1. Introduction
"Soil Carbon Balance" (SCB) refers to the amount of carbon stored or released from soil over time through organic matter input and decomposition. Forest soils are a significant carbon reservoir. When organic matter input exceeds decomposition, soil acts as a C sink, mitigating climate change (CC). However, when losses exceed input, the soil becomes a source of C, contributing to global warming. Deforestation, degradation, or poor management practices can disturb soil balance, resulting in a net C release into the atmosphere.
Soil organic carbon (SOC) sequestration is affected by various factors such as climate, soil type, tree species, soil management, and chemical composition of soil organic matter. The dominant tree species determines these factors. Understanding the interactions of SCB with these factors is crucial for sustainable land use and mitigating the effects of CC.
The accumulation of soil C is influenced by tree species productivity, leaf litter quality and quantity, nitrogen, and C deposition. Litter and underground necromass are the main contributors to soil C, with varying capacities among forest species [
1]. Soil C dynamics can be modified through treatments like species selection, thinning, harvesting, and fertilization [
2]. Annual leaf fall and herbaceous material contribute the most C to the soil. Reforestation of former cropland generally increases soil C stocks, while former grasslands and peatlands may not show significant changes or could even experience a decrease in soil C stocks [
3].
Understanding soil carbon dynamics (SCB) and its role in mitigating global warming is crucial. However, in tropical regions, studies on soil C are relatively scarce compared to temperate soils. Measuring changes in soil C is challenging due to high spatial variability and slow accumulation processes [
4]. Soil respiration to the atmosphere, a component of C loss, remains one of the least understood aspects of the terrestrial C cycle. Both components are influenced by numerous factors that vary significantly in time and space, leading to imprecise reporting estimates for forests.
Forest ecosystems are more effective at capturing and storing large amounts of carbon (C) than any other land use [
5]. Soils in these ecosystems serve as crucial C sinks, absorbing CO2 and storing it as soil organic matter [
6], making it essential to acquire accurate and comparable data on soil carbon stocks and greenhouse gas emissions. Forest soils hold the largest terrestrial reserve of C globally [
7]. Still, their C storage capacity is declining at an estimated annual loss of 75,000 million tons of soil globally [
8].
In natural forests, soil C is usually in equilibrium, but deforestation or reforestation disrupts this balance. Each year, an estimated 15 to 17 million hectares are deforested, primarily in the tropics [
8]. These activities often result in the loss of organic C, leading to significant CO2 emissions.
The aim of this study was to 1) Assess the effects of four tree species (S. macrophylla, S. mahogany, P. occidentalis, and P. caribaea) on SOC reserves and CO2 Eq fluxes; 2) measure SOC reserves in forest litter and mineral soil up to a depth of 30 cm and quantify the fluxes of the three most significant greenhouse gases (CO2, CH4, and N2O) converted to CO2 Eq units; 3) compare these fluxes and reserves to determine the SCB and estimate its magnitude. Additionally, we examined the temporal dynamics in CO2 Eq reserves and diurnal and temporal dynamics in fluxes. Our hypotheses include differences in the magnitude of OC reserves in soils under coniferous and broadleaved species and greater fluxes from the soil in broadleaf species.
2. Materials and Methods
The data collected for this study comes from sixteen stands, four for each of the species
S. macrophylla,
S. mahagony,
P. occidentalis, and
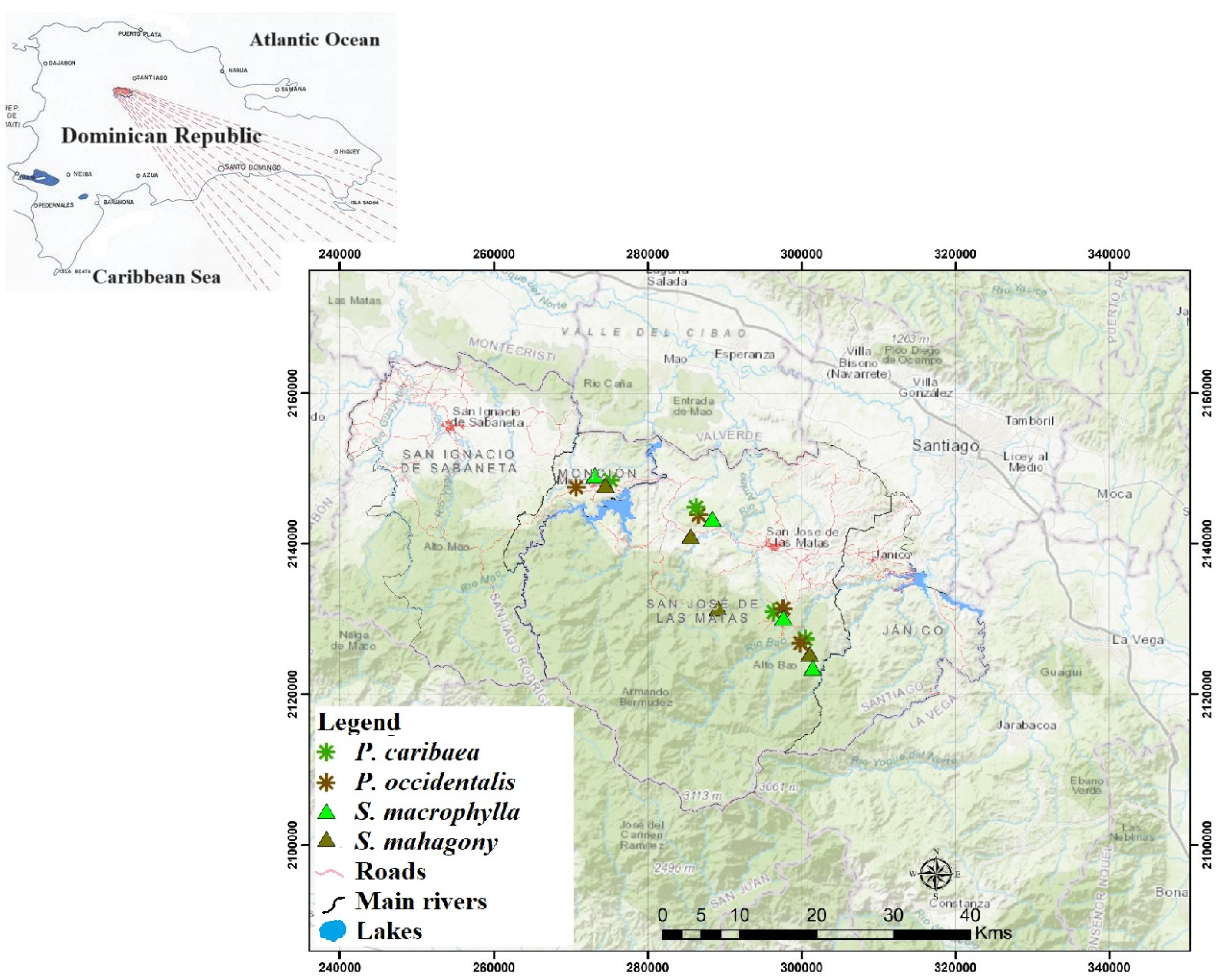
P. caribaea, located in La Sierra, Dominican Republic, with ages between 5 and 34 years (
Figure 1). La Sierra is located between UTM coordinates 251748 m - E - 325795 m E y 2116888 m N - 2156996 m N. It has an area of 1,800 km
2, where slopes range from zero to 70 percent; altitude above sea level varies from 400 m to 1600 m; the average annual temperature is 24 ºC, with a variation between maximum and minimum of less than 10 ºC; and the average annual precipitation range is between 800 to 1600 mm [
9].
