Submitted:
16 July 2024
Posted:
16 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Producing MNTs with a Truncated Carrier Module
2.3. MNT Expression and Purification
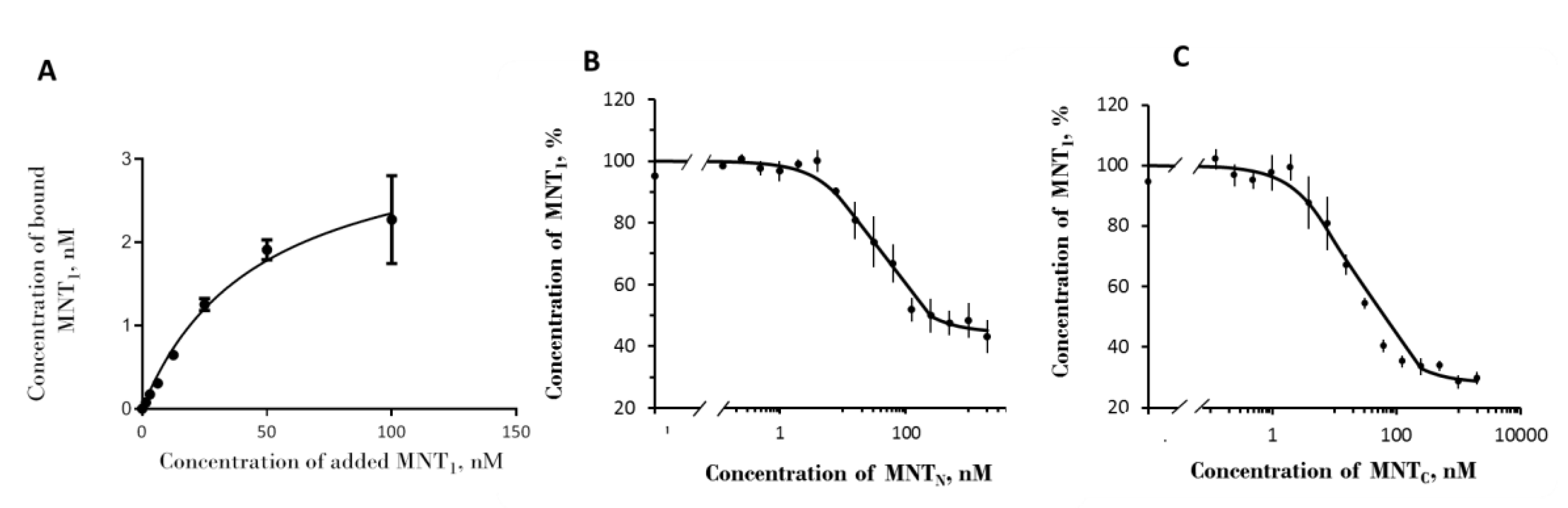
2.4. Binding Assay
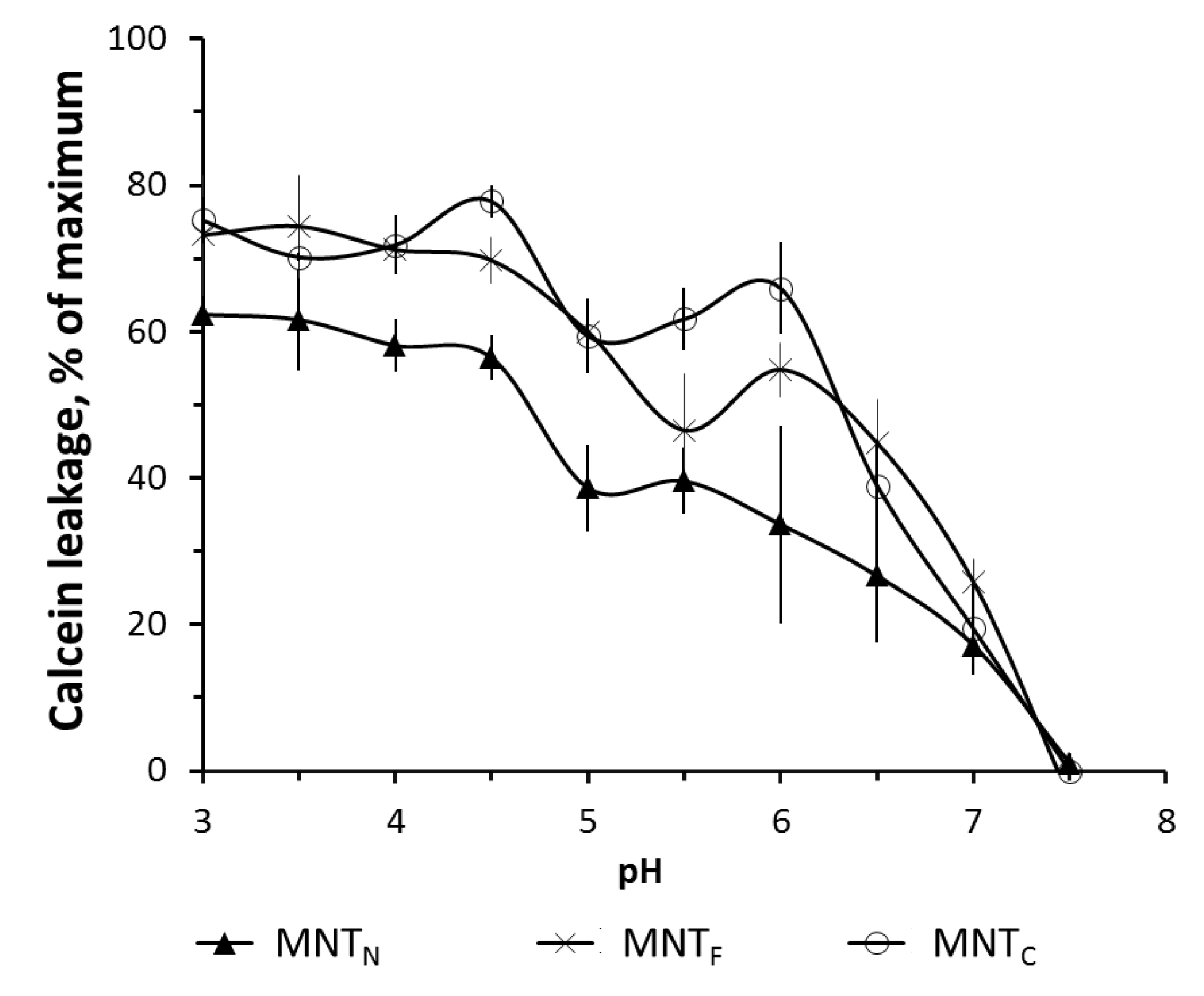
2.4. Liposome Leakage Assay
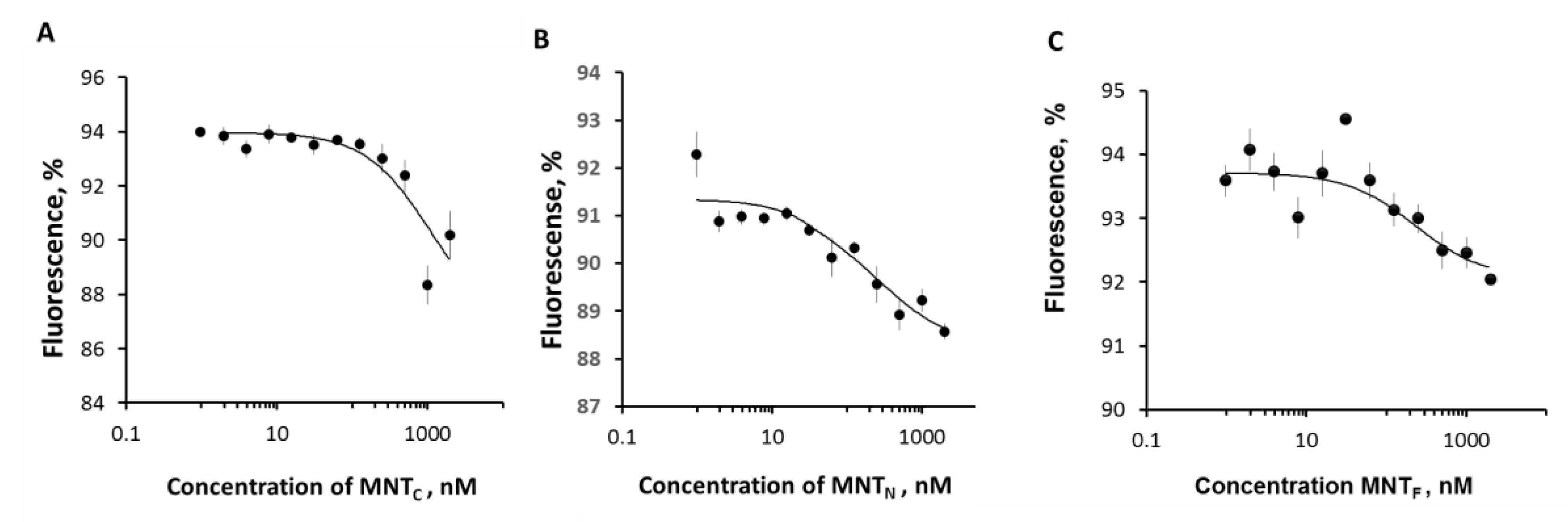
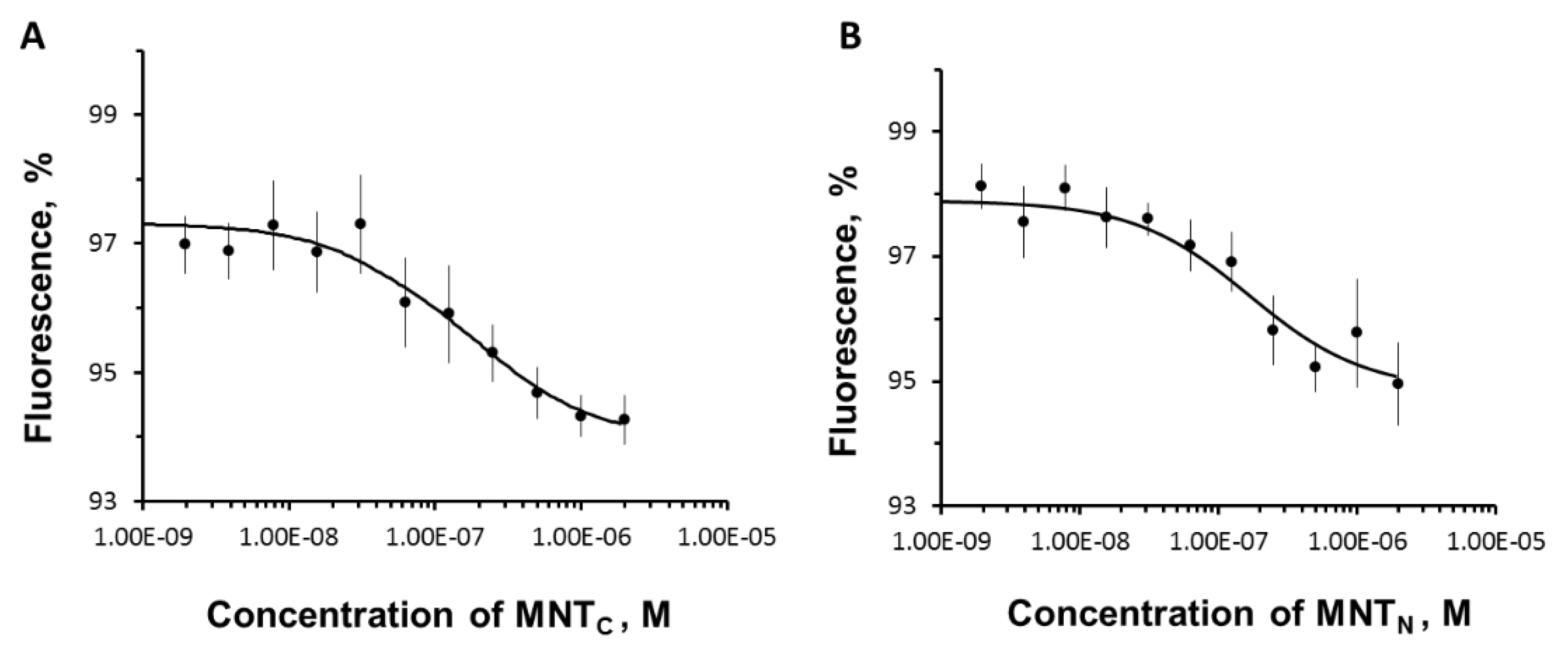
2.5. Thermophoresis
2.7. Photocytotoxicity
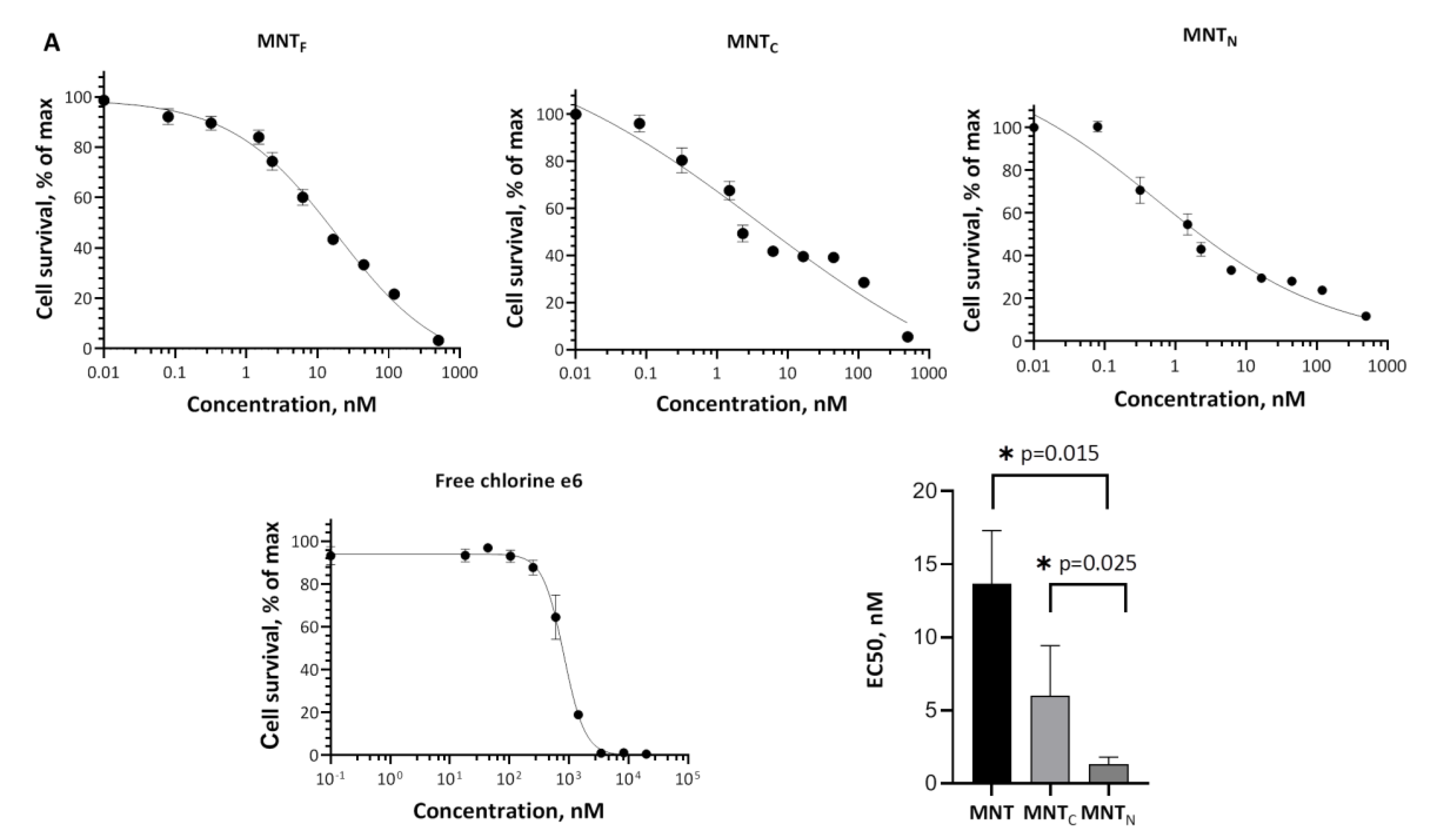
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, Y. T.; Tan, Y. J.; Oon, C. E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Manzari, M. T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D. A. Targeted drug delivery strategies for precision medicines. Nat. Rev. Mater. 2021, 6(4), 351–370. [Google Scholar] [CrossRef] [PubMed]
