Introduction
Coronavirus disease 2019 (Covid-19) pandemic dramatically reshaped oncological care, revealing the heightened vulnerability of patients with cancer who exhibit high likelihood of severe Covid-19 complications and increased mortality [
1,
2,
3]. However, there is limited understanding regarding which specific cancer pre-conditions aggravate the course of Covid-19 infection, underscoring the need for identifying patients with cancer at risk requiring prompt management and close surveillance.
SARS-CoV-2 infection leads to a broad spectrum of disease severities. Immune changes associated with severe disease include a pronounced pro-inflammatory cytokine storm and an expansion of immature myeloid populations, with neutrophils playing a pivotal role [
4,
5,
6,
7,
8,
9,
10]. This inflammatory cascade, marked by a suite of indicators such as neutrophilia, the derived neutrophil-to-lymphocyte ratio (dNLR), c-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-alpha), is essential not only to the innate immune defense against Covid-19, but also in the context of cancer [
11,
12]. Moreover, neutrophil dysfunction, including the formation of neutrophil extracellular traps (NETs), has been linked to acute respiratory distress syndrome (ARDS), thromboembolic events, and increased mortality in Covid-19 [
6], reflecting the adverse outcomes often associated with neutrophils in patients with cancer [
12].
This study investigates the underexplored critical influence of pre-existing cancer-induced inflammation on clinical outcomes of Covid-19. It hypothesizes that a cancer-induced pro-inflammatory state may intensify the inflammatory response to SARS-CoV-2, adversely impacting Covid-19 outcomes. The findings may have significant implications for the management strategies of patients with cancer and Covid-19 infection.
Methods
Study Design, Study Population, and Data Collection
Retrospective Clinical Cohort
We conducted a multi-center, international, retrospective cohort study involving 524 cancer patients with confirmed SARS-CoV-2 infection, identified between March 2020 and December 2020. Inclusion criteria were: (1) age ≥18 years; (2) a history of solid tumor malignancy prior to or during the Covid-19 disease course; and (3) SARS-CoV-2 infection confirmed by reverse transcription polymerase chain reaction (RT-PCR) on a nasopharyngeal swab. The cohort included patients with active malignancies and patients in cancer remission. Participating centers are listed in Supplementary Table S1. Clinical pseudonymized data, including laboratory and radiologic results, were retrospectively collected from electronic medical reports. The retrospective nature of the study allowed for a waiver of prospective informed consent, with pseudonymized data collection as per standard of care protocols.
Prospective Exploratory Cohort
In parallel, a prospective exploratory cohort study was conducted at Hospital Clínic de Barcelona from July 2020 to May 2021. Patients with cancer newly diagnosed with SARS-CoV-2 infection were included. The eligibility criteria, as well as clinical, laboratory, and radiological data collected, were the same as the retrospective cohort. Informed consent was obtained for prospective blood collection at various timepoints during Covid-19 infection, enabling dynamic immunological assessment. The immunophenotype of circulating immune cells was assessed in fresh peripheral blood at each timepoint using four distinct flow cytometry panels (Supplementary Table S2). In parallel, a multiplex cytokine assay (ProcartaPlex Multiplex Cytokine Assay from Thermo Fisher) was applied to measure 20 different cytokines in these same blood samples (Supplementary Table S3). Healthy volunteers and cancer patients without SARS-CoV-2 infection also participated as control groups after providing informed consent. The same four flow cytometry panels and cytokine assay were assessed per each sample
Data Collection
Clinical data encompassed patients’ demographics, comorbidities, oncological history, and detailed Covid-19 features. The latter included symptom presentation, radiological findings, length of hospitalization, therapeutic interventions, oxygen requirements, intensive care unit admissions, secondary complications, and 30-day mortality. Laboratory data, specifically circulating inflammatory markers such as dNLR, were collected at two distinct timepoints: pre-SARS-CoV-2 infection (15-45 days prior to diagnosis) and at the time of Covid-19 diagnosis. dNLR was defined as [neutrophils]/[leucocytes-neutrophils]) and was considered high if >3, consistent with previous literature [
13].
Endpoints
Primary endpoints were 30-day mortality and severity of Covid-19 disease: (a) mild Covid-19 was defined as the presence of only mild symptoms without any requirement for supplemental oxygen; (b) moderate Covid-19 was defined as the condition where supplemental oxygen was required but at a flow rate of less than 15 liters per minute (L/min); (c) severe COVID-19 was defined as the condition where supplemental oxygen was delivered at a flow rate of 15 L/min or more.
Immunophenotyping
A multiparametric flow cytometry analysis was performed with whole-blood samples collected into EDTA, by staining of cell surface markers and standard flow cytometry methods. Briefly, 100µL of heparinized whole blood was incubated for 15 min at room temperature (RT) with the appropriate concentration of monoclonal antibodies (mAbs). Cells were then incubated with 2 ml of BD lysing solution 1x (BD Bioscience, United States) for 15 min at RT to lyse erythrocytes and fix cells. Finally, cells were washed two times with phosphate-buffered saline (PBS) 1X. Peripheral blood leukocytes were stained as indicated with the following combinations of antibodies: 1) for enumeration, CD45-APCH7, CD16-BV510, CD3-APC, CD4-FITC, CD8- PercpCy5.5, CD19-PECy7, CD244-BV421, CD56-PE; 2) neutrophils subsets, CD15-PE, CD14- PercpCy5.5, CD16-PECy7, CD10-BV421, LOX 1 FITC, CD11b-BV510, CD64-APCH7, CD62L-APC; 3) monocytes subsets, CD16-BV510, CD14-PercpCy5.5, CCR5-BB515, CD44-PECy7, CD71-APCH7, CD33-APC, HLA-DR-V450, CD163-PE; 4) T lymphocytes subsets, TCRγδ-PE, CD3-APC-H7, CD4-FITC, CD8-PercPCy5.5, CD45RA-PECy7, CCR7-BV510, HLA-DR-APC, PD-1-BV421. All monoclonal antibodies used in the panels were obtained from BD Biosciences (Franklin Lakes, New Jersey, United States), except for LOX1, which was sourced from ThermoFisher. All samples studied with flow cytometry were acquired using a FACSCanto-II (BD Bioscience) cytometer. A minimum of 200,000 events were acquired for the different populations studied. FCS files were exported from BD FACSDiva Software in a 3.0 format. Analysis was performed using Infinicyt software (Cytognos SL, Salamanca, Spain).
