1. Introduction
Ensuring safe care involves proactively preventing errors and adverse events, with a primary driver of medication errors often being the incomplete or incorrect transfer of medication information [
1]. Recent studies in clinical practice underscore the significance of accurately collecting the Best Possible Medication History (BPMH) and identifying high-risk patients through an effective medication review program, resulting in a noteworthy reduction in medication errors [
1].
Throughout hospitalisation, these errors can occur in various processes [
2]. These discrepancies can be categorised into documented intentional discrepancies, undocumented intentional discrepancies, and unintentional discrepancies [
1,
4].
In this context of medication errors, drug-related problems (DRPs) are notably prevalent among elderly patients, encompassing issues like drug ineffectiveness, adverse effects, inappropriate treatment, insufficient monitoring, nonadherence, and drug interactions [
4,
5].
Polytherapy significantly adds to the intricacies of the pharmacological regimen, often leading to discrepancies [
6,
7,
8]. Evidence showed that patients with MRCI scores of 25 or with a number of 8 drugs were more likely to develop adverse events [
8]. The medication review process, comprising BPMH collection, verification of information accuracy, Medication Reconciliation (MR) with a review of prescribed therapy, and communication of accurate information, allows prescribers to make informed decisions on continuing, changing, or discontinuing drug treatments [
9,
10,
11,
12].
However, the effectiveness of this process is frequently compromised by clinicians’ relatively limited expertise in pharmacokinetics and pharmacodynamics [
6,
12]. In pre-hospitalization settings, involving pharmacists has demonstrated improved medication reviews and error reduction [
13,
14,
15,
16,
17]. Evidence suggests that multidisciplinary approaches, including clinical pharmacists, effectively reduce the frequency and severity of postoperative complications [
18,
19]. Prevention of surgical complications is pivotal for patient safety and can be addressed at various levels during hospitalisation.
In this context, the medication reconciliation process should be approached with a multidisciplinary perspective [
20,
21]. The objective of our study was to identify, classify, and evaluate different types of discrepancies and DRPs, exploring the potential contribution of clinical pharmacists in this regard.
2. Materials and Methods
Ethics Approval
This study involves human participants and was approved by the Local Ethics Committee of the Padova Province (local code AOP2394). All participants gave informed consent to participate in the study before taking part in it.
The research was conducted in accordance with the Good Clinical Practices (GCP) using the guidance documents and practices offered by the International Conference on Harmonization and the European directives 2001/20/CE and ISO 14155, and in agreement with the local regulations.
Inclusion/Exclusion Criteria and Study Design
This prospective observational study was conducted in two surgery wards in a University Hospital in Padua, Italy. The patients were recruited between May 30th and November 30th, 2021. Patients aged ≥ 18, who gave consent and undergoing scheduled General Surgery and Endocrinological Surgery were enrolled in the study. In particular, the general surgery department comprises 22 beds; in 2021, the department performed a total of 1390 surgeries, with 914 occurring during the specific study period. In the Endocrine Surgery department (with 8 beds), there were 480 admissions in 2021, of which 315 took place during the study period.
Exclusions encompassed patients discharged or transferred to another ward/hospital post-admission, those undergoing emergency surgery, and pregnant individuals.
