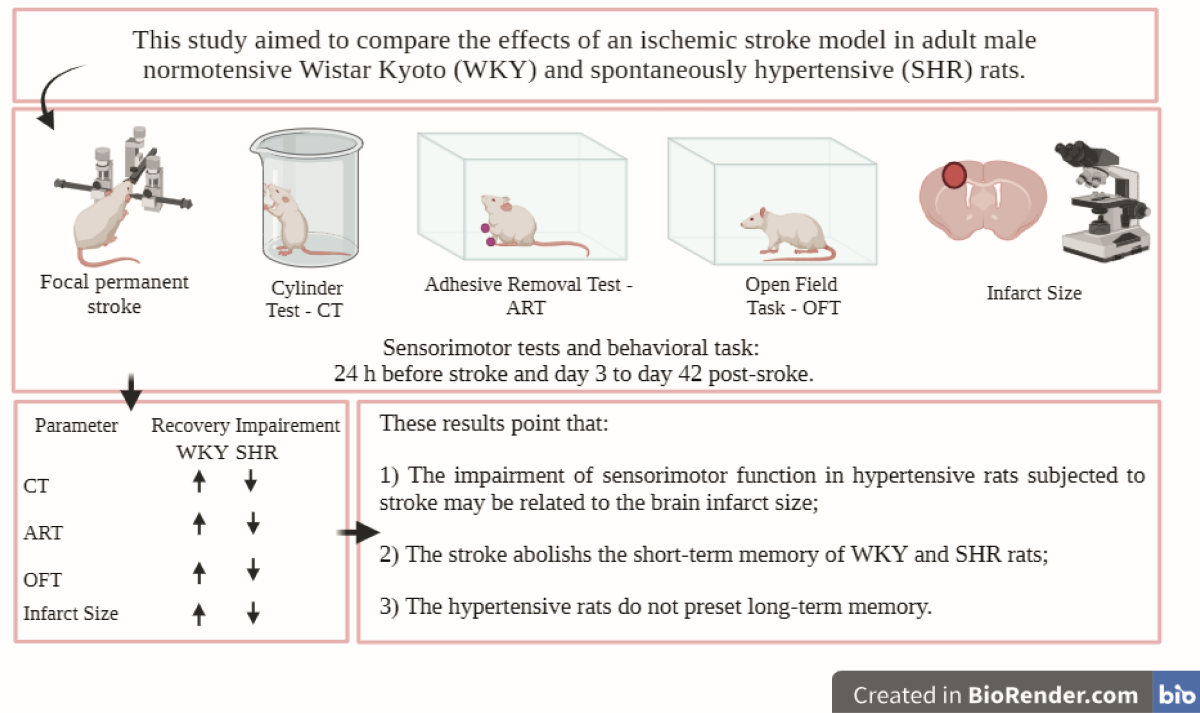
1. INTRODUCTION
1.1. Brain Stroke
Approximately 17 million individuals worldwide experience stroke each year, which is a leading cause of adverse outcomes and mortality (Chapman et al., 2023; Feigin et al., 2022; Lauder et al., 2023; Tsao et al., 2022). Stroke is categorized into two main types: hemorrhagic and ischemic strokes (Campbell et al., 2019; Campbell and Khatri, 2020). Ischemic stroke is the most prevalent type, accounting for approximately 80 percent of all stroke cases(Saini et al., 2021).
In the brain affected by ischemic stroke, the central area of damage, known as nuclear infarction, causes cell death and irreversible tissue lesions (Tuo et al., 2022; Zhang et al., 2022). The region surrounding the nucleus infarction, referred to as the penumbra in humans or the peri-infarct zone in animals, is affected, but can potentially recover. Recovery from this region is the primary goal of ischemic stroke treatment strategies for ischemic stroke(Mao et al., 2022). Delays in intervention can lead to poor patient outcomes(Kijpaisalratana et al., 2023; McCann and Lawrence, 2020; Mead et al., 2023).
Systemic arterial hypertension (SAH) is a multifactorial medical condition characterized by a persistent increase in blood pressure, affecting several individuals worldwide(“First WHO report details devastating impact of hypertension and ways to stop it,” 2023; Mead et al., 2023; Oparil et al., 2018). SAH causes mechanical stress on the arterial walls, resulting in remodeling of the neurovascular structure and cerebral blood flow(Koundal et al., 2019). Thus, SAH is the main risk factor for stroke(Mead et al., 2023; Staehr et al., 2023) and contributes to worse outcomes(Kim, 2023; Majesky and Weiser-Evans, 2022; Santisteban et al., 2023; Wu et al., 2023). As most preclinical studies on stroke have been conducted on healthy young animals, it is essential to develop studies that consider age and relevant comorbidities, such as SAH(McCann and Lawrence, 2020; Rexrode et al., 2022).
One of the most utilized animal models in these studies is the spontaneously hypertensive rat (SHR)(Coatl-Cuaya et al., 2022; Li et al., 2023; Szlęzak et al., 2021; Tchekalarova et al., 2023), a model of genetic SAH developed by the selective breeding of Wistar Kyoto rats (WKY), which serve as the homologous control group for SHR(Tchekalarova et al., 2023; Wang et al., 2021).
This study evaluated the behavioral outcomes produced by an experimental model of ischemic stroke caused by thermocoagulation of pial vessels in hypertensive (SHR) and normotensive (WKY) rats by examining sensorimotor function, short- and long-term memory, and brain lesion size. The results should lead to more effective clinical intervention strategies.
2. MATERIALS AND METHODS
2.1. Ethical Aspects
All procedures involving animals followed the guidelines recommended by the National Guidelines for Animal Experimentation, according to Brazilian law 11,794 of 08/10/2008 (Estabelecendo procedimentos para o uso científico de animais., 2008) and the policies of the National Council for the Control of Animal Experimentation (CONCEA)(“Conselho Nacional de Controle de Experimentação Animal - CONCEA. RESOLUÇÃO NORMATIVA N 15, DE 16 DE DEZEMBRO DE 2013,” 2013) and Guide for Care in the Use of Laboratory Animals from the National Institute of Health(Guide for the Care and Use of Laboratory Animals, 2011). The animals were euthanized following Law 714, 06/20/2002, which provides procedures and methods for the euthanasia of animals and other measures of the Federal Council of Veterinary Medicine, Law 11,794 of 10/08/2008, and the Practice Guidelines of Euthanasia at CONCEA(“Guia Brasileiro de Produção, manutenção ou Utilização de Animais em Atividades de Ensino ou Pesquisa Científica,” 2023). The authors declare that they followed the guidelines of the National Research Council's Guide for the Care and Use of Laboratory Animals(“Guide for the Care and Use of Laboratory Animals,” 2024) and made every effort to minimize animal suffering and discomfort, using the fewest animals possible to obtain consistent results. If any discomfort or suffering was observed, we implemented a humane endpoint.
