1. Introduction
Oxidative stress (OS) is defined as an imbalance between the cellular effects of reactive oxygen species (ROS) and the ability of the organism to repair such damages.[
1] OS is a topic of particular importance, because it is involved in the pathogenesis of several diseases (i.e., oncology, diabetes, ischemic diseases, surgery) and because higher levels of OS are associated with worse outcomes.[
1,
2,
3] In the context of solid cancer surgery, OS is a topic of particular interest because both the malignancy itself and the surgical intervention are major contributors to the modulation of elevated OS levels.
The development of solid tumors, driven by aberrant cell growth and intense cellular death, induces tissue hypoxia.[
4] This hypoxic environment leads to mitochondrial dysfunction and lipid peroxidation, resulting in pronounced OS.[
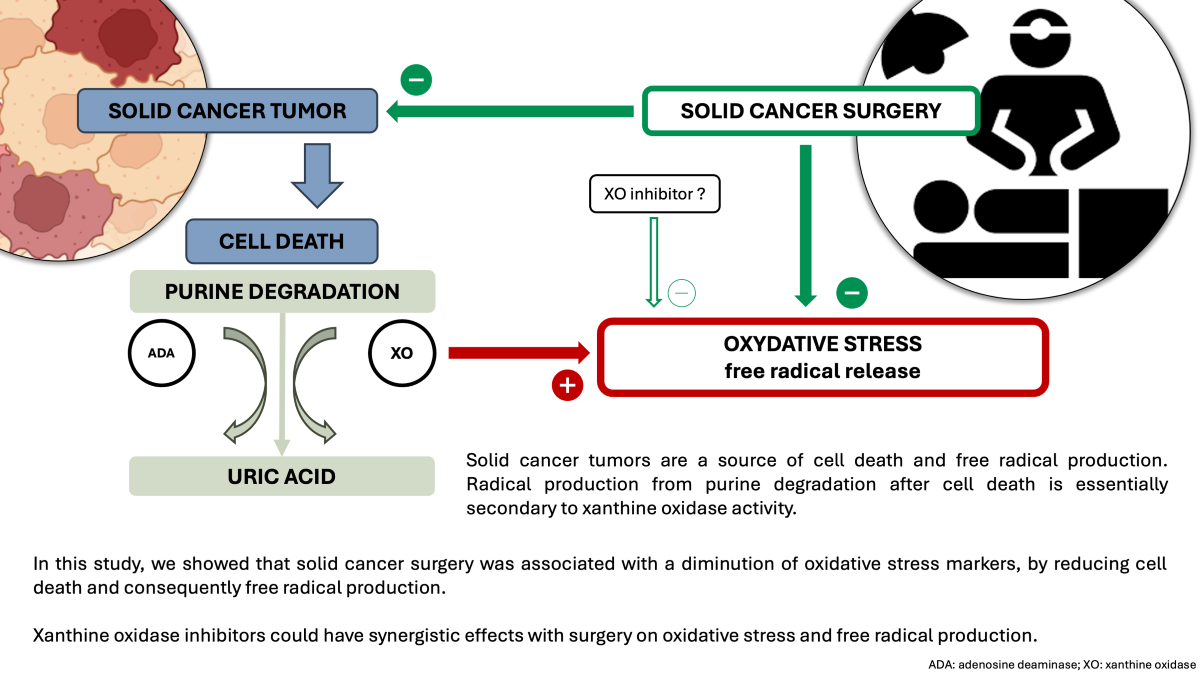
5] Therefore, oxidative stress levels can be assessed by exploring the purine nucleotide salvage pathway. This pathway, activated following cell death, allows for the transformation of nucleic acid molecules into uric acid through the degradation of purine bases. Two key enzymes are involved in this process : adenosine deaminase (ADA) and xanthine oxidase (XO) which are the flux limiting enzymes. XO is an enzyme catalyzing the oxidation of hypoxanthine to xanthine and then xanthine to uric acid, this last step being associated with radical production.
Major surgery can induce acute and profound physiological changes, leading to significant inflammation and ischemia/reperfusion injury. These conditions generate free radical, primarily reactive oxygen species (ROS), and consequently cause elevated levels of OS.[
6] The most complex and invasives surgeries are associated with higher levels of OS when compared with less invasive procedures, suggesting that a diminution of surgical stress could be beneficial in major solid cancer surgery.[
7] In this setting, OS can be assessed using less specific markers such as ischemia-modified albumin (IMA), redox potential (RP), or highly sensitive troponin-T (TnT-hs).
Several studies assessed the effect of chemotherapy or immunotherapy on OS during solid cancer treatments.[
8,
9] However, the effect of surgery (and more precisely the effect of tumor resection) on OS behavior during solid cancer treatment remains poorly documented. The aim of this study was to evaluate the effects of solid cancer major surgery on OS levels.
2. Materials and Methods
2.1. Patients
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by an institutional review board (Comité de Protection des Personnes SUD-EST VI, reference 2018-A02511-54) on December 9, 2019. Written informed consent was obtained from all subjects involved in the study.
We performed a prospective, comparative, and observational single-center cohort study, between May 2021 and May 2023. Inclusion criteria were :
High-risk surgery patients were defined as adult patients over 50 years of age, with an American Society of Anesthesiologists (ASA) score
2 (clinical score performed by an anesthesiologist for assessing the fitness of a patient before surgery). Elective surgery was defined as intra-abdominal or thoracic surgery with a duration of at least 90 minutes and requiring the use of invasive blood pressure monitoring.[
10]
Exclusion criteria were :
Inability to understand the study and/or sign an informed consent, refusal to sign an informed consent
Hypoalbuminemia, defined by an albumin level < 30 g.L-1
Pre-existent inflammatory disease, defined by a C reactive-protein (CRP) level > 20 mg.L-1.
