0. Introduction
The aim of breast-conserving surgery is complete tumor removal with satisfying cosmetic results and adequate oncologic radicality, characterized by safe surgical margins [
1] Clear communication among surgeons, pathologists, and radiologists is crucial, along with the proper marking of resection margins [
2,
3]. Ambiguous specimen orientation is a quality control issue, hindering correct re-excision, potentially reducing oncologic accuracy, and increasing local recurrence [
4]. Surgical guidelines emphasize the importance of a specimen marking protocol for clear orientation, requiring accurate information about the primary surgery’s anatomical extent and tumor location within the specimen. A national survey prompted the development of a new tool for accurate reconstruction of the in vivo situation
1. Materials and Methods
A review was conducted to assess the techniques used for breast specimen orientation from the operating field to microscopic sectioning in Hungarian breast cancer treatment departments. The review also evaluated the satisfaction of radiologists, pathologists, and surgeons with these techniques based on the questionnaire provided below.
Online questionnaire for surgeons, radiologists, and pathologists about their breast cancer specimen orientation protocols in Hungary:
-
What type of marking is used at your institution to orient specimens for breast-conserving surgery? Multiple answers are possible.
Please briefly describe the type of marking used at your institution. (For example, in the case of suture marking: double, short suture= superior; single, long suture= lateral)
-
In the case of non-palpable breast tumors, is the orientation of the specimen always unambiguous to you based on the intraoperative specimen mammogram image?
