Submitted:
15 July 2024
Posted:
16 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
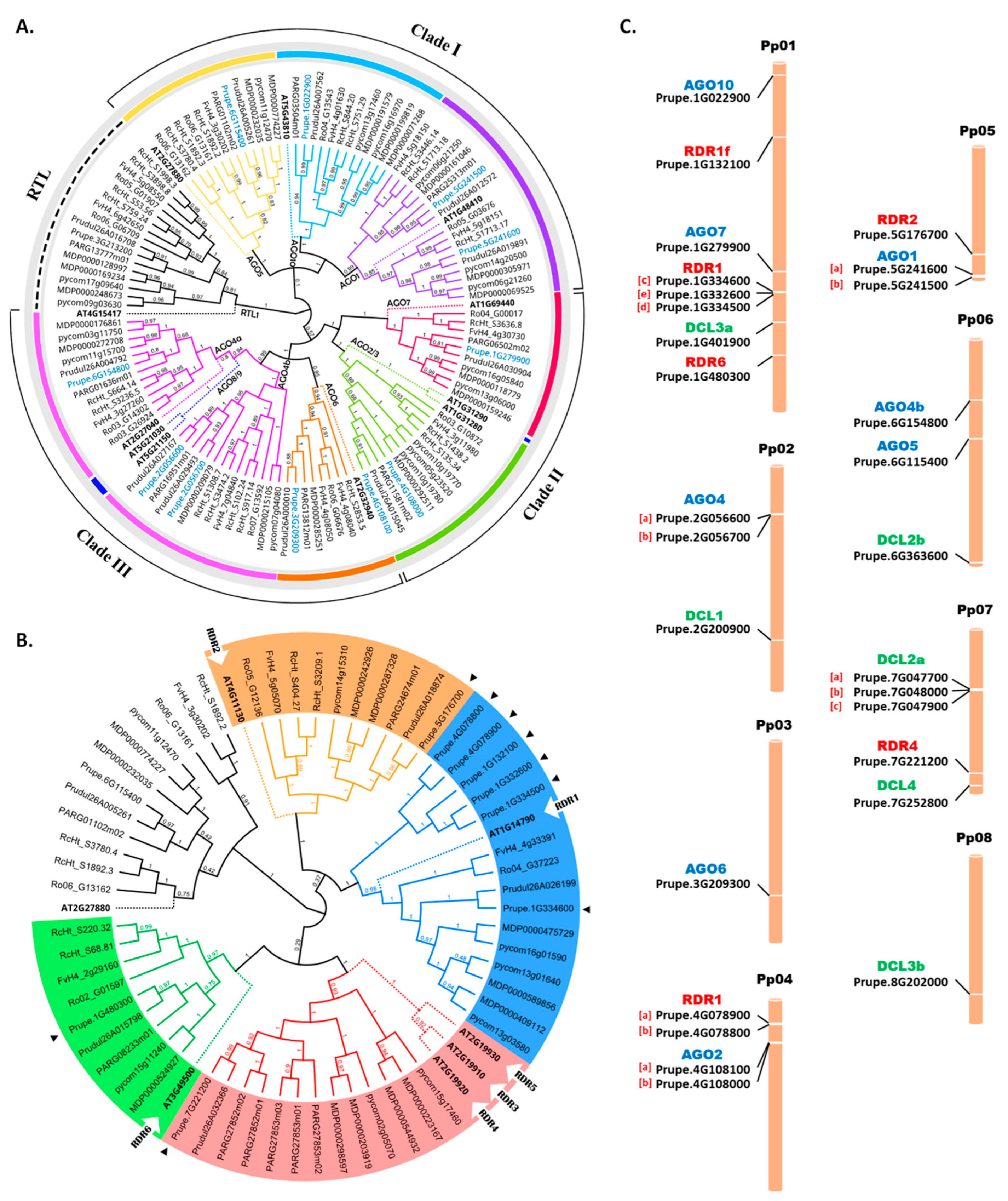
2.1. Identification and Characterization of RNAi-Related Genes in Rosaceae
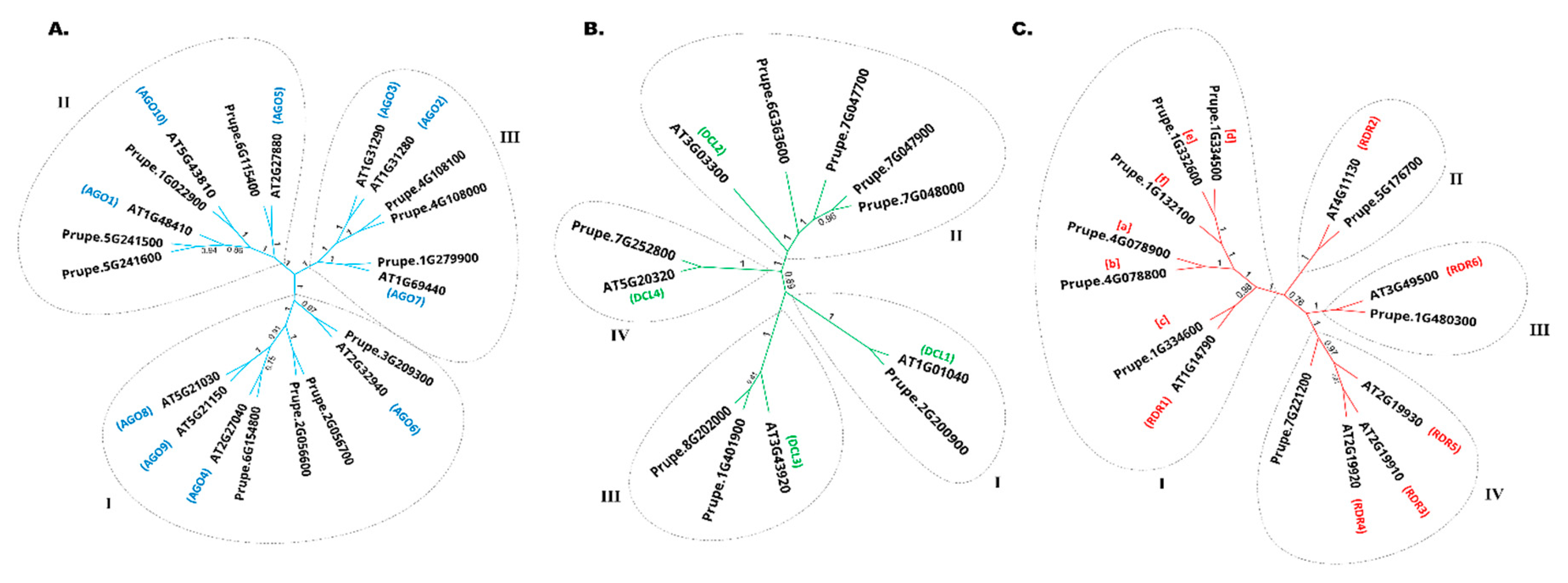
2.2. Chromosomal Localization, and Evolutionary Analysis in P. persica
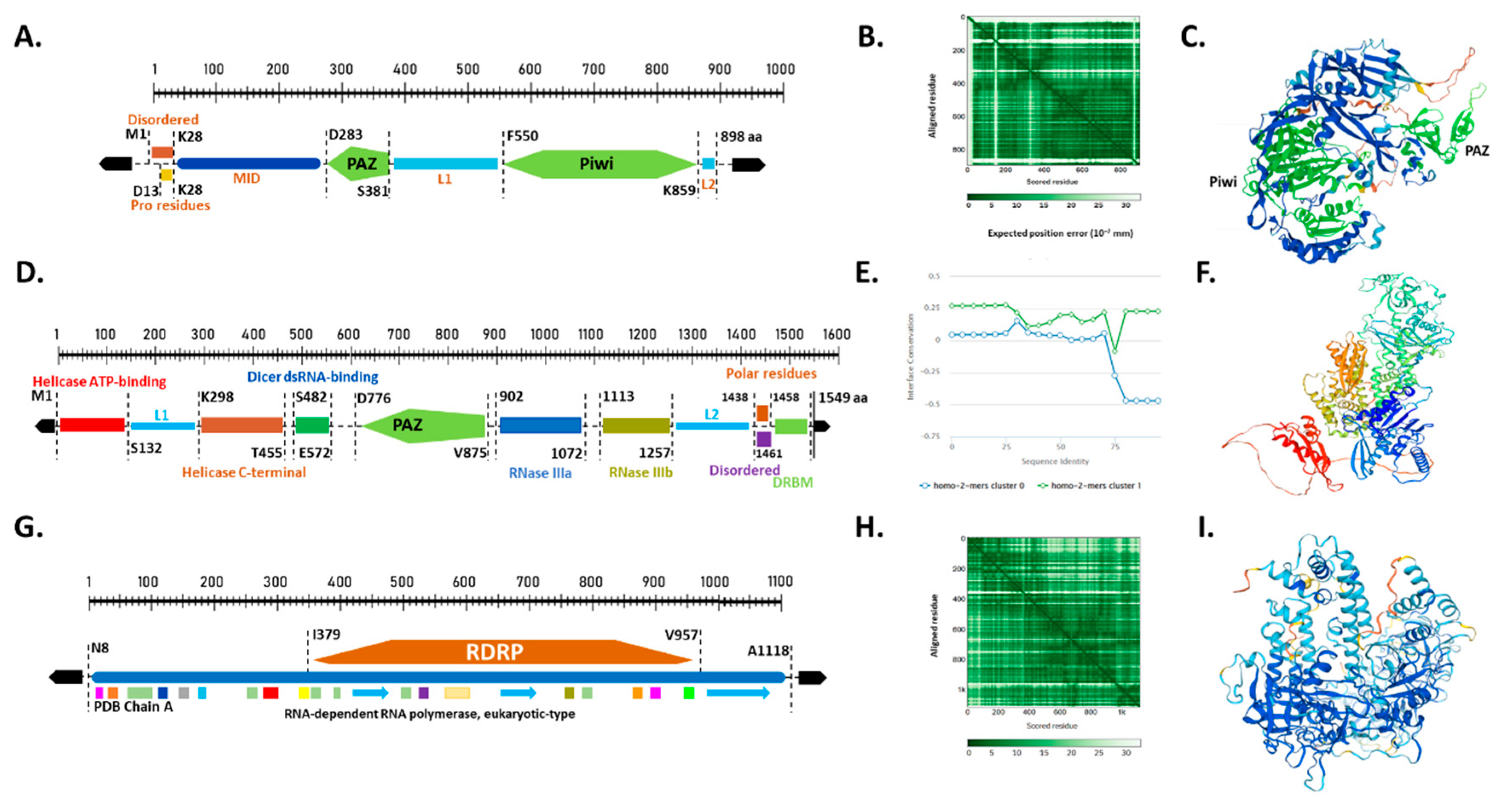
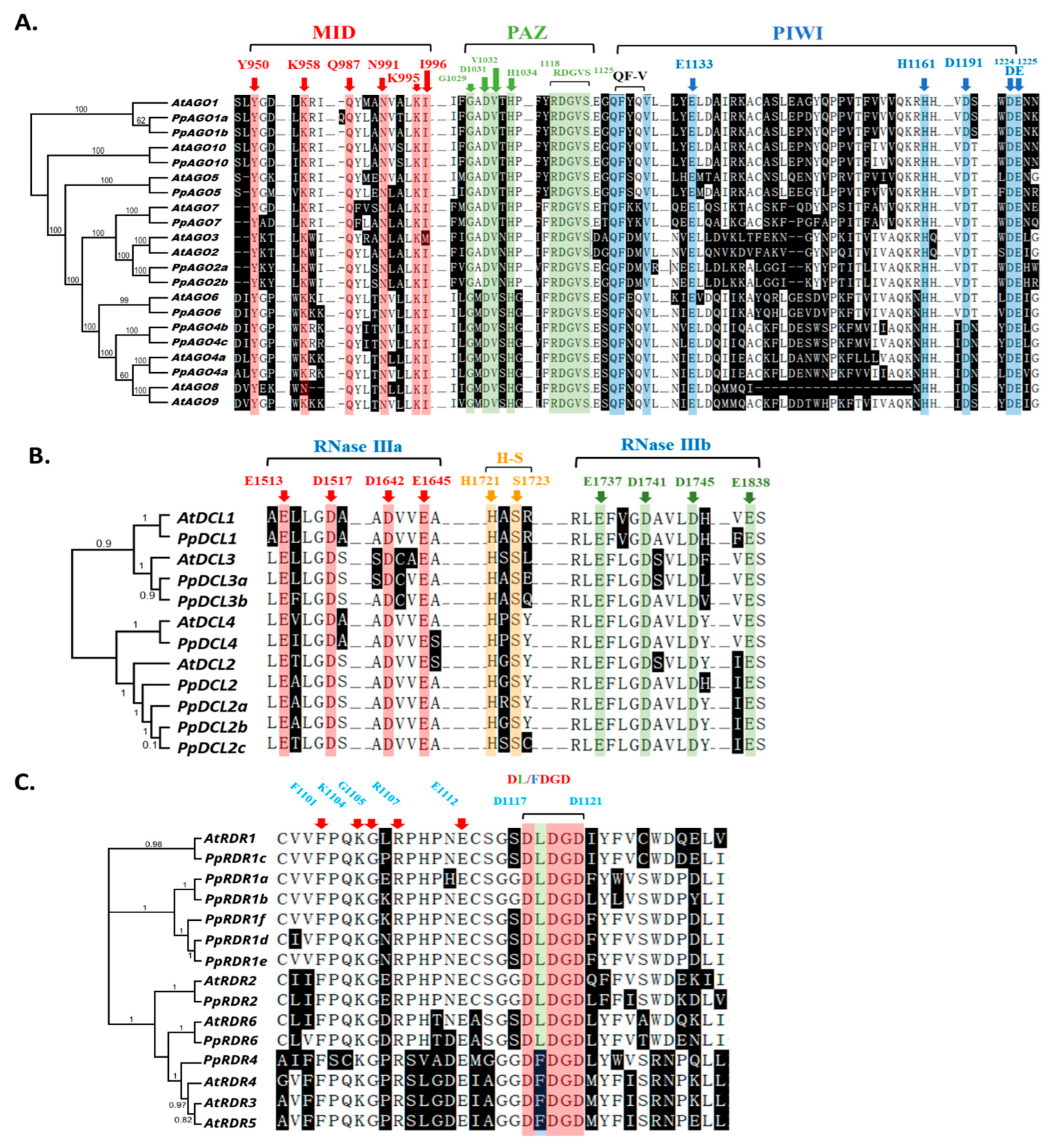
2.3. Gene structure and Motif Analysis in P. persica
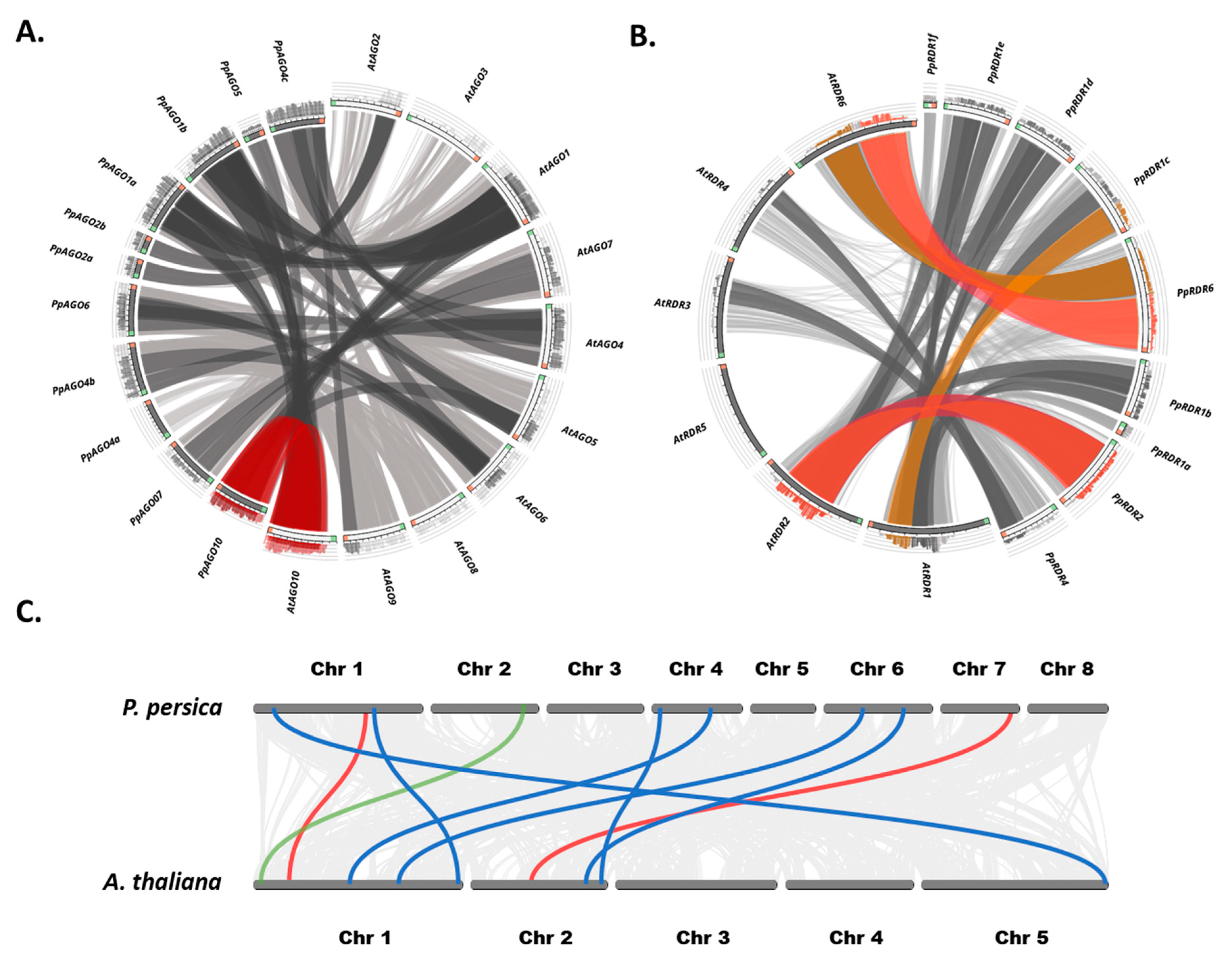
2.4. Orthologous Similarity and Collinearity Analysis for Non-Coding RNA Genes
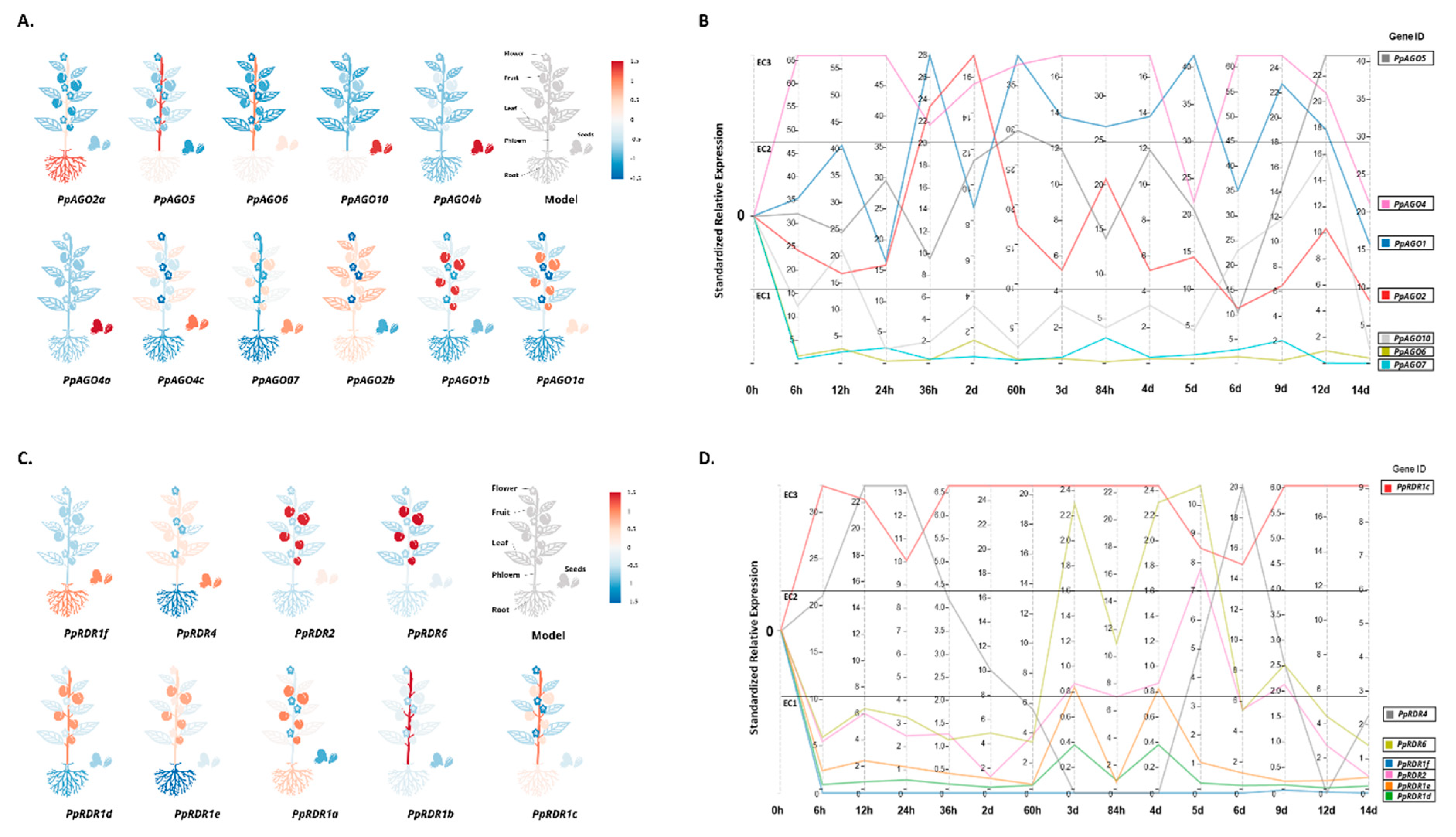
2.6. Expression Patterns of AGO, DCL and RDR Genes in Peach under Drought Stress
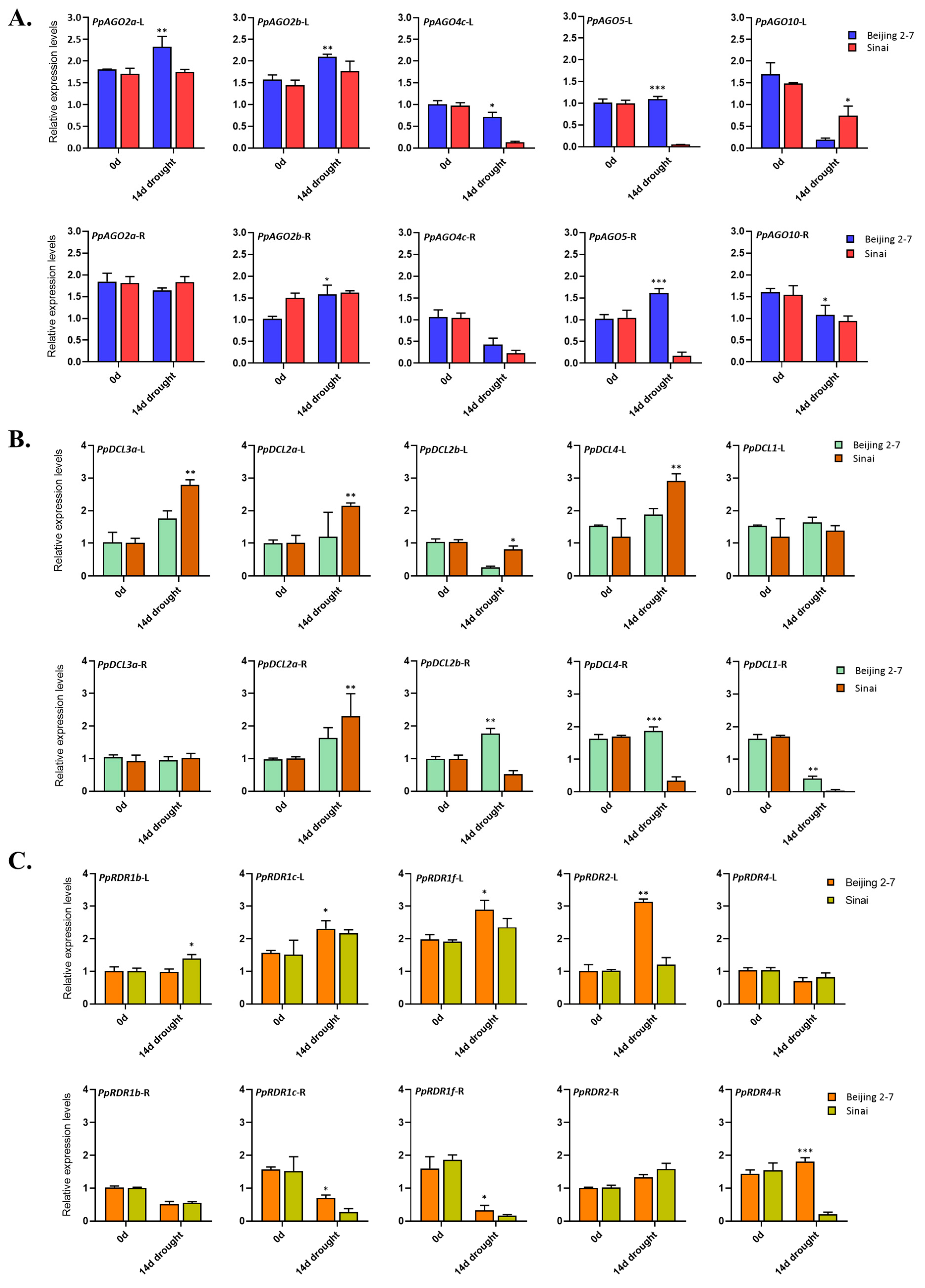
2.7. Quantitative Real-Time PCR (qRT-PCR) Validation of AGO, DCL, and RDR Genes
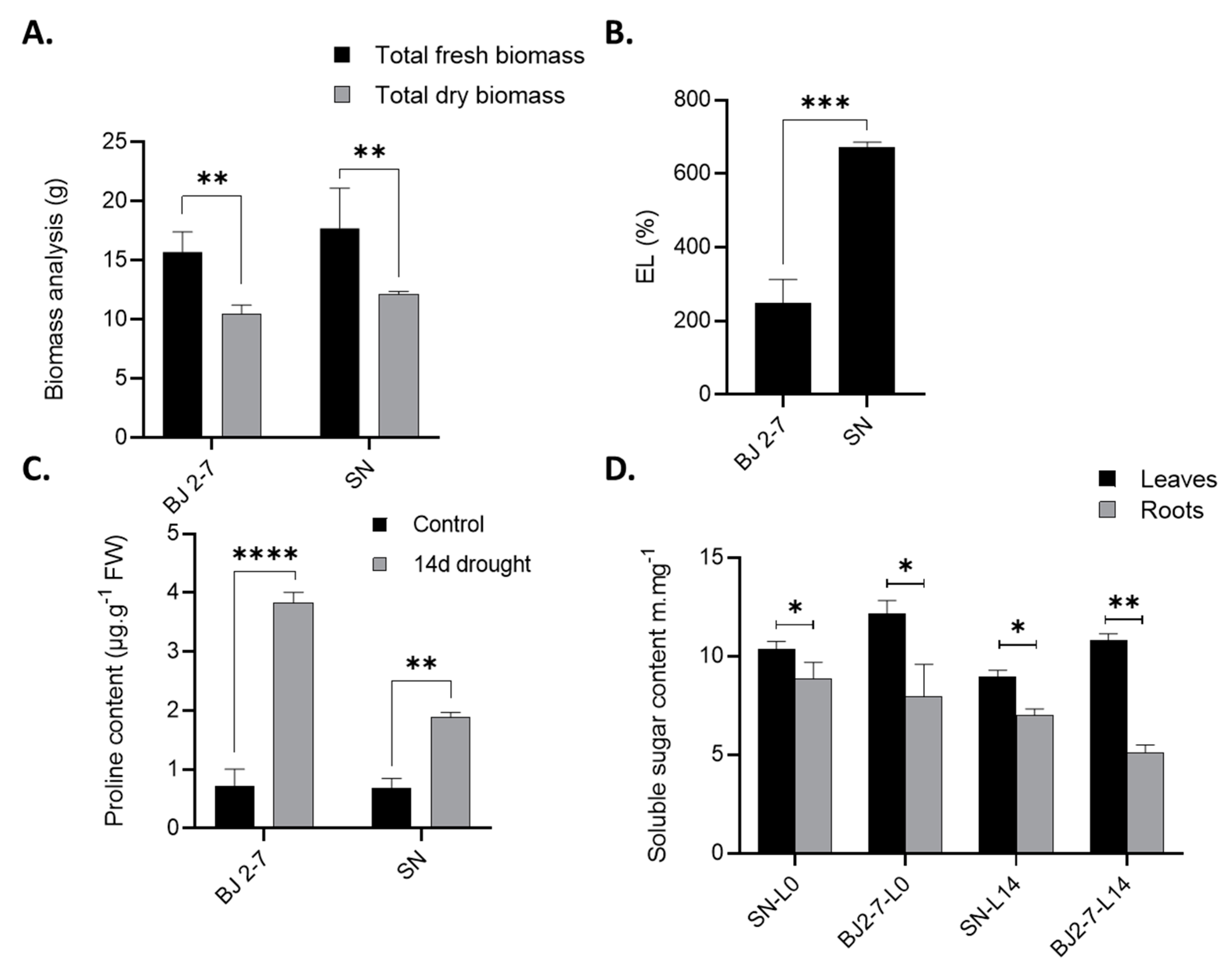
2.8. Analysis of Plant Biomass, Electrolyte Leakage (EL), Proline Content, and Total Soluble Sugars Content in Peach Cultivars under Drought Stress
3. Discussion
4. Materials and Methods
4.1. Identification and Characterization of AGO, DCL and RDR Genes in Rosaceae
4.2. Multiple Sequence Alignments and Phylogenetic Analysis of AGO, DCL and RDR Genes in Peach
