Introduction
Aging is inherently variable, with progressive physiological, motor, and cognitive decline accompanying the process. The global demographic shift, especially in industrial societies, underscores the importance of maintaining health and physical capacity in older adults. As the incidence and prevalence of chronic non-communicable diseases (NCD) correlate with age, strategies aimed at extending individual health span are receiving growing attention to preserve quality of life and ease the socioeconomic impact (Stiebler et al., 2021).
Central to this challenge is the maintenance of physical and cognitive fitness, which play a pivotal role in preserving autonomy and enhancing overall well-being in older adults (Bangsbo et al., 2019). Currently, the World Health Organization recommends a minimum of 150-300 minutes of moderate or 75-150 minutes of intense aerobic physical activity (PA) per week, along with targeted muscle strengthening twice a week (World Health Organization, 2020).
Markers of physical fitness include, but are not limited to, cardiorespiratory fitness (as measured by maximal oxygen uptake [VO2max]), overall strength (handgrip strength), lower limb muscular strength (sit-to-stand test) and heart-rate variability (HRV). These parameters add a valuable dimension to our understanding of health management and can help drive decision-making based on objective measures.
Overall, the evidence highlights the link between PA and the risk for age-related diseases (Warburton et al., 2017). Physical exercise, as a subcategory of physical activity, is deemed a structured and repetitive effort in order to improve or maintain physical fitness (Caspersen et al., 1985). Even when started at old age, physical exercise improves physical capacity, markers of cardiometabolic health and neuroplasticity, and reduces the risk of all-cause mortality (Momma et al., 2022). Conversely, age-related decreases in physical fitness are associated with increased risk of dementia, cardiovascular disease and other NCD (Sabia et al., 2019; Gottesman et al., 2017; Brain et al., 2023).
Structured exercise programs present a variety of direct health benefits, for example in mitigating sarcopenia and delaying age-related functional disability (Marzetti et al., 2017). Adopting a lifestyle that meets the weekly recommendations of 150 min of moderate-intensity aerobic activity lowers the risk of cardiovascular diseases, their associated mortalities and the incidence of Type 2 diabetes (Sheri et al., 2016; Lee et al., 2022). Moreover, increased leisure-time PA is associated with lower risks of many cancer types independent of body weight or smoking history (Moore et al., 2016). In addition, evidence from epidemiological cross-sectional and intervention studies also suggests an association between PA and a reduced risk of dementia and mortality (Erickson et al., 2019).
However, it remains unclear which type of exercise (e.g., aerobic, resistance, motor-coordinative) is most effective in preserving cognitive and physical fitness (Valenzuela et al., 2023). Overall, studies from animal models indicate that physical activity combined with a varied environment including social contact can be most effective for inducing neuroplasticity (Kempermann et al., 2010).
A potential equivalent to the combination of physical activity and social enrichment is dancing. Dance, by nature, requires coordination, rhythm, and cognitive engagement, offering a multifaceted approach to exercise that may yield significant benefits beyond those of traditional physical activities. The performance profile of dancing is characterized by acyclic-coordinated movements and a variety of cognitive processes, including the continuous storage and retrieval of new movement patterns. This involves and trains motor, sensory, and cognitive skills. Additionally, dancing promotes social interactions (Brustio et al., 2018).
Based on previous work (Müller et al., 2017; Rehfeld et al., 2018), multi-modal approaches, such as Dance/Movement Training, aim to impact both cognition and physical functioning in older adults. By improving both cardiorespiratory (VO2max) and muscular fitness (muscle hypertrophy and functional ability) within a single exercise regimen these studies have shown promising results in young and older populations (Douka et al., 2019; Fong Yan et al., 2018).
In addition, findings indicate that multimodal dance training can reduce the risk of dementia and make an important contribution to neuroplasticity and fall prevention, and thus preserve autonomy in old age; for example, Verghese and colleagues (2003) report, in an analysis of 469 healthy seniors aged over 75 years, a 76% reduction in the risk of dementia through dancing.
Among the myriad challenges faced by older adults, cognitive decline stands out. Particularly MCI as a transitional stage between the expected cognitive decline of normal aging and dementia (Müller et al., 2021). Age-related cognitive decline and MCI are estimated to affect one in six people worldwide by 2050 (World Health Organization, 2018). The conversion rate from MCI to dementia is estimated to be around 10%-15% per year (Eshkoor et al., 2015; Roberts et al., 2013). But unlike dementia, daily living abilities are still preserved (Roberts et al., 2013). The protective effects of physical activity on dementia and overall cognitive function have been well-documented, underscoring the importance of maintaining an active lifestyle to combat the cognitive decline associated with aging (Stillman et al., 2020; Erickson et al., 2019).
For example, during one year of aerobic exercise training Erickson et al. (2009; 2011) were able to show that cardiorespiratory fitness increases the size of the hippocampus and improves memory in older adults. Furthermore, Colcombe et al. (2003) have shown that cardiorespiratory fitness is associated with reduced brain tissue loss in aging humans.
However, despite the recognized value of physical activity in reducing the risk of all-cause dementia and improving various markers of physical fitness, the literature on the specific impact of dance training on physical fitness parameters in older adults diagnosed with MCI remains sparse. This gap highlights the necessity for more targeted studies to elucidate the effects of dance on improving both cardiorespiratory and muscular fitness, thereby supporting autonomy and reducing the risk of mortality and morbidity in this vulnerable population (Voss et al., 2019).
Here, we report the findings of a randomized controlled trial examining the effects of a six-month dance intervention on parameters of physical fitness in older adults with MCI. By focusing on dance as a multimodal exercise regimen, this study aims to shed light on its potential to enhance several physical fitness markers in older adults with MCI, offering new insights into effective strategies for managing age-related decline and improving quality of life among this population.
Materials and Methods
Participants
Older adults with mild cognitive impairment (MCI) were recruited as part of the “DiADEM-Dance Against DEMentia” research project through advertisements in local newspapers, flyers, posters, word of mouth, and by using existing databases. During recruitment, the individuals were screened for eligibility based on the following inclusion criteria: (i) 50 to 80 years old, (ii) native German-speaking, and (iii) able to manage everyday activities independently. Individuals who had poor or uncorrected vision/hearing or color weakness/blindness, and/or suffer from (a) severe psychiatric disorders (e.g., bipolar disorder) or depression (assessed via the Geriatric Depression Scale (GDS; 15 items; cut-off score ≥ 6), (b) severe orthopedic diseases (e.g., a bone fracture in last six months, herniated vertebral disc), (c) severe muscular diseases (e.g., myositis, tendovaginitis), (d) severe cardiovascular diseases (e.g., heart insufficiency), (e) severe endocrinologic diseases (e.g., manifest hypothyroidism or hyperthyroidism, insulin dependent diabetes mellitus type II, BMI > 30), (f) neurological diseases other than MCI (e.g., stroke, epilepsy, multiple sclerosis), (g) major injury or had major surgery in the last six months, and/or use neuroleptics, narcotic analgesics, benzodiazepines, or psychoactive medications, and/or consume illegal intoxicants and/or have an alcohol abuse, and (h) are pregnant were excluded.