2.2. Anesthesia Protocol
Anesthesia and surgery strategies were not modified because of the study protocol. All patients in the study received anesthesia in accordance with a pre-established protocol, described as follows. Total-intravenous anesthesia (TIVA), with target-controlled infusion (TCI) of propofol and remifentanil was performed. Usual starting doses were 6 – 8 µg.mL-1 and 6 – 8 ng.mL-1, respectively. Individual dosage was afterwards guided with bispectral index neurological monitoring. Neuromuscular blockade was achieved using a non-depolarizing neuromuscular blocking drug and its effect was monitored using a quantitative train-of-four technique. Epidural analgesia was systematically performed, unless contraindicated, when patient had an open approach surgery. Usual drug mixture was mixture of ropivacaine 1.6 mg.L-1 and sufentanil 0.4 µg.mL-1, administered via patient-controlled epidural analgesia (PCEA), with a continuous administration of 4 – 6 mL.h-1 and the possibility of a patient administration of 4 – 6 mL every 20 min. Multimodal analgesia was performed using acetaminophen, non-steroidal anti-inflammatory drugs, nefopam and morphine, unless contraindicated. Invasive hemodynamic monitoring was performed with an arterial line and pulse-contour analysis for continuous cardiac output monitoring. A mean arterial pressure of 10% the usual patient value was targeted, and (if needed) diluted norepinephrine (16µg.mL-1) was used to reach the target. Fluid loading was guided by dynamic fluid responsiveness monitoring.
2.3. Clinical Data Collection
The following data were collected for all patients, using our electronic health record:
Demographics: age, gender, ASA score, height, weight, body mass index (BMI)
Medical background and Charlson Comorbidity Index (a scoring system for comorbidities, a score of zero means no comorbidities and a higher score means more comorbidities)[
11]
Peri-operative characteristics: type of surgery, anesthesia and surgery length, use of an epidural analgesia, mean norepinephrine dosage and fluid balance
Outcomes : length of hospital stay, day-30 mortality rate, Comprehensive Complication Index (CCI, a scoring system for postoperative complications, a score of zero means no complications and a higher score mean more complications, with a maximum at 100 meaning the patient died) at day-5 and day-15 postoperative. [
12]
2.4. Samples Collection and Biological Data
Blood samples were collected for the measurement of the following biological variables :
at the onset of surgery: in the operating room, via an arterial line, after induction of anesthesia and before surgical incision
at the end of surgery: in the post-operative care unit, via an arterial line, after patient awaking and tracheal extubation.
The following biomarkers were measured :
2.4.1. Adenosine Deaminase Measurement
ADA was measured as described previously.[
13,
14] Adenosine deaminase catalyzes the deamination of adenosine into inosine with the formation of ammonium (NH4+) in a stoichiometric manner. Briefly, adenosine (750 μL, 28 mM) was incubated with plasma (125 μL) in saline (125 μL, 7% Bovine Serum Albumin (BSA)) for 37 min at 37°C. The reaction was started by adding adenosine and stopped in ice water. Ammonia resulting from adenosine degradation by ADA was measured using a Synchron LX 20 analyzer (Beckman Coulter) and expressed as international units (mIU) per mL.
2.4.2. Xanthine Oxidase Measurement
XO was evaluated as described previously.[
15] Briefly, 200 µL of xanthine (0.5 mmol.L
-1 dissolved in water, Sigma-Aldrich) and 100 µL of adenosine triphosphate (300 μmol.L
-1 dissolved in water) were mixed with 0.3 mL of serum. After incubation (37 °C, for 30 min), uric acid was evaluated using a DX Beckman Coulter apparatus. The results were expressed as IU.
2.4.3. Ischemia Modified Albumin Measurement
IMA was evaluated as described previously.[
16,
17] Briefly, the albumin cobalt binding (ACB) test is a quantitative in vitro diagnostic test that detects IMA by measuring the cobalt binding capacity of albumin in human serum (Ischemia Technologies, Denver, CO). Human serum, including that collected in serum separator tubes, is the only specimen type for the ACB-test and was performed on a Synchron LX 20 analyzer (Beckman Coulter, Villepinte, France). The test sample and a fixed amount of cobalt in the form of cobalt chloride were incubated. Dithiothreitol was added to react with the cobalt not bound to the N-terminus to form a measurable color. The absorbance was measured by spectrophotometer at 510 nm. In these conditions, the inter-assay coefficient of variation was less than 10%. The results were expressed as arbitrary units (AU).
2.4.4. Redox Potential Measurement
We used the probe SEN 0464 (DFROBOT, Gotronic, France, Indicator electrode Platinium, reference electrode silver-silver chloride). Automatic three points calibration, Range -2000 to +2000 millivolts (mV). Intra essay coefficient of variation < 10mV. Internal resistance <10 kOhms following the constructor recommendations. Measurement was performed on whole heparinized blood. The results were expressed in mV.
2.4.5. Highly Sensitive Troponin T Measurement
TnT-hs was measured on COBAS-8000 Roche, using an immunological sandwich method (detection threshold: 5 pg.mL-1, range: 5 – 50 ng, intra-assay variation < 10%, intra-assay range between 2 and 4 %, 99th percentile : 14 pg.mL-1). The results were expressed in ng.mL-1.
2.5. Controls
Data from thirty healthy controls (matched on gender, age, and BMI) were extracted from an existing database of biological samples, drawn from the staff of our lab. We extracted the ADA, XO, IMA and redox potential levels.
2.6. Statistical analyses
We planned to sample and analyze data from 40 patients. An a posteriori sample size calculation, using the mean and standard deviation (SD) values of our cohort and the control cohort, showed that a population between 6 and 33 patients (depending on the biomarker) was sufficient to demonstrate significant difference between these cohorts with a power of 90% and an alpha risk of 5%. Categorical variables were expressed as count (%), and continuous variables as median (25th - 75th quartile range) or mean (± standard deviation), contingent on variable distribution confirmed by the Shapiro-Wilk and Kolmogorov-Smirnov tests. Descriptive statistics summarized baseline characteristics.
Two-tailed t-tests compared normally distributed continuous variables between groups, while the Mann-Whitney U test handled non-normally distributed variables.
Pearson’s or Spearman’s correlation coefficients were computed based on variable distribution for examining relationships. Significance was set at a p-value < 0.05. Analyses were conducted using Jamovi version 2.3.19.0 and Prism version 10.1.0.
3. Results
3.1. Clinical Data
Characteristics of patients and controls are listed in
Table 1. Thirty-six patients were included overall. All patients had ASA score of 2 or 3 (mild to moderate chronic condition) and underwent surgery for a solid cancer. Thirteen (36%), 4 (11%) and 22 (61%) patients had diabetes, myocardial infarction, and hypertension.
Peroperative characteristics and outcomes are presented
Table 2.
