1. Introduction
Human haircare practices have long been influenced by culture and the environment, with diverse populations worldwide embracing distinct approaches to maintaining and styling their hair. The unique hair characteristics among different ethnic groups have always been a fascinating and controversial subject for the public and researchers alike. Ethnically, human scalp hair is classified into three major groups based on macroscopic characteristics, namely, Asian, European, and African hair [
1]. However, these categories have been considered limiting, with no representation of diversity and variability seen within and between genetically distinct groups [
2]. A recent more detailed study on hair growth, diameter, colour, and shape in young adults from 24 different ethnic groups recorded in the five continents significantly extended hair classification beyond traditional types [
3].
Afro-textured hair faces several major challenges in hair care due to inadequate knowledge and understanding of its unique properties. This lack of understanding often leads to inappropriate hair care products and practices, which can have detrimental effects on both hair and scalp [
4]. Studies have shown that hair care practices such as thermal or chemical hair straightening, hair braiding, or weaving can cause various "traumatic" alopecias in African-American women [
5]. Additionally, tight hairstyles and hair extensions can lead to traction alopecia, a type of hair loss caused by prolonged mechanical stress and tension on the hair follicles [
6].
Studies have also shown that hair loss is a common problem among African women, with a documented direct relationship between hair care practices and hair loss in Nigeria [
7]. It is also important to consider the hair care practices among women of African descent when treating specific hair and scalp disorders [
8]. Addressing these challenges requires a multi-faceted approach. Scientific and clinical partnerships between industry, academia, and public healthcare sectors play a crucial role in translating new scientific findings on Afro-textured hair and skin into practical knowledge that can be shared with the community [
9]. These collaborations can also help bridge the gap between research and everyday hair care practices, leading to more effective solutions based on understanding African hair's biology and specific requirements.
This review discusses these challenges in the context of known factors that play crucial roles in hair anatomy and biology. We summarise the unique organisation of African textured hair, particularly the involvement of hair follicle shape and chemical bonds in the hair fibre, which determines the growth patterns and mechanical properties of the hair. The phenotype of African textured hair is moreover likely associated with genetic factors. Several of them are affected by variations through single nucleotide polymorphism (SNPs), pointing to the likely significant differences in the expression of important traits linked to hair shaft diameter, keratinisation, hair follicle patterning and transcriptional control of hair development. We propose that more detailed studies of the factors responsible for characteristics of African textured hair can aid the formulation of next-generation hair care products compatible with this hair type.
2. Hair as a Composite Molecular System
2.1. Structure and Anatomy of Human Hair
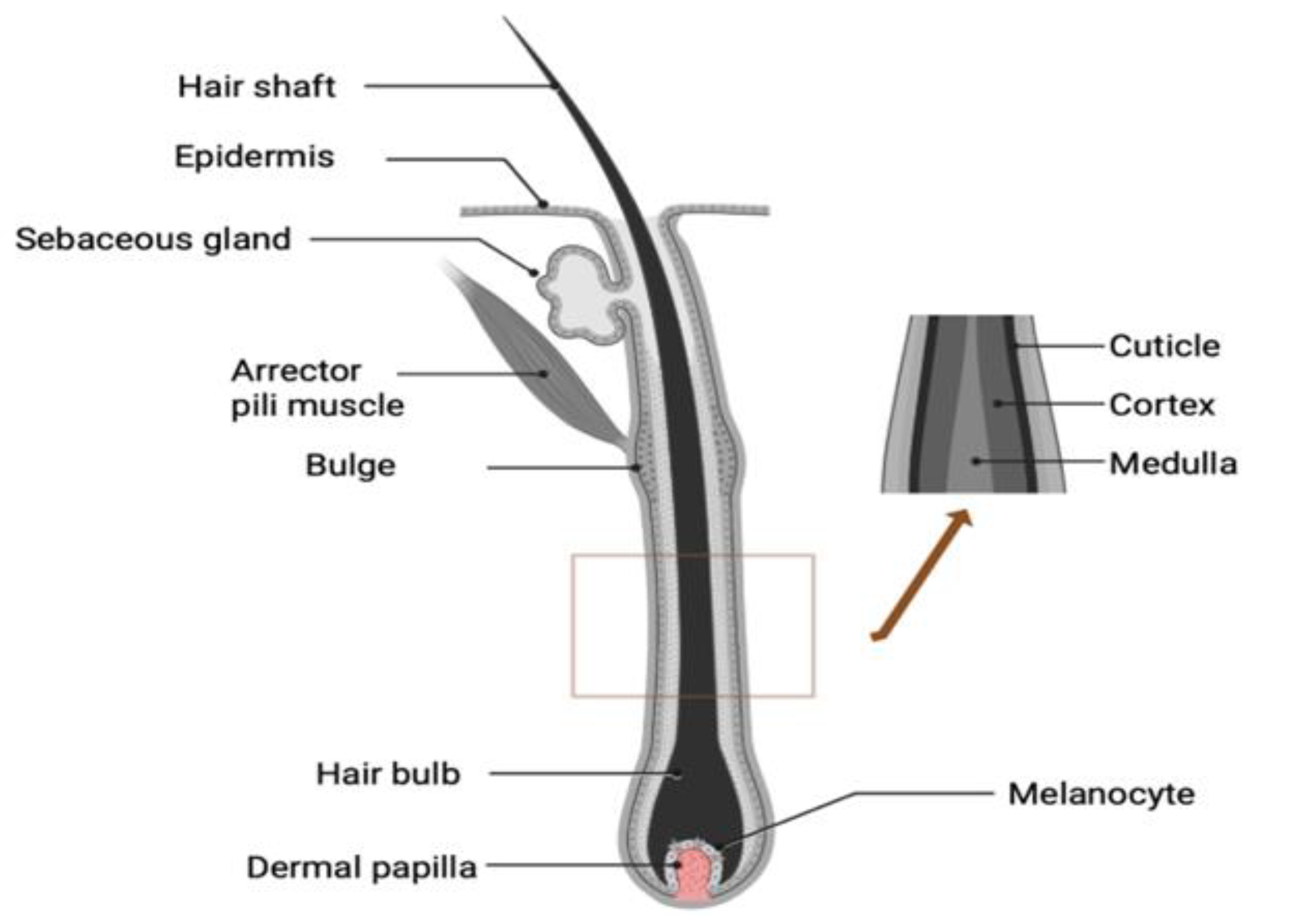
Human hair constitutes intricate fibres composed of various structural components, a perfect example of a natural composite system. The primary building blocks of hair are keratins, fibrous, cysteine-rich proteins that belong to the intermediate filament protein superfamily.
Structurally, the hair fibre is split into three primary sections: cuticle, cortex, and medulla (
Figure 1).
The medulla is centrally positioned within the hair fibre core, which is loosely arranged and may or may not be present depending on hair thickness [
10]. The cortex constitutes the most substantial portion of the hair fibre. It consists of macro fibrils formed from intermediate filaments made up of α-keratins responsible for the mechanical support in their α-helical configuration [
11]. Keratins are divided into acidic type I keratins and neutral type II keratins. These two types combine to form pairs of α-helical protofilaments. Seven to ten pairs of these protofilaments come together to create intermediate filaments (IF) [
12].
Cortical cell filaments are also demarcated by a cell membrane complex comprised of keratin-associated proteins (KAPs) with a high cysteine content and lacking specific spatial arrangements. Coiled ionic forces, hydrogen bonds, van-der-Waals forces, and disulphide bonds hold together the keratin α-helices. The cortex also contains melanosomes responsible for hair colour determined by either pheomelanin (red) or eumelanin (brown/black) present within melanosomes [
13].
The cuticle encloses the cortex and features several stacked sub-lamellar layers (epicuticle, A-layer, exocuticle, and endocuticle), predominantly cross-linked by cysteines and arranged in a scale-like pattern. The outermost epicuticle layer regulates the lubrication of the hair while serving as a barrier against penetration of the molecules from the environment into the hair structure. Surface lipids and protein interactions play vital roles in determining the overall structure of human hair fibres [
10].
2.2. The Role of Chemical Bonds in Hair Structure
Hair proteins form interactions of varying strengths. There are three types of chemical bonds responsible for the overall structure and shape of hair fibres: disulphide, hydrogen, and salt bonds.