4.3. Chromosomal Localization, Gene Structure, and Motif Analysis in Peach
4.4. Orthologous Similarity, Collinearity, and Cis-regulatory Elements Analysis for RNAi-Related Genes in Peach
4.5. Expression Analysis and qRT-PCR Validation
4.6. Plants Physiological and Biochemical Evaluations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verde, I.; Abbott, A.G.; Scalabrin, S.; Jung, S.; Shu, S.; Marroni, F.; Zhebentyayeva, T.; Dettori, M.T.; Grimwood, J.; Cattonaro, F.; et al. The high-quality draft genome of peach (Prunus persica) identifies unique patterns of genetic diversity, domestication and genome evolution. Nat Genet 2013, 45, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Zheng, Z.; Wang, L.; Liu, X.; Zhu, G.; Fang, W.; Cheng, S.; Zeng, P.; Chen, C.; Wang, X. Comparative population genomics reveals the domestication history of the peach, Prunus persica, and human influences on perennial fruit crops. Genome Biol. 2014, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Byrne, D.H.; Raseira, M.B.; Bassi, D.; Piagnani, M.C.; Gasic, K.; Reighard, G.L.; Moreno, M.A.; Pérez, S. Peach. Fruit Breed. 2012, 505–569. [Google Scholar]
- Arús, P.; Verde, I.; Sosinski, B.; Zhebentyayeva, T.; Abbott, A.G. The peach genome. Tree Genet. Genomes 2012, 8, 531–547. [Google Scholar] [CrossRef]