3.2. Enzymatic Activities, IMA, and Redox Potential
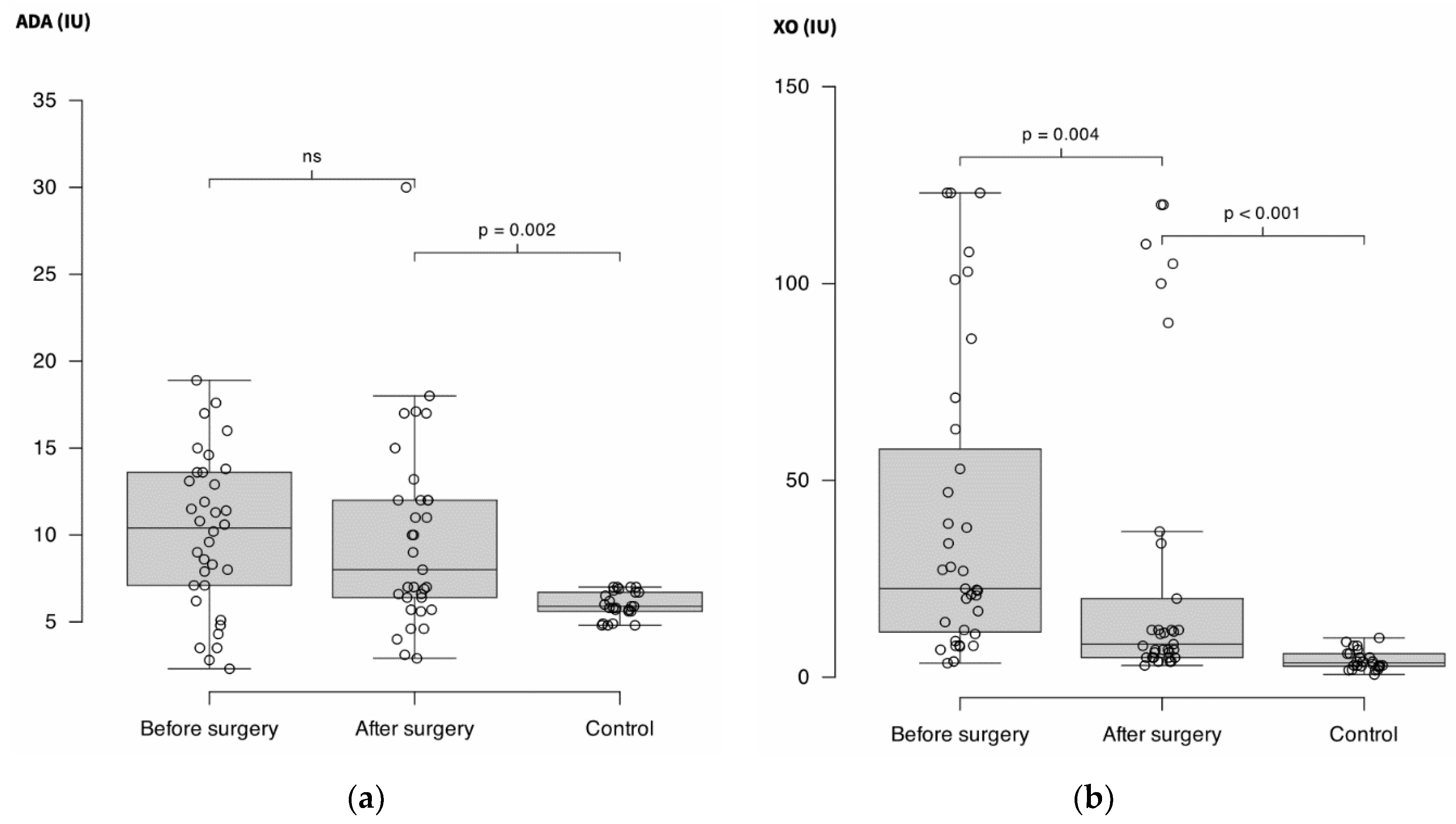
After the surgical procedure, ADA did not decrease significantly (10.4 IU (6.9 – 13.7) vs. 8 IU (6 – 12); p = 0.47) but remained higher than in controls (5.9 IU (5.6 – 6.8); p = 0.002).
XO decreased significantly after surgery (22.5 IU (11 – 63) vs. 8.4 IU (5 – 27) (-73%); p = 0.004) but remained higher than in controls (3.7 IU (2.7 – 6); p < 0.001).
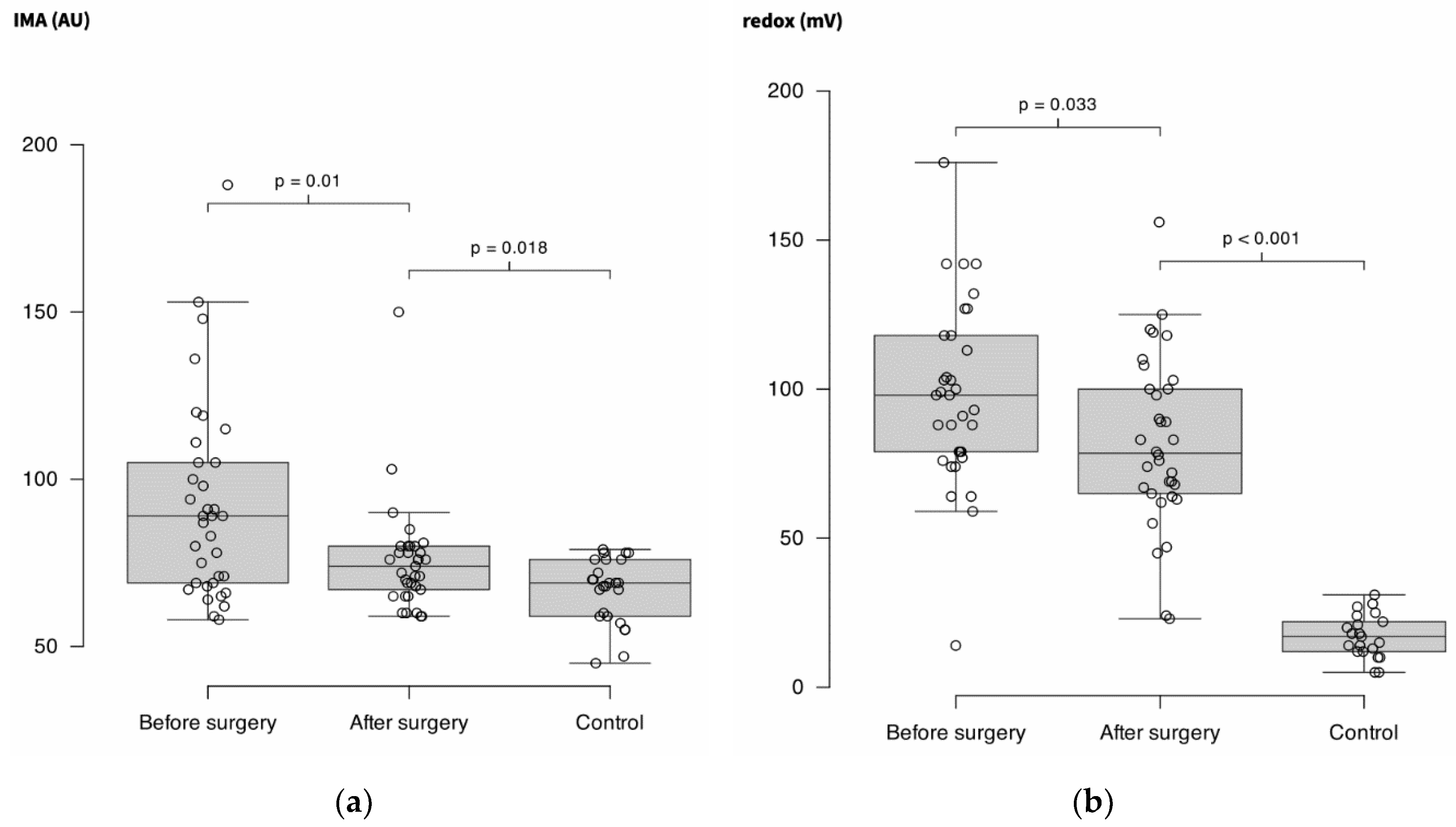