With an effect size of partial η²=0.14 and a power of .8, 52 participants would be needed to detect significant effects of interaction in a mixed ANOVA (group x time) with a significance level of α=5% (Hemmerich, 2020).
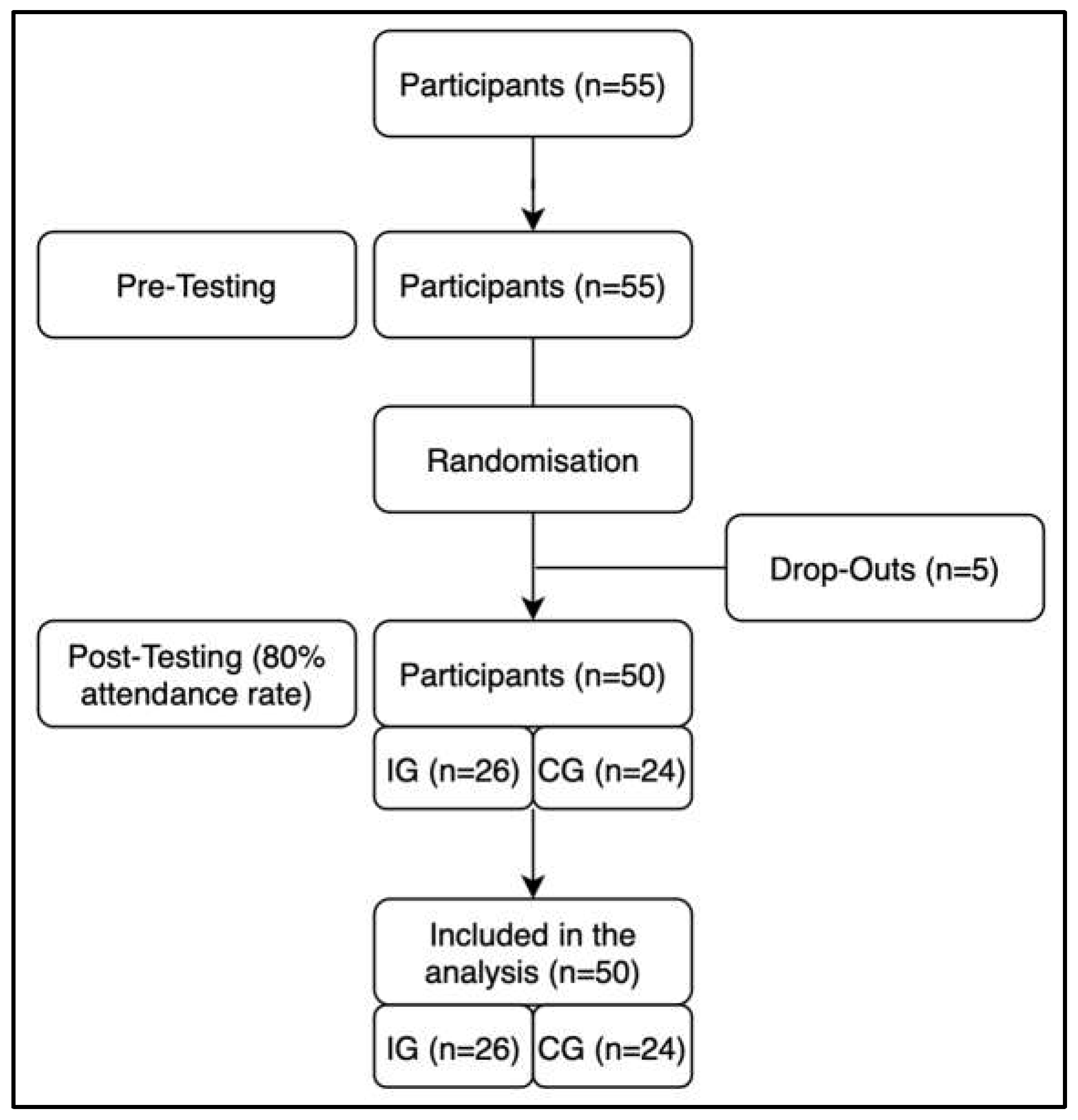
55 participants were split into two cohorts and then blindly randomized through stratified sampling (age and gender) into either an inactive control group or an intervention group participating in the dance training intervention. One adult from each group swapped their originally assigned group to ensure that a married couple could participate in the same group. Five participants dropped out during the intervention period. All other participants demonstrated an attendance of at least 80% and were thus included in the post-measurement phase (
Figure 1).
Prior to the assessment, participants were briefed about the experimental procedure and informed of possible risks and benefits associated with the study. All participants provided written consent to participate and received financial compensation. All study procedures were in accordance with the latest version of the Declaration of Helsinki and had been approved by the local ethics committee of the medical faculty of the Otto-von-Guericke University Magdeburg (reference number: 17/20). The intervention was registered in the German Clinical Trials Register (DRKS-ID: DRKS00022575) on 5th of August 2020 and added to the Cochrane Central Register of Controlled Trials on 30th of November 2020 (CENTRAL-ID: CN-02186572).
Intervention
During the six-month intervention period, the sportive dance training group performed two 90 minutes training sessions per week. In order to control the training intensity (i.e., cardiovascular strain), the heart rate of each individual participant was measured with the Polar Team Pro System (Polar Electro GmbH, Büttelborn, Germany) during all training sessions. Thereto, the participants were asked to wear a heart rate sensor (Polar Pro Sensor, Polar Electro GmbH, Büttelborn, Germany), that was attached to their body with a chest strap just below the chest muscles. Average heart rate for each training session was recorded for all participants. At the beginning of each session, all participants sat quietly on the bench for 2 minutes to establish a baseline. The length of the chest strap was individually adjusted, and the participants wore the same sensor in each training session for the entire duration of the intervention. To individualize the sensors, the respective data (a) gender, (b) date of birth, (c) height, (d) age, (e) training volume per week, (f) maximal heart rate, (g) resting heart rate, (h) VO2max, (i) aerobic threshold and (j) anaerobic threshold were entered in the system, of which parameters (f) to (j) were assessed through CPET.