- Rahmati, M.; Vercambre, G.; Davarynejad, G.; Bannayan, M.; Azizi, M.; Genard, M. Water scarcity conditions affect peach fruit size and polyphenol contents more severely than other fruit quality traits. J Sci Food Agric 2014, 95, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.S.; Kurjogi, M.M.; Khalil-ur-Rehman, M.; Pervez, T.; Songtao, J.; Fiaz, M.; Jogaiah, S.; Wang, C.; Fang, J. Drought stress revealed physiological, biochemical and gene-expressional variations in ‘Yoshihime’ peach (Prunus Persica L) cultivar. J. Plant Interact. 2018, 13, 83–90. [Google Scholar] [CrossRef]
- Belal, M.A.; El-Alakmy, H.A.; Abdelhameed, A.A.; Sourour, M.M.J.S.J.o.A.S. Influence of reducing irrigation rate and addition of super absorbent polymer on peach trees growth under north sinai conditions. 2017, 6, 249–258.
- Usman, M.; Bokhari, S.A.M.; Fatima, B.; Rashid, B.; Nadeem, F.; Sarwar, M.B.; Nawaz-ul-Rehman, M.S.; Shahid, M.; Ayub, C.M. Drought stress mitigating morphological, physiological, biochemical, and molecular responses of guava (psidium guajava L.) cultivars. Front. Plant Sci. 2022, 13, 878616. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ni, L.; Hua, J.; Liu, L.; Yin, Y.; Li, H.; Gu, C. Transcriptome analysis reveals regulatory framework for salt and drought tolerance in Hibiscus hamabo siebold & zuccarini. Forests 2021, 12, 454. [Google Scholar] [CrossRef]