IMA decreased significantly after surgery (89 AU (69 – 105) vs. 74 AU (66 – 80) (-15%); p = 0.01)but remained higher than in controls (69 AU (59 – 76); p = 0.018).
Redox potential decreased significantly after surgery (98 mV (78 – 118) vs. 79 mV (65 – 101) (-10%); p = 0.033) but remained higher than in controls (17 mV (12 – 23); p < 0.001).
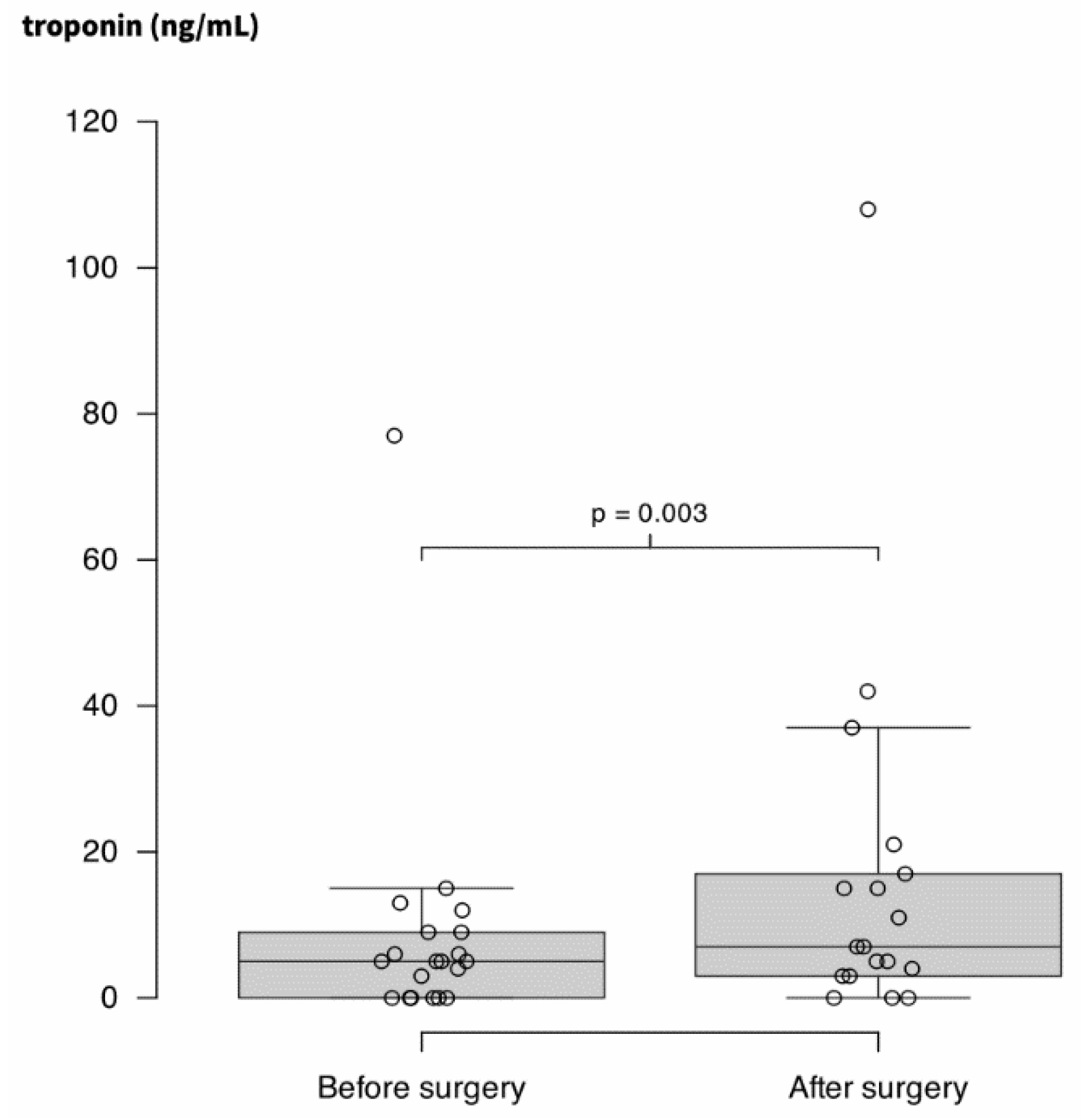
Finally, TnT-hs increased weakly but significantly after surgery (5 ng.L-1 (0 – 9) vs. 7 ng.L-1 (3 – 18); p = 0.003). Results are presented through
Figure 1a to
3.