A qualified instructor who held a master’s degree in sport science and was experienced in dance with older adults supervised each training session. The program of the sportive dance training focused on continuous learning of new movement patterns and choreographies, including the training of coordination, and learning of specific dance skills. The participants performed the dance movements in order to memorize the movement combinations prior to dancing the step sequences. In addition, the orientation in space and the intensity were important in execution of the dance routines, specifically the speed of movement, which was controlled by the instructors and progressively increased by adjusting the speed of the music. A multitude of individual dance genres was utilized, including line dance, jazz dance, square dance, and Latin-American dances. A training session was divided into four parts. The beginning was a warm-up with mobilization exercises for the whole body and the implementation of a coordinative part using various arm-leg combinations with increasing complexity. In addition, the dances that had already been rehearsed were gradually integrated into the warm-up during the program to activate the cardiovascular system and further the development of endurance stimuli. The main part was divided into two sections. The first section was strongly characterized by dance combinations that focused on complex coordinative tasks. Some of the choreographies in this part were danced with small equipment such as sticks, towels, or physio balls as drums. The second part focused on the strength-endurance aspects of dancing. Choreographies were employed and small fitness equipment such as brasils, fitness tires (1.2 kg) and small dumbbells were used for dancing to emphasize the physical demands of dancing. The fourth part of each training was a cool-down phase including a feet-to-head stretching routine and a sequence of low-intensity mobility exercises that was maintained over the entirety of the intervention. The participants therefore could internalize the routine sequences and execution of the exercises. The aim of the training sessions was to constantly challenge the participants in a controlled manner with medium to high exercise intensity as determined by the selected music. Intensity was controlled by beats per minute of the selected music. The majority (80%) of the training was accompanied by music with an appropriate tempo, while the cool-down phase was typically performed with relaxing music. Different genres of music and rhythms were offered so that the training remained varied and different musical preferences catered to. Challenging dance steps were broken down into progressions and put together piece by piece to form the entire choreography and dance sequence. When most of the participants had clearly internalized all dance steps of the sequence, tempo was, when possible, progressively increased until the intended, original tempo was reached.
Cardiopulmonary Exercise Testing
All participants completed an incremental step test to voluntary exhaustion to determine their individual VO2max on a bicycle ergometer. This test is safe to perform in older adults (Scardovi et al., 2007) and has shown to have high reliability (Barron et al., 2014; Scardovi et al., 2007). The seat height was adjusted to attain a bend of 5° in the knee joint at the lowest position in the pedal revolution. Warming up included three-minute unloaded pedaling at 0 W. The resistance increased by 25 W every three minutes. Participants were required to keep the cadence between 60 and 70 revolutions per minute. During the incremental cycling test, breath-by-breath pulmonary gas-exchange data (MetaSoft, Studio: Cortex Biophysik GmbH Leipzig, Germany), heart rate (Custo med 100, custo med GmbH, Ottobrunn, Germany) and lactate levels (Lactate Scout 4, EKF Diagnostic, Barleben, Germany) were assessed. VO2max and power output were normalized for body weight, to account for anthropometric differences in the group of subjects using the following formulas: VO2 (ml/min)/kg and P (W)/kg. Ratings of perceived exertion were collected after each step using a 6–20 Borg Scale (Borg, 1970). The highest stated value on the Borg Scale was used to measure maximum perceived exertion. The cardiopulmonary exercise testing (CPET) concluded when (i) the respiratory exchange ratio was above 1.10, (ii) a plateau in VO2 occurred (despite increasing workload) or (iii) the rating of perceived exertion was 18 or higher on the Borg Scale. CPET was terminated prematurely in the case of major electrocardiographic abnormalities, excessive blood pressure increase (≥230 mmHg systolic and/or ≥110 mmHg diastolic), or individual request (Klingenheben et al., 2018).
Heart Rate Variability
The measurement of HRV was performed as follows: Subjects were seated comfortably in a chair with their knees bent at a 90° angle, and their hands resting on their thighs. They were instructed to relax and breathe normally throughout the procedure. To minimize artifacts, participants were instructed not to speak or move during the recording. A stabilization period of five minutes was implemented to ensure a relaxed state. Subsequently, the resting state measurement was recorded for another five minutes (Laborde et al., 2017).
Electrocardiographic data were captured using a three-channel Holter-Electroencephalogram device, with a sampling rate of 1000Hz (Medilog AR12plus, Schiller Medizintechnik GmbH, Baar, Switzerland). The raw electrocardiographic data were then uploaded to the Medilog Darwin 2 analysis software package (Schiller Medizintechnik GmbH, Baar, Switzerland) for automatic analysis. A healthcare professional visually examined the data for any clinical abnormalities. Text files containing consecutive NN intervals were generated for further analysis.
The HRV analysis was performed using the Kubios premium 3.3 software package (University of Kuopio, Kuopio, Finland). Artifact correction was conducted with an artifact identification threshold of 0.3 seconds. The detrending of NN intervals utilized the smoothness priors method with parameters Lambda = 500 and fc = 0.035 Hz, following national guidelines and relevant literature (Malik et al., 1996; Sammito et al., 2015).
The HRV analysis encompassed time-domain, frequency-domain, and non-linear parameters. Mean heart rate (mHR) was measured in beats per minute. The time-domain index, RMSSD (root mean square of successive differences), as well as SDNN (standard deviation of all NN intervals) were measured in milliseconds. HF (high frequency power) is given in ms2. As non-linear measures have been relatively underutilized in the assessment of cardiac autonomic control, the non-linear index D2 was employed as a parameter reflecting the heart rate complexity. Higher D2 values indicate greater complexity and adaptability of the cardiac system, while lower values suggest a shift toward sympathetic dominance (Fernandes De Godoy, 2016).
Handgrip Strength
The assessment of maximal handgrip strength was conducted following the Southampton protocol (Roberts et al., 2011). Measurements of handgrip strength obtained by dynamometry show good to excellent relative reliability, but more variability is seen in absolute reliability, hence relatively large percentage changes would be necessary to indicate significant changes over time (Bohannon, 2017). To measure maximal handgrip strength, participants were asked: (i) to seat in a chair with their feet flat on the ground, (ii) to adduct their shoulders and remain their arm in a position with neutral rotation, (iii) to flex their elbow at a 90-degree angle, while the wrist maintained a neutral position (i.e., thumb facing upward), (iv) to exert maximum force by squeezing their hand as hard as possible for a duration of three seconds.
For each hand, participants performed three trials, switching hands after completing one trial (McGrath et al., 2020; Cawthon et al., 2019; Herold et al., 2021). The trial that yielded the highest absolute value for handgrip strength (measured in kg) was used for further statistical analysis.
To account for the influence of body composition, the maximum handgrip strength was then normalized to the body weight of the subject using the following formula: normalized handgrip strength = absolute handgrip strength (in kg)/body weight (in kg) (Dulac et al., 2016).
Sit-to-Stand Test
Functional strength of the lower body was quantified using the 30-second sit-to-stand test, which is a reliable and valid tool for the quantification of lower body strength in populations of active, older adults and is often incorporated into fall risk assessments (Rikli & Jones, 2013). During the 30-second sit-to-stand test, the participants were instructed to rise from a chair of standard height (43 cm) without armrests and sit down afterward. They were required to perform the task for thirty seconds, aiming to complete as many repetitions as possible, while keeping their arms folded in front of their chest. The test was conducted without wearing shoes, and the performance was quantified in the number of completed repetitions during the 30 seconds, from the first initial seated position to the final seated position.
Statistical Analysis
Statistical analysis was performed using SPSS (IBM SPSS Statistics for Windows, Version 28.0. Armonk, NY: IBM Corp). Normal distribution was tested using the Shapiro-Wilk test as well as visually inspected in Q-Q plots. Outliers were detected via boxplot diagrams, checked for likelihood of occurrence by deviation and dismissed when deemed a measurement error. Descriptive statistics (mean ± standard deviation [SD]) were calculated for all included parameters. For inferential statistics, repeated measures analysis of variance (ANOVA) with a 2x2 configuration with time (pre, post) as the within-subject factor and group (control, intervention) as the between-subject factor were performed. Levene’s test was used to assess the homogeneity of error variances. In case of heterogeneity, a power transform via box-cox-transformation was used to stabilize the data. Effect size was calculated as partial η² and interpreted as follows: ≥0.01 to <0.6: small effect; ≥0.06 to <0.14: medium effect; ≥0.14: large effect (Barros et al., 2018). For all tests, the significance level was set at α=5%.