Serum Analyses
Quantification of cytokines including sE-Selectin; GM-CSF; ICAM-1/CD54; IFN alpha; IFN gamma; IL-1 alpha; IL-1 beta; IL-4; IL-6; IL-8; IL-10; IL-12p70; IL-13; IL-17A/CTLA-8; IP-10/CXCL10; MCP-1/CCL2; MIP-1alpha/CCL3; MIP-1 beta/CCL4; sP-Selectin; TNF alpha was performed using the Inflammation 20-plex Human ProcartaPlex-Panel (ThermoFisher). Plasma samples were thawed at RT, diluted 1:2 with Universal Assay Buffer (provided by the manufacturer), and assayed according to the manufacturer's instructions. Measurements were done with a Bio-Plex 200 System, acquisitions and analyses were performed by using Luminex 100/200.
Statistical Analysis
Categorical variables are stated as numbers (n) and percentages (%). Continuous variables are shown as median with interquartile ranges (IQR) unless indicated otherwise.
Group comparisons utilized Chi-square or Fisher’s exact tests for categorical variables and the unpaired t-test, Wilcoxon rank-sum test, or ANOVA for continuous variables. Survival functions were estimated using the Kaplan-Meier method, with the log-rank test for comparisons. Median follow-up was calculated using the reverse Kaplan-Meier method.
Factors associated with 30-day mortality and Covid-19 severity were tested with logistic regression in univariate and multivariate analyses. Subsets identified from both patients and controls were represented through Principal Component Analysis (PCA) and visualized as heatmaps using the web tool ClustVis.
All P-values inferior to 0.05 were considered statistically significant. Statistical analyses were performed using R software.
Results
Study Population
The characteristics of the study populations, including 524 patients in the retrospective cohort and 27 in the prospective cohort, are detailed in Supplementary Table S4.
Retrospective Clinical Cohort
In the retrospective cohort, median duration of follow up was 84 days (95% CI 78-90). Patients were predominantly male (52%) with median age of 69 years (range: 35-98 years). Hypertension was present in 49%, while 20% had cardiovascular disease. With respect to cancer, 70% had active disease, 64% were at an advanced stage, and 78% with baseline Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) of less than or equal to 1. The most common cancer types were thoracic (26%), gastrointestinal (24%), breast (19%), and genitourinary (14%). Most patients (57%) were under systemic therapy, including chemotherapy (62%), endocrine therapy (22%) and immunotherapy (14%). At the time of Covid-19 diagnosis, 41% presented with moderate to severe symptoms, with fever (68%), cough (53%), and dyspnea (48%) being the most common ones. The majority (90%) required hospitalization, with a median length of stay of 13.5 days (range 1-73). Intermediate or intensive care was necessary for 12% of patients. Patients received treatment with antibiotics (74%), hydroxychloroquine (65%), antiviral therapy (31%), corticosteroids (22%) and other immunomodulatory drugs (10%). Overall mortality was 29%, with a 25% incidence of severe acute respiratory failure (SARF). In those requiring higher levels of care, mortality was 48%, and SARF was 90%. Mortality rates correlated with baseline PS, with 19% for PS <1 and 51% for PS >2 (p<0.001).
Prospective Exploratory Cohort
In the prospective cohort, patients were predominantly female (52%) with median age of 65 years (range: 49-82 years). Hypertension was reported in 52% of patients and cardiovascular disease in 15%. With respect to cancer, 96% had active disease, with 82% at an advanced stage, and 56% had a baseline PS of less than or equal to 1. The most frequent cancer types included genitourinary (26%), gastrointestinal (22%), thoracic (19%), and breast (15%). Most patients (89%) were undergoing systemic therapy, predominantly chemotherapy (67%) and immunotherapy (15%). At the onset of Covid-19, 70% had moderate to severe symptoms, with fever (78%), cough (37%), and dyspnea (33%) as common presentations. All patients were hospitalized, with a median stay of 17 days (range 3-71). Intermediate or intensive care was required for 15% of patients. The treatment regimen included antibiotics (67%), antiviral drugs (56%), corticosteroids (56%), plasma therapy (26%), and other immunomodulatory drugs (33%). The overall mortality rate stood at 15%, with an 18% rate of SARF. In the subset admitted to higher levels of care, mortality and SARF rates were 50% and 75%, respectively. Mortality rates varied with baseline PS, with 0% for PS <1 and 33% for PS >2 (p=0.057).