This study's sampling units (SU) are in igneous and metamorphic soils, with hilly to very hilly topography. Common parent materials include limestone, acid sandstone shale, and acid diorite quartz. Based on the Soil Taxonomy System (US Soil Conservation Service), they correspond to Entick Hapludolls, typical Ustorthents, and typical Haplustolls [
10].
2.1. Soil Sampling to Confirm Parent Material and Physicochemical Properties of the Soil
Temporary plots of 350 m2 were deployed in each of the sixteen SU. Litter and mineral soil samples to a depth of 30 cm were carefully packed in sealed plastic bags, labeled, and registered in a database for further analysis in laboratories. Independent soil samples were taken at the beginning and end of the study to assess physicochemical properties. Additional independent samples taken at 30 and 50 cm depth in each SU were used to determine soil texture and verify similar edaphic parental material for the species.
2.2. Determination of OC Reserves in Litter
Throughout the study, we collected litter and small branches from the soil surface in each SU during four periods (September 2020, April and October 2021, and March 2022). Within each, we randomly selected three one-m2 plots for collection, resulting in a total of 64 composite samples (CSs). We disregarded larger branches, weeds, and grass. All the material from the three plots was weighed in the field, completely mixed, and considered as litter biomass in field conditions.
From each of the 64 CSs, we collected 2-kilogram subsamples, paced and sealed them in plastic bags, and transported them to our laboratory for dry biomass determination. Subsamples were dried at 110 ºC for 24 hours until they reached a constant weight. The relationship between fresh weight and oven-dried weight was determined. Two oven-dried subsamples from each CS were sent to Ward Laboratories in Nebraska, USA (
http://www.wardlab.com) for OC determination. SOC reserves were calculated for each of the 128 subsamples by factoring in litter biomass under field conditions, the oven-dried weight/fresh weight ratio, and OC concentration as:
where,
: OC reserves in litter (t. ha-1)
: litter biomass in field conditions (t. ha-1)
: OC concentration (%)
2.3. Determination of SOC Reserves in Mineral Soil
During four evaluation periods, mineral soil was randomly sampled at three depths (0-10 cm, 10-20 cm, and 20-30 cm) in each SU to estimate SOC. A composite sample (CS) was formed for each specific depth, resulting in 192 samples. Each collection of samples was conducted with a minimum distance of 1 meter between each sampling point to avoid proximity to previously sampled areas. SOC concentration was determined by Junta Agroempresarial Dominicana (JAD) soil laboratory.
Measurements of soil bulk density (BD) in each of the 16 SUs and four evaluation periods, were made by collecting three independent soil samples at each soil depth being evaluated, using an AMS sliding hammer (⌀ interior = 4.8 cm; V = 182.77 cm3). 192 samples were processed in the laboratory, and BD was determined by fresh volume and oven-dried weights (110 ºC for 24 hours) ratios.
OC concentration from the 192 CSs was reported as a percentage. This percentage was transformed into concentration units (g C kg
-1) and converted into a significant estimate by multiplying this concentration by BD (kg. m
-3) and the volume of soil contained in one hectare, (10,000 m
2) and depth of each layer evaluated (0.10 m), to obtain SOC reserves (t. CO ha
-1). SOC reserves were calculated for each fixed depth of 0.10 m as:
where,
: SOC reserves (g CO ha-1), converted to t CO , multiplying by 106 factor (amount of g in one metric ton).
: Soil Bulk Density (kg. m-3).
: Soil OC Concentration (g C kg-1).
: Soil volume in one hectare with 0.10 m thickness (
: 0.10 m = Thickness of each layer evaluated.
2.4. CO2 Equivalent Fluxes from the Soil
Throughout all four measurement periods, we monitored CO2-equivalent (CO2 Eq.) fluxes from the ground using a G2508 spectrometer (Picarro Inc. in Sunnyvale, California, United States), which can simultaneously measure CO2, N2O, and CH4 fluxes. The spectrometer is coupled to a multiplexer and three automatic chambers (Eosense Environmental Gas Monitoring, Canada). The system's integrated software records the gases every 10 minutes. Fluxes can be recorded in multiple units, including t. ha-1 year-1.
Over 10 hours, CO2-Eq. fluxes were measured in the 16 different SUs. 2,587 measurements were taken and averaged to get 640 hourly averages (10 hours x 4 stands x 4 species x 4 periods). To express fluxes in CO2 Eq. units, CO
2, N
2O, and CH
4 fluxes were converted according to IPCC standards for each gas (CO
2 = 1; N
2O = 298 and CH
4 = 24) over a 100-year time horizon [
11]. We also considered the molar mass ratio of C, obtained by dividing CO
2 molar mass = (12.0107 + (15.9994 * 2) = 44.0095 g/mol by the molar mass of C = 12.0107, equaling to 3.67.
2.5. Carbon Dioxide Equivalent Balance
BCO2 Eq. for each SU was calculated by comparing data from OC inputs (t. ha-1) and outputs in fluxes (t. ha-1 year-1). The ecosystem boundary considered for our BCO2 Eq. assessment included litter in the soil surface and mineral soil to a 30 cm depth. Specific components were excluded from flux estimation, as they had a negligible impact on the loss of OC from the soil. These components are as follows: Non- CO2 losses, such as C monoxide, fluxes of volatile organic compounds, and herbivores.
To calculate BCO2 Eq., we used a commonly recognized empirical model for predicting changes in SOC stocks [
12], where negative values indicate a decrease in atmospheric C. To determine the change in C reserves, the model is provided below.
where:
= Periodic change in SOC reserves (t ha-1);
= SOC reserves at moment 0, (t ha-1).
= CO2 Eq. fluxes at moment 0, (t ha-1 year-1).
= SOC reserves at moment T, (t ha-1).
= CO2 Eq. fluxes at moment T, (t ha-1 year-1).
T = Time between first and last assessment of interest.
2.6. Calculations and Statistical Analysis
To conduct all statistical analyses, we used SPSS [
13]. Descriptive statistics were applied to calculate means, ranges, standard error, standard deviation, and percentiles (10th and 90th) based on four SUs for each species. Unless expressly stated otherwise, our accepted probability level was set at α = 0.05.
Each of the four stands for each species was considered an SU to measure variation in variables of interest. Univariate ANOVA (P < 0.05) was employed to analyze the effects of the species and diurnal hours on OC reserves and CO2 Eq. flux datasets. If significant effects were found, pairwise comparisons through Bonferroni's post hoc test (P ≤ 0.05) were conducted.
To assess the variation in OC reserves in litter and mineral soil and CO2 Eq. fluxes, considering forest type (broadleaves and conifers) as a fixed factor, independent sample t-tests (α = 0.05) were employed.