Figure 1.
(a) Adenosine deaminase activity (ADA) measured in plasma of 36 patients before and after surgery for solid cancer and in 30 controls. Units are international units (IU).
(b) Xanthine oxidase (XO) measured in the same conditions than
Figure 1A. ns = non-significant.
Figure 1.
(a) Adenosine deaminase activity (ADA) measured in plasma of 36 patients before and after surgery for solid cancer and in 30 controls. Units are international units (IU).
(b) Xanthine oxidase (XO) measured in the same conditions than
Figure 1A. ns = non-significant.
Figure 2.
(a) Ischemia modified albumin (IMA) measured in plasma of 36 patients before and after surgery for solid cancer and in 30 controls. Units are arbitrary units (AU) (b) Redox potential evaluated in the same conditions than in A. Units are millivolts (mV).
Figure 2.
(a) Ischemia modified albumin (IMA) measured in plasma of 36 patients before and after surgery for solid cancer and in 30 controls. Units are arbitrary units (AU) (b) Redox potential evaluated in the same conditions than in A. Units are millivolts (mV).
Figure 3.
Concentration in highly-sensitive troponin T (TnT-hs) in plasma of 36 patients before and after surgery.
Figure 3.
Concentration in highly-sensitive troponin T (TnT-hs) in plasma of 36 patients before and after surgery.
3.3. Correlation Data
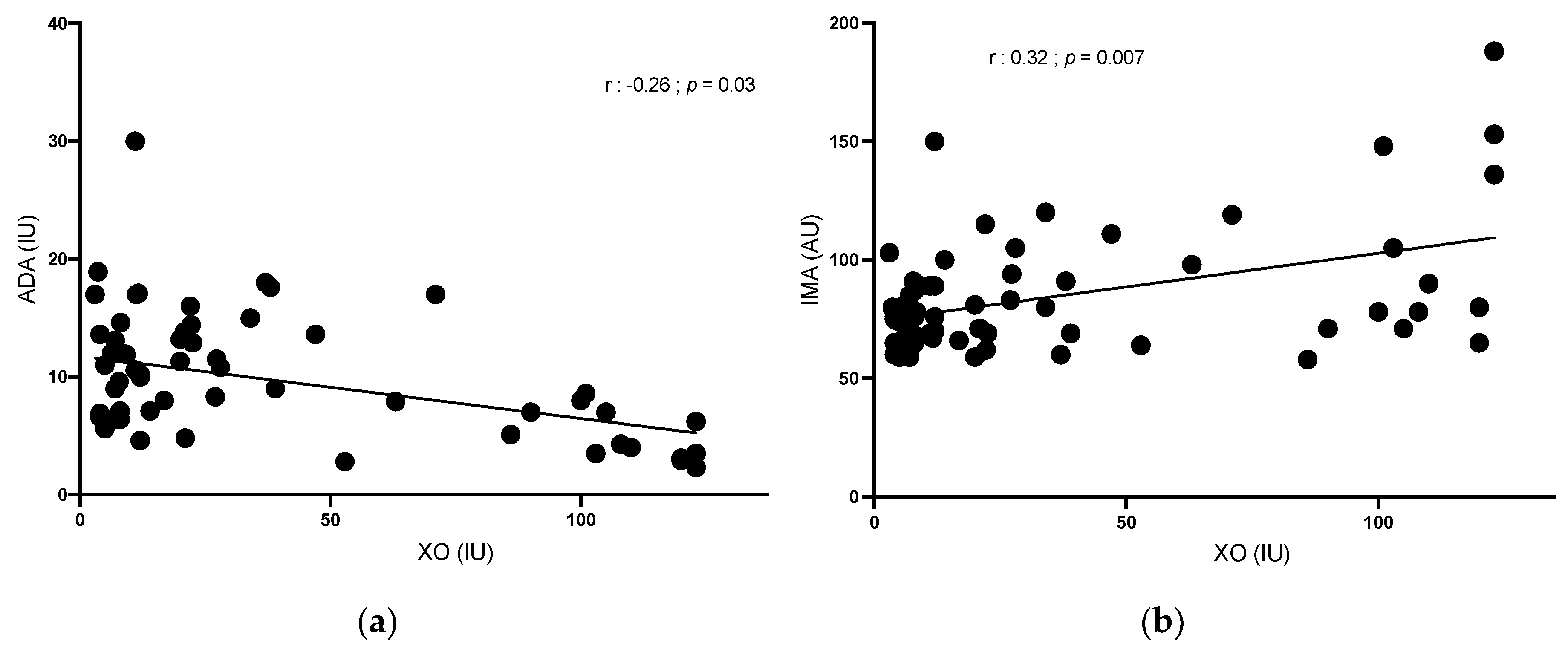
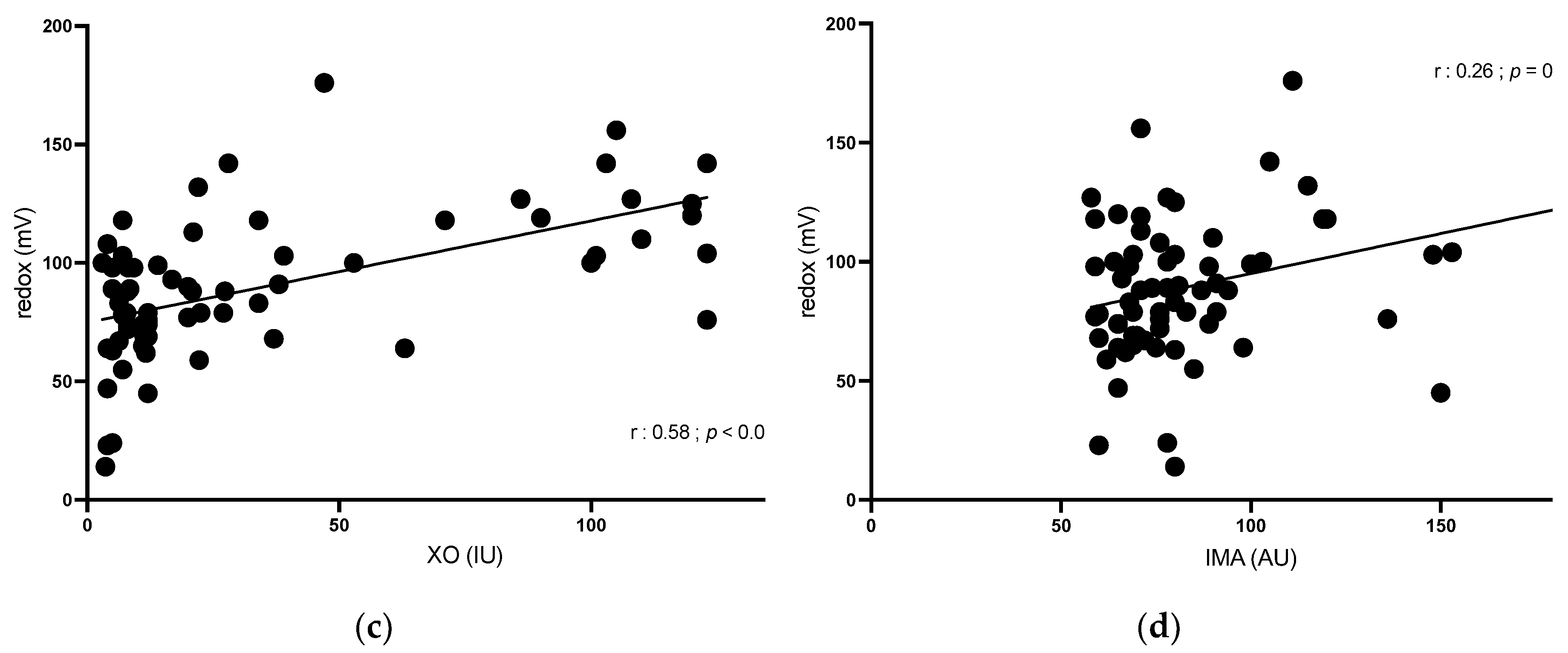
XO was positively correlated with IMA (r = 0.32; p = 0.007) and redox potential (r = 0.58; p < 0.001) and was negatively correlated with ADA (r = -0.26; p 0.03).
IMA was positively correlated with redox potential (r = 0.26;
p = 0.04). ADA was not correlated with other OS parameters (
Figure 4).
4. Discussion
The main result of this study is that solid cancer is associated with high OS, and that surgery leads to its significant decrease. The main source of OS in this cohort of surgical oncology patients seems to be the solid tumor by itself, since XO activity was correlated with IMA and redox potential, both being non-specific global markers of OS. The part of OS associated with major surgery seems to be present, as shown by the troponin T elevation after surgery, but does not counterbalance the effect OS diminution associated with the removal of the tumor.
We found high XO activity in basal conditions that decreased after surgery. XO catalyzes one of the flow-limiting steps in the purine base degradation pathway. In particular, the transformation of hypoxanthine into xanthine and then xanthine into uric acid is associated with major radical production, mostly by generating hydrogen peroxide.[
18,
19] This last step is catalyzed by the XO oxidoreductase, which may attain two inter-convertible forms, called xanthine dehydrogenase or XO. The latter uses molecular oxygen as electron acceptor and generates superoxide anion and other reactive oxygen products.[
19] In addition to uric acid, XO products comprise nitrogen species that have many biological effects, including inflammation, endothelial dysfunction, and cytotoxicity.[
20] To our knowledge, XO expression has not been associated with high malignity. However, the level of XO expression was found to be sometimes associated with poor outcome in cancer with low XO expressing cells, in relation to the inflammatory response elicited through the tissue damage.[
20] While XO activity is mostly present at the intracellular level, mostly in liver, intense XO activity is also found in plasma.[
15] Because solid cancer is accompanied by intense cells death, informative molecules as DNAs and RNAs joint purine degradation cycle and finally are irreversibly transformed into uric acid. In this context XO is the final effective enzyme of this catabolic pathway, and the high XO activity might lend weight to the use of XO inhibitors as adjuvant therapy in solid cancer.
We found high ADA levels in basal conditions that did not change during surgery. ADA catalyzes the deamination of adenosine (that mostly comes from ATP dephosphorylation) into inosine. ADA belongs to the purine salvage pathway and is well known to modulate inflammation through the regulation of extra cellular adenosine level and via the modulation of cytokines.[