-
If not, what is the approximate discrepancy?
-
In which directions or planes is the intraoperative specimen mammography taken?
-
If only bi-directional (cranio-caudal and latero-medial images are taken), would you find an image in antero-posterior direction (coronal or frontal plane) useful to accurately determine the direction of re-excision if needed?
-
In the case of non-palpable tumors, if preoperative localization is required, what is more important in determining the direction of a possible re-excision?
-
If re-excision is required due to inadequate or compromised surgical margins, would you consider an intraoperative image of the excision cavity and specimen to be helpful in the orientation of the re-resection?
-
Is the orientation protocol uniform at your institution? If not, would you consider a uniform orientation method useful?
-
Would you find it useful to have a nationally uniform marking and orientation method?
Surgeons, radiologists, and pathologists from the top 20 breast cancer centers in Hungary (population: 9.7 million, with an estimated breast cancer incidence of 8233 in 2019 [
5]) were surveyed via an online questionnaire about their departments’ breast cancer specimen orientation protocols. (Regional Science and Research Ethics Committee permission: 76-1-19/2021) Twelve out of twenty departments responded as shown below. All centers agreed to have their reported practices evaluated anonymously. Responses are presented by center.
List of the major breast cancer centers that participated in the survey. From each center, a breast surgeon, a mammologist-radiologist, and a pathologist, responsible for breast cancer diagnosis also completed the questionnaire, representing their departmental practices:
Department of Surgery, Petz Aladár University Teaching Hospital, Győr, Hungary
Department of Breast and Sarcoma Surgery, National Institute of Oncology, Budapest, Hungary
Department of Surgery, Transplantation and Gastroenterology, Semmelweis University, Faculty of Medicine, Budapest, Hungary
Department of Surgery, University of Pécs, Budapest, Hungary
Department of Surgery, Uzsoki Street Hospital, Budapest, Hungary
Department of Surgery, Hungarian Defense Forces Medical Centre, Budapest, Hungary
Department of Plastic Surgery, South Buda Central Hospital Szent Imre University Teaching Hospital, Budapest, Hungary
Department of Surgery, University of Szeged, Szeged, Hungary
Department of Surgery, University of Debrecen, Debrecen, Hungary
Department of Obstetrics and Gynecology, Central Hospital of Southern Pest National Institute of Hematology and Infectious Diseases, Szent István Hospital, Budapest, Hungary
Department of Surgery, Bajcsy-Zsilinszky Hospital and Clinic, Budapest, Hungary
Surgical Center, Ferenc Csolnoky County Hospital Veszprém, Hungary
2. Results
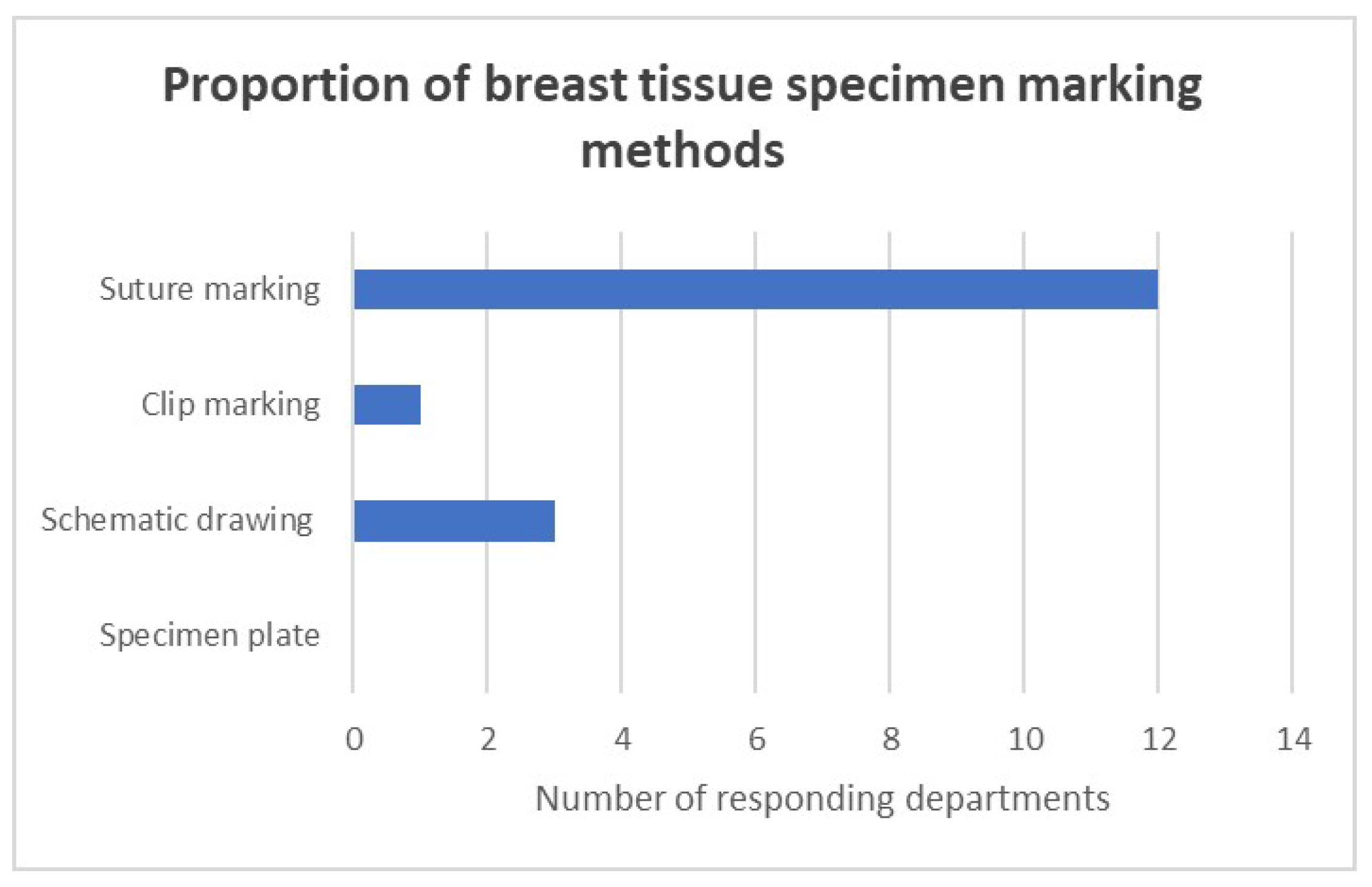
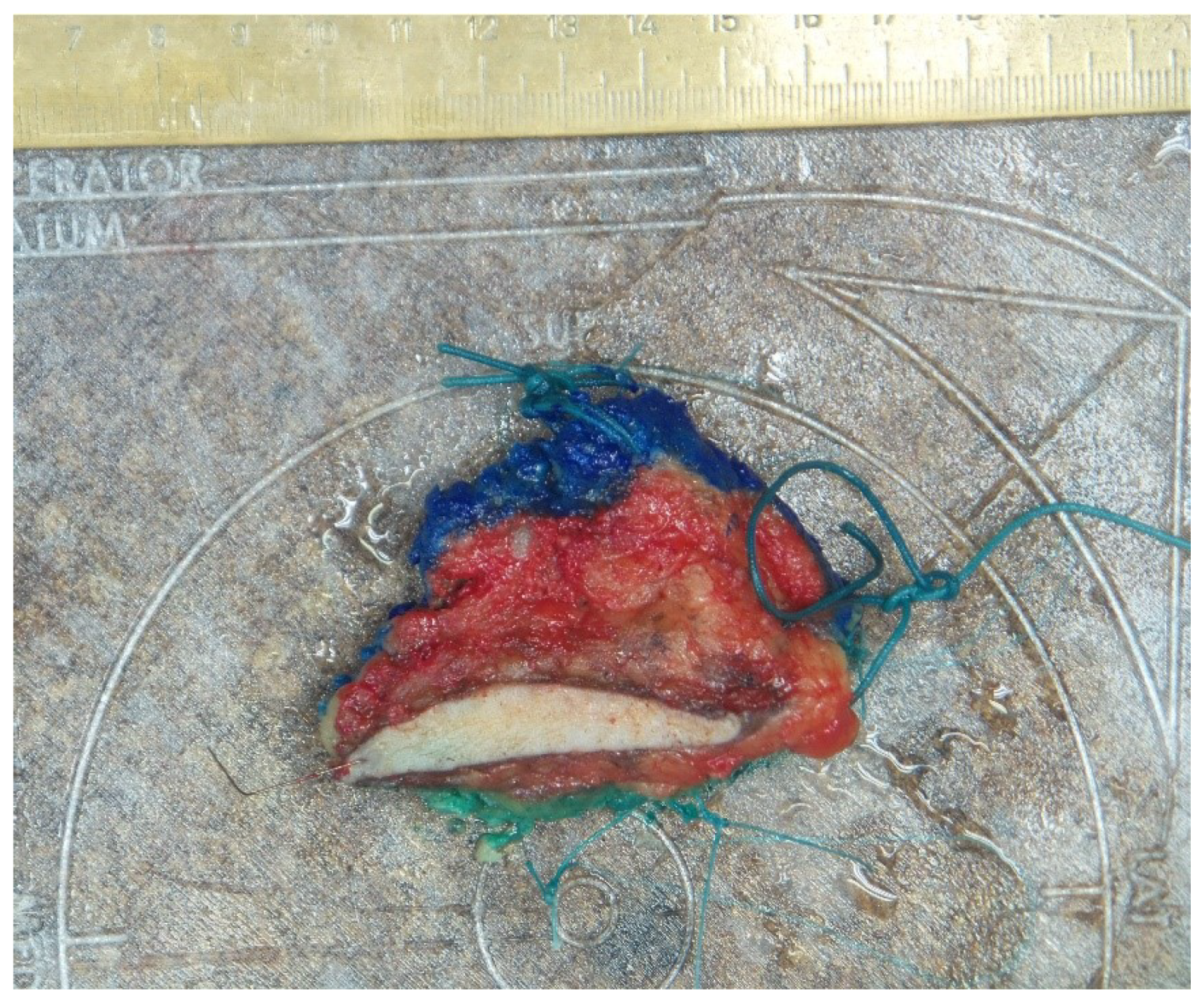
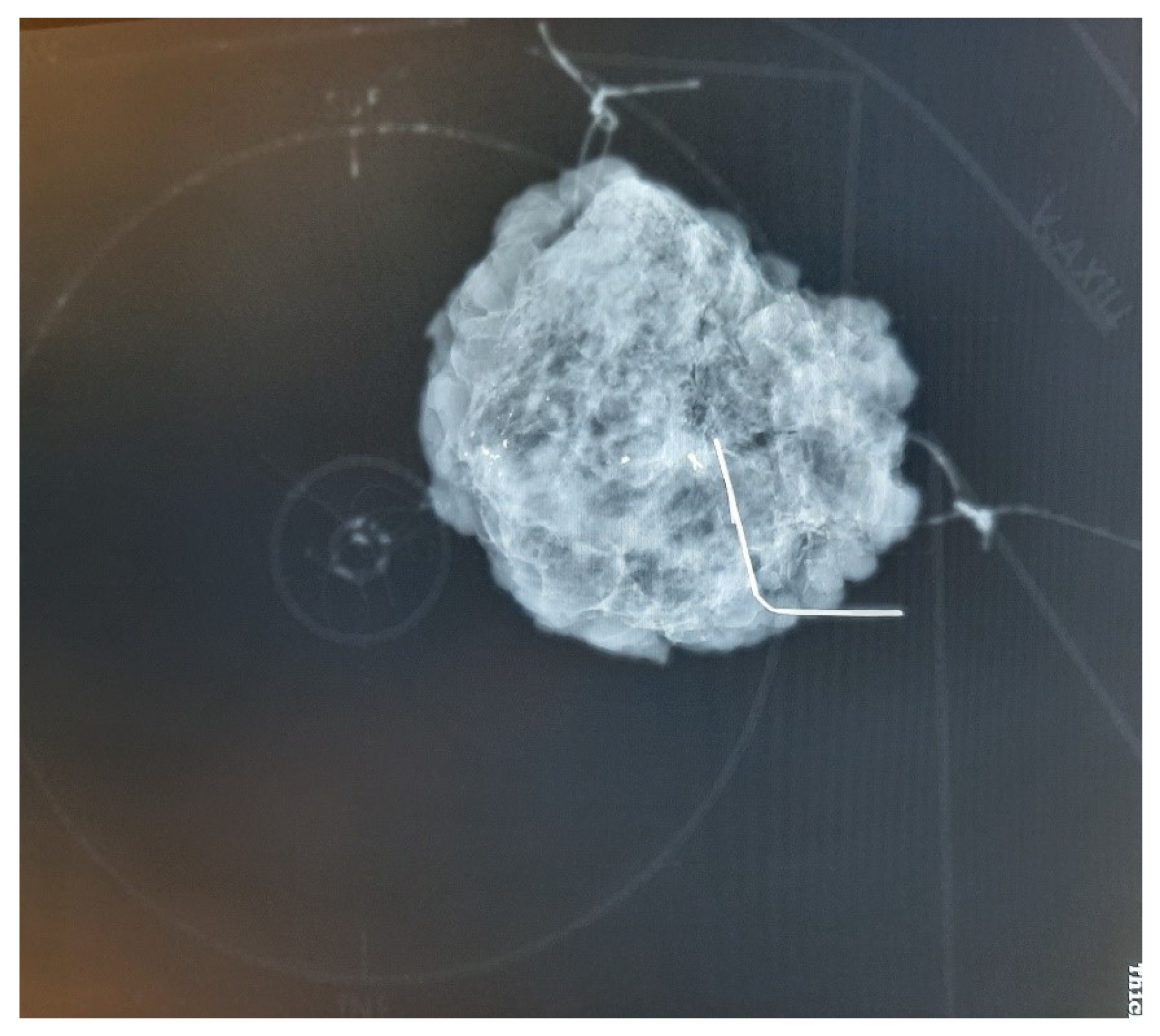
Marking with orientation sutures indicating the four anatomical directions (caudal-cranial, lateral, medial; length of strand signaling each of the courses) was the standard procedure used in all responding surgical departments (
Figure 1). Three-quarters of the responding centers (9/12) use a uniform system for orienting and marking breast tissue. In a quarter of the departments, an orienting schematic drawing accompanies the specimen from the OP to the path lab.