dNLR in Patients with Cancer and Covid-19 Infection
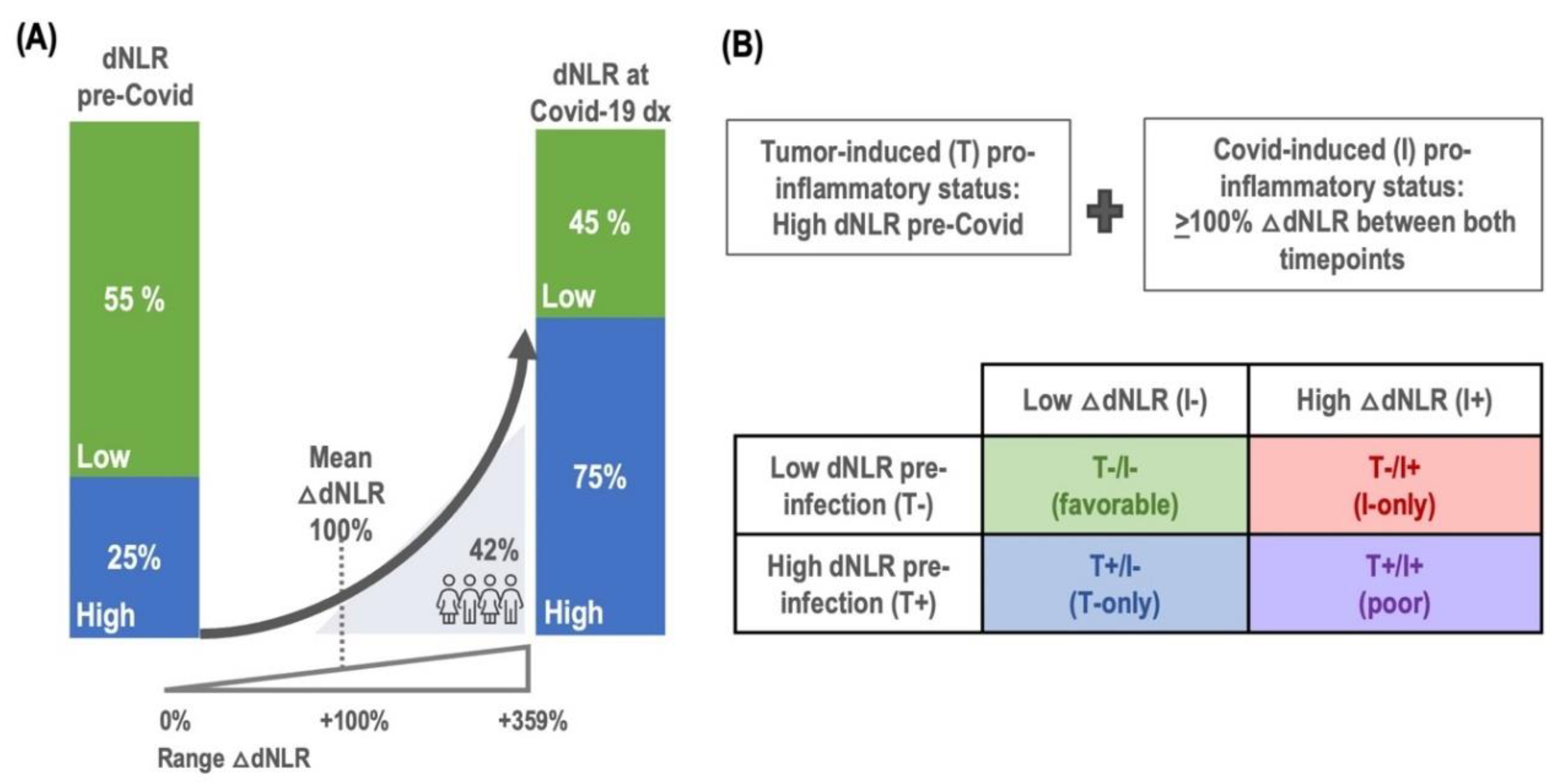
In our retrospective clinical cohort of patients with cancer and Covid-19 infection, a pre-infection pro-inflammatory status, indicated by an elevated baseline dNLR (>3), was observed in 25% of the patients. At the timepoint of Covid-19 diagnosis, the incidence of high dNLR had increased to 55%, suggesting an increase in inflammatory response upon viral infection. The change in dNLR, referred to as Delta dNLR (△dNLR), represents the percentage increase from the baseline to the Covid-19 diagnosis timepoint. The median Delta dNLR, was +70% (IQR: 0-349%), with an average increase of +100%. Notably, 42% of patients experienced a doubling (100% increase) in dNLR, as depicted in
Figure 1A, indicative of a significant inflammatory response associated with Covid-19 infection (
Figure 1A).
Building of the FLARE Score
To develop the FLARE score, we first established criteria for tumor-induced pro-inflammatory status
(T+), defined by a high dNLR pre-Covid-19 infection (at baseline), and Covid-induced pro-inflammatory status
(I+), characterized by a dNLR increase of +100% between baseline and Covid-19 diagnosis. This score, combining both tumor-induced and Covid-induced inflammation, facilitated stratification of patients into four subgroups based on inflammation status:
T+/I+ (poor), for concurrent tumor and infection-related inflammation;
T+/I- (T-only), for tumor-related inflammation only;
T/I+ (I-only), for Covid-related inflammation only; and
T-/I- (favorable), if there was no inflammation (
Figure 1B). The distribution of patients was as follows: 5% were categorized as poor (n=19), 20% as T-only (n=74), 37% as I-only (n=136), and 38% as favorable (n=140). The characteristics of patients and Covid-19 outcomes within these FLARE categories are summarized in
Table 1A,B.
Circulating Neutrophils in Patients with Cancer and Covid-19 Infection
In the prospective exploratory cohort, the immunophenotype of circulating immune cells was examined in 27 cancer patients at different timepoints of Covid-19 infection, with a total of 37 samples analyzed. Patients’ characteristics of this exploratory cohort are described in Supplementary Table S4. This group was compared to a control group of 10 patients with cancer without Covid-19 infection (Cancer Control, CC) and 6 healthy volunteers (HV). The severity of Covid-19 in these 37 samples varied, and the distribution was as follows: 16% baseline/asymptomatic (n=6; Covid-19 diagnosis but with no symptoms), 22% mild (n=8, Covid-19 symptoms but no oxygen requirements), 22% moderate (n=8, oxygen requirements <15ml/min), 30% severe (n=11, oxygen requirements >15ml/min) and were in the recovery phase post-infection (n=4).
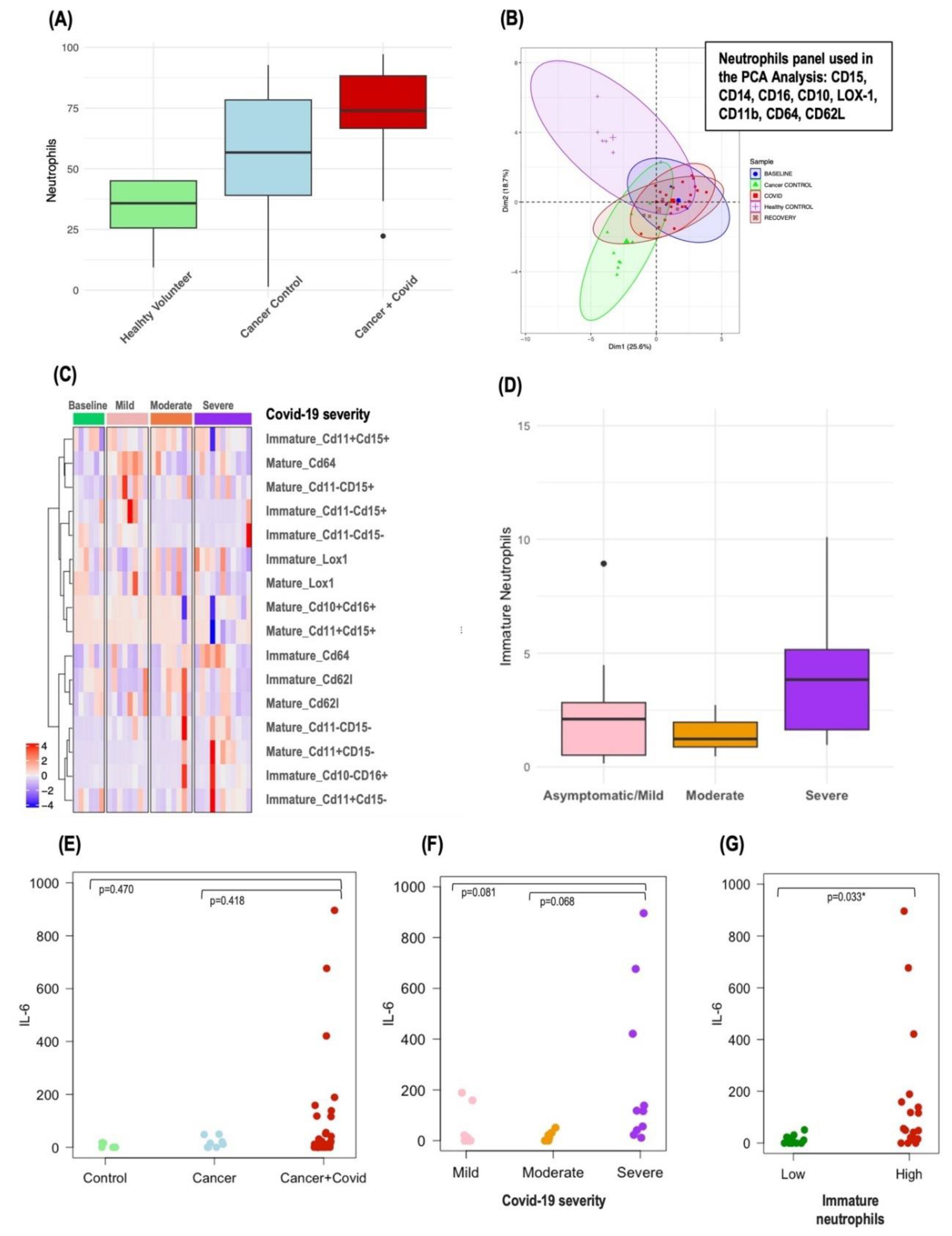
Significant differences in various immune cell populations, including lymphocytes, monocytes, eosinophils, and macrophages, were identified between the different Covid-19 severity groups, cancer controls and healthy volunteers. Of particular interest were the circulating neutrophils. Patients with cancer had a median neutrophil percentage of 56.7% (39-78.4%), which was higher than that of healthy volunteers at 35.8% (25.6-45%). This percentage increased dramatically in patients with both cancer and Covid-19 infection to 73.9% (66.7-88.3%) (p=0.0003) as shown in
Figure 3A. The increase was even more pronounced in patients with cancer and severe Covid-19 infection, where the median neutrophil percentage escalated to 88.4% (84.5-95.8%).