- Craik, D. J.; Fairlie, D. P.; Liras, S.; Price, D. The future of peptide-based drugs. Chem. Biol. Drug Des 2013, 81(1), 136–147. [Google Scholar] [CrossRef] [PubMed]
- Muttenthaler, M.; King, G. F.; Adams, D. J.; Alewood, P. F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 2021, 20(4), 309–325. [Google Scholar] [CrossRef] [PubMed]
- Vargason, A. M.; Anselmo, A. C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng 2021, 5(9), 951–967. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: current applications and future directions. Signal. Transduct. Target Ther. 2022, 7(1), 48. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, A. S. The Delivery of Biologically Active Agents into the Nuclei of Target Cells for the Purposes of Translational Medicine. Acta Naturae. 2020, 12(4), 47–56. [Google Scholar] [CrossRef] [PubMed]
- Slastnikova, T. A.; Rosenkranz, A. A.; Gulak, P. V.; Schiffelers, R. M.; Lupanova, T. N.; Khramtsov, Y. V.; Zalutsky, M. R.; Sobolev, A. S. Modular nanotransporters: a multipurpose in vivo working platform for targeted drug delivery. Int. J. Nanomedicine. 2012, 7, 467–482. [Google Scholar] [PubMed]
- Slastnikova, T. A.; Rosenkranz, A. A.; Lupanova, T. N.; Gulak, P. V.; Gnuchev, N. V.; Sobolev, A. S. Study of efficiency of the modular nanotransporter for targeted delivery of photosensitizers to melanoma cell nuclei in vivo. Dokl. Biochem. Biophys. 2012, 446, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, A. A.; Slastnikova, T. A.; Karmakova, T. A.; Vorontsova, M. S.; Morozova, N. B.; Petriev, V. M.; Abrosimov, A. S.; Khramtsov, Y. V.; Lupanova, T. N.; Ulasov, A. V.; Yakubovskaya, R. I.; Georgiev, G. P.; Sobolev, A. S. Antitumor Activity of Auger Electron Emitter (111)In Delivered by Modular Nanotransporter for Treatment of Bladder Cancer With EGFR Overexpression. Front Pharmacol. 2018, 9, 1331. [Google Scholar] [CrossRef] [PubMed]
- Slastnikova, T. A.; Koumarianou, E.; Rosenkranz, A. A.; Vaidyanathan, G.; Lupanova, T. N.; Sobolev, A. S.; Zalutsky, M. R. Modular nanotransporters: a versatile approach for enhancing nuclear delivery and cytotoxicity of Auger electron-emitting 125I. EJNMMI. Res. 2012, 2(1), 59. [Google Scholar] [CrossRef] [PubMed]
- Correia, J. H.; Rodrigues, J. A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics. 2021, 13 (9).
- Li, X.; Lovell, J. F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17(11), 657–674. [Google Scholar] [CrossRef] [PubMed]
- Kim, T. E.; Chang, J. E. Recent Studies in Photodynamic Therapy for Cancer Treatment: From Basic Research to Clinical Trials. Pharmaceutics. 2023, 15 (9).
- Agostinis, P.; Berg, K.; Cengel, K. A.; Foster, T. H.; Girotti, A. W.; Gollnick, S. O.; Hahn, S. M.; Hamblin, M. R.; Juzeniene, A.; Kessel, D.; Korbelik, M.; Moan, J.; Mroz, P.; Nowis, D.; Piette, J.; Wilson, B. C.; Golab, J. Photodynamic therapy of cancer: an update. CA Cancer J. Clin. 2011, 61(4), 250–281. [Google Scholar] [CrossRef] [PubMed]
- Huis In ’t Veld RV; Heuts, J.; Ma, S.; Cruz, L. J.; Ossendorp, F. A.; Jager, M. J. Current Challenges and Opportunities of Photodynamic Therapy against Cancer. Pharmaceutics. 2023, 15 (2).