Results
Out of all recruits, 55 were eligible to participate in the study. Five participants dropped out during the intervention period because of non-intervention-related occurrence of back pain (n=1), diseases of the heart (n=1), or lack of time/interest (n=3). No participants were excluded due to low attendance below 80%. In all, a total of 50 participants were eligible for final analysis, with 26 participants in the intervention group and 24 participants in the control group (
Table 1). For the analysis of CPET, four participants did not attend the testing of handgrip strength and Sit-to-stand performance, leaving 46 complete datasets for the analysis. For heart rate variability, ten participants were dismissed for skewed results. Borg values of the CPET were excluded from the ANOVA since they did not show homogeneity of mean error variances. No falls or other adverse events occurred during the training intervention.
Exercise Intensity
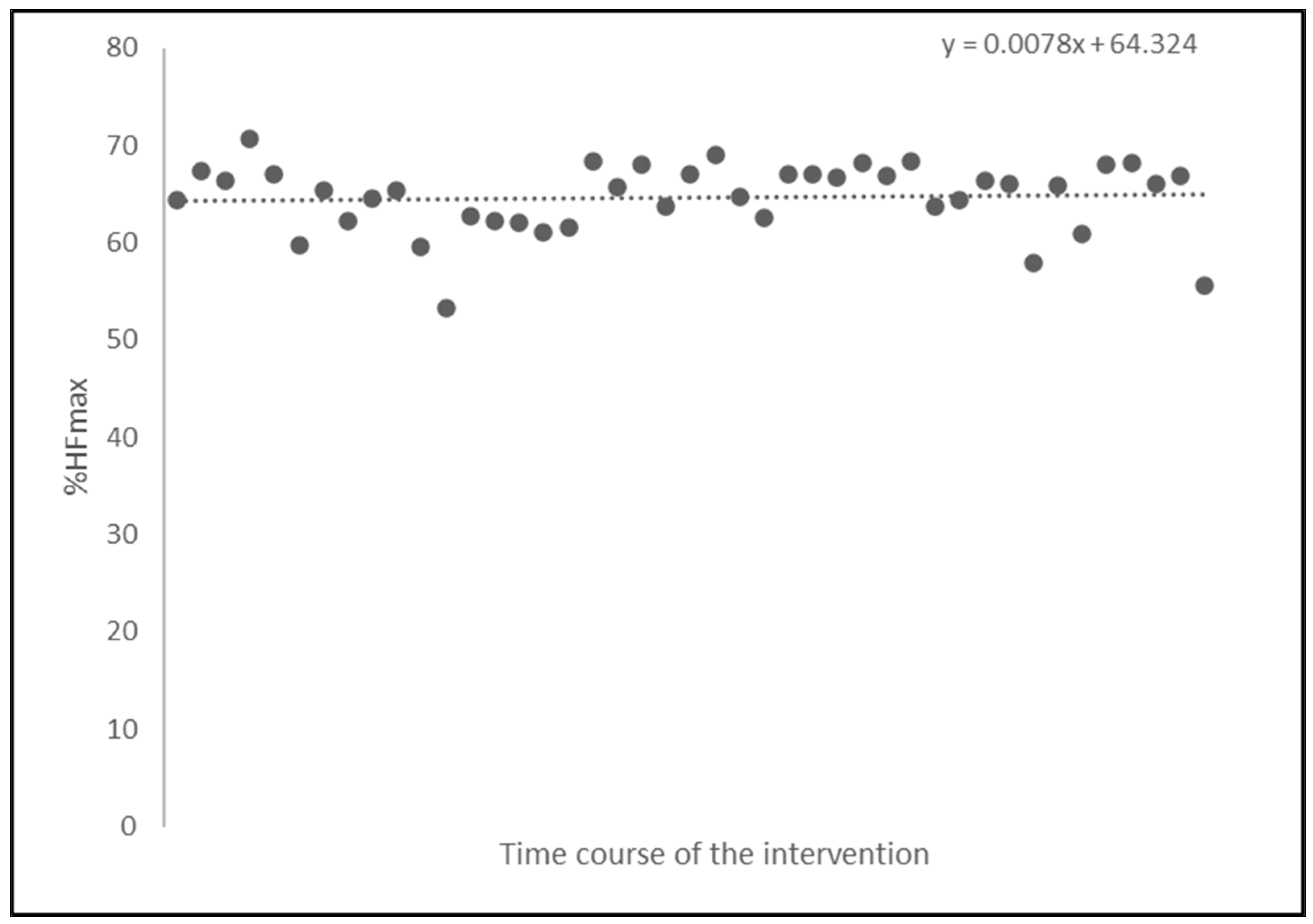
Heart rate was monitored during each 90-minute training session to gauge exercise intensity and guide the modification of the exercise intensity through the adjustments of the external load in the subsequent dance training sessions. Exercise intensity, i.e., internal load, was determined as the average percentage of the maximum heart frequency (%HF
max obtained during the CPET), across all training sessions for each participant of the intervention group. The average heart frequency measured throughout all training sessions for all participants was 97±13.49 beats per minute. The average maximal heart rate for the participants was 149.3±16.7 bpm (as determined by CPET). The average exercise intensity for the intervention group remained stable at %HF
max = 64.32% throughout the intervention (
Figure 1).
Figure 2.
Development of average exercise intensity calculated as %HFmax following the time course of the intervention over 43 training sessions.
Figure 2.
Development of average exercise intensity calculated as %HFmax following the time course of the intervention over 43 training sessions.
Cardiopulmonary Exercise Testing
There was a statistically significant interaction between time and group for VO2max: F(1, 35) = 4.326; p = .045, partial η² = .110. Regarding the control group, cardiorespiratory fitness, operationalized by VO2max, decreased from pretest to posttest (F(1, 14) = 9.448, p = .008, partial η² = .403), while such an effect of time was not noticed in the intervention group (F(1, 21) = 0.346, p = .563, partial η² = .016).
There was no statistically significant interaction between time and group for any other parameter (Pmax: F(1, 35) = 1.013, p = .321, partial η² = .028, HFmax: F(1, 35) = 0.015, p = .903, partial η² = .000, Lactate: F(1, 35) = 0.703, p = .408, partial η² = .020).
There was a statistically significant main effect for time for Pmax: F(1, 35) = 5.250, p = .028, partial η² = .130, but not for HFmax: F(1, 35) = 1.682, p = .203, partial η² = .046 or Lactate: F(1, 35) = 1.063, p = .310, partial η² = .029.
There was no significant main effect for group for the parameters Pmax: F(1, 35) = 0.230, p = .634, partial η² = .007, HFmax: F(1, 35) = 0.929, p = .342, partial η² = .026 and Lactate: F(1, 35) = 0.081, p = .777, partial η² = .002.