This study was approved by the Ethics Committee on Animal Use of the Institute of Cardiology/University Foundation of Cardiology and registered under protocols UP 5806/20 and UP 5850/20.
2.2. Animals
This study used male SHR and WKY rats aged–90-120 days obtained from the vivarium of the Experimental Cardiology Center of the Institute of Cardiology/University Foundation of Cardiology. The animals were kept at a temperature of 22 ± 2 ºC in a controlled light ambiance (light-dark cycle of 12/12 h), with water and standard animal feed ad libitum. Animals were housed in polycarbonate boxes lined with wooden shavings.
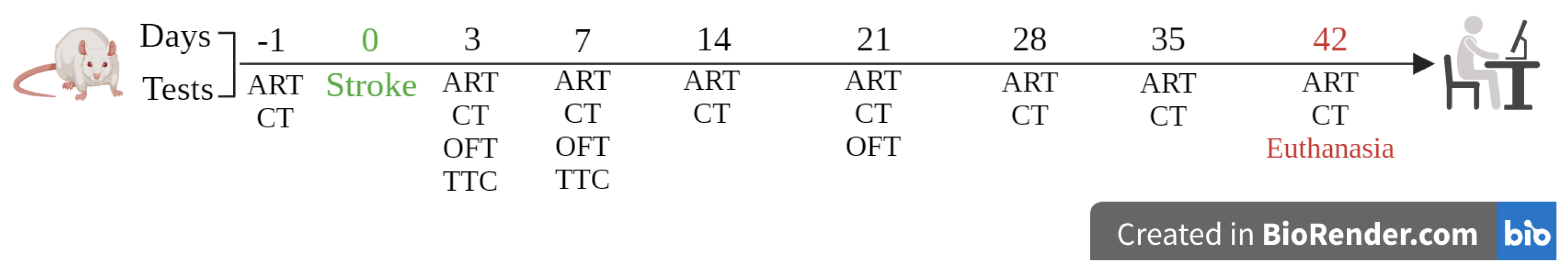
The rats were divided into two main experimental groups: (1) hypertensive (SHR) and (2) normotensive (WKY). Each group was further subdivided into (1) animals subjected to ischemic stroke by thermocoagulation of the pial vessels (ISC), (2) animals subjected to craniectomy without ischemic stroke by thermocoagulation of the pial vessels (sham), and (3) controls without surgical intervention (naive). A schematic of the procedure is shown in FIGURE XX
2.3. Focal Permanent Stroke.
Stroke is induced by thermocoagulation of pial blood vessels(Hansel et al., 2015; Müller et al., 2021; Nonose et al., 2018; Rohden et al., 2021; Teixeira et al., 2018). The animals were anesthetized with ketamine hydrochloride (90 mg/kg, 450 µL/kg i.p.) and xylazine hydrochloride (10 mg/kg, 300 µL/kg i.p.) and then placed in stereotaxic apparatus. A craniectomy was performed by exposing the left parietal cortex [+2 to –6 mm AP (anteroposterior) and –2 to –4 mm ML (medium-lateral) from the bregma].
The pial vessels were thermocoagulated for 2 min using a warm probe. The skin was then sutured with a mono-nylon thread using simple isolated stitches and the animals were kept on a thermal mattress (37 ºC) until recovery from anesthesia.
2.4. Sensorimotor Functions Evaluation
2.4.1. Cylinder Test
Symmetry of the front paws was assessed using the cylinder test (CT)(Rohden et al., 2021; Teixeira et al., 2018). The animals were placed inside a glass cylinder with a diameter of 20 cm and a height of 30 cm. An observer recorded the first 20 touches made by the front paws of the animals on a cylindrical wall. These touches were categorized as ipsilateral, contralateral (relative to the side of the brain injury), or both. After each session, the apparatus was thoroughly cleaned with 70% alcohol.
The asymmetry for each animal was calculated using the formula: asymmetry = (% of ipsilateral paw touches = ipsilateral paw touches/total sum of touches) – (% of contralateral paw touches = % of contralateral paw touches/total sum of touches). Subsequently, asymmetry was converted to % symmetry (100% asymmetry)(Magno et al., 2019; Rohden et al., 2021; Truong et al., 2023). The animals were evaluated 1 day before surgery (day -1) and 3, 7, 14, 21, 28, 35, and 42 days after the stroke.
2.4.2. Adhesive Removal Test
The adhesive removal test (ART) is one of the most efficient behavioral tests for identifying sensorimotor deficits(Mehta et al., 2024; Yilmaz et al., 2024). To administer the test, a paper sticker with a diameter of 13 mm was attached to the underside of the front paws of each animal. The animals were then placed in an acrylic experimental box measuring 30 cm in length × 22 cm in width × 22 cm in height for 60 s. The time taken to remove the adhesive from each paw (referred to as removal latency) was recorded. The test was repeated five times with a five-minute interval between trials. To calculate the latency of adhesive removal for both the contralateral and ipsilateral paws, the average of the two shortest removal times was determined from five tests(Yilmaz et al., 2024). The animals were evaluated 1 day before surgery (day -1) and 3, 7, 14, 21, 28, 35, and 42 days after surgery.
2.5. Memory Evaluation - Open Field Task
This task was designed to evaluate short- and long-term novelty habituation memories. The animals were placed in a non-transparent black box measuring 50 cm length × 50 cm width × 50 cm height. The locomotor activity of the animals was recorded for 10 min using a camera installed above the box and the distance walked per minute was calculated using ANY-Maze software (Stoelting Co., Wood Dale, IL, USA). At the end of each session, the apparatus was cleaned using 70% alcohol. Evaluations were conducted 7 and 21 days after surgery.
Short-term memory is defined as a decrease in locomotion from the 1st to the 5th minute of the first exposure, whereas long-term memory is defined as a decrease in locomotion in the 1st minute of successive exposure (Rohden et al., 2021; Teixeira et al., 2018).
2.6. Infarct Size Evaluation
The infarct size was calculated on the 3rd or 7th day post-surgery. Animals were anesthetized with ketamine hydrochloride (90 mg/kg, 450 µl/kg i.p.) and xylazine hydrochloride (10 mg/kg, 300 µl/kg i.p.). Subsequently, the brain was quickly removed from the skull, frozen, and sectioned in the coronal plane into slices of 2 mm thickness. The slices were immersed in a 2% solution of 2,3,5-triphenyl tetrazolium chloride (TTC), a red color dye, for 30 min at 37 ºC, followed by fixation in 4% PFA solution for 24 h(Nonose et al., 2018; Rohden et al., 2021; Teixeira et al., 2018). The slices were then placed in the dark, and images were captured. Areas without red coloration were considered necrotic (infarcted) and represented lesions. The total brain and lesion volumes were measured using ImageJ software. The measurements were then used to calculate the percentage of lesion volume(Rohden et al., 2021; Teixeira et al., 2018).