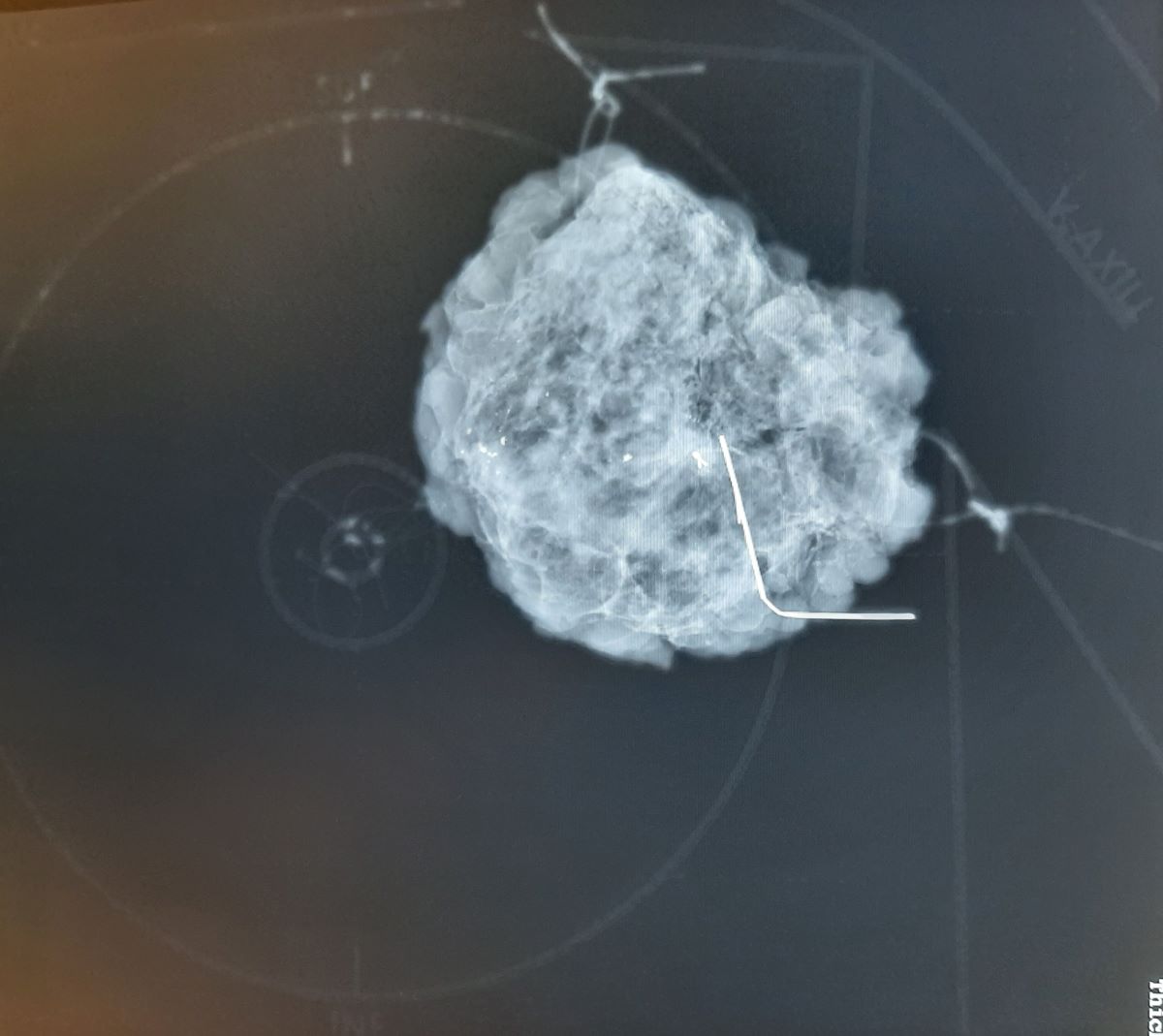
Specimen mammography images were unambiguous for only half of the responding surgeons, while obvious for all radiologists. None of the reporting centers take coronal plane specimen mammography images according to the radiologists’ response. More than 75% of responding pathological departments (9/12 respondents to this question) experience difficulty or ambiguity in the orientation of breast tissue specimens in around 5-10% of the cases. From the pathologists’ point of view, in half of the cases, the reason for orientation errors is that the way of marking the excisions is ambiguous. Suture length is a common source of misunderstanding. In the remaining ambiguous cases, the directions of the markings are not clear. Many reported failures due to loss of suture during the specimen mammography and transport to pathology. Ten of the twelve responding pathology departments and 75% of the responding surgical teams believe that coronal plane specimen mammography would be useful, especially in cases of non-palpable tumor resections. In two reporting centers, pathologists do not have access to specimen mammography images.