- Talebizadeh, Z. Regulation of Gene Expression by Small RNAs. Am. J. Hum. Genet. 2010, 86, 328–330. [Google Scholar] [CrossRef]
- Hutvágner, G.; Zamore, P.D. RNAi: nature abhors a double-strand. Curr. Opin. Genet. Dev. 2002, 12, 225–232. [Google Scholar] [CrossRef]
- Fang, X.; Qi, Y. RNAi in plants: an argonaute-centered view. Plant Cell 2016, 28, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Khare, T.; Shriram, V.; Kumar, V. RNAi technology: the role in development of abiotic stress-tolerant crops. In Biochemical, physiological and molecular avenues for combating abiotic stress tolerance in plants; Elsevier: 2018; pp. 117–133.
- Bai, M.; Yang, G.S.; Chen, W.T.; Mao, Z.C.; Kang, H.X.; Chen, G.H.; Yang, Y.H.; Xie, B.Y. Genome-wide identification of Dicer-like, Argonaute and RNA-dependent RNA polymerase gene families and their expression analyses in response to viral infection and abiotic stresses in Solanum lycopersicum. Gene 2012, 501, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.; Martienssen, R.A. The expanding world of small RNAs in plants. Nat. Rev. Mol. Cell Biol. 2015, 16, 727–741. [Google Scholar] [CrossRef]