2.8. Statistical Analysis
The effects of stroke on the behavioral parameters of the rats (TC, TRA, and OFT) were analyzed using a two-way ANOVA, followed by Sidak's multiple comparisons. A two-way ANOVA followed by Sidak’s multiple comparison test was also used to compare brain lesion volumes between SHR and WKY rats over time. Data were expressed as mean ± SEM for behavioral assessments and mean ± SD for lesion volume. An alpha value (significance level) of 0.05 was used for all the tests. All analyses were performed using Graph Pad Prism 9.0 software.
3. Results
3.1. Cylinder Test (CT)
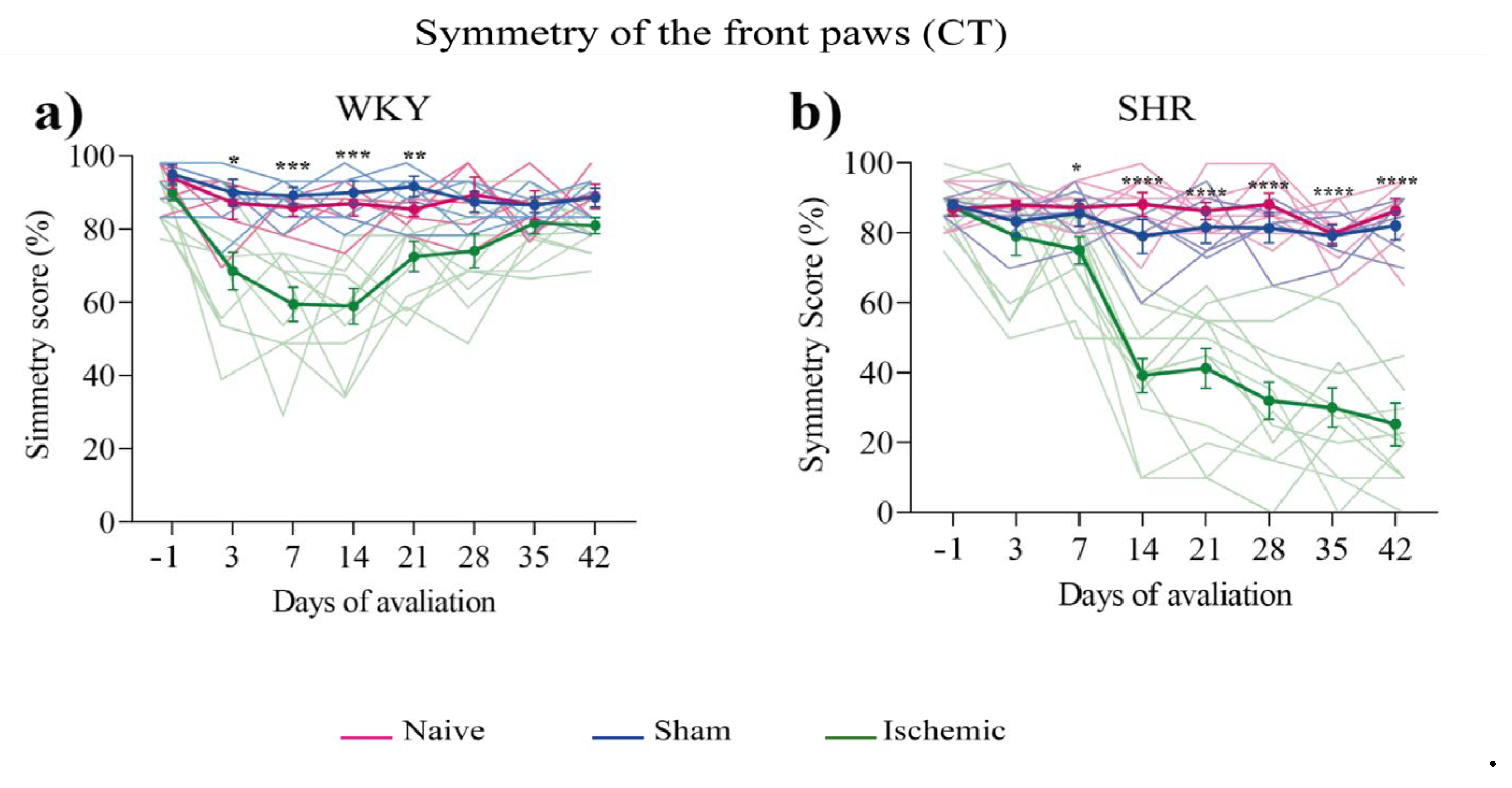
A CT was performed one day before surgery (day –1) and once a week from the 3rd to the 42nd day (end of the experiment) post-surgery. The ISC WKY group exhibited a significant symmetry reduction in CT from the 3rd day that spontaneously recovered on the 21st day post-ischemia (Figure 1a). The ISC group exhibited a significant reduction only from the 7th day post-ischemia that was intensified until the end of the experiment (Figure 1b). No alterations in symmetry were observed in the naive or sham groups.
Figure 1.
Symmetry of the front paws of WKY (a) and SHR (b) groups in the CT. Data expressed as mean ± SEM, analyzed by two-way ANOVA, followed by Sidak's multiple comparisons test. The *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ISC groups compared to naive and sham groups. WKY groups: naive (n=5), sham (n=5), ISC (n=10); SHR groups: naive (n=5), sham (n=5), ISC (n=14).
Figure 1.
Symmetry of the front paws of WKY (a) and SHR (b) groups in the CT. Data expressed as mean ± SEM, analyzed by two-way ANOVA, followed by Sidak's multiple comparisons test. The *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ISC groups compared to naive and sham groups. WKY groups: naive (n=5), sham (n=5), ISC (n=10); SHR groups: naive (n=5), sham (n=5), ISC (n=14).