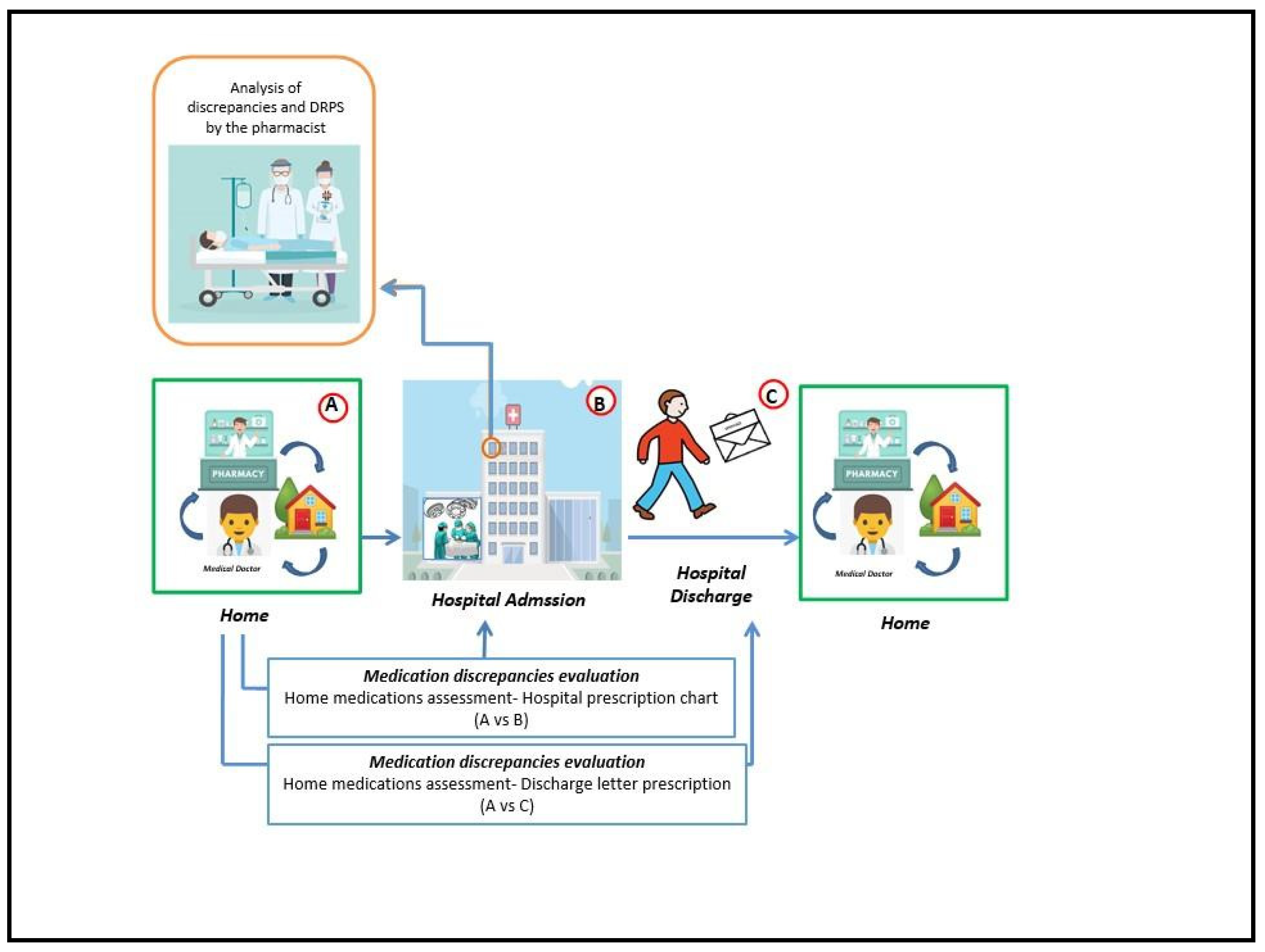
To scrutinise the characteristics of the enrolled patients, demographic and clinical data were systematically recorded. Following the acquisition of informed consent, the pharmacist gathered details regarding the home drug therapy through interviews with the patient or caregiver, creating the dataset labelled as “A.”
Within 24 to 72 hours of patient hospitalization, the pharmacist examined the hospital charts of patients. The medication review process effectively identified inconsistencies discrepancies and DRPs. The discrepancies were categorized into omissions, suspensions, drug duplications, overdoses, underdoses, and discrepancies related to the frequency of administration. Concerning the identified DRPs, they were categorised into three major groups: prescriptions not aligned with current guidelines, not-declared off-label drug prescriptions, and drug interactions. This involved comparing the home therapy with the drug therapy prescribed by the surgeon during the hospitalisation (dataset “B”) and at discharge (dataset “C”). To evaluate the incidence of drug interactions (a type of DRP) in inpatients taking multiple medications, the INTERCheck® software was employed [
22]. In this software the interactions are distinguished by their clinical relevance as “class A” (minor, no clinical relevance), “class B” (moderate, interaction associated with an uncertain or variable event), “class C” (major, interaction associated with a serious event, but which can be managed) and “class D” (contraindicated or very serious, interaction associated with a serious event for which it is appropriate to avoid co-administration or to establish careful monitoring) [
22,
23].
In our study, this application was selected as a supportive tool for prescribing, given its suitability for assessing the complex therapies administered to patients with polypharmacy.
Medications were classified according to the Anatomical Therapeutic Chemical system (ATC-System). All information related to patient characteristics, therapies and the types of discrepancies and DRPs detected has been recorded in the database. Refer to
Figure 1 for a visual representation of the medication review process and the analysed endpoints.