- Noronha Fernandes-Brum, C.; Marinho Rezende, P.; Cherubino Ribeiro, T.H.; Ricon de Oliveira, R.; Cunha de Sousa Cardoso, T.; Rodrigues do Amaral, L.; de Souza Gomes, M.; Chalfun-Junior, A. A genome-wide analysis of the RNA-guided silencing pathway in coffee reveals insights into its regulatory mechanisms. PLoS One 2017, 12, e0176333. [Google Scholar] [CrossRef]
- Ruiz-Ferrer, V.; Voinnet, O. Roles of plant small RNAs in biotic stress responses. Annu Rev Plant Biol 2009, 60, 485–510. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Swetha, C.; Pachamuthu, K.; Nair, A.; Shivaprasad, P.V. Loss of function of Oryza sativa Argonaute 18 induces male sterility and reduction in phased small RNAs. Plant Reprod 2020, 33, 59–73. [Google Scholar] [CrossRef]
- Li, Z.; Li, W.; Guo, M.; Liu, S.; Liu, L.; Yu, Y.; Mo, B.; Chen, X.; Gao, L. Origin, evolution and diversification of plant ARGONAUTE proteins. Plant J 2022, 109, 1086–1097. [Google Scholar] [CrossRef]
- Gelaw, T.A.; Sanan-Mishra, N. Non-coding RNAs in response to drought stress. Int. J. Mol. Sci. 2021, 22, 12519. [Google Scholar] [CrossRef]
- Budak, H.; Zhang, B. MicroRNAs in model and complex organisms. 2017, 17, 121–124.