3.2. Adhesive Removal Test (ART)
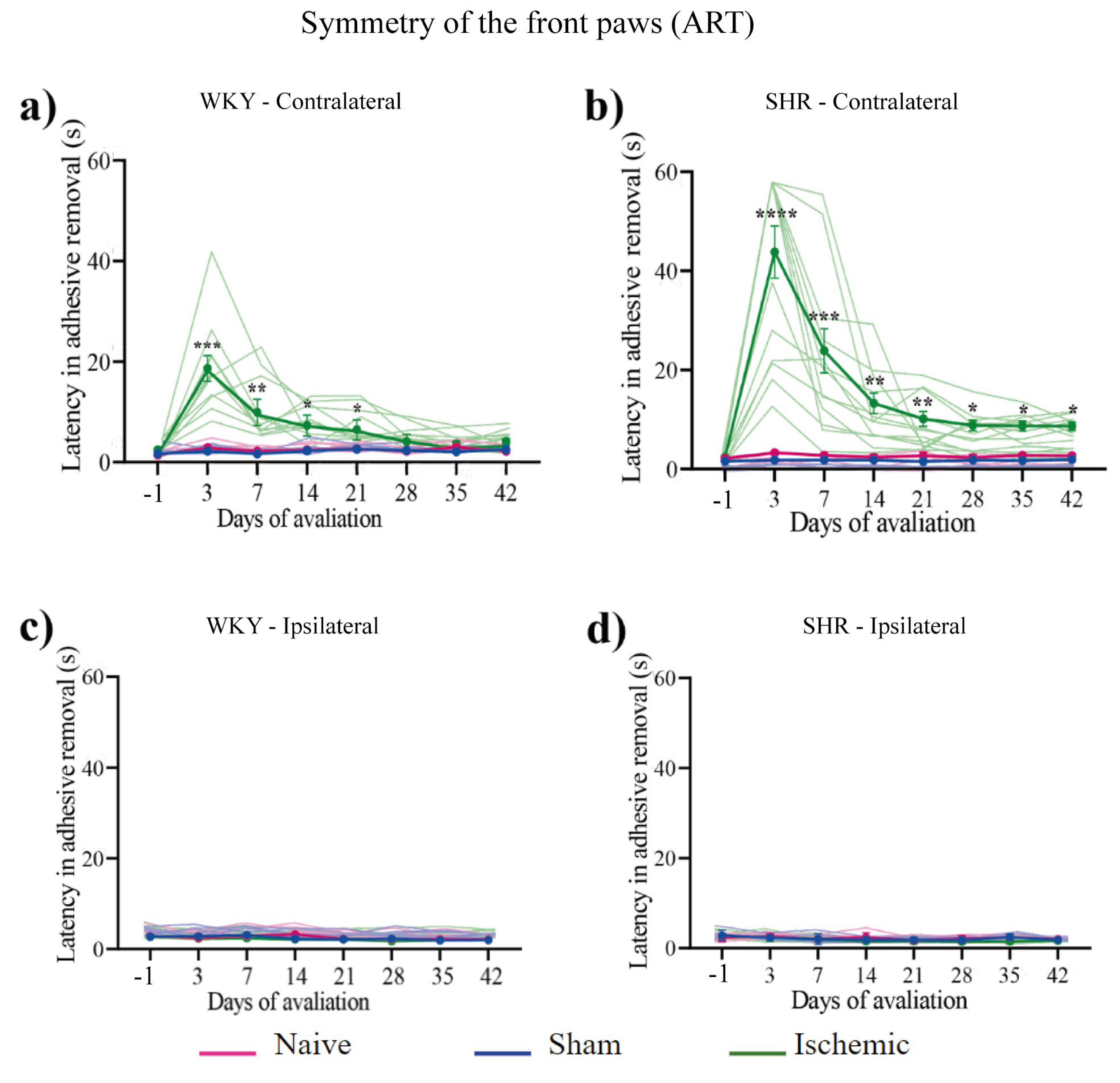
Adhesive removal of the ipsilateral and contralateral paws was evaluated on day –1 and once a week from the 3rd to the 42nd day post-surgery (end of the experiment). The ISC WKY and SHR groups exhibited a significantly prolonged adhesive removal latency from the contralateral paw on the 3rd day post-stroke. In the ISC WKY group, this effect spontaneously disappeared on the 28th day (Figure 2a), whereas in the ISC SHR group, the prolonged adhesive removal latency persisted until the end of the experiment (Figure 2b). No alterations in symmetry were observed in the naive or sham groups.
Figure 2.
Latency of adhesive removal from front paws of WKY (Figures a and c) and SHR (Figures b and d) groups. Data expressed as mean ± SEM, analyzed by two-way ANOVA followed by Sidak's multiple comparisons. The *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ISC groups compared to naive and sham groups. WKY: naive (n=5), sham (n=5), ISC (n=12); SHR: naive (n=5), sham (n=5), and ISC (n=12).
Figure 2.
Latency of adhesive removal from front paws of WKY (Figures a and c) and SHR (Figures b and d) groups. Data expressed as mean ± SEM, analyzed by two-way ANOVA followed by Sidak's multiple comparisons. The *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ISC groups compared to naive and sham groups. WKY: naive (n=5), sham (n=5), ISC (n=12); SHR: naive (n=5), sham (n=5), and ISC (n=12).