Data Collection
The collected data has been entered into Microsoft Excel™. Upon enrolment, each patient was assigned a unique and anonymized identification code, which is recorded in the database. Additionally, a dedicated form has been created for obtaining informed consent, to be presented to the patient before their participation in the study.
Statistical Analysis
The mean and standard deviation were utilised for the descriptive analysis of normally distributed data, while the median and range (Min-Max) or the interquartile range (IQR) were employed for non-normally distributed data. Additionally, for categorical variables, the number and percentage within each variable were reported.
The sample size was calculated through G*Power, version 3.1.9.7 (2020). Specifically, anticipating a potential presence of discrepancies and DRP in 70% of the selected patients, a sample size of 130 patients was deemed sufficient. This sample size would provide a 95% confidence level, with an accuracy of the discrepancy/DRP frequency at 8%, surpassing 50% where the need for intervention was evident.
Statistically significant differences between the two settings were analysed using either the Mann-Whitney Rank Sum Test or the Chi-square Test, depending on the variables studied. The threshold for statistical significance was set at p < 0.05.
3. Results
3.1. Baseline Characteristics of the Enrolled Patients
During the study period, 197 patients were included. 73 patients (37%) were recruited for General Surgery and 124 patients (63%) for Endocrinological Surgery.
As shown in
Table 1, the patients’ median age was 63 years (54-71), and 68% of them were female. These data reveal significant statistical differences between the populations in the two departments, particularly in terms of median age and gender with a higher prevalence of younger women observed in the endocrinology setting.
Regarding comorbidities, patients presented with a mean of 2.40 (SD 1.57) comorbidities. The most frequent ones were associated with the cardiovascular system (64.5%), followed by metabolic disorders (33.0%) and osteoporosis/osteoarthritis (24.9%). The only statistically significant difference between the two settings with regard to comorbidities relates precisely to diseases of the cardiovascular system, which are much more common in the General Surgery group (80.8% vs 54.8%, p<0.05).
No statistically significant difference was observed in terms of drug use. In Endocrinological Surgery, patients were taking an average of 3.58 (SD 2.64) drugs before hospitalisation. Similarly, patients in General Surgery had a comparable drugs’ amount of 3.88 (SD 2.10).