- Tiwari, R.; Rajam, M.V. RNA-and miRNA-interference to enhance abiotic stress tolerance in plants. J. Plant Biochem. Biotechnol. 2022, 31, 689–704. [Google Scholar] [CrossRef]
- Saplaoura, E.; Kragler, F. Mobile Transcripts and Intercellular Communication in Plants. Enzymes 2016, 40, 1–29. [Google Scholar] [CrossRef]
- Kehr, J.; Kragler, F. Long distance RNA movement. New Phytol 2018, 218, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, M.; Nabih, A.; Spies, D.; Hermes, V.; Bodak, M.; Wischnewski, H.; Stalder, P.; Ngondo, R.P.; Liechti, L.A.; Sajic, T.; et al. Global and precise identification of functional miRNA targets in mESCs by integrative analysis. EMBO Rep 2022, 23, e54762. [Google Scholar] [CrossRef]
- Sokolowska, A.; Rugala, M.; Oracz, K. [ARGONAUTE proteins in cell biology and plant development]. Postep. Biochem 2022, 68, 310–320. [Google Scholar] [CrossRef]
- Vaucheret, H. Post-transcriptional small RNA pathways in plants: mechanisms and regulations. Genes Dev. 2006, 20, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Chapman, E.J.; Carrington, J.C. Specialization and evolution of endogenous small RNA pathways. Nat. Rev. Genet. 2007, 8, 884–896. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Arora, R.; Lama, T.; Nijhawan, A.; Khurana, J.P.; Tyagi, A.K.; Kapoor, S. Genome-wide identification, organization and phylogenetic analysis of Dicer-like, Argonaute and RNA-dependent RNA Polymerase gene families and their expression analysis during reproductive development and stress in rice. BMC Genom. 2008, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Ye, X.; Morris, T.J. Arabidopsis DRB4, AGO1, AGO7, and RDR6 participate in a DCL4-initiated antiviral RNA silencing pathway negatively regulated by DCL1. Proc Natl Acad Sci U S A 2008, 105, 14732–14737. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, X.; Jiang, L. Integrative analysis of the RNA interference toolbox in two Salicaceae willow species, and their roles in stress response in poplar (Populus trichocarpa Torr. & Gray). Int J Biol Macromol 2020, 162, 1127–1139. [Google Scholar] [CrossRef]
- Balassa, G.; Balassa, K.; Janda, T.; Rudnóy, S. Expression Pattern of RNA Interference Genes During Drought Stressand MDMV Infection in Maize. J. Plant Growth Regul. 2022, 41, 2048–2058. [Google Scholar] [CrossRef]
- Belal, M.A.; Ezzat, M.; Zhang, Y.; Xu, Z.; Cao, Y.; Han, Y. Integrative Analysis of the DICER-like (DCL) Genes From Peach (Prunus persica): A Critical Role in Response to Drought Stress. Front. Ecol. Evol. 2022, 10, 923166. [Google Scholar] [CrossRef]
- Ahmed, F.F.; Hossen, M.I.; Sarkar, M.A.R.; Konak, J.N.; Zohra, F.T.; Shoyeb, M.; Mondal, S. Genome-wide identification of DCL, AGO and RDR gene families and their associated functional regulatory elements analyses in banana (Musa acuminata). PLoS One 2021, 16, e0256873. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Jia, C.; Miao, H.; Zhang, J.; Liu, J.; Xu, B.; Jin, Z. Genome-wide identification and transcript analysis of TCP gene family in Banana (Musa acuminata L.). Biochem. Genet. 2022, 60, 204–222. [Google Scholar] [CrossRef]
- Parker, J.S.; Barford, D. Argonaute: a scaffold for the function of short regulatory RNAs. Trends Biochem. Sci. 2006, 31, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Vaucheret, H. Plant argonautes. Trends Plant Sci. 2008, 13, 350–358. [Google Scholar] [CrossRef]
- Hamar, E.; Szaker, H.M.; Kis, A.; Dalmadi, A.; Miloro, F.; Szittya, G.; Taller, J.; Gyula, P.; Csorba, T.; Havelda, Z. Genome-Wide Identification of RNA Silencing-Related Genes and Their Expressional Analysis in Response to Heat Stress in Barley (Hordeum vulgare L.). Biomolecules 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Hutvagner, G.; Simard, M.J. Argonaute proteins: key players in RNA silencing. Nat. Rev. Mol. Cell Biol. 2008, 9, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Kwak, P.B.; Tomari, Y. The N domain of Argonaute drives duplex unwinding during RISC assembly. Nat. Struct. Mol. Biol. 2012, 19, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Arribas-Hernández, L.; Marchais, A.; Poulsen, C.; Haase, B.; Hauptmann, J.; Benes, V.; Meister, G.; Brodersen, P. The slicer activity of ARGONAUTE1 is required specifically for the phasing, not production, of trans-acting short interfering RNAs in Arabidopsis. Plant Cell 2016, 28, 1563–1580. [Google Scholar] [CrossRef]
- Cerutti, L.; Mian, N.; Bateman, A. Domains in gene silencing and cell differentiation proteins: the novel PAZ domain and redefinition of the Piwi domain. Trends Biochem. Sci. 2000, 25, 481–482. [Google Scholar] [CrossRef]
- Jinek, M.; Doudna, J.A. A three-dimensional view of the molecular machinery of RNA interference. nature 2009, 457, 405–412. [Google Scholar] [CrossRef]
- Moazed, D. Small RNAs in transcriptional gene silencing and genome defence. Nature 2009, 457, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Simon, B.; Kirkpatrick, J.P.; Eckhardt, S.; Reuter, M.; Rocha, E.A.; Andrade-Navarro, M.A.; Sehr, P.; Pillai, R.S.; Carlomagno, T. Recognition of 2′-O-methylated 3′-end of piRNA by the PAZ domain of a Piwi protein. Structure 2011, 19, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Rivas, F.V.; Tolia, N.H.; Song, J.-J.; Aragon, J.P.; Liu, J.; Hannon, G.J.; Joshua-Tor, L. Purified Argonaute2 and an siRNA form recombinant human RISC. Nat. Struct. Mol. Biol. 2005, 12, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Höck, J.; Meister, G. The Argonaute protein family. Genome Biol. 2008, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Cai, T.; Hu, Y.; Chen, Y.; Hodges, E.; Ni, F.; Wu, L.; Li, S.; Zhou, H.; Long, C. Sorting of small RNAs into Arabidopsis argonaute complexes is directed by the 5′ terminal nucleotide. Cell 2008, 133, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Frank, F.; Hauver, J.; Sonenberg, N.; Nagar, B. Arabidopsis Argonaute MID domains use their nucleotide specificity loop to sort small RNAs. EMBO J. 2012, 31, 3588–3595. [Google Scholar] [CrossRef] [PubMed]