3. Results
Table 1 shows the average fertility levels observed at the beginning and end of the study and an evaluation of soil texture to confirm similar edaphic parental material in the studied areas. Results show that most physicochemical properties had higher values in
S. mahogany stands. Additionally, all soils under the species, except for
S. mahogany (sandy loam), had a loamy sand texture.
3.1. OC in Forest Litter Under The Different Species
Table 2 displays the average values, standard error of the mean, and percentiles (10th and 90th) for dry biomass (t ha
−1), C concentration (%), C reserves (t ha
−1), and CO2 Eq. (t ha
−1) for forest stands occupied by the species. For the four evaluation periods, the mean dry biomass content in litter ranged from 18.38±1.73 to 23.07±1.70 t ha
-1, with these values being observed in
P. occidentalis and
S. mahogany, respectively. No significant differences were found among species for this variable (F (3, 60) = 1.064,
P=0.371).
The organic carbon fraction showed a range of means ± standard errors of 0.38±0.01 (%) and 0.47±0.01 (%), respectively, with the lowest value found in S. macrophylla and the highest in P. occidentalis. The average C fraction value for P. caribaea was very similar to that of P. occidentalis, differing only by 0.0025%. There were statistically significant differences observed for this variable between the stands of the different species (F (3, 60) = 9.887, P≤0.000).
According to the Bonferroni tests for multiple comparisons, there was a significant variation in the average C fraction in litter between S. macrophylla and P. occidentalis. The mean difference (MD) was -0.086 (P=0.001, 95% C.I=-0.143, -0.028). Similarly, there was a significant difference between S. macrophylla and P. caribaea (MD=-0.083, P=0.001, 95% C.I.=-0.141, -0.026). Furthermore, there were significant differences between P. occidentalis and S. mahogany (MD= 0.079, P=0.002, 95% C.I.=0.021, 0.136), and between P. caribaea and S. mahogany (MD=0.076, P=0.004, 95% C.I.=0.019, 0.134).
The results showed that S. macrophylla had (average ± standard deviation) 6.91±0.95 t ha−1, P. occidentalis had 8.65±0.83 t ha−1, P. caribaea had 8.55±0.84 t ha−1, and S. mahogany had 8.99±0.71 t ha−1 OC reserves in the litter. No significant differences were found in this variable (F (3, 60) = 1.227, P=0.308). These values are expressed in CO2 Eq. units (t ha−1), were 25.38±3.50, 31.75±3.06, 31.99±3.07, and 32.99±2.59, respectively. Additionally, the average range of litter dry biomass was between 18.38±1.73 to 23.07±1.70 t ha-1.
3.2. OC Stocks in Mineral Soils under the Different Species
The following data is presented in
Table 3: averages, standard errors, and percentiles (10th and 90th) for 192 different soil CSs where bulk density (BD) (kg m
−3), C concentration (g kg
-1), and OC reserves (t ha
−1) were calculated. The statistics are shown for three different soil depths: P1 (0-10 cm), P2 (10-20 cm), and P3 (20-30 cm). The average OC concentration (g kg
−1) in
S. mahagony soils (P1) was 18.62±2.27, which was significantly higher than in
P. caribaea soils (P3) with 4.28±0.59. There was no significant difference observed at P1 (F (3, 60) = 2.691,
P=0.054), but statistically significant differences were found at P2 (F (3, 60) = 3.950,
P= 0.012) and P3 (F (3, 60) = 2.943,
P=0.040).
In P2, the average concentration of OC (g kg-1) differed significantly between S. mahagony and P. occidentalis (mean difference = 6.539, P=0.022, 95% C.I. = 0.649, 12.429) and between S. mahagony and P. caribaea (mean difference = 6.281, P=0.030, 95% C.I. = 0.391, 12.170). In P3, the average OC concentration was significantly different between S. mahagony and P. caribaea (mean difference = 3.905, P= 0.026, 95% C.I. = 0.319, 7.491).
Average BD (kg m−3) showed a monotonic increase steadily with soil depth in S. macrophylla (1,377.56 to 1,608.19) and P. caribaea (1,412.50 to 1,585.19). BD was lowest at P1 (1,459.44) and highest at P2 (1,533.75) for soils under P. occidentalis. Under S. mahagony, BD was lowest at P2 (1,412.50) and highest at P3 (1,605.13). Statistically significant differences in BD were observed in soils under P. caribaea and S. mahagony at P2, being soils under P. caribaea denser on average (F (3, 60) = 2.994, P=0.038. The mean difference was 137.50 kg m−3.
SOC reserves (t ha
-1) varied across soil depths, with the highest values found at P1 under
S. mahagony (28.46±3.68) and the lowest at P3 under
P. caribaea (6.68±0.94) (
Table 3). Statistically significant differences in SOC stock averages were observed between
S. mahagony and
S. macrophylla at P1 (F (3, 60) = 3.559,
P=0.019) and between
S. mahagony and
P. caribaea at P3 (F (3, 60) = 3.492,
P=0.021), with mean differences of 11.153±4.039 t SOC ha-1 and 6.584±2.045 t ha
-1, respectively.
At a depth of 30 cm, statistically significant differences in SOC stock averages were observed between S. mahagony and other species (F (3, 60) = 5.677, P=0.002). SOC reserves were significantly higher in S. mahagony compared to S. macrophylla (P= 0.030, 95% I.C. = 1.218, 37.866), P. occidentalis (P=0.011, 95% I.C. = 3.657, 40.305), and P. caribaea (P=0.003, 95% I.C. = 6.693, 43.342). Mean differences ± standard (t ha-1) error were 19.543±6.716, 21.981±6.716, and 25.017±6.716, respectively.