Table 2.
Mean and standard deviation of cardiorespiratory fitness in both groups.
Table 2.
Mean and standard deviation of cardiorespiratory fitness in both groups.
| Variables |
CG |
IG |
ANOVA (Group x Time) |
| Pre (mean±SD) |
Post (mean±SD) |
Pre (mean±SD) |
Post (mean±SD) |
F |
p |
| Pmax (normalized, A.u.) |
133.33±40.83 |
121.67±42.11 |
123.86±35.76 |
119.32±36.13 |
1.013 |
.321 |
| VO2max (normalized, A.u.) |
26.47±6.51 |
24.4±5.97 |
25.45±5.99 |
26.00±6.98 |
4.326 |
.045* |
| HFmax (bpm) |
152.27±17.29 |
149.33±17.17 |
147.68±15.27 |
144.14±17.96 |
0.015 |
.903 |
| Lactate (mmol/l) |
6.26±2.36 |
6.20±3.21 |
6.30±2.30 |
5.72±2.04 |
0.703 |
.408 |
| Borg (A.u.) |
17.00±1.81 |
17.60±1.40 |
16.95±1.46 |
16.32±2.08 |
n.c. |
Sit-to-Stand Test
There was no statistically significant interaction between time and group, F(1, 44) = 3.053, p = .088, partial η² = .065. However, we noticed a significant main effect for time: F(1, 44) = 19.535, p < .001, partial η² = .307, whereby both groups showed increased performance from pretest to posttest. No significant main effect for group emerged: F(1, 44) = 18.47, p = .270, partial η² = .28.
Table 3.
Mean and standard deviation of number of repetitions during Sit-to-stand test in both groups.
Table 3.
Mean and standard deviation of number of repetitions during Sit-to-stand test in both groups.
| Variable |
CG |
IG |
ANOVA (Group x Time) |
| Pre (mean±SD) |
Post (mean±SD) |
Pre (mean±SD) |
Post (mean±SD) |
F |
p |
| Repetitions |
12.30±3.03 |
13.20±2.53 |
12.62±2.70 |
14.69±3.39 |
3.053 |
.088 |
Heart Rate Variability
There was no statistically significant interaction between time and group for any parameter (mHR: F(1, 37) = 2.210, p = .146, partial η² = .056, SDNN: F(1, 37) = 0.924, p = .566, partial η² = .000, RMSSD: F(1, 37) = 0.004, p = .948, partial η² = .000, HF: F(1, 37) = 0.698, p = .566, partial η² = .004, D2: F(1, 37) = 0.222, p = .641, partial η² = .006).
There was no significant main effect for time for any parameter (mHR: F(1, 37) = 2.625, p = .114, partial η² = .066, SDNN: F(1, 37) = 1.799, p = .188, partial η² = .046, RMSSD: F(1, 37) = 3.496, p = .069, partial η² = .086, HF: F(1, 37) = 0.153, p = .698, partial η² = .004, D2: F(1, 37) = 2.419, p = .128, partial η² = .061).
There was no significant main effect for group for any parameter (mHR: F(1, 37) = 0.148, p = .703, partial η² = .004, SDNN: F(1, 37) = 0.019, p = .891, partial η² = .001, RMSSD: F(1, 37) = 0.001, p = .977, partial η² = .000, HF: F(1, 37) = 0.153, p = .698, partial η² = .004, D2: F(1, 37) = 0.152, p = .698, partial η² = .004).
Table 4.
Mean and standard deviation of heart rate variability in both groups.
Table 4.
Mean and standard deviation of heart rate variability in both groups.
| Variables |
CG |
IG |
ANOVA (Group x Time) |
| Pre (mean±SD) |
Post (mean±SD) |
Pre (mean±SD) |
Post (mean±SD) |
F |
p |
| mHR (bpm) |
68.31±11.32 |
64.83±8.48 |
65.51±9.30 |
65.36±9.74 |
2.210 |
.146 |
| SDNN (ms) |
25.51±14.58 |
22.93±9.56 |
24.80±15.75 |
22.57±11.69 |
0.924 |
.566 |
| RMSSD (ms) |
27.22±16.93 |
23.02±12.24 |
27.51±20.73 |
23.00±14.80 |
0.004 |
.948 |
| HF (ms2) |
251.35±310.47 |
227.89±236.24 |
336.22±467.33 |
263.51±312.19 |
0.698 |
.566 |
| D2 (A.u.) |
0.71±1.21 |
0.52±0.85 |
0.93±1.48 |
0.57±1.05 |
0.222 |
.641 |
Handgrip Strength
There was no statistically significant interaction between time and group, F(1, 44) = 0.335, p = .566, partial η² = .008. A significant main effect for time was observed with F(1, 44) = 4.409, p = .042, partial η² = .091, considering that both groups showed increased handgrip strength from pretest to posttest. Handgrip strength was typically higher for the right hand, as indicated by a significant main effect for hand: F(1, 44) = 7.917, p = .007; partial η² = .152. There was no significant main effect for group, F(1, 44) = 0.708, p = .405, partial η² = .016.
Table 5.
Mean and standard deviation of handgrip strength in both groups.
Table 5.
Mean and standard deviation of handgrip strength in both groups.
| Variables |
CG |
IG |
ANOVA (Group x Time) |
| Pre (mean±SD) |
Post (mean±SD) |
Pre (mean±SD) |
Post (mean±SD) |
F |
p |
| Handgrip Strength - Left Hand (normalized, A.u.) |
1.35±0.46 |
1.41±0.42 |
1.26±0.39 |
1.29±0.39 |
0.335 |
.566 |
| Handgrip Strength - Right Hand (normalized, A.u.) |
1.40±0.45 |
1.46±0.45 |
1.31±0.40 |
1.35±0.36 |
Discussion
This study examined the effects of a six-month sportive dance intervention on parameters of physical fitness in older adults with MCI. Our findings contribute to the growing body of evidence supporting the benefits of structured forms of physical activity, particularly dance, in mitigating age-related physical decline.
Overall, the intervention group demonstrated mostly improvements or stabilization in general fitness parameters compared to the control group.
Cardiorespiratory Fitness
Notably, participants in the intervention group showed significant increases in VO2max, when compared to the control group, as evidenced by CPET. This aligns with previous research indicating that an endurance-based dance training can enhance cardiorespiratory fitness (Fong Yan et al., 2018; Rodrigues-Krause et al., 2016). However, no significant improvements were observed in HFmax, Pmax and lactate levels, which could be attributed to the moderate intensity of the dance training with an average of 64% HFmax. The step sequences of the dances, for example, needed to be trained at slower speed first in order to memorize the correct order, resulting in a lower training intensity. Karlsen et al. (2017) describe the importance of training at a high training intensity in order to elicit maximum changes in cardiorespiratory fitness.
Sit-to-Stand Test
Functional strength of the lower body, assessed using the 30-second sit-to-stand test, showed higher, although nonsignificant improvements in the intervention group, when compared to the control group. Previous literature on the sit-to-stand test in older populations has shown variability in performance levels, but our results align with studies that have observed better performance after sportive dance training interventions (Franco et al., 2020; Sooktho et al., 2022).