Disulphide bonds formed between cysteine residues of the hair keratins are the strongest and mechanically most important for the maintenance of hair shape. These interactions can only be modified or dissolved by harsh processes such as perming or relaxing. The strength of the disulphide bonds is additionally higher when the thiol groups are close to each other, resulting in easier bond formation and ultimately, curlier hair [
10,
13]. The hair shaft of curly hair is also more elliptical, and the shape of the hair follicle is more curved. This leads to a greater number of disulphide bonds between the hair fibres, resulting in more pronounced curls [
14].
Hydrogen bonds are relatively weaker than disulphide bonds but are nevertheless essential in stabilizing the structure of the keratin α-helices that make up the intermediate filaments [
15]. Hydrogen bonds not only play a role in hair elasticity but are also responsible for the hair fibre’s moisture properties, particularly moisture exchange with the environment where the bonds can stretch and reform just by simply wetting the hair [
13,
15,
16].
Salt bonds are formed between two amino acid chains and are affected by changes in pH. These types of bonds are the weakest and responsible for approximately one-third of the hair’s strength [
17].
2.3. Afro-Textured Hair Follicle Structure
Afro-textured hair displays remarkable structural variations when compared to other hair types. Afro-textured hair is not necessarily intrinsically weaker than other hair types but more vulnerable due to its high curvature [
18]. Its cross-sectional appearance is elliptical, with varying shapes and diameters, and it exhibits retro curvature at the hair bulb, resulting in an asymmetrical S-shaped hair follicle [
19]. Research has shown that across all ethnicities, curly hair results from a curly follicle and some form of asymmetry in the mitotic zone around the dermal papilla in the follicle [
14]. These unique features make African hair less resistant to mechanical extension and more prone to premature failure and breakage. The differences in curls and bonds between African and European hair can be attributed to the composition of cysteine residues in disulphide bonds. Afro hair has a higher density of disulphide bonds, contributing to its unique structure and texture [
18]. Cysteine residues play a crucial role in the formation of disulphide bonds, as they contain sulphur atoms that can form covalent bonds with other cysteine residues [
20]. The presence of more cysteine residues in African hair may contribute to the higher number of disulphide bonds, resulting in its characteristic tight curls and reduced elasticity [
18].
3. Hair Growth Cycle
Throughout a person's lifetime, hair follicles undergo regular growth cycles that involve changes in the morphology and activity of the dermal papillae located at the base of the hair follicles, the generation of new hair shafts, and the shedding of old hair [
21]. The hair cycle consists of three phases: the growth phase (anagen), the regression phase (catagen), and the quiescence phase (telogen) [
22]. A fourth phase called the exogen (shedding) has been additionally postulated by some authors [
23].
3.1. Anagen
The anagen phase is characterized by the production of hair fibre, marked by an increase in epithelial cell growth, leading to thickening, elongation, and pigmentation of the hair shaft [
22]. This phase is also distinguished by the development of the onion-like shape of the hair follicle [
24]. The anagen phase is divided further into two phases; pro-anagen (anagen I – V) and metanagen (anagen VI) [
25]. During pro-anagen, hair progenitor cells actively proliferate and initiate the process of differentiation. The new hair shaft subsequently emerges on the skin's surface in the metanagen phase. The anagen phase on the scalp typically lasts for 2 to 5 years, in contrast to the eyebrows and eyelashes, which may only require a few months to grow [
24,
26]. This phase is also associated with the production of melanin in the hair bulb. Immature melanocytes located in the stem cell niche of the hair follicle are stimulated and migrate to repopulate the hair bulb. Melanocytes also undergo morphological and biochemical changes during anagen, becoming highly dendritic and transferring mature melanosomes into keratinocytes [
27].
3.2. Catagen
The catagen phase, also known as the regression phase, is the shortest of all the phases, lasting 2-4 weeks in the human scalp. Catagen is marked by the termination of cell divisions in the hair matrix and apoptosis leading to regression of the inferior portion of the follicle and formation of a club hair [
22,
26,
28]. Tyrosinase is no longer detected in the hair bulb, and melanin production ceases. This brief period may result in a 70% loss of active hair follicles [
27].
3.3. Telogen
Telogen is the third stage of a hair growth cycle, lasting up to four months and characterised by dormant hair follicles. Depending on the hair location, telogen can last from a few weeks to almost a year, with 10-15% of all hair follicles remaining in this phase at any given time. Melanocyte precursors during telogen do not synthesize melanin or express relevant melanogenesis-related proteins. Crucially, despite being relatively quiescent, telogen has been described to possess a significant biochemical activity, which is functionally relevant to the preparation of hair follicles for the next regeneration cycle [
24,
27]. Afro-textured hair has also been found to have the slowest growth rate as more of the hair fibres are found in the telogen phase [
29], (
Table 1).
3.4. Exogen
Exogen is the fourth and often overlooked stage of the hair follicle growth cycle, which is believed to occur before or during the transition from telogen to anagen [
30]. This stage is considered to be essential for the overall health of the scalp, as it helps to remove old hair and replace it with new hair. The shedding process can last up to two months and is usually complete within five months [
31].
The timing and synchronisation of the hair growth cycle phases may differ between Afro-textured and European hair. This can be due to intrinsic and environmental factors, which can cause changes to the growth cycle within the follicular unit [
19,
32].
4. Variations in Afro-Textured Hair in Comparison to Other Ethnic Populations
While many studies have been conducted on human hair, there is only a limited amount of literature available that explores the molecular basis and the genetics behind the structural properties and texture of Afro-type hair and scalp. Although all hair types share some physical and chemical similarities, African hair has nevertheless distinct biological and physical properties compared to other hair types [
29].
4.1. Lipid and Moisture Content
The lipid molecules in hair, such as fatty acids, ceramides, glycolipids, and cholesterols, are the major components forming a laminated structure that provides a barrier to protect against external factors [
33,
34]. This barrier function is due to both internal lipids produced within hair matrix cells and external lipids from surface sebaceous lipids [
35]. Lipids contribute to the properties and morphology of both brown and white hair fibres of different ethnic groups [
36,
37].
The integral hair lipids located in the hair cuticle layers are responsible for maintaining hair integrity, hydrophobicity, moisture, and stiffness [
38]
.
Previous research analysing the lipid composition and distribution among the different ethnic hair types has found that African hair exhibits the lowest radial swelling percentage in water because of its high apolar lipid levels compared to Asian and European hair [
35,
39]. In agreement with the potential variation in lipid content among ethnic hair types, Afro-textured hair has the highest overall lipid content, with the quantities estimated to be 2.5 and 3.2 times higher compared to European and Asian hair respectively [
40]
. Afro-textured hair has also internal lipid contents 1.7 times higher than the other two ethnic groups [
33]. This type of hair additionally contains the highest quantities of free fatty acids, sterol, and polar lipids, which can modify the arrangement of keratin fibres and lead to diverse hair morphologies [
35].
One important difference is the origin of hair lipids, with sebaceous lipids contributing predominantly to Afro-textured hair and internal lipids contributing mainly to European and Asian hair types [
40]. Such lipid distribution could be a crucial factor contributing to the physical and chemical properties of hair shafts. For example, resistance to damage from ultraviolet radiation (UVR) could be linked to the integral lipids content, which is most pronounced in
Asian hair compared to the other two hair types [
35]
.
Other studies demonstrate that the internal lipids of European hair fibres have a higher unsaturated lipid content than their external counterparts, resulting in a lower permeability of the fibre to water [
41,
42,
43]. Consequently, compared to other hair types, European hair also has the highest hydration levels. Such distinctive characteristics may be due to a lower diffusion coefficient, resulting in a decreased permeability compared to Afro-textured hair [
35]. The optimal permeability is crucial for the prevention of hair fibre from losing moisture due to rapid changes in water absorption and desorption. This is supported by an observation that lipid extraction can result in a significant decrease in hair hydration, which is most pronounced in European hair and thought to be related to the degree of lipid saturation or the fraction of internal lipids rather than the total lipid amount [
35,
36,
40,
41,
42,
43], (
Table 2).
Despite its higher lipid content, Afro-textured hair is often characterised as dry or very dry, and this has been attributed to its structure. The distinct biomechanical characteristics of African hair, such as its curvature and spiral hair follicles, create areas of weakness [
45]. These structural traits make the hair more fragile and prone to breakage and ultimately contribute to its dryness. Recognising the influence of fibre curvature on hair dryness is crucial for developing effective hair care strategies and products tailored to individuals with African hair types. By considering the unique properties of African hair, particularly its structural features, targeted approaches can be developed to meet its specific mechanical strength requirements and enhance moisture retention.