3.3. Periodic Dynamics of CO2 Eq. Reserves
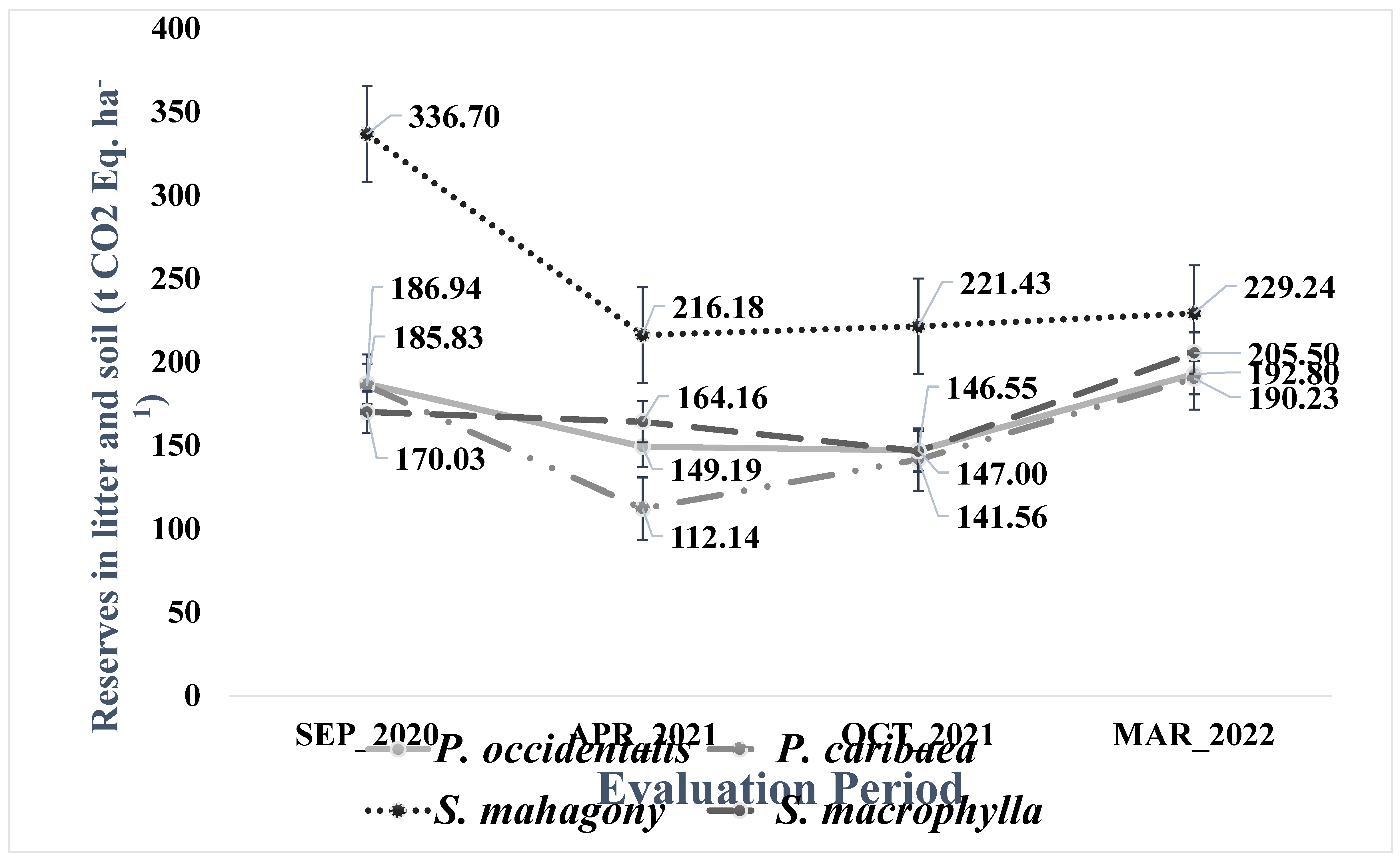
Figure 2 illustrates the changes in CO2 Eq reserves in soils under different species during four evaluation periods. The study found that the measurement period significantly impacted CO2 Eq stocks under
P. caribaea. However, no notable differences were observed in the reserves of
S. macrophylla,
P. occidentalis, and
S. mahogany stands. In April 2021, CO2 Eq reserves under
P. caribaea were lower compared to September 2020, with an average difference of -73.68 t ha
-1 (
P=0.047) and lower than in March 2022 with a mean difference of -78.19 t. ha
-1 (
P=0.033).
3.4. CO2 Equivalent Fluxes from the Soil under the Species
Diurnal soil respiration (t CO2 Eq. ha−1 year-1) was on average (± standard error) -0.0598±0.1567 for S. macrophylla, 0.2803±0.1155 for P. occidentalis, -0.5408±0.1187 for P. caribaea, and 0.9227±0.1218 for S. mahagony. Comparing the effect of species on annual fluxes from soils, we found statistically significant differences (F (3, 2579) = 22.848, P<0.001). They are lowest in P. caribaea and greatest in S. mahagony to other species.
Average CO2 Eq. fluxes were significantly different among the tree species as follows:
P. caribaea vs. S. macrophylla: Mean difference = -0.481 (P=0.049, 95% C.I. = 0.0016, 0.9603).
P. caribaea vs. P. occidentalis: Mean difference = -0.821 (P<0.001, 95% C.I. = 0.343, 1.299).
P. caribaea vs. S. mahagony: Mean difference = -1.463, (P<0.001, 95% C.I. = -1.943, -0.984).
S. mahagony showed higher average fluxes compared to:
P. occidentalis: Mean difference = 0.642 (P=0.003, 95% C.I. = -1.126, -0.159).
Diurnal and Periodic Dynamics of CO2 Eq. Fluxes