- Thieme, C.J.; Schudoma, C.; May, P.; Walther, D. Give it AGO: The search for miRNA-Argonaute sorting signals in Arabidopsis thaliana indicates a relevance of sequence positions other than the 5′-position alone. Front. Plant Sci. 2012, 3, 272. [Google Scholar] [CrossRef]
- Niaz, S. The AGO proteins: an overview. Biol. Chem. 2018, 399, 525–547. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, E.; Caudy, A.A.; Hammond, S.M.; Hannon, G.J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 2001, 409, 363–366. [Google Scholar] [CrossRef]
- Zhang, H.; Kolb, F.A.; Jaskiewicz, L.; Westhof, E.; Filipowicz, W. Single processing center models for human Dicer and bacterial RNase III. Cell 2004, 118, 57–68. [Google Scholar] [CrossRef]
- Wei, X.; Ke, H.; Wen, A.; Gao, B.; Shi, J.; Feng, Y. Structural basis of microRNA processing by Dicer-like 1. Nat. Plants 2021, 7, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Baumberger, N.; Baulcombe, D. Arabidopsis ARGONAUTE1 is an RNA Slicer that selectively recruits microRNAs and short interfering RNAs. Proc. Natl. Acad. Sci. 2005, 102, 11928–11933. [Google Scholar] [CrossRef] [PubMed]
- Vaucheret, H.; Vazquez, F.; Crété, P.; Bartel, D.P. The action of ARGONAUTE1 in the miRNA pathway and its regulation by the miRNA pathway are crucial for plant development. Genes Dev. 2004, 18, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.; Liu, Z.-W.; Wu, Z.-J.; Li, H.; Wang, W.-L.; Cui, X.; Zhuang, J. Genome-wide identification and expression analysis of GRAS family transcription factors in tea plant (Camellia sinensis). Sci. Rep. 2018, 8, 3949. [Google Scholar] [CrossRef]
- Banerjee, J.; Sahoo, D.K.; Dey, N.; Houtz, R.L.; Maiti, I.B. An intergenic region shared by At4g35985 and At4g35987 in Arabidopsis thaliana is a tissue specific and stress inducible bidirectional promoter analyzed in transgenic Arabidopsis and tobacco plants. PLoS One 2013, 8, e79622. [Google Scholar] [CrossRef]
- Younger, P. Stedman's Medical Dictionary. Ref. Rev. 2007, 21, 41–42. [Google Scholar]
- Mainz, D.; Quadt, I.; Stranzenbach, A.K.; Voss, D.; Guarino, L.A.; Knebel-Mörsdorf, D. Expression and nuclear localization of the TATA-box-binding protein during baculovirus infection. J. Gen. Virol. 2014, 95, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Howell, M.D.; Fahlgren, N.; Chapman, E.J.; Cumbie, J.S.; Sullivan, C.M.; Givan, S.A.; Kasschau, K.D.; Carrington, J.C. Genome-wide analysis of the RNA-DEPENDENT RNA POLYMERASE6/DICER-LIKE4 pathway in Arabidopsis reveals dependency on miRNA- and tasiRNA-directed targeting. Plant Cell 2007, 19, 926–942. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, H.; Takeda, A.; Chapman, E.J.; Sullivan, C.M.; Fahlgren, N.; Brempelis, K.J.; Carrington, J.C. Arabidopsis RNA-dependent RNA polymerases and dicer-like proteins in antiviral defense and small interfering RNA biogenesis during Turnip Mosaic Virus infection. Plant Cell 2010, 22, 481–496. [Google Scholar] [CrossRef]
- Martínez de Alba, A.E.; Moreno, A.B.; Gabriel, M.; Mallory, A.C.; Christ, A.; Bounon, R.; Balzergue, S.; Aubourg, S.; Gautheret, D.; Crespi, M.D. In plants, decapping prevents RDR6-dependent production of small interfering RNAs from endogenous mRNAs. Nucleic Acids Res. 2015, 43, 2902–2913. [Google Scholar] [CrossRef]
- Polydore, S.; Axtell, M.J. Analysis of RDR1/RDR2/RDR6-independent small RNAs in Arabidopsis thaliana improves MIRNA annotations and reveals unexplained types of short interfering RNA loci. Plant J 2018, 94, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Ham, B.K. The Mobile Small RNAs: Important Messengers for Long-Distance Communication in Plants. Front Plant Sci 2022, 13, 928729. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Fang, Y.; Chen, L.; Wang, J.; Chen, X. Role of non-coding RNAs in plant immunity. Plant Commun. 2021, 2, 100180. [Google Scholar] [CrossRef] [PubMed]
- Brant, E.J.; Budak, H. Plant small non-coding RNAs and their roles in biotic stresses. Front. Plant Sci. 2018, 9, 1038. [Google Scholar] [CrossRef]
- Jing, X.; Xu, L.; Huai, X.; Zhang, H.; Zhao, F.; Qiao, Y. Genome-Wide Identification and Characterization of Argonaute, Dicer-like and RNA-Dependent RNA Polymerase Gene Families and Their Expression Analyses in Fragaria spp. Genes 2023, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Krishnatreya, D.B.; Baruah, P.M.; Dowarah, B.; Chowrasia, S.; Mondal, T.K.; Agarwala, N. Genome-wide identification, evolutionary relationship and expression analysis of AGO, DCL and RDR family genes in tea. Sci Rep 2021, 11, 8679. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.L.; Meng, J.Y.; Ren, X.Y.; Yue, J.J.; Fu, H.Y.; Huang, M.T.; Zhang, Q.Q.; Gao, S.J. Genome-wide identification and characterization of DCL, AGO and RDR gene families in Saccharum spontaneum. Sci Rep 2020, 10, 13202. [Google Scholar] [CrossRef] [PubMed]
- Sabbione, A.; Daurelio, L.; Vegetti, A.; Talon, M.; Tadeo, F.; Dotto, M. Genome-wide analysis of AGO, DCL and RDR gene families reveals RNA-directed DNA methylation is involved in fruit abscission in Citrus sinensis. BMC Plant Biol 2019, 19, 401. [Google Scholar] [CrossRef]
- Qin, L.; Mo, N.; Muhammad, T.; Liang, Y. Genome-Wide Analysis of DCL, AGO, and RDR Gene Families in Pepper (Capsicum Annuum L.). Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef]
- Jing, X.; Xu, L.; Huai, X.; Zhang, H.; Zhao, F.; Qiao, Y. Genome-Wide Identification and Characterization of Argonaute, Dicer-like and RNA-Dependent RNA Polymerase Gene Families and Their Expression Analyses in Fragaria spp. Genes 2023, 14, 121. [Google Scholar] [CrossRef]
- Lin, W.; Huang, J.; Xue, M.; Wang, X.; Wang, C.J.M.D.P.B. Characterization of the complete chloroplast genome of Chinese rose, Rosa chinensis (Rosaceae: Rosa). 2019, 4, 2984–2985.
- Hibrand Saint-Oyant, L.; Ruttink, T.; Hamama, L.; Kirov, I.; Lakhwani, D.; Zhou, N.-N.; Bourke, P.; Daccord, N.; Leus, L.; Schulz, D.J.N.p. A high-quality genome sequence of Rosa chinensis to elucidate ornamental traits. 2018, 4, 473–484.