4.2. Alopecia in the Black Population
Alopecia, the medical word for hair loss, is a prevalent issue that affects both men and women of all races and ethnicities, but the aetiology differs greatly between various groups. In Black women, many forms of alopecia are associated with hair care practices such as thermal or chemical hair straightening and hair braiding. These types of alopecia include traction alopecia, trichorrhexis nodosa, and central centrifugal cicatricial alopecia [
5].
Originally described in 1968 as “hot comb alopecia”, central centrifugal cicatricial alopecia (CCCA) is a primary lymphocytic alopecia with the highest prevalence among Black women [
46]. Clinically, CCCA is characterised by hair loss that begins on the vertex of the scalp and progresses centrifugally, eventually replacing healthy hair follicles with fibrous tracts [
47]. The pathogenesis of CCCA is thought to involve an intricate array of factors such as genetic susceptibility, gene expression variants, ethnic hair care practices, and disruption of the equilibrium between proinflammatory and anti-inflammatory factors [
48].
A study suggested that mutations in the gene that encodes an enzyme peptidyl arginine deiminase, type III (PADI3) have been associated with the pathogenesis of CCCA [
49]. PADI3 is expressed primarily in the epidermis and hair follicles and is responsible for mediating the alteration of proteins essential for hair shaft formation and the maintenance of its structure, such as trichohyalin, and can additionally play a role in the interfollicular epidermal differentiation [
48,
49].
Patients with CCCA demonstrate an increase in gene expression related to fibroproliferative disorders (FPDs) in the affected scalp compared to an unaffected scalp. Specifically, the upregulation of FPD-associated genes such as platelet-derived growth factor (PDGF), collagen I (COL I), collagen III (COL III), matrix metallopeptidase 1 (MMP1), matrix metallopeptidase 2 (MMP2), matrix metallopeptidase (MMP7), and matrix metallopeptidase 9 gene (MMP9) have been observed [
48,
50].
Most individuals with CCCA present with non-inflammatory conditions with symptoms similar to androgenetic alopecia (AGA) [
49]. AGA is a prevalent type of alopecia affecting up to 50% of males and females and is distinguished by the gradual conversion of terminal hairs into vellus hairs, also known as peach fuzz [
51,
52]. Some of the trichoscopic traits of AGA include hair diameter diversity (HDD), perifollicular pigmentation and yellow spots [
53]. AGA has a clear hereditary basis and is most likely caused by an overreaction to androgen [
54]. The first signs of AGA usually appear during adolescence and progress to hair loss with a specific pattern distribution [
51]. Males experience the most prominent hair loss in the vertex and frontotemporal regions. In contrast, females experience widespread hair loss in the crown and top of the head, resulting in a broader centre part [
54]. AGA becomes more common with age, affecting up to 80% of European men and 42% of women [
51].
Although the pathophysiology of AGA is not entirely understood, increased expressions of 5-dihydrotestosterone and androgen receptors could be the causative factors [
55]. Injectable platelet-rich fibrin, Dutasteride-containing solutions and low-level laser therapies have been also found to be effective and safe in treating certain cases of AGA [
56,
57,
58].
5. Genetics of Curly Hair
The genetic traits behind the distinctive phenotypes of curly hair have been a focus of long-term investigations, frequently challenged due to the variability and complexity of Afro hair fibre. Previously conducted genome-wide association studies (GWAS) identified genes with the potential involvement in variations in human scalp hair fibre shape across different ethnic groups [
14,
59,
60]. The genes are documented to contain single nucleotide polymorphism (SNP), it is likely that these biomarkers only account for a small portion of the hair shape variation and heritability of the traits (
Table 3).
5.1. Trichohyalin (TCHH)
TCHH is expressed in the inner root sheath and medulla of the hair. The protein is involved in cross-linking keratin filaments into rigid structures, providing hair follicles with mechanical strength [
61]. TCHH presents one of the most dominant polymorphic variations associated with curly hair, located within a cluster of fifty other genes linked to keratinocyte renewal and differentiation. Within Europe, about 6% of hair curl and morphology variations are accounted for by TCHH variants, with some contributing to uncombable hair syndromes [
14,
59,
62,
63].
5.2. EGF Receptor Feedback Inhibitor 1 (ERRFI1)
Expression of ERRFI1 is regulated by mechanical force and oxidative stress. The biomarker acts as an adapter protein in control of several signalling pathways associated with skin morphogenesis and the balance between proliferation and differentiation programs in keratinocytes [
64]. ERRFI1 has a genome-wide significant association with hair follicle development and hair shape [
59].
5.3. Peroxisomal Biogenesis Factor 14 (PEX14)
PEX14 is an integral component of peroxisomes, facilitating protein import and peroxisome motility through microtubules [
65]. PEX14 locus is involved in the control of hair shape in Europe [
59].
5.4. Peptidyl Arginine Deiminase 3 (PADI3)
PADI3 catalyses the deamination of structural proteins, such as filaggrin and trichohyalin in hair follicles, modulating their folding and activity. The protein controls the terminal differentiation of keratinocytes and hair shaft formation; PADI3 mutations are also linked to the prevalence of scarring alopecia in African women [
66,
67,
68].
5.5. Transforming Growth Factor Alpha (TGFA)
TGFα is a member of the epidermal growth factor (EGF) family produced by keratinocytes and central to the proliferation and differentiation signalling pathways driving epithelial development. Gene deficiency is linked to abnormalities in the structure of hair follicles [
69,
70].
5.6. Wingless-Type MMTV Integration Site Family, Member 10A (WNT10A)
WNT10A encodes signalling proteins central to epithelial proliferation and differentiation in hair follicle cycling and development through the Wnt/β-catenin pathway. The protein is implicated in hair follicle stem cell self-renewal, hair follicle size, duration of anagen phase and hair thickness [
71]. Variations in the WNT10A gene have been associated with abnormally tightly coiled hair (woolly hair syndrome) and the inability to grow long scalp hair linked to short anagen hair (SAH) syndrome [
72,
73].
5.7. Fraser Extracellular Matrix Complex Subunit 1 (FRAS1)
FRAS1 gene codes for an extracellular matrix protein with a role in the cell adhesion and integrity of the epidermal basement membrane [
74]. Hair shape variation linked to genome-wide association with FRAS1 has also been reported [
59].
5.8. GATA Binding Protein 3 (GATA3)
The GATA3 gene encodes a transcription factor required for the development of lymphoid cells and is involved in innate immune responses of the skin through the secretion of interleukins. The protein controls hair follicles and interfollicular epidermal keratinocytes through the induction of early and late differentiation markers [
75,
76].
5.9. Leucine-Rich Repeat-Containing G Protein-Coupled Receptor 4 (LGR4)
LGR4 is the biomarker with a role in the hair follicle cycle and hair growth through activation of the Wnt/β-catenin signalling pathway. The protein also plays a role in the negative regulation of innate immunity through inhibition of pro-inflammatory cytokines. LGR4 stimulates the proliferation of hair follicle stem cells anagen phase [
59,
77].
5.10. Keratin Associated Protein (KRTAP)/Keratin (KRT)
KRTAP and KRT gene products are the major structural components of hair, with a major function in keratinisation of the hair shaft. The proteins are extensively cross-linked via disulfide bonds and play significant roles in diverse morphological characteristics of hair, including the curly hair phenotypes. KRT71 and KRT74 have also been associated with woolly hair syndrome [
14,
20].
5.11. Protein Tyrosine Kinase 6 (PTK6)
PTK6 is expressed in the stratified differentiated layers of the epidermis. The protein has a role in signal transduction in non-dividing cells; one major activity involves responses and sensitivity to ultraviolet radiation (UVB range) and inflammation [
78].
5.12. Ectodysplasin A receptor (EDAR)
The EDAR gene product is related to the tumour necrosis factor (TNF) receptor family, with a crucial role in the developmental processes of the skin and its appendages such as hair follicles and sweat glands. The protein is involved in the control of the diameter of hair shaft and hair follicle patterning [
79,
80].