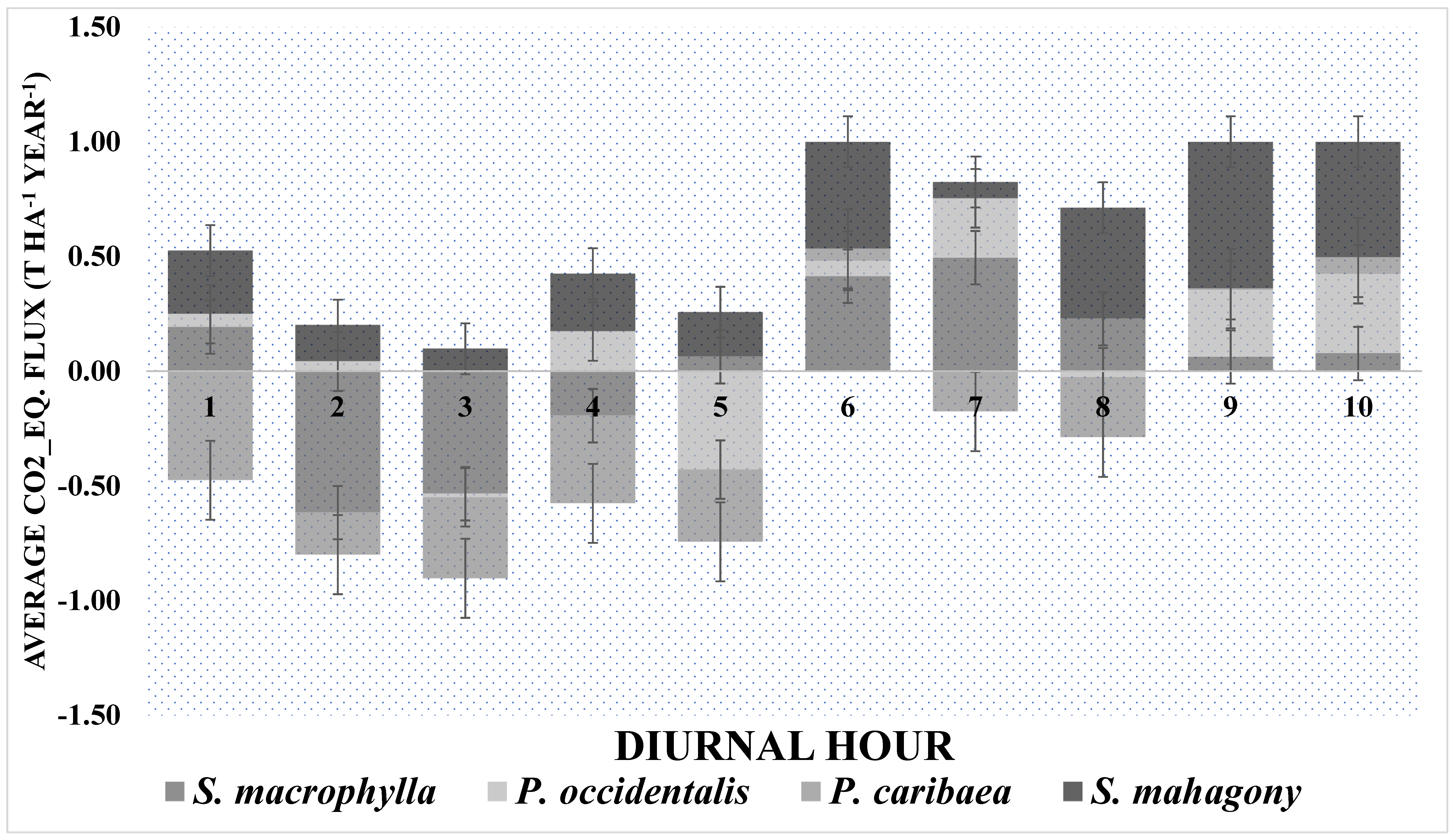
Figure 3 shows the average CO2 Eq. flux (t ha-1 year-1) during 10 hours of measurement in each of the 16 SUs. Throughout the 10 daytime hours,
S. mahagony soils consistently released CO2 Eq. into the atmosphere, whereas
P. caribaea soils were generally a source of CO2 Eq., except during hours 6 (11:00-12:00), 9 (15:00-16:00), and 10 (16:00-17:00). Similarly,
P. occidentalis soils always released CO2 Eq. except during hours 3 (9:00-10:00), 5 (10:00-11:00), and 8 (14:00-15:00). In contrast,
S. macrophylla soils consistently released CO2 Eq., except from hours 2 (8:00-9:00) to 4 (10:00-11:00). Periodic dynamics of fluxes are shown in
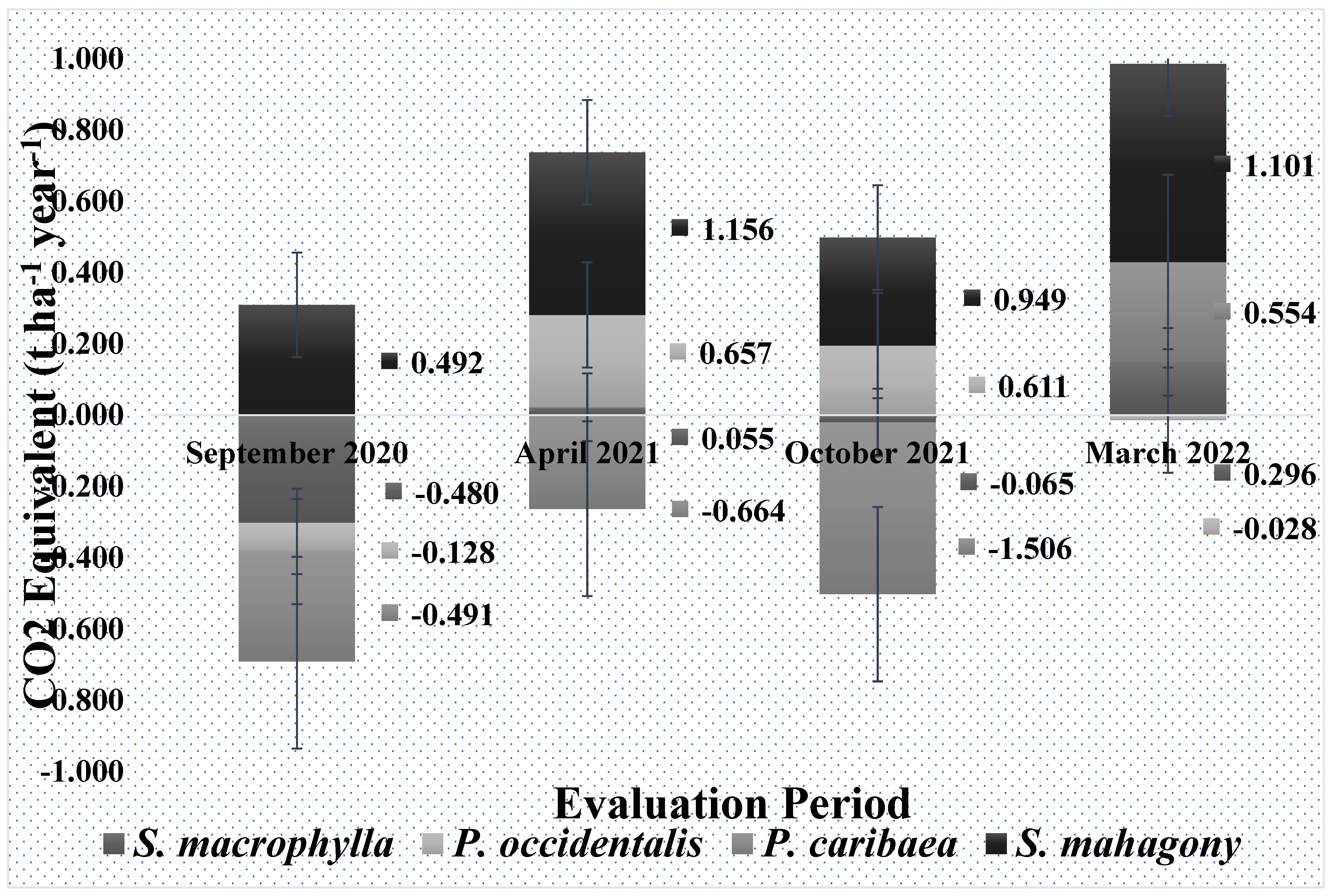
Figure 4. In September 2020, the soil under
S. mahagony behaved as a source, while the other species behaved as sinks. In April 2021 only soils under
P. caribaea behave as a sink. In October 2021,
S. mahagony and
P. occidentalis were still a source; in March 2022, the latter species became slightly a sink. For
S. macrophylla,
P. occidentalis, and
S. mahagony, there were no statistically significant differences in fluxes during the four periods. However, in
P. caribaea, we observed the following statistically significant differences:
Sep_2020 vs. Oct_2021: Mean difference = 1.015 t ha-1 year-1, P=0.010.
Mar_2022 vs. Sep_2020: Mean difference = 1.045 t ha-1 year-1, P=0.009.
Mar_2022 vs. Abr_2021: Mean difference = 1.219 t ha-1 year-1, P=0.002.
Mar_2022 vs. Oct_2021: Mean difference = 2.060 t ha-1 year-1, P<0.0001.
3.5. CO2 Equivalent Balance
Based on the data presented in
Table 4, it can be observed that BCO2 Eq. at the soil level varied among the four species in the evaluated 1.5 years.