21] Interestingly, we found that ADA and XO were inversely correlated suggesting that the production of free radicals by XO may in turn inhibit ADA activity. Everything happens as if the radical production induced by XO exerted negative feedback on ADA activity, to reduce the production of substrate (hypoxanthine and xanthine). The goal could be a retrocontrol of free radical release since it is established that ROS and nitrous oxide (NO) production secondary to XO activity may act as a second messenger in cells signaling.[
22,
23] However, this hypothesis needs further investigations.
We found high IMA levels in basal conditions that decreased after surgery. IMA results from the modification of the N-terminus cobalt-binding sites of albumin caused by the release of free radicals from hypoxic tissue.[
24] Thus, it is likely that the high serum IMA concentration that we reported were partly due to blood flowing through hypoxic tumor regions. High IMA have been reported during experimental hypoxia and in cancer, where it was associated with ROS production.[
17,
25,
26] High IMA have also been described during myocardial ischemia.[
27,
28] A perioperative myocardial ischemia, labeled MINS (myocardial injury after non-cardiac surgery) occurs in patients undergoing major surgery, and there is a growing interest in the literature since its presence is associated with poor long-term outcomes.[
29,
30,
31]
We found high redox potential in the plasma of patients that decreased after surgery. Redox potential disturbance promotes various pathological processes including hallmarks of cancer.[
32] Thus, redox development of cancer is strongly influenced by the redox conditions, while disruption of redox homeostasis is detrimental to cancer cells. Mitochondria are the major source of ROS due to their role in the respiratory chain during oxidative phosphorylation. Many enzymes are also implicated in ROS production, including nicotinamide adenine dinucleotide phosphate oxidase (NOX), but also cyclooxygenases (COX), while superoxide dismutase (SOD) belongs to the reduction system and have anti ROS properties. Here we evaluated the enzymes from the purines catabolism for the reasons explained above. ROS and redox potential are linked and ROS levels in cancer are significantly higher than in healthy cells while ROS are destructive to cancer cells.[
33] However, cancer cells, notable during metastatic processes, may escape this effect.[
23,
34] Therefore, malignant, or metastatic cells utilize reductive stress to promote their viability. NrF2 pathway is a common signaling cascade that regulates its multiple downstream cytoprotective genes leading to the resistance of oxidative stress and that is used by cancer cells.[
34] High serum concentration of ROS also enhances immunosuppressive effects and increases secretion of cytokines like TNF alpha in tumor microenvironment.[
35] Here we found that surgery decreased the redox potential, confirming that solid cancer by itself was a source of high potential redox likely via ROS production, ROS production being linked to redox potential and IMA.[
33,
36]
While our study offers valuable insights, inherent limitations are associated with its design. The single-center design restricts the generalizability of our findings to a broader population. Retrospective data collection introduces the risk of incomplete or potentially biased information, but since data were retrieved from our electronic health records system, this risk has been minimized. The absence of randomization or proper control group in our study design prevents us from establishing causation, emphasizing the need for cautious interpretation of associations identified. Despite these limitations, our study provides a fundamental understanding of the effects of surgical oncology on oxidative and prompts the necessity for future studies in this setting.