5.13. Homeobox C13 (HOXC13)
HOXC13 gene codes for the transcription factor with a conserved role in the developmental processes governing the formation and cycling of hair follicles. In hair follicles, the biomarker is expressed in the nuclei of dermal papillary cells as well as matrix and cortex cells during the anagen phase [
59,
82].
5.14. Serine Protease 53 (PRSS53)
PRSS53 gene product is a serine-type endopeptidase which co-localises with TCHH. The protein expression is high in the inner root sheath of the hair follicle and its activity is modulated during the differentiation of hair fibre [
83,
84].
5.15. Orofacial Cleft 1 Candidate 1 (OFCC1)
OFCC1 gene plays a role in hair morphology [
63].
5.16. Late Cornified Envelope 3E (LCE3E)
LCE3E gene product has a role in keratinisation and formation of the cornified envelope in the stratum corneum. It has been documented that LCE3E also affects curly hair shape [
14,
59].
5.17. Protein-Protein Interactive Network
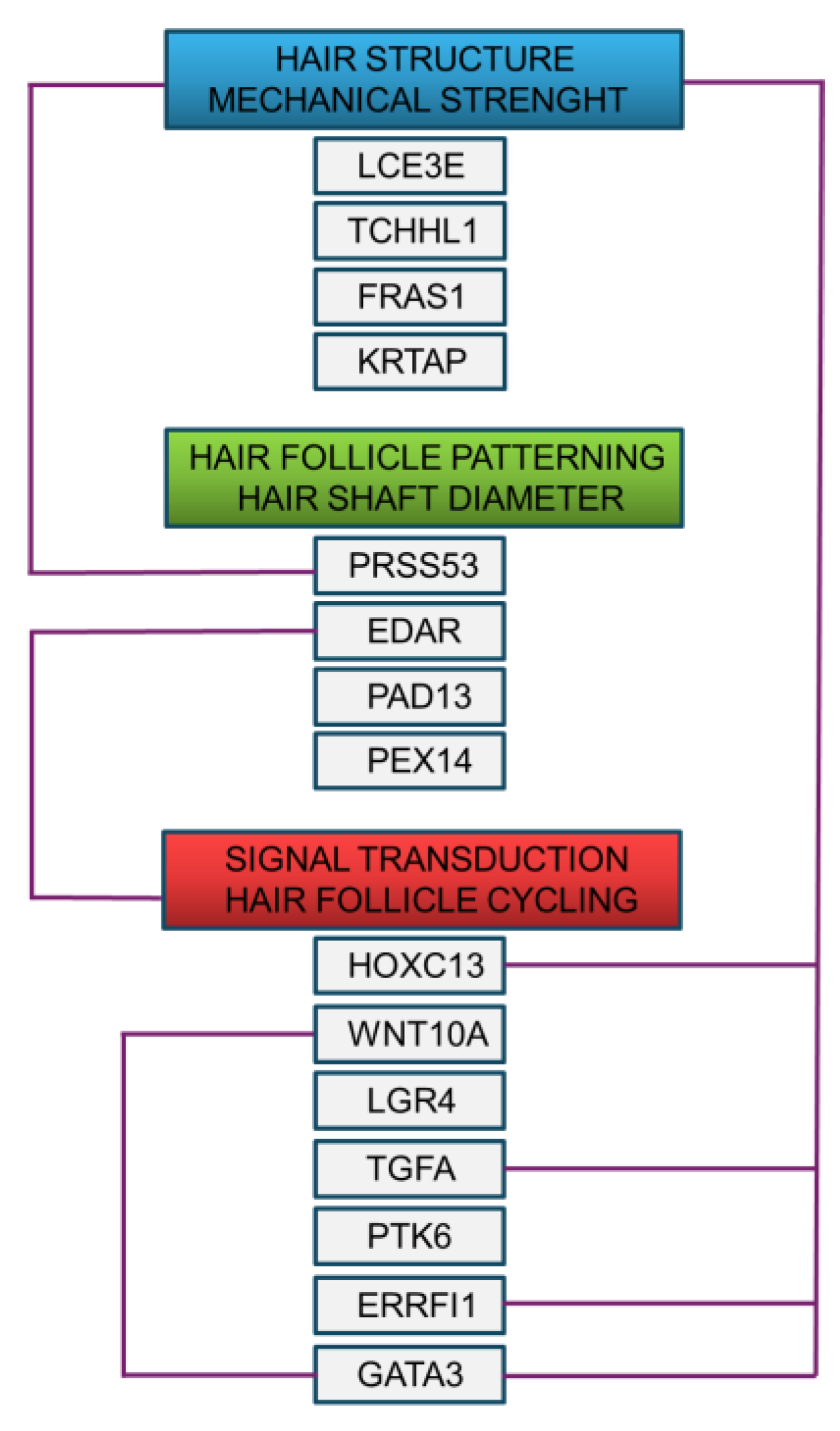
The curly hair biomarkers could be visualised in three major groups, based on the known functions in hair biology and structure. Further functional associations between the biomarkers could be derived from the data available through text mining of the existing literature (
Figure 2).
Notably, three functional groups could be identified through this analysis. The first group involves the genes controlling hair structure through keratinocyte stratification, cross-linking of keratin filaments and integrity of dermal-epidermal interactions, ensuring mechanical strength of hair follicle and hair shaft. The biomarkers of this group are LCE3E, TCHHL1, FRAS1 and KRTAP. The second group involves the genes with the activity in tissue remodelling and protein folding, responsible for hair follicle patterning and hair shaft diameter and formation. The biomarkers of this group are PRSS53, EDAR, PAD13 and PEX14. The third group is comprised of the genes with roles in control of transcription and signal transduction, regulating cell proliferation and innate immunity during hair follicle cycling. The biomarkers of this group are HOXC13, WNT10A, TGFA, LGR4, PTK6, ERRFI1 and GATA3. Several biomarkers fulfil a role at the interface of signal transduction, hair follicle patterning and cycling and hair structure. For example, PRSS53 co-localizes with TCHH in the hair follicle inner root sheath, which is believed to be associated with hair shape [
83,
84]. EDAR has a major involvement in hair follicle development and cycling through signal transduction [
85]. On the other hand, the HOXC13 transcription factor controls the expression of hair keratins and ensures the mechanical strength of the hair shaft [
82]. Deficiency in the TNFA has a severe effect on hair structure, similarly, ERRFI1 also plays a major role in the development of hair structure and shape [
59,
86]. Finally, GATA3 is an important determinant of the hair shaft structure, orchestrated with Wnt-β-catenin signalling in the inner root sheath [
87].
Based on this analysis, it is plausible that the genetics of curly hair links to biomechanics, tissue remodelling and signal transduction; the polymorphic variations through SNPs, and the synergistic effects and overlaps of these closely related genes, would affect the expression and activity of relevant gene products and phenotypes of hair.
6. The Need for Personalisation in Afro-Textured Hair Care
The need for personalised haircare products for Afro-textured hair stems from several various factors. Firstly, societal beauty standards significantly influence the choices and use of hair products among Black women. These standards may have a profound impact, as they perpetuate narrow beauty ideals that may not align with the natural characteristics of Afro-textured hair [
88]. Secondly, Afro-textured hair has unique characteristics requiring specialised care and products to maintain health and manageability effectively. With its tightly coiled structure, Afro-textured hair tends to be more prone to dryness, breakage, and damage, necessitating more robust moisturising and nourishing ingredients to keep it hydrated and minimise frizz [
89]. Lastly, it is crucial to consider the potential health risks associated with certain hair care products. Recent studies have shown a close correlation between the use of specific products containing endocrine-disrupting chemicals (EDCs) and an increased risk of breast cancer among Black women [
89,
90]. Therefore, personalised hair care products tailored to Afro-textured hair are essential to address the specific needs while minimizing potential health risks associated with harmful ingredients [
90].
6.1. Curly and Coily Hair
For years, hair typing systems have been used to classify scalp hair based on observed features such as texture or curl pattern. In the natural hair community, these methods are often used to assist individuals in understanding their hair type and selecting appropriate hair care products and styling procedures.
The most used typing system is the Walker Typing system developed in the 1990s and considered the gold standard for classifying curly and kinky/coily hair [
91]. This system categorises hair into four main types (Type 1 – 4) based on similar characteristics/curl pattern or lack thereof, with each category further subdivided into three (a, b, c) subtypes based on curl pattern, texture, and volume.