Further principal component analysis (PCA) differentiated the immunophenotypes of circulating neutrophils in patients with cancer and Covid-19 infection from those in CC and HV, as depicted in
Figure 3B. Interestingly, cancer patients across all Covid-19 severity categories—mild, moderate, and severe—tended to cluster together in the PCA plot.
Circulating Immature Neutrophils in Patients with Cancer and Covid-19 Infection
To assess the relevance of specific neutrophil subpopulations in patients with cancer and severe Covid-19 infection, we focused particularly on immature neutrophils due to their known aggressiveness and association with severe Covid-19 infection. These immature neutrophils were characterized as
CD10 negative/CD16 positive, with additional markers CD11b and CD15 expressed variably (CD11bpos/neg, CD15pos/neg), whereas mature neutrophils were identified as
CD10 positive/CD16 positive, as detailed in Supplementary Figure S1. PCA did not reveal distinct profiles in immature neutrophils that correlated with Covid-19 severity (Supplementary Figure S2A). However, a heatmap analysis of immature and mature neutrophil subpopulation exposed distinct clusters, differentiating primarily the severe and moderate Covid-19 cases from the milder forms (
Figure 3C). Notably, patients with cancer and severe Covid-19 infection had a higher median percentage of circulating immature neutrophils (4.64% [3.02-10.1%]) compared to those with asymptomatic/mild (2.1% [0.51-2.82%]) or moderate disease (1.42% [0.92-2.43%]) (p=0.360) (
Figure 3D). Yet, no definitive link was established between levels of immature neutrophils and 30-day mortality, although this observation was limited by the small number of events (n=3 for immature neutrophils data).
Correlation between Circulating Inflammatory Cytokines, Circulating Immature Neutrophils, and Circulating Inflammatory Markers (dNLR) in Patients with Cancer and Covid-19 Infection
In our investigation, we utilized a multiplex cytokine assay to measure twenty key circulating inflammatory cytokines, analyzing their levels in relation to the proportion of immature neutrophils and established circulating inflammatory biomarkers such as dNLR. Among the cytokines examined, IL-6 stood out, showing the most pronounced variations across Covid-19 severity groups (
Figure 3F), with a discernible trend when comparing patients with cancer and Covid-19 to both CC and HV (
Figure 3E). Patients with a higher proportion of immature neutrophils also demonstrated elevated IL-6 levels (
Figure 3G), and a robust correlation was observed between IL-6 concentrations and dNLR (corr=0.705, p<0.001). Detailed patient characteristics from the exploratory cohort—including dNLR, the proportion of immature neutrophils, IL-6 levels, and Covid-19 outcomes—are presented in
Table 2, enabling a comprehensive visual assessment of the correlations. However, it is important to note that no association was found between IL-6 levels and 30-day mortality, an observation that was limited by the small number of events (n=3 for IL-6 data).
Monitoring of Circulating Neutrophils and IL-6
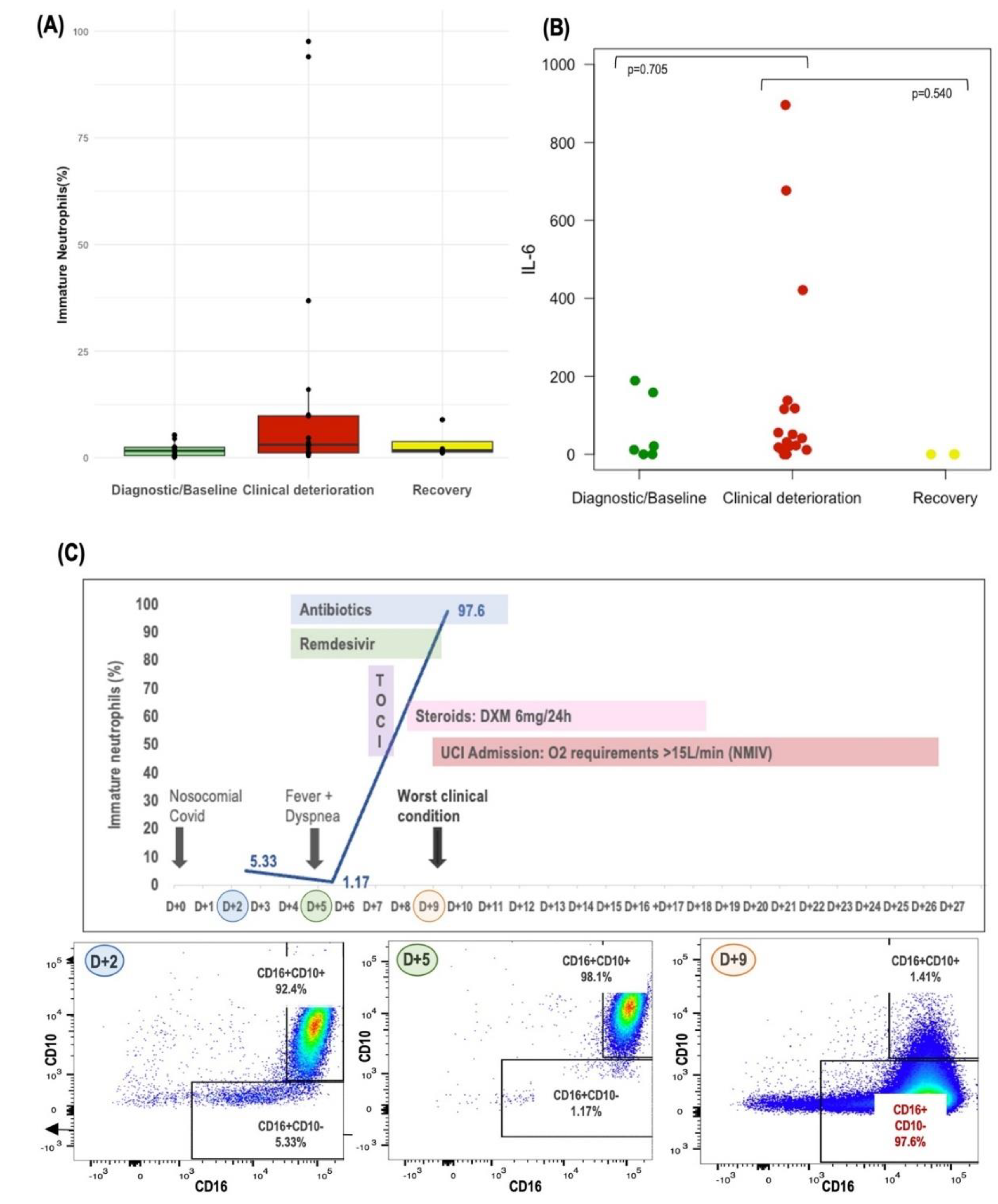
Lastly, we tracked the evolution of circulating neutrophils in patients with sequential samples available throughout the evolution of Covid-19 infection. This dynamic analysis allowed us to discern patterns and highlighted how the emergence of immature neutrophils during Covid-19 evolution could lead to development of severe Covid-19 disease. Notably, we observed a consistent trend towards a higher proportion of immature neutrophils, alongside elevated IL-6 levels, in samples collected during periods of clinical deterioration as compared to those from baseline or recovery phases, as depicted in Figure 5A,B. Additionally, the case study of FLARE #15 is presented in Figure 5C, which graphically depicts the evolution of circulating immature neutrophils, illustrating the potential impact of these cells on the severity of the patient's Covid-19 infection.
Discussion