S. macrophylla,
P. occidentalis, and
P. caribaea exhibited negative BCO2 Eq. values (-23.196, -3.838, and -2.299 t ha
-1), respectively. This indicates CO2 Eq. absorption from the atmosphere. On the other hand,
S. mahogany had a positive BCO2 Eq. value of 0.982 t ha
-1, suggesting that its soils emitted CO2 Eq. into the atmosphere. The soil under
S. macrophylla absorbed the highest amount of CO2 Eq. (∆CO2 Eq.=-23.196 t ha
-1). Conversely, the soil under S. mahogany emitted the most CO2 Eq. (∆ CO2 Eq.=0.982 t ha
-1). The calculations for BCO2 Eq. made for the four species were:
4. Discussion
This research studied two types of species: coniferous (
P. occidentalis and
P. caribaea) and broadleaved (
S. macrophylla and
S. mahogany). With a few exceptions, SOC concentration and reserves decreased with soil depth while soil BD increased.
P. occidentalis had the highest BD at P2, and
S. mahagony had the lowest BD at P2 (
Table 3). SOC sequestration potential differs from sites, soil depths, and tree growth stages [
14]. Wei et al. [
15] reported more significant OC accumulation in the 0–10 cm depth than in the 10–80 cm depth. Huang et al. [
16] also reported SOC concentration decreases with soil depth and is significantly higher in the 0-10 cm soil layer than in other soil layers.
The 0-10 cm layer usually has the highest SOC concentration because of the direct input of organic materials and because decomposition processes are also more active near the surface due to higher microbial activity and better aeration [
17].
Our study found that 40% of the average total carbon reserves (t ha
−1) in the soil were stored in the upper mineral layer (0-10 cm), followed by 24% in the second layer (10-20 cm), and 20% in the third layer (20-30 cm). According to Wei et al. [
15], forest-derived OC in afforested soils accounted for 52-86% of the total OC in the 0-10 cm depth, 36-61% of the total OC in the 10-20 cm depth, and 11-50% of the total OC in the 20-80 cm depth. In 30-year-old
Pinus elliotti Engelm.,
Schima superba, and
Pinus massoniana plantations, subtropical China, the 0-15 cm soil layer accounted for 32.1-34.1% of the total SOC density. It was significantly higher than other soil layers [
16].
The soil BD percent increases between P1 and P3 in
S. macrophylla,
P. occidentalis,
P. caribaea, and
S. mahagony were 16.74%, 3.01%, 12.23%, and 5.04%. Other researchers have reported contradictory findings on soil BD patterns. Some authors reported it to decrease with depth [
18,
19], while others reported increases with depth [
20]. Wu et al. [
21] reported that bulk density decreased by 4.3% at deeper soil layers compared to surface layers in his study. Azeez et al. [
20] noted bulk density to increase at 20-40 and 40-60 cm depths under some tree plantations.
The lowest average reserves were found in the litter layer, with only 16% of the total. Subashree et al. [
22], who studied tropical coniferous and broadleaf forests, reported reserves distributed as follows: 44.2% in the 0-10 cm layer, 32.0% in the 10-20 cm layer, and 23.8% in the 20-30 cm layer. Combining both reservoirs, the average ± standard error of OC reserves was higher in broadleaved species (57.55±4.79 t ha
−1) than conifers (44.48±2.13 t ha
−1).
The observed OC reserves in our study are low compared to studies in temperate latitudes. For instance, Uri et al. [
23] reported 83.67 t ha
−1 in the top 30 cm of soils under Pinus sylvestris L. in Estonia, and Berhongaray et al. [
2] found soil OC reserves in short-rotation woody crops with average values of 140 t ha
−1 in Belgium.
In the mineral soil (30 cm), the average ± standard error of OC reserve values were 49.60±4.57 t ha−1 for broadleaved and 35.87±2.03 t ha−1 for conifers. The average ± standard error of OC reserve in litter was 7.95±0.61 t ha−1 for broadleaved and 8.60±0.58 t ha−1 for conifers. There were no significant differences in the C fraction in litter among conifers or broadleaves, and there were no statistically significant differences between conifers and broadleaves for OC reserves in the litter (t (61.827) = - 0.769, p = 0.434). However, differences were observed in SOC reserves (30 cm), with a mean difference of 13.73 t ha−1 (t (42.774) = 2.747, p = 0.021), favoring broadleaf species.
Schulp et al. [
24], as cited in Garrett et al. [
25], found higher SOC stocks to a depth of 20 cm under coniferous forests (76.44 t C ha
-1) than under broadleaved forests (67.45 t C ha
-1). This trend was also reported by Laganière et al. [
26], who, at 15 cm soil depth, found higher SOC stocks under
Picea mariana (Mill.) (46.3 t C ha
-1) than under
Populus tremuloides Michx (34.7 t C ha
-1). In agreement with our findings, Wang et al. [
27] found SOC stocks in the top 10 cm of soil under
Pinus massoniana, which were lower than under the species
Castanopsis hystrix,
Michelia macclurei, and
Mytilaria laosensis, reporting 29.2 t C ha
-1 and 32.6.34.9 t C ha
-1, respectively. Vesterdal et al. [
28] found consistent effects of species on soil organic carbon.
Considering both litter and mineral soil reservoirs, total reserves of OC (t ha
−1) were highest in
S. mahagony with an average ± standard deviation of 68.36±32.00, followed by the reserves in
S. macrophylla (46.75±15.53),
P. occidentalis (46.05±12.22), and
P. caribaea (42.91±12.11). Converted to CO2 Eq. units, these are, respectively, 250.89±117.45; 171.56±56.99, 168.99±44.86, and 157.47±44.43 t ha
−1. Our results are consistent with previous studies by Vesterdal et al. [
28] and Lee et al. [
29], which also found higher OC reserves in coniferous litter and higher OC reserves in the mineral soil under broadleaf species. This trend is attributed to the slower rate of decomposition in conifers and the more active soil fauna in broadleaf species, leading to greater incorporation of organic matter in soil aggregates [
30,
31].
Other studies have reported both higher and lower values for litter biomass. Lee et al. [
29] reported litter OC reserves of 3.28±0.13 t ha
−1 for broadleaved and 4.63±0.18 t ha
−1 for conifers in South Korea, while our study estimated values 2.42 and 1.85 times higher, respectively. In the mineral soil, they reported 44.11±1.54 t ha
−1 for broadleaved and 33.96±1.62 t ha
−1 for conifers. Cook et al. [
32] reported values of 38.3±1.9 t ha
−1 and 36.0±1.6 t ha
−1 for broadleaf and coniferous OC reserves, respectively.
Cha et al. [
33] suggest that the higher difficulty in decomposition of the litter layer in coniferous forests compared to broadleaf forests could explain the observed differences in litter biomass. Additionally, the lower pH levels in coniferous forest soils (average pH 4.32) compared to broadleaf forests (average pH 5.16) (
Table 1), result in reduced microorganism activity and retention of fresher leaves in the soil under conifers [
29].
Different species and types of forests have different rates of litterfall and decomposition, which can have varying impacts on carbon dynamics. Harris et al. [
34] estimate that global forests were a net carbon sink of −7.6 E+09 ± 4.9 E+10 t CO2 Eq. yr−1, reflecting a balance between gross carbon removals (−1.56 E+10 ± 4.9 t CO2 Eq. yr−1) and gross emissions from deforestation and other disturbances (8.1 E+09 ± 2.5 E+09 t CO2 Eq. yr−1). In our study, conducted in a subtropical region, the daily flux magnitudes ranged from uptakes of -0.0598±0.1567 to emissions of 0.9227±0.1218 t CO2 Eq. ha
-1 yr
-1 for
S. macrophylla and
S. mahogany, respectively. These quantities at our sites could be considered negligible as compared to reports from other latitudes. Rubaiyata et al. [
35] found CO2 Eq. uptakes of approximately -6.58 x 10
-2 tons per hectare in temperate forest stands in Goettingen, Germany. Duan et al. [
36] studied three types of larch boreal forest (
Larix gmelinii) in the Daxing’an Mountains, Northeast China, with fluxes averaging 2.91 x 10
1 t CO2 Eq ha
-1 yr
-1.