Two-thirds of the surgeons believe that an intraoperative photograph of the excision cavity and specimen would be helpful for orienting the re-resection. The most common sources of inadequate markings or even errors in existing (homemade) orientation techniques, such as suture or clip markings, were reported in the notes section of the questionnaire. Three-way suture or clip markers become dislodged due to the irregular shape and consistency of the resected breast tissue. This may result in displacement or even loss of the marker. The orientation of the mammographic image is often unclear to the surgeon, leading to incorrect site identification. As a result of the survey, the basic requirements for an unbiased presentation platform were identified. It must securely fix the specimen and reproduce the original, inpatient position of the removed tissue mass with a high degree of reproducibility. The platform must be radio-translucent, as specimen mammography is an integral part of the protocol. Ideally, it is sterile so that intraoperative maneuvers are trouble-free and the work of the scrub nurse is eased.
In order to meet the above detailed requirements, a 3D printed (
Appendix A) plastic specimen plate was built (
Figure 2,
Figure 3,
Figure 4 and
Figure 5), which allows the oriented anchoring of the breast tissue specimen representing the original in vivo situation. This way the removed specimen is completely consistent with the original layout in the patient. The plate is made to mimic the female breast and axillary scheme so that the location and direction of the specimen can be unambiguously determined.
This scheme can be clearly seen in mammographic images, but the plate does not have a disturbing X-ray positive shadow (
Figure 3 and
Figure 5). This plate provides mammographic images of breast specimens in the coronal plane, which is easier to understand and more dependable for surgeons and pathologists.
3. Discussion
After breast-conserving surgery, re-excision is required in approximately 25% of patients with invasive breast carcinoma and around 30% of patients with DCIS. About half of these re-operations are performed in patients with negative surgical margins [
6,
7,
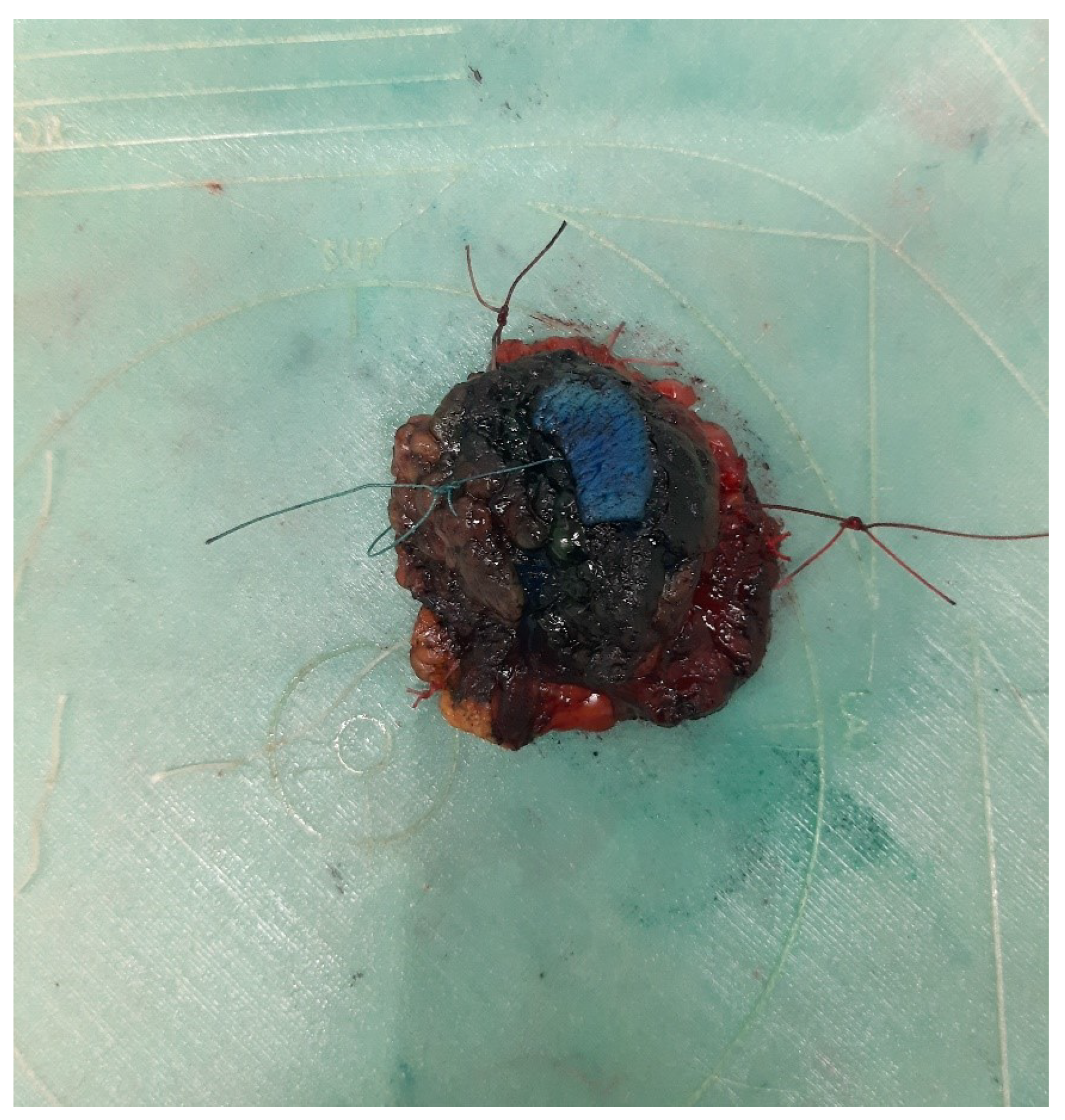
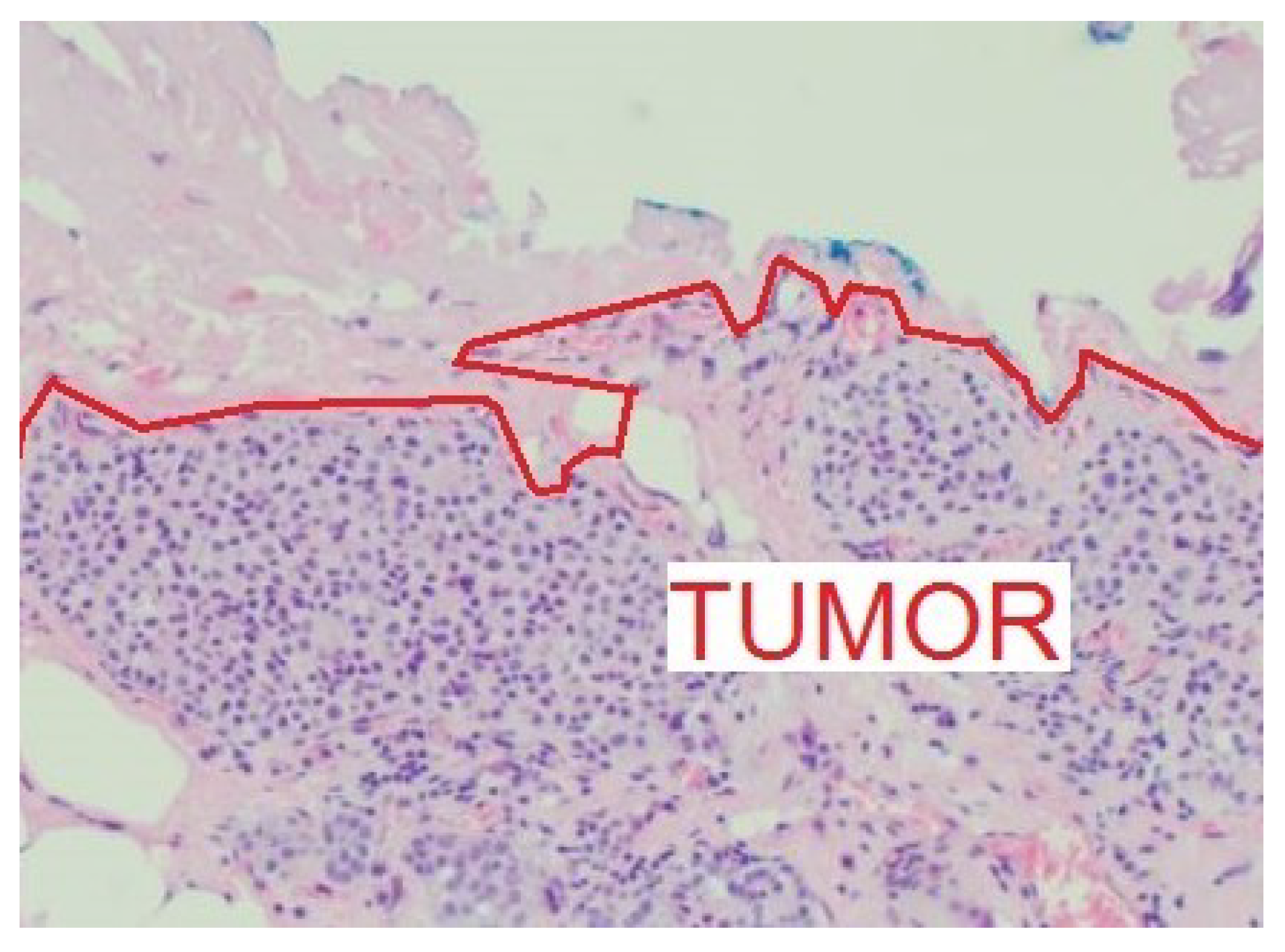
8]. A negative surgical margin is defined as no tumor on ink, where the ink is used to mark a side of the histological specimen (
Figure 6).