- Gilyazova, D. G.; Rosenkranz, A. A.; Gulak, P. V.; Lunin, V. G.; Sergienko, O. V.; Khramtsov, Y. V.; Timofeyev, K. N.; Grin, M. A.; Mironov, A. F.; Rubin, A. B.; Georgiev, G. P.; Sobolev, A. S. Targeting cancer cells by novel engineered modular transporters. Cancer Res. 2006, 66(21), 10534–10540. [Google Scholar] [CrossRef]
- Khramtsov, Y. V.; Ulasov, A. V.; Slastnikova, T. A.; Rosenkranz, A. A.; Lupanova, T. N.; Georgiev, G. P.; Sobolev, A. S. Modular Nanotransporters Delivering Biologically Active Molecules to the Surface of Mitochondria. Pharmaceutics. 2023, 15 (12).
- Ilari, A.; Bonamore, A.; Farina, A.; Johnson, K. A.; Boffi, A. The X-ray structure of ferric Escherichia coli flavohemoglobin reveals an unexpected geometry of the distal heme pocket. J. Biol. Chem. 2002, 277(26), 23725–23732. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M. R.; Liu, W.; Michelich, C. R.; Dewhirst, M. W.; Yuan, F.; Chilkoti, A. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J. Natl. Cancer Inst. 2006, 98(5), 335–344. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Takahashi, J.; Yamamoto, M. Molecular Basis of the KEAP1-NRF2 Signaling Pathway. Mol. Cells 2023, 46(3), 133–141. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kensler, T. W.; Motohashi, H. The KEAP1-NRF2 System: a Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol Rev. 2018, 98(3), 1169–1203. [Google Scholar] [CrossRef]
- Hayes, J. D.; Dinkova-Kostova, A. T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39(4), 199–218. [Google Scholar] [CrossRef] [PubMed]
- Kopacz, A.; Kloska, D.; Forman, H. J.; Jozkowicz, A.; Grochot-Przeczek, A. Beyond repression of Nrf2: An update on Keap1. Free Radic. Biol. Med. 2020, 157, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Lo, S. C.; Hannink, M. PGAM5 tethers a ternary complex containing Keap1 and Nrf2 to mitochondria. Exp. Cell Res. 2008, 314(8), 1789–1803. [Google Scholar] [CrossRef] [PubMed]
- Trower, M. K. A rapid PCR-based colony screening protocol for cloned inserts. Methods Mol. Biol. 1996, 58, 329–333. [Google Scholar] [PubMed]
- Ota, M.; Asamura, H.; Oki, T.; Sada, M. Restriction enzyme analysis of PCR products. Methods Mol. Biol. 2009, 578, 405–414. [Google Scholar] [PubMed]
- Sanger, F.; Nicklen, S.; Coulson, A. R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. U. S. A 1977, 74(12), 5463–5467. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Slastnikova, T. A.; Rosenkranz, A. A.; Ulasov, A. V.; Khramtsov, Y. V.; Lupanova, T. N.; Georgiev, G. P.; Sobolev, A. S. Mouse Syngeneic Melanoma Model with Human Epidermal Growth Factor Receptor Expression. Pharmaceutics. 2022, 14 (11).
- Szoka, F., Jr.; Papahadjopoulos, D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc. Natl. Acad. Sci. U. S. A 1978, 75(9), 4194–4198. [Google Scholar] [CrossRef] [PubMed]
- Karyagina, T. S.; Ulasov, A. V.; Slastnikova, T. A.; Rosenkranz, A. A.; Lupanova, T. N.; Khramtsov, Y. V.; Georgiev, G. P.; Sobolev, A. S. Targeted Delivery of (111)In Into the Nuclei of EGFR Overexpressing Cells via Modular Nanotransporters With Anti-EGFR Affibody. Front Pharmacol. 2020, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Ladokhin, A. S. pH-triggered conformational switching along the membrane insertion pathway of the diphtheria toxin T-domain. Toxins. (Basel) 2013, 5(8), 1362–1380. [Google Scholar] [CrossRef] [PubMed]
- Leka, O.; Vallese, F.; Pirazzini, M.; Berto, P.; Montecucco, C.; Zanotti, G. Diphtheria toxin conformational switching at acidic pH. FEBS J 2014, 281(9), 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Rodnin, M. V.; Kyrychenko, A.; Kienker, P.; Sharma, O.; Posokhov, Y. O.; Collier, R. J.; Finkelstein, A.; Ladokhin, A. S. Conformational switching of the diphtheria toxin T domain. J Mol. Biol. 2010, 402(1), 1–7. [Google Scholar] [CrossRef] [PubMed]