3.3. Open Field Task (OFT)
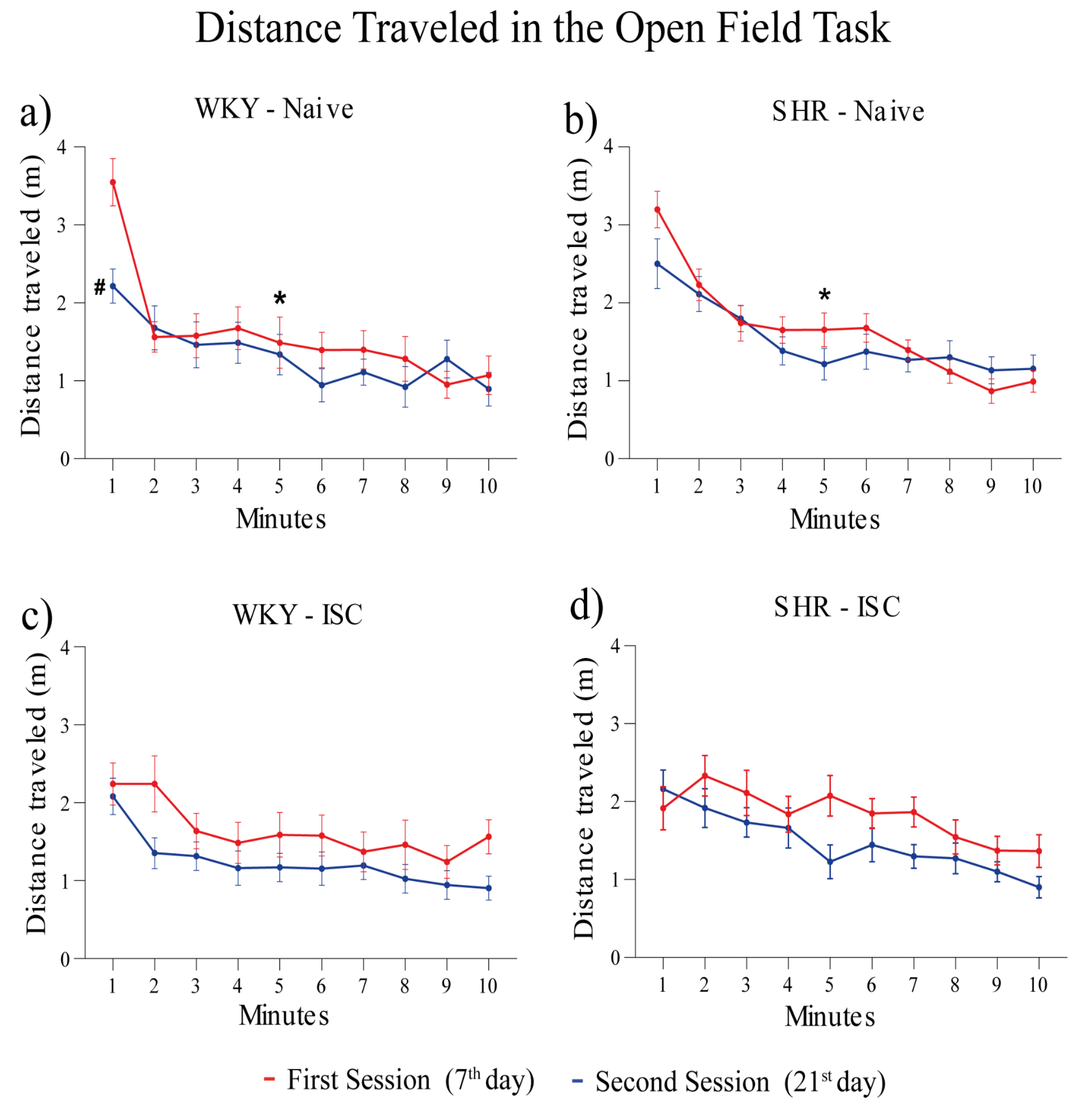
The distance traveled in two successive sessions of the OFT is shown in Figure 3 for the WKY (Naive, ISC) groups and SHR (Naive, ISC) groups. The naive WKY (Figure 3a) and naïve SHR (Figure 3b) groups showed a significant decrease in the distance traveled in the first session from the 1st to the 5th minute, indicating short-term memory(Kraeuter et al., 2019). However, this difference was not observed in the ISC WKY (Figure 3c) and ISC SHR groups (Figure 3d), indicating that stroke affects the short-term memory of habituation to novelty(Kraeuter et al., 2019). Moreover, only naïve WKY rats (Figure 3a) presented long-term memory of habituation to novelty, as evidenced by a decrease in locomotion when comparing the 1st minute of the first session with the 1st minute of the second session, indicating that stroke and/or HAS affected the long-term memory of habituation to novelty.
Figure 3.
Distance traveled in the OFT on the 7th (first session) and 21st (second session) day after stroke. Naive WKY (a) and naive SHR (b) groups; ISC WKY (c) and ISC SHR (d) groups. Data expressed as mean ± SEM analyzed by two-way ANOVA followed by Sidak's multiple comparisons. The *p<0.05, comparing the 1st min with the 5th min in the first session (short-term memory); #p<0.05, comparing the 1st min of the first session with the 1st min of the second session (long-term memory). Naive WKY (n=10), Naive SHR (n=10), ISC WKY (n=12), and ISC SHR (n=12).
Figure 3.
Distance traveled in the OFT on the 7th (first session) and 21st (second session) day after stroke. Naive WKY (a) and naive SHR (b) groups; ISC WKY (c) and ISC SHR (d) groups. Data expressed as mean ± SEM analyzed by two-way ANOVA followed by Sidak's multiple comparisons. The *p<0.05, comparing the 1st min with the 5th min in the first session (short-term memory); #p<0.05, comparing the 1st min of the first session with the 1st min of the second session (long-term memory). Naive WKY (n=10), Naive SHR (n=10), ISC WKY (n=12), and ISC SHR (n=12).
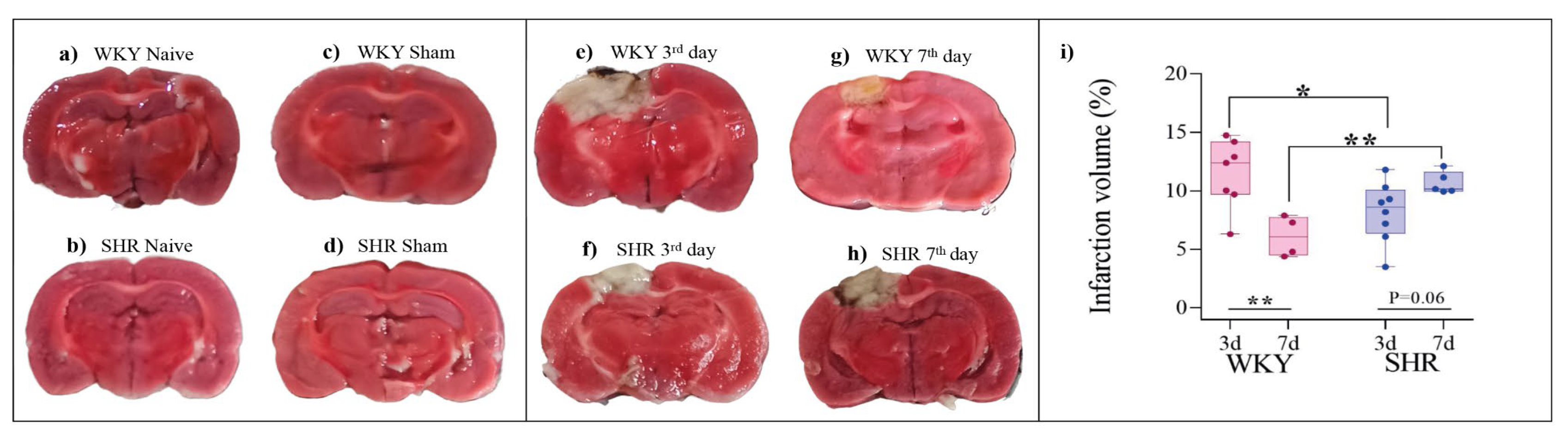
3.4. Infarct Size
On the 3rd day post-stroke, the infarct size in the ISC WKY group (Figure 4e) was significantly larger than that in the ISC SHR (Figure 4f). On the 7th day post-stroke, the infarct size in the ISC WKY (Figure 4g) group decreased compared to that on the 3rd day (Figure 4e), whereas in the ISC SHR group on the 7th day post-stroke, it remained the same as that on the 3rd day (Figure 4f and 4h). Statistical analysis of infarct size is shown in Figure 4i. No ischemic lesions were detected in the brains of WKY and SHR rats in either the naive or sham groups (Figures 4a-d). The smaller infarct size on the 3rd day in SHR compared to WKY rats could be correlated with the absence of asymmetry that was only observed in the SHR on the CT conducted on the 3rd day after stroke (Figure 1b).