Afro-textured hair, also known as Type 4 hair, encompasses a diverse range of textures and patterns that fall under the kinky/coily category. This hair type is defined by its natural tight coils, making it unique and beautiful. Afro hair can be further classified into subtypes, including tight corkscrew curls (4a), wiry curls with sharp angles (4b), and extremely kinky hair with a zigzag pattern (4c) [
91]. It is important to note that Afro hair is often prone to dryness and requires proper moisturisation and care. This hair type has significant shrinkage, which means that its true length may not be immediately visible.
Despite its practical applications, the Andre Walker Typing system has received several criticisms ranging from lack of inclusivity to reinforcement of European beauty standards.
Firstly, it has been observed that the system tends to depend on qualitative measures, which can be imprecise and unclear, thus posing challenges in comprehensively understanding the range of phenotypic variations [
92]. The system has also received significant criticism for displaying a preference for looser curl patterns (Type 2 and 3) over kinky/coily (Type 4) textures. This bias is evident not only in the system's output but also in the way we commonly discuss and categorize different hair textures [
93].
Studies tend to focus more on curly hair than coily hair due to several reasons. One reason is that curly hair is more prevalent in the population, making it easier to recruit participants for the studies [
92]. Coily and curly hair are two different hair types that have unique characteristics and require different hair care practices. Coily hair is defined by tight, springy curls that are prone to dryness and breakage, while curly hair has looser, more defined curls that are also prone to dryness and frizz [
92].
Curly hair has been the target for many beauty and hair care products, making it a more commercially viable research area [
94]. Furthermore, molecular markers and genetic factors have been associated with curly hair, making it easier to study [
95]. However, the need to study coily hair is increasingly recognised as well, given its unique characteristics that require different hair care practices than curly hair [
60].
6.2. Increased Sensitivity of Afro-Textured Hair to Chemical Relaxers and Hair Dye Products
A widely used product in Afro-textured hair management is the chemical relaxer, with sodium hydroxide (NaOH) as one of the most common ingredients. Chemical hair relaxers are a popular method of hair straightening among African-American women due to difficulties that arise from managing their natural hair. However, studies have shown that the use of these relaxers can lead to various health risks. One study has found that the use of hair relaxers may increase the risk of uterine leiomyomata in African-American women [
96]. Another study showed that chemical hair relaxers damage hair and can cause hair loss, with CCCA type of hair loss common among African-American women [
97]. Although the relationship between chemical relaxers and CCCA is unclear, most other forms of hair grooming methods used by African Americans, including the use of braids, weaves, and chemical relaxers, have been linked to the development of CCCA [
98]. However, there is no evidence to suggest that the use of chemical hair relaxers increases the risk of breast cancer [
99].
African-American women often use chemical relaxers to improve the manageability and appearance of their hair as well as to reduce styling time. However, if used incorrectly, relaxers can cause scalp irritation, burning, and hair loss [
19]. In another study, aimed to investigate the effects of chemical straighteners on the hair shaft and scalp, the researchers found that the application of chemical straighteners to the hair can cause structural damage, such as porosity, and a reduction in hair strength [
99]. Another study measured the pH levels of various lye and no-lye hair relaxers and found that all relaxers tested had a pH level that was corrosive to the skin [
100].
Another product with the potential to cause more damage to Afro-textured hair is hair dye. In Europe and the United States, over 33% of women aged 18 and above, as well as more than 10% of men over 40 years old use hair dye [
101].
According to the recently published work, hair dyes contain a variety of chemicals, including aromatic amines, nitrosamines, phenylenediamines, and para-toluene diamines, all of which have been associated with skin irritation and allergies. The study also found that some hair dyes contain heavy metals, such as lead and arsenic, which can cause toxicity [
101]. Another study found that scalp burns can be caused by the use of bleaching agents containing hydrogen peroxide [
100].
Permanent hair dyes contain p-phenylenediamines (PPD) and m-aminophenols, which serve as primary intermediates and couplers during hair dyeing [
103]. PPD has been linked to allergies and contact dermatitis, with skin reactions along the neck, hairline, and ears being the most common clinical pattern of hair dye contact allergy observed [
104,
105,
106]. Multiple studies have also indicated potential hazards associated with the use of hair dyes especially among populations with Afro-textured hair [
107,
108].
In conclusion, because of the likely increased sensitivity, the use of chemical relaxers and hair dyes may lead to side effects ranging from mild to extreme conditions, further emphasizing the need for new products that can facilitate easy management of Afro-textured hair.
7. Discussion
In this manuscript, we summarised the current knowledge and highlighted the topics of interest in the area of Afro-textured hair. Such interest is underlined by the growing demand for the personalisation of cosmetic products, particularly those designed for darker skin tones and curly or coily hair types associated with Black skin.
Current knowledge of the genetics and molecular interactions characterising Afro-textured hair, particularly its distinct structure and patterns of growth, is still limited. To address this, we performed a literature search describing differentially expressed genes alongside the genes harbouring single nucleotide polymorphism (SNP), focusing on the biomarkers associated with curly or African hair type. This approach also allowed distinguishing and highlighting likely genetic differences between Afro-textured hair and hair types from other major ethnic groups, namely Asian and European.
There is substantial research available in the area of SNP, such polymorphism is thought to frequently affect the expression or activity of the respective gene products or their combination [
109]. Our analysis of the genes representing some of the likely biomarkers of Afro-textured hair revealed several clusters corresponding to the structure-function biological interaction networks. This approach identified three major genetic groups characterising the curly and Afro-textured hair, pointing to biomechanics, tissue remodelling and signal transduction in control of hair follicle cycling and hair shaft. The main contributing factors appear to be those linked to keratinocyte stratification, cross-linking of keratin filaments and protein folding as well as the control of transcription, signal transduction and cell proliferation. Such contribution points to the importance of proper control and maintenance of the processes associated with the growth rate, diameter and mechanical strength of hair shafts, which might be unique to Afro-textured hair type and likely affected by gene polymorphism causing variation.
This interpretation is supported by the experimental and epidemiology data available in the literature, pointing to the additional influence of extrinsic or environmental factors affecting the follicular unit and hair shaft. Compared to Asian and European hair, African hair has a relatively slower growth rate that can be estimated at around 70%-75% compared with other ethnicities (
Table 1). More hair fibres are also found in the resting (telogen) phase, which would contribute to the likely differences in hair growth cycles between European and Afro-textured hair. Furthermore, Afro-textured hair not only grows at a different rate compared to other hair types, but the hair care practices and products commonly used for this hair type also increase the risk of early hair loss. The higher prevalence of central centrifugal cicatricial alopecia (CCCA) among Black women is linked to both genetic susceptibility and hair care practices. Changes in the hair follicle regenerative cycles and hair shaft formation in CCCA, likely caused by higher sensitivities to external thermal and mechanical factors, are indicated by similarities with fibroproliferative disorders and androgenic alopecia. Such changes would include the miniaturisation of hair follicles and expression of pro-fibrotic genes as well as a marked decrease in hair shaft diameter and changes in pigmentation.
The increased sensitivities of Afro-textured hair to extrinsic factors can be related to the unique structural variations observed in curly and coily hair compared to straight hair of European and Asian origin. The most prominent feature is the high curvature of the hair shaft, resulting in varying diameters within elliptical cross-sections, which makes the hair more prone to breakage upon mechanical stress. The additional important factors contributing to the unique morphology and elasticity of Afro-textured hair are disulphide bonds composed of cysteine residues, which occur in higher numbers compared to European hair.
The hair shaft is well protected from the environment by a barrier formed by apolar lipid molecules originating mostly within sebaceous glands and forming an external layer in Afro-textured hair. The specific morphology of hair can also be maintained by polar lipids and free fatty acids, which affect the organisation of keratin fibres and are abundant in Afro-textured hair.
The lipid fraction contributes to the physical properties of hair fibre such as moisture. The relatively high content of internal lipid fraction, particularly unsaturated lipids, can be associated with low water permeability. Internal lipids are more pronounced in European and Asian hair, contributing to moisture retention and UVR resistance of hair fibre. Compared to other hair types, Afro-textured hair has relatively low hydration levels and frequently becomes dry, therefore development of hair care products designed to target the areas of structural weakness whilst enhancing moisture retention could address this.