Pathologically negative surgical resection margins depend on several factors, including the number of sections examined, and the direction and technique of pathological sectioning. The type of breast cancer, its stromal invasion, for instance the lack of E-cadherin, or its intraductal spread can significantly affect the assessment of resection margins due to the irregularities of the tumor edge [
9]. Other important factors include type of transport and storage until pathological processing, orientation techniques, and mammography with specimen compression. In a compressed breast tissue specimen, the specimen dimension is reduced in the compression direction to approximately half the normal width and doubled in the perpendicular direction. It is estimated that a complete examination of the edge surfaces of an average spherical lumpectomy specimen would require 3000 sections [
10].
The complexity of the procedure explains why nearly half of all re-excisions result in negative surgical margins in the final report. Current practice is that, despite the consensus recommendations, a surgical margin of around or less than 1 mm indicates a re-excision for certain R0 status. The expectation is that a larger negative margin will improve disease outcome [
11,
12]. Naturally, local recurrence and prognosis are not only related to surgical margins, but also to the size of the primary tumor, grade, lymph node metastases, stage, proliferative activity, and estrogen receptor expression [
13].
According to the current guidelines, a breast tissue specimen marking protocol is required for clear orientation of the excised specimen during breast cancer surgery [
14]. In case of inadequate or positive surgical margins, re-excision in the direction of the affected margin is required. This results in better cosmetic outcomes due to significantly less intact tissue removal compared to complete wound cavity excision [
15]. It allows larger volume removal in the required direction [
16]. This requires accurate information about the anatomical extent and dimensions of the primary surgery and the location of the tumor within the specimen [
17] Therefore, specimen orientation procedures are of exceptional importance.
The survey revealed a general dissatisfaction with the status quo in the OP-radiology-pathology chain. There are identifiable general patterns. There is a common pattern that paves the road for the solution. The keywords are recognizable topographical points in accordance with the actual anatomy of the individual patient and non-radio opacity of the platform. Multi-directional intraoperative mammography, supplemented by an antero-posterior image, in the coronal plane, is the optimal basis for pathological and surgical orientation. The task is to unambiguously present the excised tissue mass to the pathologist.
Since the excisions normally need to be done from the chest wall to the pectoral muscle, including the fascia, and up to the subcutaneous tissue, it’s important to mark and locate the tumor in the coronal plane. Demonstration plates intend to reduce information loss and increase patient/procedural safety in the surgeon-pathologist-oncologist chain [
2]. However, this type of specimen presentation has not been available in breast oncology up to now. The presented concept and method (patent pending) developed in accordance with the clinical needs of the daily practice explored in our survey seems to be a viable solution to the problem described.
4. Conclusions
A need was identified to improve quality control in an important subset of breast cancer surgery. The orientation of the specimen mammography image in the coronal plane, supplemented with the breast scheme of the plate, may provide a better understanding for pathologists and is an orientation tool in case of reoperation for the breast surgeon, if needed. The specimen plate developed aims at higher accuracy and increased synchronization of mammography-surgical-pathological processing. This plate effectively addresses the limitations of traditional orientation methods, enhancing the overall accuracy and reliability of breast cancer specimen analysis.
Author Contributions
Conceptualization, A. D., T.F.M; methodology, A.D, GY.K., T.SZ; software, G.K; validation, A.D, T.F.M; analysis, A.D., D.K; investigation, A.D., T.SZ; resources, A.D., GY.K; data curation, A.D., T.F.M; writing—original draft preparation, A.D; writing—review and editing, A.D., D.K; visualization, GY.K; supervision, T.F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Regional Science and Research Ethics Committee (protocol code 76-1-19/2021, 24-06-2021).
Informed Consent Statement
Patient consent was waived due to the use of anonymized data and the lack of risk to participants, attributed to the concurrent application of the old method.
Data Availability Statement
Data is contained within the article or supplementary material.