Specifically, 36.9% of patients in general surgery presented with a medication count greater than or equal to 5, while in the endocrinological surgery department, 29.8% had an equal or higher number of 5 medications. The average age of polypharmacy patients in general surgery was approximately 71 years, while in endocrinological surgery, it was 68 years.
3.2. Assessment of Discrepancies and DRPs
Although no significant disparities were found in all the baseline population characteristics, noteworthy findings emerged during the examination of discrepancies and DRPs: compared to the operational flow depicted in
Figure 1, the discrepancies and DRPs observed are the sum of those identified through the A vs B and A vs C comparisons.
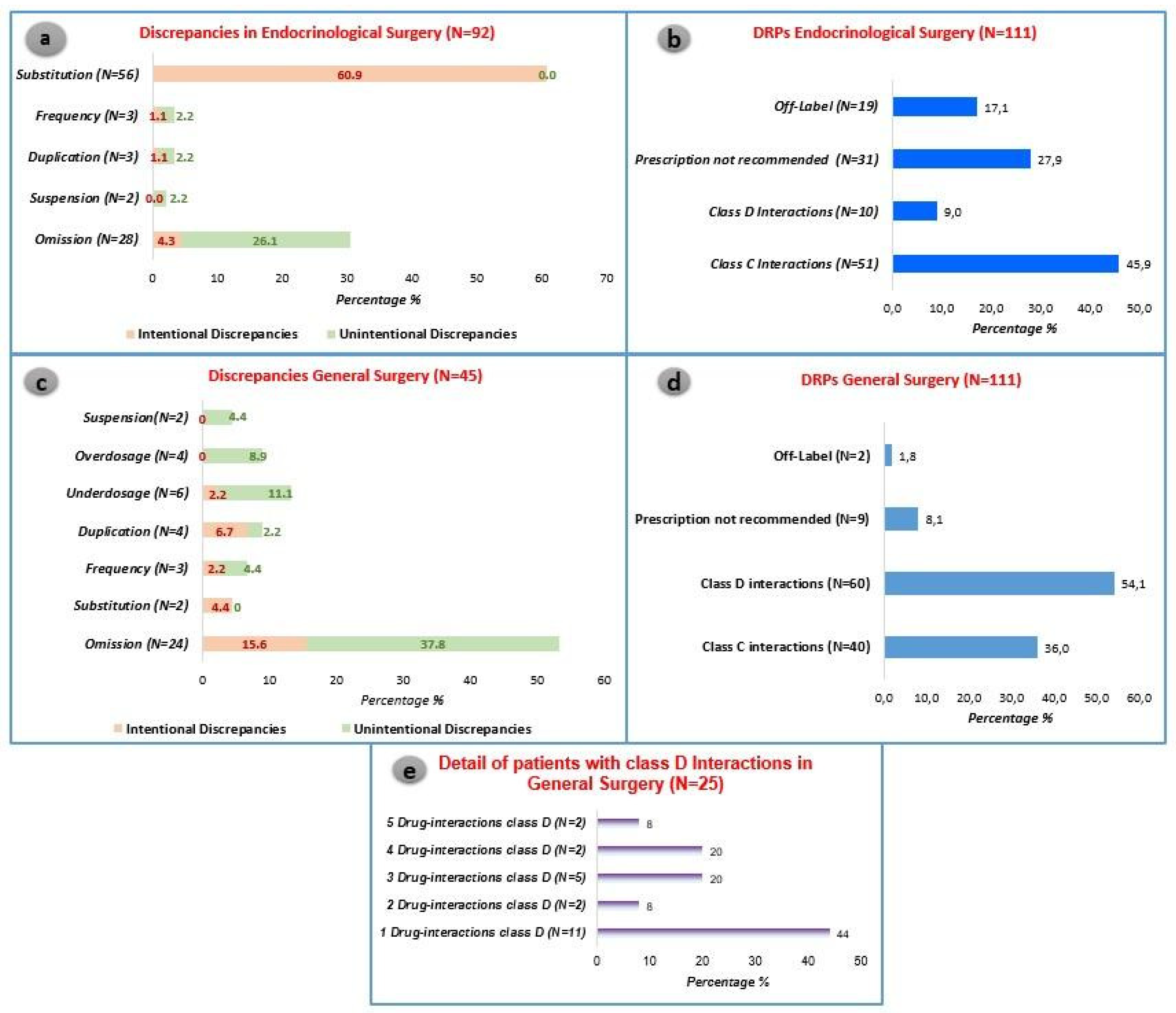
During the medication review, the analysis revealed 92 discrepancies and 111 DRPs in Endocrinological Surgery. These findings were identified in 106 patients, constituting 85.5% of the admitted patients resulting in a mean of 0.88 discrepancies and 1.05 DRPs per patient. Moreover, 45 discrepancies and 111 DRPs were documented within 61 patients in General Surgery, accounting for 83.6% of admitted patients. In total, the pharmacist-led medication review revealed a total of 222 DRPs and 137 discrepancies across a cohort of 197 patients.
In Endocrinological Surgery, intentional discrepancies, particularly substitutions, were the most prevalent, comprising 60.9% of cases. Notably, the drug Levothyroxine was frequently substituted with a non-equivalent alternative. On the other hand, in General Surgery, the most frequent discrepancies were omissions, occurring in 53.4% of the prescriptions. See
Figure 2 for further information. The latter were further subdivided into class C and class D interactions.
The predominant DRP observed in both settings revolves around drug interactions. Specifically, in the endocrinology setting, class C interactions constitute 45.9% of DRPs, while in General Surgery, class D interactions account for 54.1% of DRPs. Although no statistically significant differences were noted between the two settings regarding class C interactions, a significant disparity emerged in the study for more severe interactions. These severe interactions were notably more prevalent in General Surgery (54.1% vs. 9.0%, p<0.05).
The endocrinology scenario is significantly impacted by the high incidence of thyroidectomies, which necessitate hormone replacement therapy using levothyroxine. This active ingredient, due to its interactions with various pharmacological classes, can result in a diminished therapeutic effect of levothyroxine and the development of hypothyroidism goitre. On the other hand, the most frequent class D interactions in general surgery are associated with the risk of QT interval prolongation. Further information is available on
Table 2 and
Table 3.