Figure 4.
Infarct size in WKY and SHR. Representative images from brain slices stained with TTC (a-h). Comparison of infarct size on the 3rd day with the 7th day post-stroke in WKY and SHR (i). Data are expressed as mean ± SD, analyzed by two-way ANOVA, followed by Sidak's multiple comparisons test. The *p<0.05 and **p<0.01. Naive (n=5), sham (n=5), WKY ISC 3 days (n=7), WKY ISC 7 days (n=4), SHR ISC 3 days (n=8), and SHR ISC 7 days (n=5).
Figure 4.
Infarct size in WKY and SHR. Representative images from brain slices stained with TTC (a-h). Comparison of infarct size on the 3rd day with the 7th day post-stroke in WKY and SHR (i). Data are expressed as mean ± SD, analyzed by two-way ANOVA, followed by Sidak's multiple comparisons test. The *p<0.05 and **p<0.01. Naive (n=5), sham (n=5), WKY ISC 3 days (n=7), WKY ISC 7 days (n=4), SHR ISC 3 days (n=8), and SHR ISC 7 days (n=5).
4. DISCUSSION
Animal models of cerebral ischemia are essential tools for understanding the pathophysiology of stroke and for developing new therapeutic and protective strategies. In the present study, we evaluated the effects of a stroke model in rats spontaneously presenting with SAH, which is considered the main risk factor for stroke based on previous studies where the SHR exhibited cerebrovascular alterations similar to those observed in humans with SAH(Coatl-Cuaya et al., 2022; Li et al., 2023; Szlęzak et al., 2021; Tchekalarova et al., 2023).
Here, hypertensive rats (SHR), subjected to a permanent model of focal ischemic stroke by thermocoagulation of the pial vessels, were compared to normotensive rats (WYK) in terms of i) loss in the recovery of post-ischemic sensorimotor function (CT and ART), ii) impaired short- and long-term memory (OFT), and iii) increased brain lesion size.
The SHR group subjected to stroke showed a decrease in the symmetry of the front paws compared to the WKY group, which lasted until the end of the experiment. These findings are consistent with those of previous studies using various cerebral ischemia models and/or different animal species, which consistently indicated greater neurological impairment in hypertensive rats than in normotensive rats(Sayed et al., 2020; Thakkar et al., 2019). Tchekalarova et al. 2023, demonstrated that WKY rats achieved full functional recovery much earlier than SHR(Tchekalarova et al., 2023). Investigations by our group using the same stroke model in Wistar rats indicated the recovery of front paw symmetry up to the 42nd day post-stroke(Rohden et al., 2021; Teixeira et al., 2018). The observed impairment in post-stroke sensorimotor recovery in SHR can be explained by the neurovascular changes that these animals present due to SAH, such as remodeling of cerebral blood vessels, dysfunction of the blood-brain barrier, impaired self-regulation of cerebral blood flow, and functional hyperemia(Koundal et al., 2019; Raz et al., 2019). These findings highlight the divergent post-ischemic functional motor responses of Wistar rats compared to those of WKY and SHR rats, highlighting the importance of conducting studies with different strains and models of cerebral ischemic injury.
In this study, the assessment of short- and long-term memory revealed notable differences between WKY rats and SHR. The naive SHR group exhibited a deficiency in long-term memory in the OFT, which agreed with previous studies that have consistently suggested that hypertensive rats exhibit memory impairment compared to normotensive rats(Matsuzaki et al., 2024; Sontag et al., 2013). These results highlight the importance of investigating the effects of hypertension on memory and provide a basis for future research aimed at better understanding these mechanisms and developing intervention strategies.
This study showed a significantly smaller infarct size in SHR at the 3rd day post-stroke point compared to WKY. However, on the 7th day post-stroke, only WKY rats exhibited a reduction in infarct volume, displaying a smaller infarct size than that in SHR. Previous research has indicated that hypertensive animals have a greater infarct size and more severe sequelae in motor function than normotensive animals(Sayed et al., 2020). Thus, our results highlight the potential role of infarct size in post-stroke sensorimotor recovery, prompting further investigation into the underlying mechanisms in normotensive and hypertensive rat models.
5. Conclusions
The results of the present study indicate a negative impact on behavioral outcomes, sensorimotor activity, and long-term memory in animals with SAH. To date, no studies have been found that subjected animals to permanent focal cerebral ischemia using the thermocoagulation model of pial vessels in SHR and WKY rats and evaluated the post-ischemia sensorimotor and memory long-term outcomes. These results reinforce the need to use animal stroke models with comorbidities, such as SAH, to improve translational perspectives for preclinical investigations of the treatment and prevention of stroke in humans.
Highlights
Stroke abolishes short-term memory in normotensive and hypertensive rats.
Stroke causes long-term sensorimotor impairment in hypertensive rats.
Brain infarct sizes vary between normotensive and hypertensive rats across time.
Long-term memory is abolished in hypertensive rats, but not in normotensive rats.
References
- Campbell, B.C.V., De Silva, D.A., Macleod, M.R., Coutts, S.B., Schwamm, L.H., Davis, S.M., Donnan, G.A., 2019. Ischaemic stroke. Nat. Rev. Dis. Primer 5, 70. [CrossRef]
- Campbell, B.C.V., Khatri, P., 2020. Stroke. The Lancet 396, 129–142. [CrossRef]
- Chapman, N., Ching, S.M., Konradi, A.O., Nuyt, A.M., Khan, T., Twumasi-Ankrah, B., Cho, E.J., Schutte, A.E., Touyz, R.M., Steckelings, U.M., Brewster, L.M., 2023. Arterial Hypertension in Women: State of the Art and Knowledge Gaps. Hypertension 80, 1140–1149. [CrossRef]
- Coatl-Cuaya, H., Tendilla-Beltrán, H., De Jesús-Vásquez, L.M., Garcés-Ramírez, L., Gómez-Villalobos, M.D.J., Flores, G., 2022. Losartan enhances cognitive and structural neuroplasticity impairments in spontaneously hypertensive rats. J. Chem. Neuroanat. 120, 102061. [CrossRef]
- Conselho Nacional de Controle de Experimentação Animal - CONCEA. RESOLUÇÃO NORMATIVA N 15, DE 16 DE DEZEMBRO DE 2013 [WWW Document], 2013. . Pub No 1179408.