Current data and the literature point to the potential risks and dangers that Black women may face when using thermal or chemical hair-strengthening and certain hair dye products. The unique characteristics of Afro-textured hair, such as increased porosity and susceptibility to damage, coupled with potentially harmful chemicals in hair formulations, can lead to adverse health effects. By addressing these specific concerns and tailoring interventions, we can work towards minimizing the risks and ensuring the health and safety of this population.
8. Conclusions
The need for personalized hair care specific to Afro-textured hair is undeniable and crucial. Afro-textured hair possesses unique characteristics that demand specialized care and attention. These hair types are more prone to dryness, breakage, and damage, making tailored products and routines essential for maintaining their health and manageability. Furthermore, societal beauty standards and cultural ideologies of beauty significantly influence the use of hair products among Black women, emphasizing the significance of personalized care to cater to individual preferences and promote self-expression. Additionally, the potential health risks associated with certain hair care ingredients underscore the importance of personalized products that address the specific needs and concerns of Afro-textured hair while minimising potential harm. By acknowledging and addressing the distinct requirements of Afro-textured hair, personalized hair care products can empower individuals to embrace and celebrate their natural hair, fostering self-confidence and overall well-being. Therefore, the need for personalisation in hair care for Afro-textured hair is crucial in promoting healthy, beautiful, and culturally affirming hair experiences.
Author Contributions
Conceptualization, D.O., E.M. and O.C.I; methodology, D.O., E.M. and O.C.I.; software, D.O. and O.C.I; validation, D.O. and E.M.; formal analysis, D.O and E.M; investigation, D.O. E.M. and O.C.I; resources, O.C.I.; data curation, D.O. and E.M.; writing—original draft preparation, D.O.; writing—review and editing, D.O. and E.M.; visualization, D.O., E.M. and O.C.I.; supervision, O.C.I.; project administration, O.C.I.; funding acquisition, O.C.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were analysed in this study. Data sharing is not applicable to this article. .
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cruz, C.F.; Costa, C.; Gomes, A.C.; et al. Human hair and the impact of cosmetic procedures: a review on cleansing and shape-modulating cosmetics. Cosmetics 2016, 3, 26-X. [CrossRef]
- De La Mettrie, R.; Saint-Léger, D.; Loussouarn, G.; et al. Shape variability and classification of human hair: a worldwide approach. Hum Biol 2007, 79, 265-281. [CrossRef]
- Loussouarn, G.; Lozano, I.; Panhard, S.; et al. Diversity in human hair growth, diameter, colour and shape. An in vivo study on young adults from 24 different ethnic groups observed in the five continents. Eur J Dermatol 2016, 26, 144-154. [CrossRef]
- Roseborough, I.E. and McMichael, A.J. Hair care practices in African-American patients. Semin Cutan Med Surg 2009, 28, 103-108. [CrossRef]
- Callender, V.D.; McMichael, A.J.; Cohen, G.F. Medical and surgical therapies for alopecias in black women. Dermatol Ther 2004, 17, 164-176. [CrossRef]
- Hall, R.R.; Francis. S.; Whitt-Glover, M.; et al. Hair care practices as a barrier to physical activity in African American women. JAMA Dermatol 2013, 149, 310-314. [CrossRef]
- Nnoruka, N.E. Hair loss: is there a relationship with hair care practices in Nigeria?. Int J Dermatol 2005, 44, 13-17. [CrossRef]
- Dadzie, O.E.; Salam, A. The hair grooming practices of women of African descent in London, United Kingdom: findings of a cross-sectional study. JEADV 2016, 30, 1021-1024. [CrossRef]
- Verschoore, M.; Dlova, N. Advances in dermatology in sub-Saharan Africa in the past 20 years from workshops to the birth of the African Society of Dermatology and Venereology. Int J Dermatol 2022, 61, 841-847. [CrossRef]
- Cruz, C.F.; Martins, M.; Egipto, J.; et al. Changing the shape of hair with keratin peptides. RSC Advances 2017, 7, 51581-51592. [CrossRef]
- Yang, W.; Yu, Y.; Ritchie, R.O.; Meyers, M.A. On the strength of hair across species. Matter 2020, 2, 136-149. [CrossRef]
- Malinauskyte, E.; Cornwell, P.A.; Reay, L.; et al. Effect of equilibrium pH on the structure and properties of bleach-damaged human hair fibers. Biopolymers 2020, 111, e23401-x. [CrossRef]
- Madnani, N. and Khan, K. Hair cosmetics. IJDVL 2013, 79, 654. [CrossRef]
- Westgate, G.E.; Ginger, R.S.; Green, M.R. The biology and genetics of curly hair. Exp Dermatol 2017, 26, 483-490. [CrossRef]
- Breakspear, S.; Noecker, B.; Popescu, C. Relevance and evaluation of hydrogen and disulfide bond contribution to the mechanics of hard α-keratin fibers. J Phys Chem B 2019, 123, 4505-4511. [CrossRef]
- Breakspear, S.; Frueh, P.; Neu, A.; et al. Learning from hair moisture sorption and hysteresis. Int J Cosmet Sci 2022, 44, 555-568. 10.1111/ics.12806.
- Tonanzi, G. How to strengthen & repair hair bonds. Curlsmith EU. Available online: https://eu.curlsmith.com/blogs/product-guides/how-to-strengthen-repair-hair-bonds. (Accessed 29 March 2023).
- Molamodi, K.; Fajuyigbe, D.; Sewraj, P.; et al. Quantifying the impact of braiding and combing on the integrity of natural African hair. Int J Cosmet Sci 2021, 43, 321-331. [CrossRef]
- Aryiku, S.A.; Salam, A.; Dadzie, O.E.; Jablonski, N.G. Clinical and anthropological perspectives on chemical relaxing of afro-textured hair. J Eur Acad Dermatol Venereol 2015, 29, 1689-1695. [CrossRef]
- Khan, I.; Maldonado, E.; Vasconcelos, V.; et al. Mammalian keratin associated proteins (Krtaps) subgenomes: disentangling hair diversity and adaptation to terrestrial and aquatic environments. BMC Genomics 2014, 15, 779. [CrossRef]
- Lin, X.; Zhu, L.; He, J. Morphogenesis, growth cycle and molecular regulation of hair follicles. Front Cell Dev Biol 2022, 10, 823. [CrossRef]
- Chen, Y.; Ding, Y.; Yang, X.; et al. Kartogenin regulates hair growth and hair cycling transition. Int J Med Sci 2022, 19, 537-546. [CrossRef]
- Grymowicz, M.; Rudnicka, E.; Podfigurna, A.; et al. Hormonal effects on hair follicles. Int J Mol Sci 2020, 21, 5342. [CrossRef]
- Hoover E, Alhajj M, Flores JL. Physiology, Hair. Updated 2021 Jul 26. StatPearls Internet. Treasure Island (FL): StatPearls Publishing.
- Buffoli B, Rinaldi F, Labanca M, et al. The human hair: from anatomy to physiology. Int J Dermatol. 2014;53(3):331-341. [CrossRef]
- Martel, J.L.; Miao, J.H.; Badri, T. Anatomy, hair follicle. In: StatPearls Internet. Treasure Island (FL): StatPearls Publishing; 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470195/.
- Fernandes, B.; Cavaco-Paulo, A.; Matamá, T.A. Comprehensive review of mammalian pigmentation: paving the way for innovative hair colour-changing cosmetics. Biology 2023, 12, 290. [CrossRef]
- Lan, S.; Liu, F.; Zhao, G.; et al. Cyclosporine A increases hair follicle growth by suppressing apoptosis-inducing factor nuclear translocation: a new mechanism. Fundam Clin Pharmacol 2015, 29, 191-203. [CrossRef]
- Salam, A.; Aryiku, S.; Dadzie, O.E. Hair and scalp disorders in women of African descent: an overview. Br J Dermatol. 2013, 169, 19-32. [CrossRef]
- Higgins, C.A.; Westgate, G.E.; Jahoda, C.A. From telogen to exogen: mechanisms underlying formation and subsequent loss of the hair club fiber. J Invest Dermatol 2009, 129, 2100-2108. [CrossRef]
- Roland, J. Stages of hair growth plus how to maintain hair health in every stage. Healthline. Available online: https://www.healthline.com/health/stages-of-hair-growth#growing-phase. (Accessed 29 March 2023).