In relation to the other categories of identified Drug-Related Problems (DRPs), notable statistically significant distinctions exist between the two cohorts. Specifically, within the endocrinology domain, a significantly elevated percentage of prescriptions deviating from guidelines was documented (27.9% vs. 8.1%, p<0.05). This discrepancy is primarily associated with non-adherence to antibiotic and antithrombotic prophylaxis recommendations. Moreover, the same setting exhibited a statistically higher percentage of off-label prescriptions (17.1% vs. 1.8%, p<0.05).
4. Discussion
Capturing and categorising medication discrepancies stands as a crucial initial step in the optimal execution of medication review practices. Our research delved into the medication review procedures within the confines of an Italian Academic hospital, focusing specifically on two surgical wards. The primary objective was to meticulously identify and classify all instances of discrepancies and DRPs.
Notably, the demographic composition of the study’s participants exhibited a discernible gender imbalance. The female demographic emerged as the predominant representation, particularly within the domain of Endocrinological Surgery. This observation aligns with epidemiological data indicating a higher prevalence of thyroid disease among women. Specifically, studies highlight a 5% prevalence rate in women compared to a 1% prevalence rate in men [
24].
This gender-specific variation within our study population serves as a noteworthy insight, potentially influencing the nature and manifestation of medication-related issues. Understanding these demographic nuances is pivotal for tailoring medication review processes to address the distinctive needs and concerns associated with different patient groups, ultimately optimising healthcare outcomes.
The literature consistently underscores the heightened vulnerability to diseases with advancing age, particularly among individuals with comorbidities who concurrently assume multiple medications [
25]. The augmented risk of drug interactions and discrepancies parallels the escalating number of medications prescribed [
26,
27]. Within the scope of our study, the average age of patients in General Surgery was 69 years, while in Endocrinological Surgery, it was 60 years.
In the context of medication discrepancies, patients hospitalised in Endocrinological Surgery were found to assume an average of 3.58 drugs at home, compared to 3.88 in General Surgery. The analysis unearthed a total of 92 discrepancies and 111 DRPs in Endocrinological Surgery. Notably, intentional discrepancies were prevalent, with the substitution for the drug Levothyroxine accounting for 60.9% of cases, underscoring the critical role of clinical pharmacists in averting such issues.
The study highlighted class C drug interactions as a significant concern in Endocrinological Surgery, constituting 45.9% of DRPs. Notably, the most frequent interaction involved the reduction of the therapeutic effect of levothyroxine (37.3%) due to co-administration with calcium. Additionally, 27.9% of discrepancies were attributed to prescriptions not aligned with guidelines, with antibiotic prophylaxis (54.8%) for thyroidectomy emerging as a major concern.
In General Surgery, where 45 discrepancies and 111 DRPs were identified, omissions were the principal discrepancy (53.4%), aligning with existing literature. Class D interactions comprised 54.1% of DRPs, primarily associated with drugs prolonging the QT interval. The absence of a dedicated pharmacist for medication review in the surgical wards highlighted a potential gap in the multidisciplinary approach recommended for the medication review process.
This study not only classified the types of discrepancies and their intentionality but also addressed the lack of a standardised classification for discrepancies in existing literature. By extending the analysis to surgical areas, a critical gap in many medication review studies was addressed. Importantly, the research shed light on drugs associated with a higher risk of discrepancies and DRPs, contributing valuable insights to the broader field.
Drawing from recent Italian findings, the study emphasised the positive impact of pharmacist involvement in reducing omissions in BPMHs collected before surgery. This underscores the potential for mitigating medication errors in pre-surgical settings, particularly in Italy where clinical pharmacists are not institutionally involved in this process [
2,
18]. The study’s meticulous classification and consideration of intentionality provide a nuanced understanding of the medication review process’s efficacy, setting a valuable precedent for future research in this domain.
Study Limitation
Several limitations are inherent in our study. Firstly, the duration was relatively brief, and the size of the patient sample under examination was modest. Additionally, the absence of an analysis of pharmacist intervention in the research methodology poses a notable limitation. Subsequent investigations could enhance our understanding by implementing a study design that divides the patient cohort into two arms, allowing for a more detailed exploration of the impact of pharmacist interventions.
Furthermore, a critical avenue for future research lies in assessing the number of ADRs for each patient. Evaluating potential correlations between the clinical pharmacist’s interventions and key clinical endpoints, such as the reduction of hospitalisation days and drug interactions, would provide valuable insights. By delving into these aspects, future studies could offer a more comprehensive and nuanced understanding of the role of clinical pharmacists in optimising patient outcomes.