Wu et al. [
37] reported emissions in the order of 15.93, 16.45, and 14.06 t CO2 Eq. ha
-1 for the conifers
Larix gmelinii,
Pinus sylvestris var. mongolica, and the broadleaved species
Betula platyphylla, respectively. Uri et al. [
23] reported 6.0 t C ha
-1 yr
-1 (22.02 t CO2 Eq. ha
-1 yr
-1) for
P. silvestris in a temperate forest in Estonia, and Pastore et al. [
38] reported 8.1 t ha
-1 yr
-1 (29.73 t CO2 Eq. ha
-1 yr
-1) for temperate coniferous stands in Ireland. Gahagan et al. [
39] reported fluxes of approximately 6.72 and 6.95 t ha
-1 yr
-1 (24.66 and 25.51 t CO2 Eq. ha
-1 yr
-1) for hardwood and
Pinus resinosa Ait temperate stands, respectively, in Northern Michigan, USA.
Mean soil CO2 Eq. fluxes differed significantly among tree species during the 1.5 years that lasted the study. Fluxes from P. caribaea forest stands significantly differed from fluxes from the other three species evaluated, P. occidentalis, S. macrophylla, and S. mahagony. There were also statistically significant differences in fluxes between S. mahagony and S. macrophylla, as well as S. mahagony and P. occidentalis. The soil under S. macrophylla exhibited the highest uptake CO2 Eq. (∆CO2 Eq.=-23.196 t ha-1). Conversely, the soil under S. mahogany emitted the most CO2 Eq. (∆ CO2 Eq.=0.982 t ha-1).
In old-growth tropical forests in Malaysian Borneo, a region that is a global hotspot for emission from forest degradation, annual soil respiration was equal to the organic carbon inputs into the soil with differences between respiration and inputs in the order of 0.66 t CO2 Eq. ha
−1 year
−1 [
40].
Betula pendula forest stands in the pole, middle-aged, and premature stages within Estonia, exhibited carbon soil budgets of -0.33, -2.13, and -2.97 t CO2 Eq. ha
−1 year
−1, respectively [
41]. Despite being in different biomes, these values are within the range of our estimated fluxes for the four species studied. Overall, forests worldwide are estimated to absorb about 7.6 billion metric tons of carbon dioxide annually, acting as a net carbon sink of roughly 1.5 times the annual emissions from the entire United States [
42].
Figure 1.
Distribution of sample units (forest stands) for P. caribaea, P. occidentalis, S. macrophylla, and S. mahagony within La Sierra, Dominican Republic.
Figure 1.
Distribution of sample units (forest stands) for P. caribaea, P. occidentalis, S. macrophylla, and S. mahagony within La Sierra, Dominican Republic.
Figure 2.
Periodic dynamics of CO2 Equivalent reserves in litter and soils (30 cm) under S. mahagony, S. macrophylla, P. occidentalis, and P. caribaea in the four measurement periods (September 2020, April 2021, October 2021, and March 2022).
Figure 2.
Periodic dynamics of CO2 Equivalent reserves in litter and soils (30 cm) under S. mahagony, S. macrophylla, P. occidentalis, and P. caribaea in the four measurement periods (September 2020, April 2021, October 2021, and March 2022).
Figure 3.
Diurnal dynamics (10 hours) of CO2 Equivalent fluxes in soils under 4 stands of S. macrophylla, P. occidentalis, P. caribaea, and S. mahagony.
Figure 3.
Diurnal dynamics (10 hours) of CO2 Equivalent fluxes in soils under 4 stands of S. macrophylla, P. occidentalis, P. caribaea, and S. mahagony.
Figure 4.
Periodic dynamics of CO2 Equivalent fluxes in the soils under 4 stands of S. macrophylla, P. occidentalis, P. caribaea, and S. mahagony. The values on the right side of the bars correspond to the average values of fluxes (t CO2 Equivalent ha-1 year-1) in each species.
Figure 4.
Periodic dynamics of CO2 Equivalent fluxes in the soils under 4 stands of S. macrophylla, P. occidentalis, P. caribaea, and S. mahagony. The values on the right side of the bars correspond to the average values of fluxes (t CO2 Equivalent ha-1 year-1) in each species.
Table 1.
Results of the analysis that characterizes the average of the physicochemical properties and the type of parent material of the soils for each of the species.
Table 1.
Results of the analysis that characterizes the average of the physicochemical properties and the type of parent material of the soils for each of the species.
| Physicochemical Property |
Unit |
S. macrophylla |
P. occidentalis |
P. caribaea |
S. mahagony |
| pH in water |
5.20 |
4.39 |
4.26 |
5.12 |
| Electrical conductivity |
mS/cm |
0.19 |
0.09 |
0.09 |
0.54 |
| Organic matter |
% |
2.45 |
2.18 |
2.15 |
2.61 |
| Nitrogen |
% |
0.12 |
0.11 |
0.11 |
0.13 |
| Phosphorus |
mg/kg |
29.80 |
4.87 |
7.72 |
65.68 |
| Removable acidity (Al + H+) |
Meq/100 |
1.07 |
19.50 |
1.88 |
1.53 |
| Calcium |
Meq/101 |
19.78 |
17.36 |
8.79 |
22.77 |
| Magnesium |
Meq/102 |
10.41 |
13.98 |
13.60 |
14.90 |
| Potassium |
Meq/103 |
0.13 |
0.13 |
0.15 |
0.99 |
| Cation Exchange Capacity |
Meq/105 |
29.13 |
50.96 |
24.41 |
39.80 |
| Calcium Saturation |
% |
58.30 |
43.37 |
37.94 |
56.45 |
| Magnesium Saturation |
% |
38.16 |
30.19 |
46.12 |
37.85 |
| Potassium Saturation |
% |
0.39 |
0.50 |
0.65 |
1.88 |
| Aluminum Saturation |
% |
4.20 |
25.94 |
7.80 |
5.10 |
| Calcium/Magnesium |
Proport. |
1.80 |
1.61 |
0.76 |
1.55 |
| Calcium/Potassium |
Proport. |
158.96 |
177.09 |
92.36 |
40.53 |
| Magnesium/Potassium |
Proport. |
114.34 |
149.41 |
208.37 |
31.83 |
| (Ca + Mg)/K |
Proport |
273.30 |
326.52 |
300.73 |
72.36 |
| Copper |
mg/kg |
2.09 |
3.53 |
2.12 |
4.38 |
| Manganese |
mg/kg |
64.40 |
72.19 |
64.81 |
93.04 |
| Iron |
mg/kg |
124.66 |
148.87 |
143.37 |
190.99 |
| Zinc |
mg/kg |
1.83 |
1.57 |
0.69 |
3.22 |
| Clay |
% |
4.50 |
4.00 |
4.00 |
6.50 |
| Silt |
% |
16.00 |
14.00 |
12.50 |
18.00 |
| Sand |
% |
79.50 |
82.00 |
83.50 |
75.50 |
| Texture |
|
sl |
Ls |
Ls |
Ls |
| sl = Sandy loam; Ls = loamy sandy; Proport. = proportion. |
Table 2.