The interplay between cancer-associated conditions and Covid-19 outcomes has been extensively studied, with factors like comorbidity burden, gender, age, and tumor stage being widely recognized as influential [
1,
2,
3]. Yet, the major role of systemic inflammation, a pathogenic mechanism shared by both cancer progression and Covid-19, remains to be fully elucidated.
Building upon the findings of Dettorre et al. and Cortellini et al. [
14,
15], which underscore the prognostic value of inflammatory markers such as the neutrophil-to-lymphocyte ratio (NLR) in predicting adverse outcomes and sequelae for patients with cancer and Covid-19 infection, our study aimed to expand the scope by examining the impact of a pre-existing tumor-induced inflammatory state. We explored whether such a state predisposes patients with cancer to an exacerbated innate immune response upon Covid-19 infection.
Numerous routine blood parameters have been investigated as potential circulating inflammatory biomarkers in patients with cancer, including elevated neutrophils, platelets, LDH and hypoalbuminemia, all of them associated with poor outcomes [
16,
17]. More recently, novel neutrophils-based ratios, such as NLR and dNLR, have also been examined. The NLR is a well-known prognostic factor in patients with cancer [
11] and has also been shown to be an independent risk factor for severe Covid-19 disease [
8]. We focused on the dNLR for its broader inclusion of granulocyte and monocytes subpopulations and because it offers a relative ratio of neutrophils while considering the total white blood cell count, thus providing a more comprehensive inflammatory profile. High dNLR has been linked to poorer outcomes across various cancer types [
18,
19,
20].
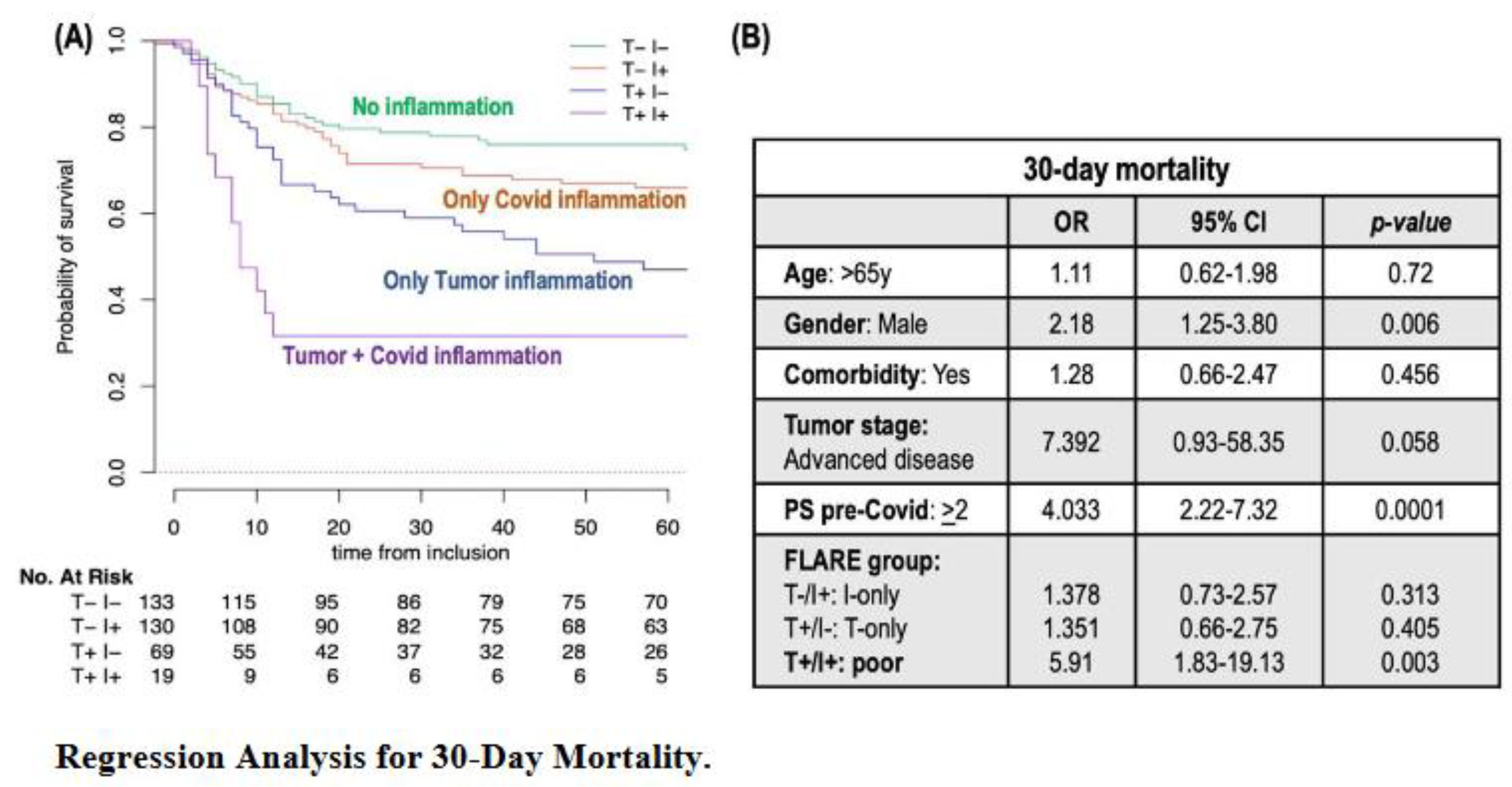
In the present work, we demonstrate how the FLARE score, derived from dNLR, helps stratify patients with cancer and Covid-19 infection into four distinct groups, identifying those at the highest risk of early mortality. Among our cohort of 524 patients, the 30-day mortality rate was 29%, in line with previous reports [
1,
2]. Notably, 5% of patients with a poor FLARE were significantly more likely to have a fatal outcome with a 30-day mortality rate of 68%, whereas patients with T-only, I-only, and favorable FLARE had early mortality rates of 39%, 33% and 23%, respectively (p<0.001). This score proved to be an independent early-mortality predictor, even after adjusting for factors such as age, gender, comorbidities, tumor stage, and baseline PS. Furthermore, the rate of Covid-19 complications also varied significantly across these groups, with 88% for poor FLARE
versus 79%, 75%, and 54% in T-only, I-only, and favorable FLARE, respectively (p<0.001). Altogether, our results warrant the FLARE score in patients with cancer and Covid-19 infection as an attractive and inexpensive predictor of both mortality and morbidity in this population.
In our exploratory analysis, we examined the immunophenotype of circulating neutrophils in a small cohort of patients with cancer and Covid-19 infection and aimed to shed light on the potential key subpopulations driving the innate immune response to SARS-CoV-2. While neutrophils have historically been viewed as homogenous, recent studies underscore their functional diversity [
21,
22]. Immature neutrophils are thought to result from premature release from the bone marrow and are known for their altered functional capacity. Their prevalence has been linked to systemic inflammatory responses and clinical deterioration in patients with sepsis and may also contribute to cancer progression [
23,
24,
25]. These immature forms can often be increased in the peripheral blood of patients with cancer due to systemic chemokines produced by either the tumor or cancer treatments [
12].
Emerging studies suggest that the shift toward immature forms, with an hyperactivation of immature CD10- subpopulations, may be a key determinant of Covid-19 severity [
26,
27,