5. Conclusions
A major source of oxidative stress in solid cancer could be xanthine oxidase, which is known to produce large quantities of free radicals, particularly ROS. Even though major surgery increases OS levels, the global effect of surgical removal of such tumor is a significant decrease in OS. We also found that ADA and XO were inversely correlate, suggesting the existence of a negative retrocontrol mechanism of free radical release, between the production of free radicals and the production of their substrate. Finally, our study highlights the potential value of XO inhibitors as adjuvant therapy in solid tumor surgery.
Supplementary Materials
no supplementary materials
Author Contributions
Conceptualization, Bruno Pastene and Régis Guieu; Data curation, Marion Marlinge; Formal analysis, Bruno Pastene and Régis Guieu; Investigation, Bruno Pastene, Marion Marlinge and Amin Ben Lassoued; Methodology, Bruno Pastene and Régis Guieu; Supervision, Marc Leone, Laurent Zieleskiewicz and Régis Guieu; Validation, Bruno Pastene and Régis Guieu; Writing – original draft, Bruno Pastene and Régis Guieu; Writing – review & editing, Julien Fromonot, Julia Dodivers, Nathalie Lalevée, Pascal-Alexandre Thomas, David Birnbaum, Marc Leone and Laurent Zieleskiewicz.
Funding
This research was supported by our employer, Hôpitaux Universitaires de Marseille (AP-HM) and Aix Marseille University (AMU)
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by an institutional review board (Comité de Protection des Personnes SUD-EST VI, reference 2018-A02511-54) on December 9, 2019.
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author
Acknowledgments
Sharma Tania for English corrections.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative Stress and Its Role in Cancer. J Cancer Res Ther 2021, 17, 22–28. [Google Scholar] [CrossRef]
- Schwarz, C.; Fitschek, F.; Bar-Or, D.; Klaus, D.A.; Tudor, B.; Fleischmann, E.; Roth, G.; Tamandl, D.; Wekerle, T.; Gnant, M.; et al. Inflammatory Response and Oxidative Stress during Liver Resection. PLoS One 2017, 12, e0185685. [Google Scholar] [CrossRef]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid Med Cell Longev 2019, 2019, 7092151. [Google Scholar] [CrossRef]
- Aboelella, N.S.; Brandle, C.; Kim, T.; Ding, Z.-C.; Zhou, G. Oxidative Stress in the Tumor Microenvironment and Its Relevance to Cancer Immunotherapy. Cancers (Basel) 2021, 13, 986. [Google Scholar] [CrossRef]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial Dysfunction and Oxidative Stress in Aging and Cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef] [PubMed]
- Toro-Pérez, J.; Rodrigo, R. Contribution of Oxidative Stress in the Mechanisms of Postoperative Complications and Multiple Organ Dysfunction Syndrome. Redox Rep 2021, 26, 35–44. [Google Scholar] [CrossRef]
- O’Leary, D.P.; Wang, J.H.; Cotter, T.G.; Redmond, H.P. Less Stress, More Success? Oncological Implications of Surgery-Induced Oxidative Stress. Gut 2013, 62, 461–470. [Google Scholar] [CrossRef]
- Jiang, H.; Zuo, J.; Li, B.; Chen, R.; Luo, K.; Xiang, X.; Lu, S.; Huang, C.; Liu, L.; Tang, J.; et al. Drug-Induced Oxidative Stress in Cancer Treatments: Angel or Devil? Redox Biol 2023, 63, 102754. [Google Scholar] [CrossRef]
- Liu, R.; Peng, L.; Zhou, L.; Huang, Z.; Zhou, C.; Huang, C. Oxidative Stress in Cancer Immunotherapy: Molecular Mechanisms and Potential Applications. Antioxidants (Basel) 2022, 11, 853. [Google Scholar] [CrossRef]
- Shoemaker, W.C.; Appel, P.L.; Kram, H.B.; Waxman, K.; Lee, T.S. Prospective Trial of Supranormal Values of Survivors as Therapeutic Goals in High-Risk Surgical Patients. Chest 1988, 94, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Carrozzino, D.; Guidi, J.; Patierno, C. Charlson Comorbidity Index: A Critical Review of Clinimetric Properties. Psychother Psychosom 2022, 91, 8–35. [Google Scholar] [CrossRef] [PubMed]
- Slankamenac, K.; Graf, R.; Barkun, J.; Puhan, M.A.; Clavien, P.-A. The Comprehensive Complication Index: A Novel Continuous Scale to Measure Surgical Morbidity. Ann. Surg. 2013, 258, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bruzzese, L.; Rostain, J.-C.; Née, L.; Condo, J.; Mottola, G.; Adjriou, N.; Mercier, L.; Berge-Lefranc, J.-L.; Fromonot, J.; Kipson, N.; et al. Effect of Hyperoxic and Hyperbaric Conditions on the Adenosinergic Pathway and CD26 Expression in Rat. J Appl Physiol (1985) 2015, 119, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Guieu, R.; Brunet, P.; Sampol, J.; Bechis, G.; Fenouillet, E.; Mege, J.L.; Capo, C.; Vitte, J.; Ibrahim, Z.; Carrega, L.; et al. Adenosine and Hemodialysis in Humans. J Investig Med 2001, 49, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Gondouin, B.; Jourde-Chiche, N.; Sallee, M.; Dou, L.; Cerini, C.; Loundou, A.; Morange, S.; Berland, Y.; Burtey, S.; Brunet, P.; et al. Plasma Xanthine Oxidase Activity Is Predictive of Cardiovascular Disease in Patients with Chronic Kidney Disease, Independently of Uric Acid Levels. Nephron 2015, 131, 167–174. [Google Scholar] [CrossRef]