Handgrip Strength
Despite these positive outcomes, the intervention did not show a significant effect on handgrip strength. Little research exists on handgrip strength following a dance intervention; Woloszyn et al. (2021) noted an improvement in handgrip strength for older wheelchair users after finishing twelve weeks of dance movement therapy, but it can be argued that the wheelchair use itself was a sufficient stimulus for increasing handgrip strength. Another study found a favorable effect for the control group after twelve weeks, while the dance group did not display significant changes (Kamegaya et al., 2014). A meta-analysis of Labott et al. (2019) concluded that multimodal training approaches generally display a stronger influence on handgrip strength than interventions adhering to a single training focus (strength, balance, flexibility or endurance). In our study, both IG and CG improved from pre-testing to post-testing, so the changes cannot be attributed to the intervention, although small training gadgets such as weights and drumsticks were used regularly.
Heart Rate Variability
Furthermore, no significant influence of the intervention on any of the parameters of heart rate variability was visible. The lack of significant changes in HRV is particularly noteworthy, as a higher HRV is generally associated with better cardiovascular health. The decrease or stabilization of the parameters of HRV in this study may reflect age-related limitations in the cardiovascular system’s adaptive capacity (Grässler et al., 2021).
Limitations
Several limitations should be considered when interpreting these findings. During CPET, psychological factors and varying levels of motivation among participants may have impacted their performance during these tests.
Additionally, the roof of the sports room used for the training sessions interfered with the GPS sensors of the heart rate sensor, whereby the recorded speed of the participants as well as the travelled distance could not be evaluated.
The COVID-19 pandemic also affected the study design and participant behavior. The need to adhere to pandemic regulations resulted in the formation of two cohorts, whereby the intended intervention length was cut in half, from twelve to six months. A longer duration could have helped to better pronounce any effects. Additionally, the pandemic regulations could have potentially increased sedentary behavior in both groups due to homebound restrictions, possibly skewing the magnitude of effect and making the results more difficult to compare to studies in an ordinary setting. Future research should aim to replicate this study under conditions that allow for consistent and unrestricted physical activity to better control for these variables.
Conclusion
In conclusion, our study supports the potential of dance interventions to improve certain aspects of physical fitness in older adults with MCI, particularly cardiorespiratory fitness (operationalized by VO2max). However, the intervention did not significantly enhance other parameters of cardiorespiratory fitness, functional lower body strength, handgrip strength or HRV. These findings partially align with existing literature, highlighting the need for further research with larger sample sizes. Future studies should explore the impact of sportive dance training interventions on balance abilities and cognitive parameters to provide a more comprehensive understanding of their benefits for older adults with MCI.
The promising results regarding physical fitness improvements suggest that dance could be a valuable addition to exercise regimens for older adults. Given its multimodal nature, combining physical, cognitive, and social elements, dance offers a holistic approach to enhancing the health and well-being of this population. Further investigation into the specific mechanisms underlying these benefits will be crucial in optimizing dance-based interventions and maximizing their impact on healthy aging.
Overall, this study underscores the potential of dance as an effective and enjoyable form of structured physical activity for older adults.
Author Contributions
Conceptualization, Stefanie Schreiber, Rüdiger Braun-Dullaeus, Patrick Müller, Notger G. Müller and Anita Hökelmann; Formal analysis, Ulrich Thiel and Marvin Stiebler; Funding acquisition, Patrick Müller and Anita Hökelmann; Investigation, Ulrich Thiel, Marvin Stiebler, Berit Labott, Johanna Bappert, Corinna Langhans, Nicole Halfpaap, Bernhard Grässler, Fabian Herold, Patrick Müller and Anita Hökelmann; Methodology, Patrick Müller, Notger G. Müller and Anita Hökelmann; Project administration, Corinna Langhans, Patrick Müller, Notger G. Müller and Anita Hökelmann; Resources, Patrick Müller, Notger G. Müller and Anita Hökelmann; Software, Bernhard Grässler; Supervision, Stefanie Schreiber, Rüdiger Braun-Dullaeus, Patrick Müller, Notger G. Müller and Anita Hökelmann; Visualization, Ulrich Thiel and Marvin Stiebler; Writing—original draft, Ulrich Thiel and Marvin Stiebler; Writing—review & editing, Ulrich Thiel, Marvin Stiebler, Berit Labott, Fabian Herold, Patrick Müller, Notger G. Müller and Anita Hökelmann.
Funding
The DiADEM project was supported by the European Regional Development Fund through the Investitionsbank Sachsen-Anhalt (ZS/2019/07/99755). Patrick Müller was supported by the Polycarb-Leporin-Programm of the University Mageburg (PLP23/5) and the German Center for Mental Health (DZPG; BMBF).
Conflict of Interest Statement
The authors declare no conflict of interests.
References
- Bangsbo J, Blackwell J, Boraxbekk CJ, Caserotti P, Dela F, Evans AB, Jespersen AP, Gliemann L, Kramer AF, Lundbye-Jensen J, Mortensen EL, Lassen AJ, Gow AJ, Harridge SDR, Hellsten Y, Kjaer M, Kujala UM, Rhodes RE, Pike ECJ, Skinner T, Skovgaard T, Troelsen J, Tulle E, Tully MA, van Uffelen JGZ, Viña J. Copenhagen Consensus statement 2019: physical activity and ageing. Br J Sports Med. 2019 Jul;53(14):856-858. Epub 2019 Feb 21. PMID: 30792257; PMCID: PMC6613739. [CrossRef]
- Barron A, Dhutia N, Mayet J, Hughes AD, Francis DP, Wensel R. Test-retest repeatability of cardiopulmonary exercise test variables in patients with cardiac or respiratory disease. Eur J Prev Cardiol. 2014 Apr;21(4):445-53. Epub 2014 Jan 7. PMID: 24398370. [CrossRef]
- Barros L.A.N., Ferrari-Piloni C., Torres E.M., Estrella C., Valladares-Neto J. Effect size: A statistical basis for clinical practice. Rev. Odonto Ciênc. 2018;33:84. [CrossRef]
- Bohannon RW. Test-Retest Reliability of Measurements of Hand-Grip Strength Obtained by Dynamometry from Older Adults: A Systematic Review of Research in the PubMed Database. J Frailty Aging. 2017;6(2):83-87. PMID: 28555708. [CrossRef]
- Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2(2):92-8. PMID: 5523831.
- Brain, J., Greene, L., Tang, E. Y. H., Louise, J., Salter, A., Beach, S., Turnbull, D., Siervo, M., Stephan, B. C. M., & Tully, P. J. (2023). Cardiovascular disease, associated risk factors, and risk of dementia: An umbrella review of meta-analyses. Frontiers in epidemiology, 3, 1095236. [CrossRef]
- Brustio PR, Liubicich ME, Chiabrero M, Rabaglietti E. Dancing in the golden age: a study on physical function, quality of life, and social engagement. Geriatr Nurs. 2018 Nov;39(6):635-639. Epub 2018 Nov 12. PMID: 29773413. [CrossRef]
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985 Mar-Apr;100(2):126-31. PMID: 3920711; PMCID: PMC1424733.
