Al Faraj, A.; Gazeau, F.; Wilhelm, C.; Devue, C.; Guerin, C.L.; Pechoux, C.; Paradis, V.; Clement, O.; Boulanger, C.M.; Rautou, P.E. Endothelial cell-derived microparticles loaded with iron oxide nanoparticles: feasibility of MR imaging monitoring in mice. Radiology 2012, 263, 169-178. [CrossRef]
- Werner, N.; Wassmann, S.; Ahlers, P.; Kosiol, S.; Nickenig, G. Circulating CD31+/annexin V+ apoptotic microparticles correlate with coronary endothelial function in patients with coronary artery disease. Arterioscler Thromb Vasc Biol 2006, 26, 112-116. [CrossRef]
- van Ierssel, S.H.; Hoymans, V.Y.; Van Craenenbroeck, E.M.; Van Tendeloo, V.F.; Vrints, C.J.; Jorens, P.G.; Conraads, V.M. Endothelial microparticles (EMP) for the assessment of endothelial function: an in vitro and in vivo study on possible interference of plasma lipids. PLoS One 2012, 7, e31496. [CrossRef]
- Amabile, N.; Heiss, C.; Real, W.M.; Minasi, P.; McGlothlin, D.; Rame, E.J.; Grossman, W.; De Marco, T.; Yeghiazarians, Y. Circulating endothelial microparticle levels predict hemodynamic severity of pulmonary hypertension. Am J Respir Crit Care Med 2008, 177, 1268-1275. [CrossRef]
- Zacharia, E.; Antonopoulos, A.S.; Oikonomou, E.; Papageorgiou, N.; Pallantza, Z.; Miliou, A.; Mystakidi, V.C.; Simantiris, S.; Kriebardis, A.; Orologas, N.; et al. Plasma signature of apoptotic microvesicles is associated with endothelial dysfunction and plaque rupture in acute coronary syndromes. J Mol Cell Cardiol 2020, 138, 110-114. [CrossRef]
- Bernal-Mizrachi, L.; Jy, W.; Fierro, C.; Macdonough, R.; Velazques, H.A.; Purow, J.; Jimenez, J.J.; Horstman, L.L.; Ferreira, A.; de Marchena, E.; et al. Endothelial microparticles correlate with high-risk angiographic lesions in acute coronary syndromes. Int J Cardiol 2004, 97, 439-446. [CrossRef]
- Yang, J.; Bi, L.; He, X.; Wang, Z.; Qian, Y.; Xiao, L.; Shi, B. Follicular Helper T Cell Derived Exosomes Promote B Cell Proliferation and Differentiation in Antibody-Mediated Rejection after Renal Transplantation. Biomed Res Int 2019, 2019, 6387924. [CrossRef]
- Ciullo, A.; Biemmi, V.; Milano, G.; Bolis, S.; Cervio, E.; Fertig, E.T.; Gherghiceanu, M.; Moccetti, T.; Camici, G.G.; Vassalli, G.; et al. Exosomal Expression of CXCR4 Targets Cardioprotective Vesicles to Myocardial Infarction and Improves Outcome after Systemic Administration. Int J Mol Sci 2019, 20. [CrossRef]
- Wan, W.; Murphy, P.M. Regulation of atherogenesis by chemokine receptor CCR6. Trends Cardiovasc Med 2011, 21, 140-144. [CrossRef]
- Arunachalam, P.; Ludewig, P.; Melich, P.; Arumugam, T.V.; Gerloff, C.; Prinz, I.; Magnus, T.; Gelderblom, M. CCR6 (CC Chemokine Receptor 6) Is Essential for the Migration of Detrimental Natural Interleukin-17-Producing gammadelta T Cells in Stroke. Stroke 2017, 48, 1957-1965. [CrossRef]
- Hofmann, U.; Frantz, S. Role of lymphocytes in myocardial injury, healing, and remodeling after myocardial infarction. Circ Res 2015, 116, 354-367. [CrossRef]
- Schumacher, D.; Liehn, E.A.; Singh, A.; Curaj, A.; Wijnands, E.; Lira, S.A.; Tacke, F.; Jankowski, J.; Biessen, E.A.L.; van der Vorst, E.P.C. CCR6 Deficiency Increases Infarct Size after Murine Acute Myocardial Infarction. Biomedicines 2021, 9. [CrossRef]
- Feng, G.; Bajpai, G.; Ma, P.; Koenig, A.; Bredemeyer, A.; Lokshina, I.; Lai, L.; Forster, I.; Leuschner, F.; Kreisel, D.; et al. CCL17 Aggravates Myocardial Injury by Suppressing Recruitment of Regulatory T Cells. Circulation 2022, 145, 765-782. [CrossRef]
| CONTROL | IAM | |
| n | 8 | 10 |
| Gender (F/M) | 5/3 (62%/38%) | 6/4 (60%/40%) |
| Age | 60 ± 2,4 | 64,8 ± 5,1 |
| Weight (kg) | 74,9 ± 5,7 | 70,1 ± 4,2 |
| Height (m) | 1,6 ± 0,1 | 1,6 ± 1,4 |
| BMI | 29,6 ±1,9 | 26,6 ±1,2* |
| Waist Circumference (cm) | 95,5 ± 4,6 | 102,6 ± 3,3 |
| Glycemia (mg/dl) | 76,4 ± 5,6 | 176,0 ± 45,0* |
| Total Cholesterol (mg/dl) | 171,7± 14,5 | 181,5± 12,7 |
| Triglycerides | 130,4 ± 7,6 | 149,6 ± 27,9 |
| LDL Cholesterol (mg/dl) | 119,3 ± 20,8 | 107,8 ± 12,4 |
| HDL Cholesterol (mg/dl) | 38,1 ± 5,1 | 43,0 ± 3,9 |
| sLox-1 | 156,7 ± 55 | 294±70,3* |
| Troponin (ng/ml) | - | 238.262 ± 72494 |
| CK-Total (U/L) | - | 2.205 ± 677 |
| CK-Mb (ng/ml) | - | 219,8 ± 64,7 |
| CRP (mg/dl) | - | 1,6 ± 0,5 |
| Previous Myocardial Infarction | ||
| Yes | - | 0 (0%) |
| No | - | 10 (100%) |
| Diagnosis | ||
| AMI with ST-segment elevation in inferior wall | - | 5 (50%) |
| AMI with ST-segment elevation in anterior wall | - | 4 (40%) |
| AMI with ST-segment elevation in anteroseptal area | - | 1 (10%) |
| TIMI Score | ||
| I | - | 0 |
| II | - | 0 |
| III | - | 10 (100%) |
| KILLIP score | ||
| I | - | 5 (50%) |
| II | - | 5 (50%) |
| III | - | 0 (0%) |
| IV | - | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