28]. In line with other studies, our results confirm a greater number or circulating neutrophils with a different immunophenotype in patients with cancer and Covid-19 compared with cancer controls and healthy volunteers, and a transition towards immature forms in patients who developed a severe Covid-19 disease. To the best of our knowledge, this is the first study to confirm this trend towards immature forms in patients with cancer and Covid-19 infection. Our understanding of immature neutrophils though remains incomplete, but this evidence warrants further research into how these cells contribute to both cancer and Covid-19 disease.
Research has shown that severe Covid-19 can be predicted not only by emergent myelopoiesis, as previously described, but also by increased levels of inflammatory biomarkers, notably IL-6 [
29]. High IL-6 levels are known to predict worse outcomes in Covid-19 [
30,
31] and contribute to the unique pattern of immune dysregulation seen in severe cases [
32]. In patients with cancer, IL-6 is recognized for its role in promoting tumor growth and treatment resistance [
33,
34,
35]. In our study, we analyzed twenty inflammatory cytokines in patients with cancer and Covid-19 and found that IL-6 stood out for showing the most significant variations across the spectrum of Covid-19 severity. Notably, IL-6 levels were elevated in patients with a higher proportion of immature neutrophils, highlighting a link with an intensified innate immune response. Moreover, the significant correlation between IL-6 and dNLR emphasizes the use of dNLR as a biomarker for tracking the inflammatory trajectory of Covid-19 in patients with cancer.
Our study does face limitations, including the retrospective nature of the multicentric cohort with missing data, and variability in treatment decisions. Moreover, immunophenotyping was restricted to a small cohort and a limited panel of surface markers. These constraints limit the strength of our conclusions. Additionally, an important limitation of our work is the uncertainty regarding whether the prognostic value of the FLARE score is specific to Covid-19 or if it could also apply to other viral infections and bacterial infections. We have not yet explored the FLARE score's applicability beyond the context of Covid-19, leaving open the question of its potential utility in predicting outcomes for patients with cancer affected by other types of infections.
Despite these limitations, our findings highlight the importance of a pre-existing tumor-induced proinflammatory status, introducing the FLARE score as a practical outcome predictor for patients with cancer and Covid-19 infection. Our findings, particularly regarding the role of immature neutrophils and their potential as prognostic biomarkers, underscore the need for further investigation to fully understand their impact on disease progression in both cancer and Covid-19 infection.
Conclusions
Our study aimed to identify circulating inflammatory biomarkers that are predictive of progression to severe Covid-19 among patients with cancer – markers that could potentially be identified before the disease reaches its peak, thereby enabling the initiation of timely therapeutic interventions. Through retrospective analysis, we discovered that a pre-existing tumor-induced inflammatory state, indicated by elevated dNLR levels prior to the diagnosis of Covid-19, was indicative of adverse outcomes in these patients. We developed the FLARE score, an integrative measure reflecting inflammation driven by both the tumor and Covid-19 infection. The FLARE score is an easy-to-use and worldwide accessible tool that effectively stratifies patients with cancer by their risk of Covid-19 complications and early mortality, aiding clinicians in identifying and prioritizing those who might benefit most from immediate and intensive treatment strategies. In our prospective exploratory cohort, the immunophenotypic analysis of circulating neutrophils revealed that the accumulation of immature neutrophils could be associated with an unfavorable progression of Covid-19 disease. This finding indicates that a more in-depth characterization of neutrophil subpopulations and improve our ability to predict outcomes for patients with cancer and Covid-19 infection.