- De Mirecki-Garrido, M.; Santana-Farré, R.; Guedes-Hernandez, N.; et al. Ginseng in hair growth and viability. In Benzie IFF, Wachtel-Galor S, Editors. Ginseng: Modern Aspects of the Famed Traditional Medicine. 2022, 69. [CrossRef]
- Cruz, C.F.; Fernandes, M.M.; Gomes, A.C.; et al. Keratins and lipids in ethnic hair. Int J Cosmet Sci 2013, 35, 244-249. [CrossRef]
- Song, S.H.; Lim, J.H.; Son, S.K.; et al. Prevention of lipid loss from hair by surface and internal modification. Sci Rep 2019, 9, 1-9. [CrossRef]
- Leerunyakul, K.; Suchonwanit, P. Asian hair: a review of structures, properties, and distinctive disorders. Clin Cosmet Investig Dermatol 2020, 13, 309-318. [CrossRef]
- Oliver, M.A.; Marti, M.; Coderch, L.; et al. Lipid loses and barrier function modifications of the brown-to-white hair transition. Skin Res Technol 2019, 25, 517-525. [CrossRef]
- Wade, M.; Tucker, I.; Cunningham, P.; et al. Investigating the origins of nanostructural variations in differential ethnic hair types using X-ray scattering techniques. Int J Cosmet Sci. 2013, 35, 430-441. [CrossRef]
- Ji, J.H.; Park, TS.; Lee, H.J.; et al. The ethnic differences of the damage of hair and integral hair lipid after ultra violet radiation. Ann Dermatol. 2013, 25, 54-60. [CrossRef]
- Franbourg, A.; Hallegot, P.; Baltenneck, F.; et al. Current research on ethnic hair. JAAD 2003, 48, S115-S119. [CrossRef]
- Martí, M.; Barba, C.; Manich, A.M.; et al. The influence of hair lipids in ethnic hair properties. Int J Cosmet Sci 2015, 38, 77-84. [CrossRef]
- Coderch, L.; Oliver, M.A.; Martínez, V.; et al. Exogenous and endogenous lipids of human hair. Skin Res Technol 2017, 23, 479-485. [CrossRef]
- Kreplak, L.; Briki, F.; Duvault, Y.; et al. Profiling lipids across Caucasian and Afro-American hair transverse cuts, using synchrotron infrared microspectrometry. Int J Cosmet Sci 2001, 23, 369-374. [CrossRef]
- Ma, X.; Jin, Z.; Jin, T. Effects of extrusion conditions on chemical properties of extruded white ginseng root hair. J Sci Food Agr 2019, 99, 3186-3191. [CrossRef]
- Coderch, L.; Oliver, M.A.; Carrer, V.; et al. External lipid function in ethnic hairs. J Cosmet Dermatol. 2019; 18, 1912-1920. [CrossRef]
- Khumalo, N.P. African hair length: The picture is clearer. JEADV 2006, 20, 556-560. [CrossRef]
- Aguh, C. and McMichael, A. Central centrifugal cicatricial alopecia. JAMA Dermatol 2020, 156, 1036-1036. [CrossRef]
- Jamerson, T.A.; Talbot, Jr, C.C.; Dina, Y. et al. Gene expression profiling suggests severe, extensive central centrifugal cicatricial alopecia may be both clinically and biologically distinct from limited disease subtypes. Exp Dermatol 2022, 31, 789-793. [CrossRef]
- Lawson, C.N.; Bakayoko, A.; Callender, V.D. Central centrifugal cicatricial alopecia: challenges and treatments. Dermatol Clin 2021, 39, 389-405. [CrossRef]
- Malki, L.; Sarig, O.; Romano, M.T.; et al. Variant PADI3 in central centrifugal cicatricial alopecia. NEJM 2019, 380, 833-841. [CrossRef]
- Aguh, C.; Dina, Y.; Talbot, Jr, C.C.; Garza, L. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. JAAD 2018, 79, 904-912. [CrossRef]
- Blumeyer, A.; Tosti, A.; Messenger, A.; et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men. JDDG 2011, 9, S1-S57. [CrossRef]
- Kutlu, Ö. Dexpanthenol may be a novel treatment for male androgenetic alopecia: Analysis of nine cases. Dermatol Ther 2020, 33, e13381. [CrossRef]
- Inui, S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol 2011, 38, 71-75. [CrossRef]
- Ho, C.H.; Sood, T.; Zito, P.M. Androgenetic alopecia. In StatPearls Internet. StatPearls Publishing; 2021.
- Premanand, A. and Reena Rajkumari, B. Androgen modulation of Wnt/β-catenin signaling in androgenetic alopecia. Arch Dermatol Res. 2018, 310, 391-399. [CrossRef]
- Shashank, B. and Bhushan, M. Injectable Platelet-Rich Fibrin (PRF): The newest biomaterial and its use in various dermatological conditions in our practice: A case series. J Cosmet Dermatol 2021, 20, 1421-1426. [CrossRef]
- Sobhy, N.; Aly, H.; El Shafee, A.; El Deeb, M. Evaluation of the effect of injection of dutasteride as mesotherapeutic tool in treatment of androgenetic alopecia in males. Our Dermatology Online. 2013, 4, 40. [CrossRef]
- Avci, P.; Gupta, G.K.; Clark, J.; et al. Low-level laser (light) therapy (LLLT) for treatment of hair loss. LSM 2014, 46, 144-151. [CrossRef]
- Liu, F.; Chen, Y.; Zhu, G.; et al. Meta-analysis of genome-wide association studies identifies 8 novel loci involved in shape variation of human head hair. Hum Mol Gen 2018, 27, 559-575. [CrossRef]
- Cloete, E.; Khumalo, N.P.; Ngoepe, M.N. The what, why and how of curly hair: a review. Proc R Soc (London) A. 2019, 475, 20190516. [CrossRef]
- Steinert, P.M.; Parry, D.A.; Marekov, L.N. Trichohyalin mechanically strengthens the hair follicle: multiple cross-bridging roles in the inner root sheath. JBC 2003, 278, 41409-41419. [CrossRef]
- Medland, S.E.; Nyholt, D.R.; Painter, J.N.; et al. Common variants in the trichohyalin gene are associated with straight hair in Europeans. AJHG 2009, 85, 750-755. [CrossRef]
- Eriksson, N.; Macpherson, J.; Tung, J.; et al. Web-based, participant-driven studies yield novel genetic associations for common traits. PloS Genet 2010, 6, e1000993. [CrossRef]
- Ferby, I.; Reschke, M.; Kudlacek, O.; et al. Mig6 is a negative regulator of EGF receptor-mediated skin morphogenesis and tumor formation. Nat Med. 2006, 12, 568-573. [CrossRef]
- Bharti, P.; Schliebs, W.; Schievelbusch, T.; et al. PEX14 is required for microtubule-based peroxisome motility in human cells. J Cell Sci 2011, 124, 1759-1768. [CrossRef]
- Vikhe Patil, K.; Mak, K.H.; Genander, M. A Hairy Cituation - PADIs in Regeneration and Alopecia. Front Cell Dev Biol 2021, 9, 789676. [CrossRef]
- Basmanav, FBÜ.; Cau, L.; Tafazzoli, A. et al. Mutations in three genes encoding proteins involved in hair shaft formation cause uncombable hair syndrome. AJHG 2016, 99, 1292-1304. [CrossRef]
- Hassan, M. and Netchiporouk, E. Autosomal-Dominant Mutation in PADI3 Responsible for up to 25% of Central Centrifugal Cicatricial Alopecia Cases. J Cutan Med Surg 2019, 23, 553. [CrossRef]
- Appleton, C.T.G.; Usmani, S.E.; Mort, J.S.; Beier, F. Rho/ROCK and MEK/ERK activation by transforming growth factor-α induces articular cartilage degradation. Lab Invest 2010, 90, 20-30. [CrossRef]
- Luetteke, N.C.; Qiu, T.H.; Peiffer, R.L.; et al. TGF alpha deficiency results in hair follicle and eye abnormalities in targeted and waved-1 mice. Cell. 1993, 73, 263-78. [CrossRef]
- Hochfeld, L.M.; Bertolini, M.; Broadley, D.; et al. Evidence for a functional interaction of WNT10A and EBF1 in male-pattern baldness. PLoS One 2021, 16, e0256846. [CrossRef]