The findings of this study underscore the pivotal role of transitions of care as crucial junctures for patient safety. The observed prevalence of discrepancies among patients in both wards highlights the urgent need for a more robust MR process to mitigate potential errors. It is noteworthy that, in both cases, the absence of a hospital pharmacist involved in the MR process adds an additional layer of concern.
To address these challenges, a paradigm shift towards a multidisciplinary approach to the MR process is essential. The inclusion of a pharmacist in the process becomes particularly relevant, given their specialised knowledge about drugs. Their expertise is instrumental in identifying discrepancies and DRPs, ultimately contributing to a more accurate and effective MR process.
Furthermore, the meticulous identification of discrepancies and DRPs holds significant promise in preventing potential complications, especially in high-risk patients. By integrating a pharmacist’s insights into the MR process, healthcare teams can proactively address medication-related issues during transitions of care, fostering a safer and more streamlined continuum of patient care.
5. Conclusions
Frequently, the medication review process is superficially carried out, introducing errors that may adversely affect patient health. This study explores the potential positive impact of involving the clinical pharmacist in identifying and managing discrepancies and DRPs. Our findings suggest that the inclusion of clinical pharmacists in the medication review process can significantly enhance the accuracy and thoroughness of medication assessments. By actively identifying and addressing discrepancies, clinical pharmacists help to minimize the risk of adverse drug events and improve overall patient safety. Furthermore, their expertise in pharmacotherapy allows for more tailored and effective medication management, which can lead to better therapeutic outcomes. This underscores the importance of integrating clinical pharmacists into multidisciplinary healthcare teams, not only to optimize medication use but also to contribute to the broader goal of enhancing the quality of patient care. Future research should continue to investigate the specific contributions of clinical pharmacists in various healthcare settings to further validate and expand upon these findings.
Author Contributions
Conceptualization, F.F.F., F.V. and N.R.; methodology, D.M.; software, F.F.F.; validation, C.B., G.S., M.I. and F.V.; formal analysis, F.F.F., D.M. and L.P.; investigation, F.F.F., L.P., F.Te. and F.To; resources, C.B., G.S., M.I. and F.V.; data curation, F.F.F. and D.M.; writing—original draft preparation, F.F.F.; writing—review and editing, D.M.; visualization, L.P.; supervision, C.B. and F.V..; project administration, N.R.; funding acquisition, not applicable. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study involves human participants and was approved by the Local Ethics Committee of the Padova Province (local code AOP2394).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Data will be provided upon request to the corresponding author.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Almanasreh E, Moles R, Chen TF. The medication reconciliation process and classification of discrepancies: a systematic review. Br J Clin Pharmacol. 2016;82(3):645-658. [CrossRef]
- Wittich CM, Burkle CM, Lanier WL. Medication errors: an overview for clinicians. Mayo Clin Proc. 2014;89(8):1116-1125. [CrossRef]
- Graabæk T, Terkildsen BG, Lauritsen KE, Almarsdóttir AB. Frequency of undocumented medication discrepancies in discharge letters after hospitalization of older patients: a clinical record review study.Ther Adv Drug Saf. 2019;10:2042098619858049. Published 2019 Jun 16. [CrossRef]
- van den Bemt PM, Egberts TC, de Jong-van den Berg LT, Brouwers JR. Drug-related problems in hospitalised patients. Drug Saf. 2000;22(4):321-333. [CrossRef]
- Qu C, Meng L, Wang N, et al. Identify and categorize drug-related problems in hospitalized surgical patients in China. Int J Clin Pharm. 2019;41(1):13-17. [CrossRef]
- Chiarelli MT, Antoniazzi S, Cortesi L, et al. Pharmacist-driven medication recognition/ reconciliation in older medical patients. Eur J Intern Med. 2021;83:39-44. [CrossRef]
- Patel CH, Zimmerman KM, Fonda JR, Linsky A. Medication Complexity, Medication Number, and Their Relationships to Medication Discrepancies. Ann Pharmacother. 2016;50(7):534-540. [CrossRef]
- Pantuzza LL, Ceccato MDGB, Silveira MR, Junqueira LMR, Reis AMM. Association between medication regimen complexity and pharmacotherapy adherence: a systematic review. Eur J Clin Pharmacol. 2017;73(11):1475-1489. [CrossRef]
- Dei Tos M, Canova C, Dalla Zuanna T. Evaluation of the medication reconciliation process and classification of discrepancies at hospital admission and discharge in Italy. Int J Clin Pharm. 2020;42(4):1061-1072. [CrossRef]
- Sadasivaiah S, Smith DE, Goldman S, Ratanawongsa N. Improving best possible medication history with vulnerable patients at an urban safety net academic hospital using pharmacy technicians. BMJ Open Qual. 2017;6(2):e000102. Published 2017 Oct 21. [CrossRef]
- Seroussi B, Ghomari MB, Guezennec G, Federspiel F, Debrix I, Bouaud J. Easy Medication Reconciliation at Hospital Admission: The EzMedRec Decision Support System. AMIA Annu Symp Proc. 2021;2020:1110-1119. Published 2021 Jan 25.