Ethic statement
The study received approval from the HCB Ethics Committee (IRB no. HCB/2020/1026). All cancer patients, whether infected with Covid-19 or not, who were participating in the exploratory prospective cohort, provided written informed consent. Additionally, samples from 6 healthy volunteers (HVs) were obtained from the immunology department, with prior consent granted for their use in research.
Declaration of Interest
E.S declares personal fees for educational events and/or material from Novartis, Pfizer, Eisai, and Daiichi Sankyo; advisory fees from Pfizer and Seagen; and travel/accommodation expenses from Gilead, Daiichi Sankyo, Novartis, and Lilly. M.G.H reports personal fees (invited speaker) from Ipsen and travel/accommodation expenses from Novartis. T.G declares personal fees (invited speaker) from GSK and travel/accommodation expenses from Bristol-Myers Squibb, Reddy’s, Pfizer and MSD. J.G declares personal fees (invited speaker) from LEO Pharma and Astellas Pharma, and travel/accommodation expenses from Lilly, Bristol-Myers Squibb/Roche, Ipsen, Novartis and Roche. J.C.T reports travel/accommodation expenses from: Merck, Pfizer, Roche, and Lily/Genentech. M.T declares travel/accommodation expenses from Eli-Lilly. C.T declares no competing non-financial interests but reports advisory and consulting fees from Novartis and AstraZeneca, lecture fees from Roche, Pfizer, Biocartis, MSD and Janssen Oncology, research from Novartis. H.A declares consulting fees from Astra Zeneca, lecture fees from Takeda and travel/accommodation expenses from Angelini Pharma, BMS, MSD, Roche, Takeda. S.P reports personal fees (invited speaker, advisory board) from AstraZeneca, Eli-Lilly, Novartis, AMGEN, Takeda, Sanofi, Bristol Myers Squibb, MSD, and Roche; and research grants from AstraZeneca, Bristol Myers Squibb, and Roche outside the submitted work. X.M. declares research grants from Bristol-Myers-Squibb; fees for educational activities from Novartis, Bristol-Myers-Squibb, Astra Zeneca, Roche, Pfizer, MSD, Takeda, Ferrer, Esteve; advisory fees from Takeda, Pfizer, MSD, Boehringer, Astra Zeneca; travel/accommodation expenses from Lilly, Boehringer, Roche and Bristol-Myers-Squibb. F.B-M. has the HER2DX patent filed (PCT/EP2022/086493), the DNADX patent filed (EP22382387.3) and the TNBCDX patent filed (EP23382703.9). A.P. reports advisory and consulting fees from Roche, Daiichi-Sankyo, AstraZeneca, Pfizer, Novartis, Guardant Health, and Peptomyc, lecture fees from Roche, Novartis, AstraZeneca, and Daiichi Sankyo, institutional financial interests from Boehringer, Novartis, Roche, AstraZeneca, Daiichi-Sankyo, MedSIR, SL, Celgene, Astellas and Pfizer; stockholder and consultant of Reveal Genomics, SL; a patent PCT/EP2016/080056, the HER2DX patent filed (PCT/EP2022/086493), the DNADX patent filed (EP22382387.3) and the TNBCDX patent filed (EP23382703.9). L.M. declares research grants from Amgen, Inivata, AstraZeneca, Gilead; fees for educational activities from Bristol-Myers Squibb, AstraZeneca, Roche, Takeda, Janssen, Pfizer, MSD, Daiichi-Sankyo, Radonova; advisory fees from Roche, Takeda, AstraZeneca, Jannsen, MSD; and travel/accommodation expenses from Bristol-Myers Squibb, Roche, Takeda, AstraZeneca, Janssen. The other authors declare no conflicts of interest.
Data sharing statement
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Code availability statement
No proprietary R codes were used for the purpose of this study and all codes are retrievable online for free.
Declaration of generative AI in scientific writing:
During the preparation of this work the author(s) used ChatGPT4 in the writing process. After using this tool, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Author Contributions
E.S. and L.M. conceived the study. E.S., D.C., J.A.C., M.G.H., M.R., N.E., J.G., J.C.T., M.T., S.P., R.L.C., X.M., C.U., J.B.B., M.V.B. and J.N.M. retrieved Institutional patients’ data. E.S, J.M.T, and E.A, performed the statistical and bioinformatic analyses. E.S and L.M. wrote the first manuscript draft. All authors contributed to the final interpretation of study data. All authors contributed to manuscript revisions and approved its final version.
Funding
This Research Project was supported by Amgen. Any views, opinions, findings, conclusions, or recommendations expressed in this material are those solely of the authors and do not necessarily reflect those of funding entities.
Acknowledgments
E.S. is supported by Contracte Clínic Recerca “Emili Letang i Josep Font” 2022 and I Predoctoral Grant in Precision Oncology, 2022 (Cátedra UB IOP). L.M. has received funding from the Beca SEOM retorno 2019; ESMO fellowship 2019-2021; Contrato Juan Rodes 2020 (ISCIII, Ministry of Health; JR20/00019); Ayuda Juan Rodés SEOM 2020; Ayuda de la Acción Estratégica en Salud- ISCIII FIS 2021 (PI21/01653); Beca SEOM para Proyectos de Investigación en Immunoncología 2021; Beca SEOM-ASTRAZENECA para Proyectos de Investigación para Grupo Emergente 2022; RADONORM, funded by European Union's Horizon 2020 Research and Innovation Programme under Grant Agreement No. 900009; Grupo Español de Tumores Huérfanos e infrecuentes (GETHI), Convocatoria Inés de Pablo Llorens 2023; Becas Gilead a la Investigación Biomédica 2023. A.P. received funding from Fundación CRIS contra el cáncer PR_EX_2021-14, Agència de Gestó d'Ajuts Universitaris i de Recerca 2021 SGR 01156, Fundación Fero BECA ONCOXXI21, Instituto de Salud Carlos III PI22/01017, Asociación Cáncer de Mama Metastásico IV Premios M. Chiara Giorgetti, Breast Cancer Research Foundation BCRF-23-198, and RESCUER, funded by European Union's Horizon 2020 Research and Innovation Programme under Grant Agreement No. 847912. Any views, opinions, findings, conclusions, or recommendations expressed in this material are those solely of the authors and do not necessarily reflect those of funding entities. Preliminary results of this study were presented at ESMO Congress 2020 (36) and ASCO 2022 (37) as poster communications.
References
- Pinato DJ, Zambelli A, Aguilar-Company J, et al. Clinical portrait of the SARS-CoV-2 epidemic in European cancer patients. Cancer Discov. 2020;10(10):1465-1474.