- Doolan, B.J.; Onoufriadis, A.; Kantaputra, P.; McGrath, J.A. WNT10A, dermatology and dentistry. BJD 2021, 185, 1105-1111. [CrossRef]
- Sun, Q.; Lee, L.W.; Hall, E.K.; et al. Hair and skin predict cardiomyopathies: Carvajal and erythrokeratodermia cardiomyopathy syndromes. Pediatr Dermatol 2020, 38, 31-38. [CrossRef]
- Short, K.; Wiradjaja, F.; Smyth, I. Let's stick together: the role of the Fras1 and Frem proteins in epidermal adhesion. IUBMB Life 2007, 59, 427-35. [CrossRef]
- Kalekar, L.A.; Cohen, J.N.; Prevel, N.; et al. Regulatory T cells in skin are uniquely poised to suppress profibrotic immune responses. Sci Immunol 2019, 4, eaaw2910. [CrossRef]
- Chikh, A.; Sayan, E.; Thibaut, S.; et al. Expression of GATA-3 in epidermis and hair follicle: relationship to p63. Biochem Biophys Res Commun 2007, 361, 1-6. [CrossRef]
- Ren, X.; Xia, W.; Xu, P.; et al. Lgr4 Deletion Delays the Hair Cycle and Inhibits the Activation of Hair Follicle Stem Cells. J Invest Dermatol 2020 140, 1706-1712. [CrossRef]
- Chastkofsky, M.I.; Bie, W.; Ball-Kell, S.M.; et al. Protein Tyrosine Kinase 6 Regulates UVB-Induced Signaling and Tumorigenesis in Mouse Skin. J Invest Dermatol 2015 135, 2492-2501. [CrossRef]
- Cui, C.Y. and Schlessinger, D. EDA signaling and skin appendage development. Cell Cycle 2006 5, 2477-2483. [CrossRef]
- Mou, C.; Jackson, B.; Schneider, P et al. Generation of the primary hair follicle pattern. PNAS 2006 103, 9075-9080. [CrossRef]
- Wu, S.; Tan, J.; Yang, Y.; et al. Genome-wide scans reveal variants at EDAR predominantly affecting hair straightness in Han Chinese and Uyghur populations. Hum Genet 2016, 135, 1279-1286. [CrossRef]
- Jave-Suarez, L.F.; Winter, H.; Langbein, L.; et al. HOXC13 is involved in the regulation of human hair keratin gene expression. JBC 2002, 277, 3718-3726. [CrossRef]
- Adhikari, K.; Fontanil, T.; Cal, S.; et al. A genome-wide association scan in admixed Latin Americans identifies loci influencing facial and scalp hair features. Nat Commun 2016, 7, 10815. [CrossRef]
- Deng, J.; Song, Y.; Liu, H.; et al. A direct link between Prss53, hair curvature, and skeletal dysplasia. bioRxiv 560847 2019. [CrossRef]
- Botchkarev, V.A.; Fessing, M.Y. Edar signaling in the control of hair follicle development. J Investig Dermatol Symp Proc. 2005, 10, 247-251. [CrossRef]
- Singh, B.; Coffey, R.J. From wavy hair to naked proteins: the role of transforming growth factor alpha in health and disease. Semin Cell Dev Biol 2014, 28, 12-21. [CrossRef]
- Kaufman, C.K.; Zhou, P.; Pasolli, H.A.; et al. GATA-3: an unexpected regulator of cell lineage determination in skin. Genes Dev 2003, 17, 2108-2122. [CrossRef]
- Bailey, J.; Ericson, M.; Tomlin-Harris, T.; et al. Abstract P6-05-39: Black Breast Cancer Survivors’ Sociocultural Perspectives of Beauty, and Use of Personal Care Products Containing Endocrine Disrupting Chemicals. Cancer Res 2023, 83(5_Suppl), P6-05. [CrossRef]
- Hawthorne, C. Making Italy: Afro-Italian entrepreneurs and the racial boundaries of citizenship. Soc Cult Geogr 2021, 22, 704-724. [CrossRef]
- Brenner, B.; Evans, S.; Miller, K.; et al. Breast cancer and the environment: Reaching multicultural communities; Advocates mentoring advocates. Environ Justice. 2015, 8, 117-125. [CrossRef]
- Washington, G. Towards creation of a curl pattern recognition system. Conference: nt’l Conf. IP, Comp. Vision and Pattern Recognition 2018, Available at: https://csce.ucmss.com>books>LFS>CSREA2018>IPC4188.
- Gaines, M.; Page, I.; Miller, N.; et al. Reimagining Hair Science: A New Approach to Classify Curly Hair Phenotypes via New Quantitative Geometrical & Structural Mechanical Parameters. Acc Chem Res. 2023, 56, 1330-1339. [CrossRef]
- Simeon, A. The controversial history of the hair typing system, Byrdie. Byrdie. Available at: https://www.byrdie.com/hair-typing-system-history-5205750. (Accessed 29 March 2023).
- Gomes, J.R.; de Almeida, F.A.S.; Adão, J.M.; et al. The Brazilian Beauty Industry and the cosmetics market for Frizzy / Curly hair. IJHSS 2019, 9, 6. [CrossRef]
- Daniels, G.; Fraser, A.; Westgate, G.E. How different is human hair? A critical appraisal of the reported differences in global hair fiber characteristics and properties toward defining a more relevant framework for hair type classification. Int J Cosmet Sci 2023, 45, 50-61. [CrossRef]
- Wise, L.A.; Palmer, J.R.; Reich, D.; et al. Hair relaxer use and risk of uterine leiomyomata in African-American women. AJE 2012, 175, 432-440. [CrossRef]
- Khumalo, N.P.; Stone, J.; Gumedze, F.; et al. 'Relaxers' damage hair: evidence from amino acid analysis. JAAD 2010, 62, 402-408. [CrossRef]
- Kyei, A.; Bergfeld, W.F.; Piliang, M.; Summers, P. Medical and environmental risk factors for the development of central centrifugal cicatricial alopecia: a population study. Arch Dermatol 2011, 147, 909-914. [CrossRef]
- Paula, J.N.H.D.; Basílio, F.M.A.; Mulinari-Brenner, F.A. Effects of chemical straighteners on the hair shaft and scalp. An Bras Dermatol 2022, 97, 193-203. [CrossRef]
- Sishi, V.N.; Van Wyk, J.C.; Khumalo, N.P. The pH of lye and no-lye hair relaxers, including those advertised for children, is at levels that are corrosive to the skin. SAMJ 2019, 109, 941-946. [CrossRef]
- He, L.; Michailidou, F.; Gahlon, H.L.; Zeng, W. Hair dye ingredients and potential health risks from exposure to hair dyeing. Chem Res Toxicol 2022, 35, 901-915. [CrossRef]
- Jeong, M.S.; Lee, C.M.; Jeong, W.J.; et al. Significant damage of the skin and hair following hair bleaching. J Dermatol 2010, 37, 882-887. [CrossRef]
- Bolt, H.M.; Golka, K. The debate on carcinogenicity of permanent hair dyes: new insights. Crit RevToxicol 2007, 37, 521-536. [CrossRef]
- Yazar, K.; Boman, A.; Lidén, C. p-Phenylenediamine and other hair dye sensitizers in Spain. Contact Dermatitis. 2012, 66, 27-32. [CrossRef]
- Venkatesan, G.; Dancik, Y.; Sinha, A.; et al. Development of novel alternative hair dyes to hazardous para-phenylenediamine. J Hazard Mater 2021, 402, 123712. [CrossRef]
- Søsted, H.; Hesse, U.; Menné, T.; et al. Contact dermatitis to hair dyes in a Danish adult population: an interview-based study. BJD 2005, 153, 132-135. 10.1111/j.1365-2133.2005.06588.x.
- Eberle, C.E., Sandler, D.P.; et al. Hair dye and chemical straightener use and breast cancer risk in a large US population of black and white women. Int J Cancer 2020, 147, 383-391. [CrossRef]
- White, A.J.; Gregoire, A.M.; Taylor, K.W.; et al. Adolescent use of hair dyes, straighteners, and perms in relation to breast cancer risk. Int J Cancer 2021, 148, 2255-2263. [CrossRef]
- Shastry, B.S. SNPs: impact on gene function and phenotype. Methods Mol Biol 2009, 578, 3-22. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).