- Estabelecendo procedimentos para o uso científico de animais., 2008.
- Feigin, V.L., Brainin, M., Norrving, B., Martins, S., Sacco, R.L., Hacke, W., Fisher, M., Pandian, J., Lindsay, P., 2022. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 17, 18–29. [CrossRef]
- First WHO report details devastating impact of hypertension and ways to stop it, 2023.
- Guia Brasileiro de Produção, manutenção ou Utilização de Animais em Atividades de Ensino ou Pesquisa Científica [WWW Document], 2023. URL https://www.ufrgs.br/ceua/documentacao/legislacao/.
- Guide for the Care and Use of Laboratory Animals, 2024.
- Guide for the Care and Use of Laboratory Animals: Eighth Edition, 2011. . National Academies Press, Washington, D.C. [CrossRef]
- Hansel, G., Tonon, A.C., Guella, F.L., Pettenuzzo, L.F., Duarte, T., Duarte, M.M.M.F., Oses, J.P., Achaval, M., Souza, D.O., 2015. Guanosine Protects Against Cortical Focal Ischemia. Involvement of Inflammatory Response. Mol. Neurobiol. 52, 1791–1803. [CrossRef]
- Kijpaisalratana, N., Ament, Z., Patki, A., Bhave, V.M., Garcia-Guarniz, A.-L., Judd, S.E., Cushman, M., Long, D.L., Irvin, M.R., Kimberly, W.T., 2023. Association of Circulating Metabolites With Racial Disparities in Hypertension and Stroke in the REGARDS Study. Neurology 100. [CrossRef]
- Kim, H.-L., 2023. Arterial stiffness and hypertension. Clin. Hypertens. 29, 31. [CrossRef]
- Koundal, S., Liu, X., Sanggaard, S., Mortensen, K., Wardlaw, J., Nedergaard, M., Benveniste, H., Lee, H., 2019. Brain Morphometry and Longitudinal Relaxation Time of Spontaneously Hypertensive Rats (SHRs) in Early and Intermediate Stages of Hypertension Investigated by 3D VFA-SPGR MRI. Neuroscience 404, 14–26. [CrossRef]
- Kraeuter, A.-K., Guest, P.C., Sarnyai, Z., 2019. The Open Field Test for Measuring Locomotor Activity and Anxiety-Like Behavior, in: Guest, P.C. (Ed.), Pre-Clinical Models, Methods in Molecular Biology. Springer New York, New York, NY, pp. 99–103. [CrossRef]
- Lauder, L., Mahfoud, F., Azizi, M., Bhatt, D.L., Ewen, S., Kario, K., Parati, G., Rossignol, P., Schlaich, M.P., Teo, K.K., Townsend, R.R., Tsioufis, C., Weber, M.A., Weber, T., Böhm, M., 2023. Hypertension management in patients with cardiovascular comorbidities. Eur. Heart J. 44, 2066–2077. [CrossRef]
- Li, X., Ren, C., Li, S., Zhao, W., Wang, P., Ji, X., 2023. The antihypertensive effect of remote ischemic conditioning in spontaneously hypertensive rats. Front. Immunol. 13, 1093262. [CrossRef]
- Magno, L.A., Collodetti, M., Tenza-Ferrer, H., Romano-Silva, M., 2019. Cylinder Test to Assess Sensory-Motor Function in a Mouse Model of Parkinson’s Disease. BIO-Protoc. 9. [CrossRef]
- Majesky, M.W., Weiser-Evans, M.C.M., 2022. The adventitia in arterial development, remodeling, and hypertension. Biochem. Pharmacol. 205, 115259. [CrossRef]
- Mao, R., Zong, N., Hu, Y., Chen, Y., Xu, Y., 2022. Neuronal Death Mechanisms and Therapeutic Strategy in Ischemic Stroke. Neurosci. Bull. 38, 1229–1247. [CrossRef]
- Matsuzaki, K., Sugimoto, N., Hossain, S., Islam, R., Sumiyoshi, E., Hashimoto, M., Kishi, H., Shido, O., 2024. Theobromine improves hyperactivity, inattention, and working memory via modulation of dopaminergic neural function in the frontal cortex of spontaneously hypertensive rats. Food Funct. 10.1039.D4FO00683F. [CrossRef]
- McCann, S.K., Lawrence, C.B., 2020. Comorbidity and age in the modelling of stroke: are we still failing to consider the characteristics of stroke patients?Comorbidity and age in the modelling of stroke: are we still failing to consider the characteristics of stroke patients? BMJ Open Sci. 44. [CrossRef]
- Mead, G.E., Sposato, L.A., Sampaio Silva, G., Yperzeele, L., Wu, S., Kutlubaev, M., Cheyne, J., Wahab, K., Urrutia, V.C., Sharma, V.K., Sylaja, P., Hill, K., Steiner, T., Liebeskind, D.S., Rabinstein, A.A., 2023. A systematic review and synthesis of global stroke guidelines on behalf of the World Stroke Organization. Int. J. Stroke 18, 499–531. [CrossRef]
- Mehta, S.L., Chelluboina, B., Morris-Blanco, K.C., Bathula, S., Jeong, S., Arruri, V., Davis, C.K., Vemuganti, R., 2024. Post-stroke brain can be protected by modulating the lncRNA FosDT. J. Cereb. Blood Flow Metab. 44, 239–251. [CrossRef]
- Müller, G.C., Loureiro, S.O., Pettenuzzo, L.F., Almeida, R.F., Ynumaru, E.Y., Guazzelli, P.A., Meyer, F.S., Pasquetti, M.V., Ganzella, M., Calcagnotto, M.E., Souza, D.O., 2021. Effects of intranasal guanosine administration on brain function in a rat model of ischemic stroke. Purinergic Signal. 17, 255–271. [CrossRef]
- Nonose, Y., Gewehr, P.E., Almeida, R.F., da Silva, J.S., Bellaver, B., Martins, L.A.M., Zimmer, E.R., Greggio, S., Venturin, G.T., Da Costa, J.C., Quincozes-Santos, A., Pellerin, L., de Souza, D.O., de Assis, A.M., 2018. Cortical Bilateral Adaptations in Rats Submitted to Focal Cerebral Ischemia: Emphasis on Glial Metabolism. Mol. Neurobiol. 55, 2025–2041. [CrossRef]