- Pinato DJ, Lee AJX, Biello F, et al. Presenting features and early mortality from SARS-CoV-2 infection in cancer patients during the initial stage of the COVID-19 pandemic in Europe. Cancers. 2020;12.
- Kuderer NM, Choueiri TK, Shah DP, et al. COVID-19 and Cancer Consortium. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907-1918.
- Meizlish ML, Pine AB, Bishai JD, et al. A neutrophil activation signature predicts critical illness and mortality in COVID-19. Blood Adv. 2021;5(5):1164-1177. [CrossRef]
- Schulte-Schrepping J, Reusch N, Paclik D, et al. Severe COVID-19 Is Marked by a Dysregulated Myeloid Cell Compartment. Cell. 2020;182(6):1419-1440.e23.
- Barnes BH, Adrover JM, Baxter-Stoltzfus A, et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med. 2020;217(6).
- Aschenbrenner AC, Mouktaroudi M, Krämer B, et al. Disease severity-specific neutrophil signatures in blood transcriptomes stratify COVID-19 patients. Genome Med. 2021;13(1):7.
- Liu J, Liu Y, Xiang P, et al. Neutrophil-to-Lymphocyte Ratio Predicts Severe Illness Patients with 2019 Novel Coronavirus in the Early Stage. J Transl Med. 2020;18(1):206.
- Hazeldine J, Lord JM. Neutrophils and COVID-19: Active Participants and Rational Therapeutic Targets. Front Immunol. 2021;12:680134.
- García de Guadiana-Romualdo L, Rodríguez Rojas C, et al. Circulating levels of calprotectin, a signature of neutrophil activation in prediction of severe respiratory failure in COVID-19 patients: a multicenter, prospective study (CalCov study). Inflamm Res. 2022;71(1):57-67.
- Templeton AJ, Mairéad G, Mc Namara MG, et al. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis. J Natl Cancer Inst. 2014;106(6).
- Mackey JB, Coffelt SB, Carlin LM. Neutrophil Maturity in Cancer. Front Immunol. 2019.
- Mezquita L, Preeshagul I, Auclin E, et al. Predicting immunotherapy outcomes under therapy in patients with advanced NSCLC using dNLR and its early dynamics. Eur J Cancer. 2021;151:211-220. [CrossRef]
- Dettorre GM, Dolly S, Loizidou A, et al. Systemic pro-inflammatory response identifies patients with cancer with adverse outcomes from SARS-CoV-2 infection: the OnCovid Inflammatory Score. J Immunother Cancer. 2021;9(3):e002277.
- Cortellini A, Gennari A, Pommeret F, et al. COVID-19 Sequelae and the Host Proinflammatory Response: An Analysis From the OnCovid Registry. JNCI 2022; 114(7): 979–987.
- Mc Millan, DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39(5):534-540.
- Charrier M, Mezquita L, Lueza B, et al. Circulating innate immune markers and outcomes in treatment-naïve advanced non-small cell lung cancer patients. Eur J Cancer. 2019;108:88-96.
- Suzuki R, Takagi T, Hikichi T, et al. Derived neutrophil/lymphocyte ratio predicts gemcitabine therapy outcome in unresectable pancreatic cancer. Oncol Lett. 2016;11(5):3441-3445.
- vanKessel KEM, de Haan LM, Fransen van de Putte EE, et al. Elevated derived neutrophil-to-lymphocyte ratio corresponds with poor outcomes in patients undergoing pre-operative chemotherapy in muscle-invasive bladder cancer. Bladder Cancer. 2016;2(3):351-360.
- Amato RJ, Flaherty A, Zhang Y, et al. Clinical prognostic factors associated with outcome in patients with renal cell cancer with prior tyrosine kinase inhibitors or immunotherapy treated with everolimus. Urol Oncol. 2014;32(3):345-354.
- Silvestre-Roig C, Fridlender ZG, Glogauer M, Scapini P. Neutrophil Diversity in Health and Disease. Trends Immunol. 2019;40(7):565-583.
- Arasanz H, Bocanegra AI, Morilla I, et al. Circulating Low Density Neutrophils Are Associated with Resistance to First Line Anti-PD1/PDL1 Immunotherapy in Non-Small Cell Lung Cancer. Cancers. 2022;14(16):3846.
- Daix T, Guerin E, Tavernier E, et al. Multicentric standardized flow cytometry routine assessment of patients with sepsis to predict clinical worsening. Chest. 2018;154(3):617-627.
- Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16(7):431-446.
- Shaul ME, Eyal O, Guglietta S, et al. Circulating neutrophil subsets in advanced lung cancer patients exhibit unique immune signature and relate to prognosis. FASEB J. 2020;34(3):4204-4218.
- Rice CM, Lewis P, Ponce-Garcia FM, et al. Neutrophils in severe COVID-19 are characterized by a hyperactive immature state and maintained CXCR2 expression. Life Sci Alliance. 2023;6(2):e202201658.
- Carissimo G, Xu W, Kwok I, et al. Whole blood immunophenotyping uncovers immature neutrophil-to-VD2 T-cell ratio as an early marker for severe COVID-19. Nat Commun. 2020;11(1):5243.
- Combadiere B, Adam L, Quentric P, et al. LOX-1+ immature neutrophils predict severe COVID-19 patients at risk of thrombotic complications. Front Immunol. 2021;12:752612.
- Townsend L, Dyer AH, Naughton A, et al. Severe COVID-19 is characterised by inflammation and immature myeloid cells early in disease progression. Heliyon. 2022;8:e09230.
- Han H, Ma Q, Li C, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9(1):1123-1130.
- Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146(1):128-136.e4.
- Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe. 2020;27:992-1000.
- Kumari N, Dwarakanath BS, Das A, Bhatt AN. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016;37(9):11553-11572.
- Weber R, Groth C, Lasser S, et al. IL-6 as a major regulator of MDSC activity and possible target for cancer immunotherapy. Cell Immunol. 2021;359:104254.
- Huseni MA, Wang L, Klementowicz JE, et al. CD8+ T cell-intrinsic IL-6 signaling promotes resistance to anti-PD-L1 immunotherapy. Cell Rep Med. 2023;4(1):100878.
- E. Seguí, E. Auclin, D. Casadevall et al. Change of circulating pro-inflammatory markers between preCOVID-19 condition and COVID-19 diagnosis predicts early death in cancer patients: The FLARE score. ESMO 2020. Ann Oncol, September 2020; 31(4):S1008.
- E. Seguí, JM Torres, E. Auclin et al. The FLARE score, circulating neutrophils, and association with COVID-19 outcomes in patients with solid tumors. ASCO 2022. Journal of Clinical Oncology, June 2022; 40(16):
suppl.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).