- Carrega, L.; Giaime, P.; Montserrat, C.; Vincente, O.; Brunet, P.; Dussol, B.; Berland, Y.; Guieu, R. Influence of the Dialysis Membrane on Markers of Tissue Ischemia. J Investig Med 2006, 54, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Joulia, F.; Coulange, M.; Lemaitre, F.; Desplantes, A.; Costalat, G.; Bruzzese, L.; Franceschi, F.; Barberon, B.; Kipson, N.; Jammes, Y.; et al. Ischaemia-Modified Albumin during Experimental Apnoea. Can J Physiol Pharmacol 2015, 93, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, E.; Leech, A. The Oxidation of Acetyl CoA. In Biochemistry for the Medical Sciences; Wiley, 1992; ISBN 0-471-90058-3. [Google Scholar]
- Glantzounis, G.K.; Tsimoyiannis, E.C.; Kappas, A.M.; Galaris, D.A. Uric Acid and Oxidative Stress. Curr Pharm Des 2005, 11, 4145–4151. [Google Scholar] [CrossRef] [PubMed]
- Battelli, M.G.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine Oxidoreductase in Cancer: More than a Differentiation Marker. Cancer Med 2016, 5, 546–557. [Google Scholar] [CrossRef]
- Antonioli, L.; Colucci, R.; La Motta, C.; Tuccori, M.; Awwad, O.; Da Settimo, F.; Blandizzi, C.; Fornai, M. Adenosine Deaminase in the Modulation of Immune System and Its Potential as a Novel Target for Treatment of Inflammatory Disorders. Curr Drug Targets 2012, 13, 842–862. [Google Scholar] [CrossRef]
- Battelli, M.G.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine Oxidoreductase-Derived Reactive Species: Physiological and Pathological Effects. Oxid Med Cell Longev 2016, 2016, 3527579. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Hu, Q.; Qin, Y.; Xu, J.; Zhang, B.; Yu, X.; Wang, W. The Relationship of Redox With Hallmarks of Cancer: The Importance of Homeostasis and Context. Front Oncol 2022, 12, 862743. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, D.; Rael, L.T.; Bar-Or, R.; Slone, D.S.; Mains, C.W.; Rao, N.K.R.; Curtis, C.G. The Cobalt-Albumin Binding Assay: Insights into Its Mode of Action. Clin Chim Acta 2008, 387, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Fidan, E.; Mentese, A.; Kavgaci, H.; Orem, A.; Fidan, S.; Uzun, A.; Ozdemir, F.; Aydin, F. Increased Ischemia-Modified Albumin Levels in Patients with Gastric Cancer. Neoplasma 2012, 59, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Ellidag, H.Y.; Eren, E.; Aydin, O.; Akgol, E.; Yalcinkaya, S.; Sezer, C.; Yilmaz, N. Ischemia Modified Albumin Levels and Oxidative Stress in Patients with Bladder Cancer. Asian Pac J Cancer Prev 2013, 14, 2759–2763. [Google Scholar] [CrossRef] [PubMed]
- Sbarouni, E.; Georgiadou, P.; Theodorakis, G.N.; Kremastinos, D.T. Ischemia-Modified Albumin in Relation to Exercise Stress Testing. J Am Coll Cardiol 2006, 48, 2482–2484. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.K.; Roy, D.; Gaze, D.C.; Collinson, P.O.; Kaski, J.C. Role of “Ischemia Modified Albumin”, a New Biochemical Marker of Myocardial Ischaemia, in the Early Diagnosis of Acute Coronary Syndromes. Emerg Med J 2004, 21, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Botto, F.; Alonso-Coello, P.; Chan, M.T.V.; Villar, J.C.; Xavier, D.; Srinathan, S.; Guyatt, G.; Cruz, P.; Graham, M.; Wang, C.Y.; et al. Myocardial Injury after Noncardiac Surgery: A Large, International, Prospective Cohort Study Establishing Diagnostic Criteria, Characteristics, Predictors, and 30-Day Outcomes. Anesthesiology 2014, 120, 564–578. [Google Scholar] [CrossRef]
- Devereaux, P.J.; Sessler, D.I. Cardiac Complications in Patients Undergoing Major Noncardiac Surgery. N Engl J Med 2015, 373, 2258–2269. [Google Scholar] [CrossRef]
- Bello, C.; Rössler, J.; Shehata, P.; Smilowitz, N.R.; Ruetzler, K. Perioperative Strategies to Reduce Risk of Myocardial Injury after Non-Cardiac Surgery (MINS): A Narrative Review. J Clin Anesth 2023, 87, 111106. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Sallam, R.M. Reactive Oxygen Species in Health and Disease. J Biomed Biotechnol 2012, 2012, 936486. [Google Scholar] [CrossRef] [PubMed]
- Adler, V.; Yin, Z.; Tew, K.D.; Ronai, Z. Role of Redox Potential and Reactive Oxygen Species in Stress Signaling. Oncogene 1999, 18, 6104–6111. [Google Scholar] [CrossRef]
- Tasdogan, A.; Ubellacker, J.M.; Morrison, S.J. Redox Regulation in Cancer Cells during Metastasis. Cancer Discov 2021, 11, 2682–2692. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zheng, W.; Liu, J.; Zhang, Y.; Qin, H.; Wu, H.; Xue, B.; Lu, Y.; Shen, P. Oxidative Stress in Malignant Melanoma Enhances Tumor Necrosis Factor-α Secretion of Tumor-Associated Macrophages That Promote Cancer Cell Invasion. Antioxid Redox Signal 2013, 19, 1337–1355. [Google Scholar] [CrossRef] [PubMed]
- Jena, I.; Nayak, S.R.; Behera, S.; Singh, B.; Ray, S.; Jena, D.; Singh, S.; Sahoo, S.K. Evaluation of Ischemia-Modified Albumin, Oxidative Stress, and Antioxidant Status in Acute Ischemic Stroke Patients. J Nat Sci Biol Med 2017, 8, 110–113. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).