- Al-Jazairi AS, Al-Suhaibani LK, Al-Mehizia RA, et al. Impact of a medication reconciliation program on cardiac surgery patients. Asian Cardiovasc Thorac Ann. 2017;25(9):579-585. [CrossRef]
- Gleason KM, McDaniel MR, Feinglass J, et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25(5):441-447. [CrossRef]
- Bansal N, Tai WT, Chen LC. Implementation of an innovative surgical pharmacy service to improve patient outcomes-Twelve-month outcomes of the Enhanced Surgical Medicines Optimization Service. J Clin Pharm Ther. 2019;44(6):904-911. [CrossRef]
- Nguyen AD, Lam A, Banakh I, Lam S, Crofts T. Improved Medication Management With Introduction of a Perioperative and Prescribing Pharmacist Service. J Pharm Pract. 2020;33(3):299-305. [CrossRef]
- Hick HL, Deady PE, Wright DJ, Silcock J. The impact of the pharmacist on an elective general surgery pre-admission clinic. Pharm World Sci. 2001;23(2):65-69. [CrossRef]
- Mengato D, Pivato L, Codato L, et al. Best Possible Medication History Collection by Clinical Pharmacist in a Preoperative Setting: An Observational Prospective Study. Pharmacy (Basel). 2023;11(5):142. Published 2023 Sep 8. [CrossRef]
- Hohn N, Langer S, Kalder J, Jacobs MJ, Marx G, Eisert A. Optimizing the pharmacotherapy of vascular surgery patients by medication reconciliation. J Cardiovasc Surg (Torino). 2014;55(2 Suppl 1):175-181.
- Bolliger M, Kroehnert JA, Molineus F, Kandioler D, Schindl M, Riss P. Experiences with the standardized classification of surgical complications (Clavien-Dindo) in general surgery patients. Eur Surg. 2018;50(6):256-261. [CrossRef]
- Leguelinel-Blache G, Arnaud F, Bouvet S, et al. Impact of admission medication reconciliation performed by clinical pharmacists on medication safety. Eur J Intern Med. 2014;25(9):808-814. [CrossRef]
- Mekonnen AB, McLachlan AJ, Brien JA. Effectiveness of pharmacist-led medication reconciliation programmes on clinical outcomes at hospital transitions: a systematic review and meta-analysis. BMJ Open. 2016;6(2):e010003. Published 2016 Feb 23. [CrossRef]
- Ghibelli S, Marengoni A, Djade CD, et al. Prevention of inappropriate prescribing in hospitalized older patients using a computerized prescription support system (INTERcheck(®)). Drugs Aging. 2013;30(10):821-828. [CrossRef]
- Martocchia A, Spuntarelli V, Aiello F, et al. Using INTERCheck® to Evaluate the Incidence of Adverse Events and Drug-Drug Interactions in Out- and Inpatients Exposed to Polypharmacy. Drugs Real World Outcomes. 2020;7(3):243-249. [CrossRef]
- Paschou SA, Vryonidou A, Goulis DG. Thyroid nodules: A guide to assessment, treatment and follow-up. Maturitas. 2017;96:1-9. [CrossRef]
- Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83. [CrossRef]
- Gujjarlamudi HB. Polytherapy and drug interactions in elderly. J Midlife Health. 2016;7(3):105-107. [CrossRef]
- Sund JK, Sletvold O, Mellingsæter TC, et al. Discrepancies in drug histories at admission to gastrointestinal surgery, internal medicine and geriatric hospital wards in Central Norway: a cross-sectional study. BMJ Open. 2017;7(9):e013427. Published 2017 Sep 24. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).