- Cawthon, P.M.; Travison, T.G.; Manini, T.M.; Patel, S.; Pencina, K.M.; Fielding, R.A.; Magaziner, J.M.; Newman, A.B.; Brown, T.; Kiel, D.P.; et al. Establishing the Link Between Lean Mass and Grip Strength Cut-points With Mobility Disability and Other Health Outcomes: Proceedings of the Sarcopenia Definition and Outcomes Consortium Conference. J. Gerontol. Ser. A 2019, 75, 1317–1323.
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003; 58: 176–180. [CrossRef]
- Douka et al. 2019. Traditional Dance Improves the Physical Fitness and Well-Being of the Elderly. Front. Aging Neurosci., 05 April 2019 Sec. Neurocognitive Aging and Behavior Volume 11 - 2019. [CrossRef]
- Dulac M, Boutros GE, Pion C, Barbat-Artigas S, Gouspillou G, Aubertin-Leheudre M. Is handgrip strength normalized to body weight a useful tool to identify dynapenia and functional incapacity in post-menopausal women? Braz J Phys Ther. 2016 Nov-Dec;20(6):510-516. Epub 2016 Sep 15. PMID: 27683834; PMCID: PMC5176197. [CrossRef]
- Erickson KI, Hillman C, Stillman CM, Ballard RM, Bloodgood B, Conroy DE, Macko R, Marquez DX, Petruzzello SJ, Powell KE; FOR 2018 PHYSICAL ACTIVITY GUIDELINES ADVISORY COMMITTEE*. Physical Activity, Cognition, and Brain Outcomes: A Review of the 2018 Physical Activity Guidelines. Med Sci Sports Exerc. 2019 Jun;51(6):1242-1251. PMID: 31095081; PMCID: PMC6527141. [CrossRef]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wójcicki TR, McAuley E, Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009; 19: 1030–1039. [CrossRef]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011; 108: 3017–3022. [CrossRef]
- Erickson KI, Hillman C, Stillman CM, Ballard RM, Bloodgood B, Conroy DE, Macko R, Marquez DX, Petruzzello SJ, Powell KE. Physical Activity, Cognition, and Brain Outcomes: A Review of the 2018 Physical Activity Guidelines. Med Sci Sports Exerc. 2019; 51: 1242–1251. [CrossRef]
- Eshkoor SA, Hamid TA, Mun CY, Ng CK. Mild cognitive impairment and its management in older people. Clin Interv Aging. 2015 Apr 10;10:687-93. PMID: 25914527; PMCID: PMC4401355. [CrossRef]
- Fernandes de Godoy M. Nonlinear Analysis of Heart Rate Variability: A Comprehensive Review. J. Cardiol. Ther. 2016;3:528–533. [CrossRef]
- Fong Yan, A., Cobley, S., Chan, C. et al. The Effectiveness of Dance Interventions on Physical Health Outcomes Compared to Other Forms of Physical Activity: A Systematic Review and Meta-Analysis. Sports Med 48, 933–951 (2018). [CrossRef]
- Franco MR, Sherrington C, Tiedemann A, Pereira LS, Perracini MR, Faria CSG, Negrão-Filho RF, Pinto RZ, Pastre CM. Effect of Senior Dance (DanSE) on Fall Risk Factors in Older Adults: A Randomized Controlled Trial. Phys Ther. 2020 Apr 17;100(4):600-608. PMID: 31899491. [CrossRef]
- Gottesman, R. F., Albert, M. S., Alonso, A., Coker, L. H., Coresh, J., Davis, S. M., Deal, J. A., McKhann, G. M., Mosley, T. H., Sharrett, A. R., Schneider, A. L. C., Windham, B. G., Wruck, L. M., & Knopman, D. S. (2017). Associations Between Midlife Vascular Risk Factors and 25-Year Incident Dementia in the Atherosclerosis Risk in Communities (ARIC) Cohort. JAMA neurology, 74(10), 1246–1254. [CrossRef]
- Grässler B, Thielmann B, Böckelmann I, Hökelmann A. Effects of different exercise interventions on heart rate variability and cardiovascular health factors in older adults: a systematic review. Eur Rev Aging Phys Act. 2021 Nov 17;18(1):24. PMID: 34789148; PMCID: PMC8597177. [CrossRef]
- Hemmerich, W. (2020). StatistikGuru: Stichprobengröße für die mixed ANOVA berechnen. Retrieved from https://statistikguru.de/rechner/stichprobengroesse-mixed-anova.html.
- Herold, F.; Behrendt, T.; Törpel, A.; Hamacher, D.; Müller, N.G.; Schega, L. Cortical hemodynamics as a function of handgrip strength and cognitive performance: A cross-sectional fNIRS study in younger adults. BMC Neurosci. 2021, 22, 10.
- Kamegaya T, Araki Y, Kigure H; Long-Term-Care Prevention Team of Maebashi City; Yamaguchi H. Twelve-week physical and leisure activity programme improved cognitive function in community-dwelling elderly subjects: a randomized controlled trial. Psychogeriatrics. 2014 Mar;14(1):47-54. Epub 2014 Feb 16. PMID: 24528600. [CrossRef]
- Karlsen T, Aamot IL, Haykowsky M, Rognmo Ø. High Intensity Interval Training for Maximizing Health Outcomes. Prog Cardiovasc Dis. 2017 Jun-Jul;60(1):67-77. Epub 2017 Apr 3. PMID: 28385556. [CrossRef]
- Kempermann G, Fabel K, Ehninger D, Babu H, Leal-Galicia P, Garthe A, Wolf SA. Why and how physical activity promotes experience-induced brain plasticity. Front Neurosci. 2010 Dec 8;4:189. PMID: 21151782; PMCID: PMC3000002. [CrossRef]
- Klingenheben, Thomas & Loellgen, Herbert & Bosch, Ralph & Trappe, H.-J. (2018). Manual zum Stellenwert der Ergometrie. Der Kardiologe. 12. [CrossRef]
- Laborde S, Mosley E, Thayer JF. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research - Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front Psychol. 2017 Feb 20;8:213. PMID: 28265249; PMCID: PMC5316555. [CrossRef]
- Labott BK, Bucht H, Morat M, Morat T, Donath L. Effects of Exercise Training on Handgrip Strength in Older Adults: A Meta-Analytical Review. Gerontology. 2019;65(6):686-698. Epub 2019 Sep 9. PMID: 31499496. [CrossRef]
- Lee, D. H., Rezende, L. F. M., Joh, H. K., Keum, N., Ferrari, G., Rey-Lopez, J. P., Rimm, E. B., Tabung, F. K., & Giovannucci, E. L. (2022). Long-Term Leisure-Time Physical Activity Intensity and All-Cause and Cause-Specific Mortality: A Prospective Cohort of US Adults. Circulation, 146(7), 523–534. [CrossRef]
- Marek Malik, J. Thomas Bigger, A. John Camm, Robert E. Kleiger, Alberto Malliani, Arthur J. Moss, Peter J. Schwartz, Heart rate variability: Standards of measurement, physiological interpretation, and clinical use, European Heart Journal, Volume 17, Issue 3, March 1996, Pages 354-381. [CrossRef]
- Marzetti E, Calvani R, Tosato M, Cesari M, Di Bari M, Cherubini A, Broccatelli M, Savera G, D’Elia M, Pahor M, Bernabei R, Landi F; SPRINTT Consortium. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin Exp Res. 2017 Feb;29(1):35-42. Epub 2017 Feb 8. [CrossRef]
- McGrath, R.; Hackney, K.J.; Ratamess, N.A.; Vincent, B.M.; Clark, B.C.; Kraemer, W.J. Absolute and Body Mass Index Normalized Handgrip Strength Percentiles by Gender, Ethnicity, and Hand Dominance in Americans. Adv. Geriatr. Med. Res. 2020, 2, e200005.