Acknowledgments
We would like to express our gratitude to the members of the Radiology Department and the Petz Aladár University Teaching Hospital for their invaluable support and collaboration.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A. 3D Printing Details
SPECIMEN PLATE - TECHNICAL SPECIFICATIONS OF PRODUCTION
Specimen plate dimensions: 209 mm x 199.5 mm x 1.2 mm
The plate is made using a Creality Ender 5 3D printer
A layer thickness of 0.1 mm was used to create a solid yet braided structure. This allows the material to be shaped like a textile, but is more flexible.
The material is TPU A95 synthetic resin. This material holds the stitches used to attach the specimen securely, maintaining the proper tensile strength even if only a small bite of the plate is taken with the stitch.
-
Printing parameters:
References
- Curigliano, G.; Burstein, H.J.; Gnant, M.; Loibl, S.; Cameron, D.; Regan, M.; St Gallen Consensus Conference Panelists 2023. Understanding breast cancer complexity to improve patient outcomes: The St Gallen International Consensus Conference for the Primary Therapy of Individuals with Early Breast Cancer 2023. Annals of Oncology: Official Journal of the European Society for Medical Oncology 2023, 34, 970–986. [Google Scholar] [CrossRef] [PubMed]
- Molnar, T.F. A new device for the identification of lymph nodes at lung cancer surgery. Eur J Cardiothorac Surg 2007, 31, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Kurniasih, D.A.A.; Setiawati, E.P.; Pradipta, I.S.; Subarnas, A. Patients’ Perspectives of Interprofessional Collaboration in Breast Cancer Unit. Healthcare 2023, 11, 332, Published 2023 Jan 23. [Google Scholar] [CrossRef] [PubMed]
- Houssami, N.; Macaskill, P.; Marinovich, M.L.; Dixon, J.M.; Irwig, L.; Brennan, M.E.; et al. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer 2010, 46, 3219–3232. [Google Scholar] [CrossRef] [PubMed]
- Kiss, Z.; Kocsis, J.; Nikolényi, A.; Horváth, Z.; Knollmajer, K.; Benedek, A.; et al. Opposite trends in incidence of breast cancer in young and old female cohorts in Hungary and the impact of the Covid-19 pandemic: a nationwide study between 2011-2020. Front Oncol 2023, 13, 1182170. [Google Scholar] [CrossRef] [PubMed]
- Morrow, M.; Jagsi, R.; Alderman, A.K.; Griggs, J.J.; Hawley, S.T.; Hamilton, A.S.; et al. JAMA 2009, 302, 1551–1556. [CrossRef]
- McCahill, L.E.; Single, R.M.; Aiello Bowles, E.J.; Feigelson, H.S.; James, T.A.; Barney, T.; et al. Variability in reexcision following breast conservation surgery. JAMA 2012, 307, 467–475. [Google Scholar] [CrossRef]
- Kong, J.; Bandyopadhyay, S.; Chen, W.; Al-Mufarrej, F.; Choi, L.; Kosir, M.A. Improved Rate of Negative Margins for Inflammatory Breast Cancer Using Intraoperative Frozen Section Analysis. Cancers 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, T.; Yasuda, M.; Ito, J.; Watanabe, K.; Gonda, K.; Abe, N.; et al. Pathological aspects of the intraductal spread of breast cancer. Breast Cancer 2013, 20, 34–40. [Google Scholar] [CrossRef]
- Carter, D. Margins of "lumpectomy" for breast cancer. Hum Pathol 1986, 17, 330–332. [Google Scholar] [CrossRef] [PubMed]
- Pilewskie, M.; Morrow, M. Margins in breast cancer: How much is enough? Cancer 2018, 124, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Akiyama, F.; Hiraoka, M.; Inaji, H.; Ohuchi, N.; Takatsuka, Y.; et al. Surgical Margin Status as a Cause of Local Failure after Breast Conserving Therapy. Breast Cancer 1999, 6, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Maishman, T.; Cutress, R.I.; Hernandez, A.; Gerty, S.; Copson, E.R.; Durcan, L.; et al. Local Recurrence and Breast Oncological Surgery in Young Women With Breast Cancer: The POSH Observational Cohort Study. Ann Surg 2017, 266, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Lázár, G.; Kelemen, P.; Kósa, C.; Maráz, R.; Paszt, A.; Pavlovics, G.; et al. IV. Emlőrák Konszenzus Konferencia – Az emlőrák korszerű sebészi kezelése. Magy Onkol 2020, 64, 329–346. Hungarian. Epub 2020 Nov 30. [PubMed]
- Gibson, G.R.; Lesnikoski, B.A.; Yoo, J.; Mott, L.A.; Cady, B.; Barth, R.J. Jr. A comparison of ink-directed and traditional whole-cavity re-excision for breast lumpectomy specimens with positive margins. Ann Surg Oncol 2001, 8, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Volleamere, A.J.; Kirwan, C.C. National survey of breast cancer specimen orientation marking systems. Eur J Surg Oncol 2013, 39, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Jenkins, S. A technique for marking oncological breast tissue specimens. Ann Med Surg (Lond) 2016, 7, 7–8. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).