- Oparil, S., Acelajado, M.C., Bakris, G.L., Berlowitz, D.R., Cífková, R., Dominiczak, A.F., Grassi, G., Jordan, J., Poulter, N.R., Rodgers, A., Whelton, P.K., 2018. Hypertension. Nat. Rev. Dis. Primer 4, 18014. [CrossRef]
- Raz, L., Bhaskar, K., Weaver, J., Marini, S., Zhang, Q., Thompson, J.F., Espinoza, C., Iqbal, S., Maphis, N.M., Weston, L., Sillerud, L.O., Caprihan, A., Pesko, J.C., Erhardt, E.B., Rosenberg, G.A., 2019. Hypoxia promotes tau hyperphosphorylation with associated neuropathology in vascular dysfunction. Neurobiol. Dis. 126, 124–136. [CrossRef]
- Rexrode, K.M., Madsen, T.E., Yu, A.Y.X., Carcel, C., Lichtman, J.H., Miller, E.C., 2022. The Impact of Sex and Gender on Stroke. Circ. Res. 130, 512–528. [CrossRef]
- Rohden, F., Teixeira, L.V., Bernardi, L.P., Ferreira, P.C.L., Colombo, M., Teixeira, G.R., De Oliveira, F.D.S., Cirne Lima, E.O., Guma, F.C.R., Souza, D.O., 2021. Functional Recovery Caused by Human Adipose Tissue Mesenchymal Stem Cell-Derived Extracellular Vesicles Administered 24 h after Stroke in Rats. Int. J. Mol. Sci. 22, 12860. [CrossRef]
- Saini, V., Guada, L., Yavagal, D.R., 2021. Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology 97. [CrossRef]
- Santisteban, M.M., Iadecola, C., Carnevale, D., 2023. Hypertension, Neurovascular Dysfunction, and Cognitive Impairment. Hypertension 80, 22–34. [CrossRef]
- Sayed, M.A., Eldahshan, W., Abdelbary, M., Pillai, B., Althomali, W., Johnson, M.H., Arbab, A.S., Ergul, A., Fagan, S.C., 2020. Stroke promotes the development of brain atrophy and delayed cell death in hypertensive rats. Sci. Rep. 10, 20233. [CrossRef]
- Sontag, T.-A., Fuermaier, A.B.M., Hauser, J., Kaunzinger, I., Tucha, O., Lange, K.W., 2013. Spatial Memory in Spontaneously Hypertensive Rats (SHR). PLoS ONE 8, e74660. [CrossRef]
- Staehr, C., Aalkjaer, C., Matchkov, V.V., 2023. The vascular Na,K-ATPase: clinical implications in stroke, migraine, and hypertension. Clin. Sci. 137, 1595–1618. [CrossRef]
- Szlęzak, D., Bronowicka-Adamska, P., Hutsch, T., Ufnal, M., Wróbel, M., 2021. Hypertension and Aging Affect Liver Sulfur Metabolism in Rats. Cells 10, 1238. [CrossRef]
- Tchekalarova, J., Krushovlieva, D., Ivanova, P., Kortenska, L., 2023. Spontaneously hypertensive rats vs. Wistar Kyoto and Wistar rats: An assessment of anxiety, motor activity, memory performance, and seizure susceptibility. Physiol. Behav. 269, 114268. [CrossRef]
- Teixeira, L.V., Almeida, R.F., Rohden, F., Martins, L.A.M., Spritzer, P.M., De Souza, D.O.G., 2018. Neuroprotective Effects of Guanosine Administration on In Vivo Cortical Focal Ischemia in Female and Male Wistar Rats. Neurochem. Res. 43, 1476–1489. [CrossRef]
- Thakkar, P., McGregor, A., Barber, P.A., Paton, J.F.R., Barrett, C., McBryde, F., 2019. Hypertensive Response to Ischemic Stroke in the Normotensive Wistar Rat: Mechanisms and Therapeutic Relevance. Stroke 50, 2522–2530. [CrossRef]
- Truong, S.H.T., Bonnici, B., Rupasinghe, S., Kemp-Harper, B.K., Samuel, C.S., Broughton, B.R.S., 2023. Post-stroke administration of H2 relaxin reduces functional deficits, neuronal apoptosis and immune cell infiltration into the mouse brain. Pharmacol. Res. 187, 106611. [CrossRef]
- Tsao, C.W., Aday, A.W., Almarzooq, Z.I., Alonso, A., Beaton, A.Z., Bittencourt, M.S., Boehme, A.K., Buxton, A.E., Carson, A.P., Commodore-Mensah, Y., Elkind, M.S.V., Evenson, K.R., Eze-Nliam, C., Ferguson, J.F., Generoso, G., Ho, J.E., Kalani, R., Khan, S.S., Kissela, B.M., Knutson, K.L., Levine, D.A., Lewis, T.T., Liu, J., Loop, M.S., Ma, J., Mussolino, M.E., Navaneethan, S.D., Perak, A.M., Poudel, R., Rezk-Hanna, M., Roth, G.A., Schroeder, E.B., Shah, S.H., Thacker, E.L., VanWagner, L.B., Virani, S.S., Voecks, J.H., Wang, N.-Y., Yaffe, K., Martin, S.S., on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee, 2022. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation 145. [CrossRef]
- Tuo, Q., Zhang, S., Lei, P., 2022. Mechanisms of neuronal cell death in ischemic stroke and their therapeutic implications. Med. Res. Rev. 42, 259–305. [CrossRef]
- Wang, Y., Zhang, T., Zhang, Y., Yu, Y., Bai, F., Zhang, H., Chi, Y., 2021. Effects of inverted photoperiods on the blood pressure and carotid artery of spontaneously hypertensive rats and Wistar–Kyoto rats. J. Hypertens. 39, 871–879. [CrossRef]
- Wu, H., Fan, Y., Zhang, M., 2023. Advanced Progress in the Role of Adipose-Derived Mesenchymal Stromal/Stem Cells in the Application of Central Nervous System Disorders. Pharmaceutics 15, 2637. [CrossRef]
- Yilmaz, U., Tanbek, K., Gul, S., Koc, A., Gul, M., Sandal, S., 2024. Intracerebroventricular BDNF infusion may reduce cerebral ischemia/reperfusion injury by promoting autophagy and suppressing apoptosis. J. Cell. Mol. Med. 28, e18246. [CrossRef]
- Zhang, Q., Jia, M., Wang, Y., Wang, Q., Wu, J., 2022. Cell Death Mechanisms in Cerebral Ischemia–Reperfusion Injury. Neurochem. Res. 47, 3525–3542. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).