- VanBuren, R.; Wai, C.M.; Colle, M.; Wang, J.; Sullivan, S.; Bushakra, J.M.; Liachko, I.; Vining, K.J.; Dossett, M.; Finn, C.E.; et al. A near complete, chromosome-scale assembly of the black raspberry (Rubus occidentalis) genome. Gigascience 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Bianco, L.; Cestaro, A.; Sargent, D.J.; Banchi, E.; Derdak, S.; Di Guardo, M.; Salvi, S.; Jansen, J.; Viola, R.; Gut, I.; et al. Development and validation of a 20K single nucleotide polymorphism (SNP) whole genome genotyping array for apple (Malus x domestica Borkh). PLoS One 2014, 9, e110377. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Li, M.; Li, L.; Yin, H.; Wu, J.; Zhang, S. Genome-wide identification and comparative analysis of the heat shock transcription factor family in Chinese white pear (Pyrus bretschneideri) and five other Rosaceae species. BMC Plant Biol 2015, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Alioto, T.; Alexiou, K.G.; Bardil, A.; Barteri, F.; Castanera, R.; Cruz, F.; Dhingra, A.; Duval, H.; Fernandez, I.M.A.; Frias, L.; et al. Transposons played a major role in the diversification between the closely related almond and peach genomes: results from the almond genome sequence. Plant J 2020, 101, 455–472. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Zhang, J.; Wang, S.; Yang, L.; Luo, Y.; Gao, S.; Zhang, M.; Wu, S.; Hu, S.; Sun, H.; et al. The apricot (Prunus armeniaca L.) genome elucidates Rosaceae evolution and beta-carotenoid synthesis. Hortic Res 2019, 6, 128. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Bateman, A.; Clements, J.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Heger, A.; Hetherington, K.; Holm, L.; Mistry, J. Pfam: the protein families database. Nucleic Acids Res. 2014, 42, D222–D230. [Google Scholar] [CrossRef] [PubMed]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: an approach using the bootstrap. evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Tajima, F.; Nei, M. Estimation of evolutionary distance between nucleotide sequences. Mol. Biol. Evol. 1984, 1, 269–285. [Google Scholar]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.; Birney, E.; Durbin, R.; Eddy, S.R.; Finn, R.D.; Sonnhammer, E.L. Pfam 3.1: 1313 multiple alignments and profile HMMs match the majority of proteins. Nucleic Acids Res. 1999, 27, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Stothard, P. The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. Biotechniques 2000, 28, 1102–1104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-h.; Jin, H.; Marler, B.; Guo, H. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Rombauts, S.; Déhais, P.; Van Montagu, M.; Rouzé, P. PlantCARE, a plant cis-acting regulatory element database. Nucleic Acids Res. 1999, 27, 295–296. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
| Protein Name | Assigned ID | Location | Genomic (bp) |
CDS (bp) |
Protein (aa) |
Mw (kDa) |
pI | Source of Sequences |
|---|---|---|---|---|---|---|---|---|
| PpAGO1b | Prupe.5G241600 | Chr5 | 7872 | 3210 | 1069 | 118.5 | 9.6 | NCBI |
| PpAGO1a | Prupe.5G241500 | Chr5 | 8521 | 3309 | 1102 | 121.6 | 9.7 | NCBI |
| PpAGO2b | Prupe.4G108100 | Chr4 | 4728 | 3192 | 1063 | 110.8 | 8.7 | NCBI |
| PpAGO2a | Prupe.4G108000 | Chr4 | 3499 | 2907 | 969 | 108.1 | 8.5 | Phytozome |
| PpAGO4c | Prupe.6G154800 | Chr6 | 8919 | 2820 | 939 | 104.2 | 8.6 | NCBI |
| PpAGO4a | Prupe.2G056700 | Chr2 | 8931 | 2736 | 911 | 101.7 | 9.5 | NCBI |
| PpAGO4b | Prupe.2G056600 | Chr2 | 8406 | 2619 | 873 | 97.8 | 9.7 | Phytozome |
| PpAGO5 X1* | Prupe.6G115400 | Chr6 | 7057 | 3012 | 1003 | 111.9 | 9.8 | NCBI |
| PpAGO5 X2 | 2718 | 905 | NCBI | |||||
| PpAGO5 X3 | 2508 | 835 | NCBI | |||||
| PpAGO6 | Prupe.3G209300 | Chr3 | 7864 | 2697 | 898 | 100.3 | 8.7 | NCBI |
| PpAGO7 X1 | Prupe.1G279900 | Chr1 | 4021 | 3042 | 1013 | 114.9 | 9.4 | NCBI |
| PpAGO7 X2 | 3039 | 1012 | NCBI | |||||
| PpAGO10 | Prupe.1G022900 | Chr1 | 9159 | 2973 | 990 | 111.2 | 9.4 | NCBI |
| PpDCL1 | Prupe.2G200900 | Chr2 | 10239 | 5916 | 1971 | 220.3 | 5.8 | NCBI |
| PpDCL2a | Prupe.7G048000 | Chr7 | 4909 | 2346 | 781 | 88.9 | 6.3 | NCBI |
| PpDCL2b | Prupe.7G047900 | Chr7 | 5286 | 2349 | 782 | 88.8 | 6.1 | NCBI |
| PpDCL2c X1 | Prupe.7G047700 | Chr7 | 23773 | 2364 | 782 | 91.6 | 6.7 | NCBI |
| PpDCL2c X2 | 2103 | 700 | NCBI | |||||
| PpDCL2 | Prupe.6G363600 | Chr6 | 10785 | 4191 | 1396 | 159.5 | 6.4 | NCBI |
| PpDCL3a X1 | Prupe.1G401900 | Chr1 | 10655 | 5073 | 1690 | 183.7 | 6.1 | NCBI |
| PpDCL3a X2 | 4935 | 1644 | NCBI | |||||
| PpDCL3a X3 | 4479 | 1492 | NCBI | |||||
| PpDCL3b | Prupe.8G202000 | Chr8 | 8675 | 5025 | 1674 | 194.6 | 6.5 | NCBI |
| PpDCL4 X1 | Prupe.7G252800 | Chr7 | 13130 | 4926 | 1641 | 183.6 | 6.0 | NCBI |
| PpDCL4 X2 | 4914 | 1637 | NCBI | |||||
| PpRDR1a X1 | Prupe.4G078900 | Chr4 | 4121 | 3435 | 1144 | 128.1 | 6.7 | NCBI |
| PpRDR1a X2 | 3354 | 1117 | NCBI | |||||
| PpRDR1b | Prupe.4G078800 | Chr4 | 4871 | 3084 | 1028 | 117.0 | 7.4 | Phytozome |
| PpRDR1c | Prupe.1G334600 | Chr1 | 5735 | 3372 | 1123 | 128.3 | 6.8 | NCBI |
| PpRDR1d | Prupe.1G334500 | Chr1 | 4636 | 3315 | 1104 | 124.4 | 6.6 | NCBI |
| PpRDR1e | Prupe.1G332600 | Chr1 | 5347 | 3774 | 1257 | 141.9 | 6.3 | NCBI |
| PpRDR1f | Prupe.1G132100 | Chr1 | 2487 | 2127 | 709 | 79.8 | 6.5 | Phytozome |
| PpRDR2 | Prupe.5G176700 | Chr5 | 7653 | 3357 | 1118 | 127.4 | 6.4 | NCBI |
| PpRDR4 | Prupe.7G221200 | Chr7 | 9707 | 3240 | 1079 | 122.0 | 7.1 | NCBI |
| PpRDR6 | Prupe.1G480300 | Chr1 | 6593 | 3591 | 1196 | 136.4 | 6.8 | NCBI |
| *X represent different isoforms | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