- Di, G. A.; Bonamore, A. Globin interactions with lipids and membranes. Methods Enzymol. 2008, 436, 239–253. [Google Scholar]
- Sui, M.; Liu, W.; Shen, Y. Nuclear drug delivery for cancer chemotherapy. J. Control Release 2011, 155(2), 227–236. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Liu, J.; Shi, J. Cancer cell nucleus-targeting nanocomposites for advanced tumor therapeutics. Chem. Soc. Rev. 2018, 47(18), 6930–6946. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Jain, P.; Asati, S.; Haider, T.; Soni, V.; Pandey, V. State-of-art based approaches for anticancer drug-targeting to nucleus. Journal of Drug Delivery Science and Technology 2018, 48, 383–392. [Google Scholar] [CrossRef]
- Goyal, P.; Malviya, R. Advances in nuclei targeted delivery of nanoparticles for the management of cancer. Biochim. Biophys. Acta Rev. Cancer 2023, 1878(3), 188881. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865(5), 721–733. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C. B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284(20), 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Dinkova-Kostova, A. T. The cytoprotective role of the Keap1-Nrf2 pathway. Arch. Toxicol. 2011, 85(4), 241–272. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Motohashi, H.; Yamamoto, M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol Sci 2013, 34(6), 340–346. [Google Scholar] [CrossRef] [PubMed]
- Guntas, G.; Lewis, S. M.; Mulvaney, K. M.; Cloer, E. W.; Tripathy, A.; Lane, T. R.; Major, M. B.; Kuhlman, B. Engineering a genetically encoded competitive inhibitor of the KEAP1-NRF2 interaction via structure-based design and phage display. Protein Eng Des Sel 2016, 29(1), 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Wen, S.; Li, Y. X.; Gao, X. X.; Zhang, X.; Jiang, Z. Y. Recent progress in Keap1-Nrf2 protein-protein interaction inhibitors. Eur. J Med Chem. 2020, 202, 112532. [Google Scholar] [CrossRef] [PubMed]
- Ulasov, A. V.; Rosenkranz, A. A.; Georgiev, G. P.; Sobolev, A. S. Nrf2/Keap1/ARE signaling: Towards specific regulation. Life Sci 2022, 291, 120111. [Google Scholar] [CrossRef] [PubMed]
- Nath, R.; Sengupta, S.; Bhattacharjee, A. Enzymatic and Non-Enzymatic Response during Nitrosative Stress in. Post-Щpy Mikrobiologii-Advancements of Microbiology 2022, 61 (2), 81-93.
- Poole, R. K.; Hughes, M. N. New functions for the ancient globin family: bacterial responses to nitric oxide and nitrosative stress. Mol. Microbiol. 2000, 36(4), 775–783. [Google Scholar] [CrossRef] [PubMed]
- Forrester, M. T.; Eyler, C. E.; Rich, J. N. Bacterial flavohemoglobin: a molecular tool to probe mammalian nitric oxide biology. Biotechniques 2011, 50(1), 41–45. [Google Scholar] [CrossRef] [PubMed]
- Mukai, M.; Mills, C. E.; Poole, R. K.; Yeh, S. R. Flavohemoglobin, a globin with a peroxidase-like catalytic site. J Biol. Chem. 2001, 276(10), 7272–7277. [Google Scholar] [CrossRef] [PubMed]
- Membrillo-Hernandez, J.; Coopamah, M. D.; Anjum, M. F.; Stevanin, T. M.; Kelly, A.; Hughes, M. N.; Poole, R. K. The flavohemoglobin of Escherichia coli confers resistance to a nitrosating agent, a "Nitric oxide Releaser," and paraquat and is essential for transcriptional responses to oxidative stress. J Biol. Chem. 1999, 274(2), 748–754. [Google Scholar] [CrossRef] [PubMed]
- Frey, A. D.; Farres, J.; Bollinger, C. J.; Kallio, P. T. Bacterial hemoglobins and flavohemoglobins for alleviation of nitrosative stress in Escherichia coli. Appl. Environ. Microbiol. 2002, 68(10), 4835–4840. [Google Scholar] [CrossRef] [PubMed]
- Buytaert, E.; Dewaele, M.; Agostinis, P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim. Biophys. Acta 2007, 1776(1), 86–107. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