- Momma H, Kawakami R, Honda T, Sawada SS. Muscle-strengthening activities are associated with lower risk and mortality in major non-communicable diseases: a systematic review and meta-analysis of cohort studies. Br J Sports Med. 2022 Jul;56(13):755-763. Epub 2022 Feb 28. PMID: 35228201; PMCID: PMC9209691. [CrossRef]
- Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, Keadle SK, Arem H, Berrington de Gonzalez A, Hartge P, Adami HO, Blair CK, Borch KB, Boyd E, Check DP, Fournier A, Freedman ND, Gunter M, Johannson M, Khaw KT, Linet MS, Orsini N, Park Y, Riboli E, Robien K, Schairer C, Sesso H, Spriggs M, Van Dusen R, Wolk A, Matthews CE, Patel AV. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern Med. 2016 Jun 1;176(6):816-25. [CrossRef]
- Müller P, Stiebler M, Schwarck S, Haghikia A, Düzel E. Physical activity, aging and brain health. Dtsch Z Sportmed. 2021;72: 327-334. [CrossRef]
- Müller P, Rehfeld K, Schmicker M, Hökelmann A, Dordevic M, Lessmann V, Brigadski T, Kaufmann J, Müller NG. Evolution of Neuroplasticity in Response to Physical Activity in Old Age: The Case for Dancing. Front Aging Neurosci. 2017; 9: 56. [CrossRef]
- Rehfeld, K., Lüders, A., Hökelmann, A., Lessmann, V., Kaufmann, J., Brigadski, T., Müller, P., & Müller, N. G. (2018). Dance training is superior to repetitive physical exercise in inducing brain plasticity in the elderly. PloS one, 13(7), e0196636. [CrossRef]
- Rikli RE, Jones CJ. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist. 2013 Apr;53(2):255-67. Epub 2012 May 20. PMID: 22613940. [CrossRef]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429.
- Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med. 2013 Nov;29(4):753-72. PMID: 24094295; PMCID: PMC3821397. [CrossRef]
- Rodrigues-Krause J, Farinha JB, Krause M, Reischak-Oliveira Á. Effects of dance interventions on cardiovascular risk with ageing: Systematic review and meta-analysis. Complement Ther Med. 2016 Dec;29:16-28. Epub 2016 Sep 4. PMID: 27912941. [CrossRef]
- Sabia, S., Fayosse, A., Dumurgier, J., Schnitzler, A., Empana, J. P., Ebmeier, K. P., Dugravot, A., Kivimäki, M., & Singh-Manoux, A. (2019). Association of ideal cardiovascular health at age 50 with incidence of dementia: 25 year follow-up of Whitehall II cohort study. BMJ (Clinical research ed.), 366, l4414. [CrossRef]
- Sammito, Stefan & Thielmann, Beatrice & Seibt, Reingard & Klussmann, A. & Weippert, Matthias & Böckelmann, Irina. Guideline for the application of heart rate and heart rate variability in occupational medicine and occupational science. ASU International. 2015. [CrossRef]
- Scardovi AB, Coletta C, De Maria R, Perna S, Aspromonte N, Feola M, Rosso G, Greggi M, Ceci V. The cardiopulmonary exercise test is safe and reliable in elderly patients with chronic heart failure. J Cardiovasc Med (Hagerstown). 2007 Aug;8(8):608-12. PMID: 17667032. [CrossRef]
- Sheri R. Colberg, Ronald J. Sigal, Jane E. Yardley, Michael C. Riddell, David W. Dunstan, Paddy C. Dempsey, Edward S. Horton, Kristin Castorino, Deborah F. Tate; Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 1 November 2016; 39 (11): 2065–2079. [CrossRef]
- Sooktho S, Songserm N, Woradet S, Suksatan W. A Meta-Analysis of the Effects of Dance Programs on Physical Performance: Appropriate Health Promotion for Healthy Older Adults. Ann Geriatr Med Res. 2022 Sep;26(3):196-207. Epub 2022 Sep 6. PMID: 36064303; PMCID: PMC9535373. [CrossRef]
- Stiebler M, Müller P, Bock M, Lechner B, Lechner K. Turning back the clock on aging? A perspective on selected mechanisms and therapeutic avenues. Dtsch Z Sportmed. 2021; 72: 335-343. [CrossRef]
- Stillman CM, Esteban-Cornejo I, Brown B, Bender CM, Erickson KI. Effects of Exercise on Brain and Cognition Across Age Groups and Health States. Trends Neurosci. 2020; 43: 533–543. [CrossRef]
- Valenzuela PL, Saco-Ledo G, Morales JS, Gallardo-Gómez D, Morales-Palomo F, López-Ortiz S, Rivas-Baeza B, Castillo-García A, Jiménez-Pavón D, Santos-Lozano A, Del Pozo Cruz B, Lucia A. Effects of physical exercise on physical function in older adults in residential care: a systematic review and network meta-analysis of randomised controlled trials. Lancet Healthy Longev. 2023 Jun;4(6):e247-e256. Epub 2023 May 11. PMID: 37182530. [CrossRef]
- Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, Kuslansky G, Ambrose AF, Sliwinski M, Buschke H. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003 Jun 19;348(25):2508-16. PMID: 12815136. [CrossRef]
- Voss MW, Soto C, Yoo S, Sodoma M, Vivar C, van Praag H. Exercise and Hippocampal Memory Systems. Trends Cogn Sci (Regul Ed ). 2019; 23: 318–333. [CrossRef]
- Warburton, D. E. R., & Bredin, S. S. D. (2017). Health benefits of physical activity: a systematic review of current systematic reviews. Current opinion in cardiology, 32(5), 541–556. [CrossRef]
- Wołoszyn, N., Wiśniowska-Szurlej, A., Grzegorczyk, J. et al. The impact of physical exercises with elements of dance movement therapy on the upper limb grip strength and functional performance of elderly wheelchair users living in nursing homes – a randomized control trial. BMC Geriatr 21, 423 (2021). [CrossRef]
- World Health Organization. Fact Sheet on Ageing. Geneva: World Health Organization; 2018.
- World Health Organization. WHO guidelines on physical activity and sedentary behaviour. Geneva: World Health Organization; 2020.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).