Mean and percentile values (10 and 90) for dry biomass (DB), carbon fraction (CF), and carbon content (CC) in the forest litter.
Table 2.
Mean and percentile values (10 and 90) for dry biomass (DB), carbon fraction (CF), and carbon content (CC) in the forest litter.
| |
Units |
Stats |
S. macrophylla |
P. occidentalis |
P. caribaea |
S. mahagony |
| |
|
N |
16 |
16 |
16 |
16 |
| Forest litter dry biomass |
t ha-1
|
Mean |
18.56 |
18.38 |
19.26 |
23.07 |
| Standard Error |
2.63 |
1.73 |
2.33 |
1.70 |
| 10th Percentile |
3.69 |
10.13 |
11.32 |
13.69 |
| 90th Percentile |
33.19 |
31.52 |
36.24 |
34.75 |
| C fraction |
|
Mean |
0.38 |
0.47 |
0.47 |
0.39 |
| Standard Error |
0.01 |
0.01 |
0.02 |
0.01 |
| 10th Percentile |
0.28 |
0.41 |
0.31 |
0.29 |
| 90th Percentile |
0.44 |
0.53 |
0.53 |
0.45 |
| C reserves |
t ha-1
|
Mean |
6.91 |
8.65 |
8.55 |
8.99 |
| Standard Error |
0.95 |
0.83 |
0.84 |
0.71 |
| 10th Percentile |
1.41 |
4.36 |
5.29 |
4.52 |
| 90th Percentile |
12.40 |
15.03 |
15.24 |
12.49 |
| CO2 Eq. stored in litter |
t ha-1
|
Mean |
25.38 |
31.75 |
31.39 |
32.99 |
| Standard Error |
3.50 |
3.06 |
3.07 |
2.59 |
| 10th Percentile |
5.16 |
16.00 |
19.42 |
16.57 |
| 90th Percentile |
45.52 |
55.17 |
55.93 |
45.86 |
Table 3.
Mean values, standard error, and percentiles (10 and 90) for organic carbon concentration (OCC), soil bulk density (BD), and organic carbon stocks (OCS) at the three assessed soil depths under the four species.
Table 3.
Mean values, standard error, and percentiles (10 and 90) for organic carbon concentration (OCC), soil bulk density (BD), and organic carbon stocks (OCS) at the three assessed soil depths under the four species.
| |
Soil Layer (cm) |
OCC (g C kg-1) |
BD (kg m-3) |
OCS (t C ha-1) |
| Specie |
Depth |
N |
Mean |
Standard Error |
P10
|
P90
|
Mean |
Standard Error |
P10
|
P90
|
Mean |
Standard Error |
P10
|
P90
|
| S. macrophylla |
0-10 |
16 |
12.89 |
1.67 |
3.75 |
23.94 |
1377.56 |
70.93 |
1012.40 |
1827.20 |
17.31 |
2.19 |
6.46 |
29.89 |
| 10-20 |
16 |
8.05 |
0.78 |
2.89 |
12.24 |
1552.50 |
37.63 |
1327.00 |
1800.00 |
12.54 |
1.25 |
4.54 |
19.61 |
| 20-30 |
16 |
6.29 |
0.76 |
3.74 |
11.93 |
1608.19 |
42.33 |
1339.80 |
1869.40 |
9.99 |
1.12 |
5.32 |
17.27 |
| P. occidentalis |
0-10 |
16 |
12.75 |
1.74 |
5.26 |
24.46 |
1459.44 |
69.40 |
1071.40 |
1886.30 |
18.42 |
2.69 |
6.66 |
35.57 |
| 10-20 |
16 |
6.28 |
0.89 |
0.70 |
10.71 |
1533.75 |
23.00 |
1421.00 |
1693.00 |
9.57 |
1.34 |
1.09 |
16.95 |
| 20-30 |
16 |
6.26 |
1.08 |
1.65 |
13.62 |
1503.31 |
41.85 |
1265.20 |
1807.40 |
9.41 |
1.67 |
2.35 |
21.58 |
| P. caribaea |
0-10 |
16 |
12.03 |
1.70 |
1.91 |
21.78 |
1412.50 |
52.62 |
1090.80 |
1675.70 |
17.46 |
2.64 |
2.31 |
34.15 |
| 10-20 |
16 |
6.53 |
0.96 |
1.88 |
12.39 |
1578.12 |
23.77 |
1449.00 |
1753.00 |
10.22 |
1.45 |
3.02 |
19.65 |
| 20-30 |
16 |
4.28 |
0.59 |
0.98 |
7.75 |
1585.19 |
56.44 |
1292.60 |
1925.70 |
6.68 |
0.94 |
1.83 |
12.58 |
| S. mahagony |
0-10 |
16 |
18.62 |
2.27 |
9.56 |
37.79 |
1528.12 |
56.06 |
1201.40 |
1838.70 |
28.46 |
3.68 |
13.91 |
62.61 |
| 10-20 |
16 |
12.82 |
2.64 |
3.61 |
31.84 |
1440.62 |
47.81 |
1144.00 |
1784.00 |
17.65 |
3.48 |
5.68 |
42.09 |
| 20-30 |
16 |
8.19 |
1.16 |
2.59 |
15.43 |
1605.13 |
44.74 |
1427.50 |
1966.20 |
13.26 |
1.85 |
3.62 |
23.65 |
Table 4.
Net balance at soil scale, between average reserves (litter and soil) and CO2 Equivalent fluxes in soils under the species S. macrophylla, P. occidentalis, P. caribaea and S. mahagony, in the 1.5-year period between the first and last assessment.
Table 4.
Net balance at soil scale, between average reserves (litter and soil) and CO2 Equivalent fluxes in soils under the species S. macrophylla, P. occidentalis, P. caribaea and S. mahagony, in the 1.5-year period between the first and last assessment.
| Species |
Reserves |
Flux |
Balance |
Reserves |
Flux |
Balance |
∆CO2 Equivalent |
| |
(t CO2 Eq. ha-1) |
(t CO2 Eq. ha-1 year-1) |
(t CO2 Eq. ha-1) |
(t CO2 Eq. ha-1) |
(t CO2 Eq. ha-1 year-1) |
(t CO2 Eq. ha-1) |
(t CO2 Eq. ha-1) |
| |
September 2020 |
March 2022 |
1.5 Years |
| S. macrophylla |
170.030 |
-0.242 |
170.272 |
205.500 |
0.433 |
205.067 |
-23.196 |
| P. occidentalis |
186.940 |
-0.075 |
187.015 |
192.800 |
0.028 |
192.772 |
-3.838 |
| P. caribaea |
185.830 |
-0.237 |
186.067 |
190.230 |
0.714 |
189.516 |
-2.299 |
| S. mahagony |
336.700 |
0.621 |
336.079 |
229.240 |
1.126 |
228.114 |
0.982 |