1. Introduction
Fruit vegetables such as peppers (
Capsicum annuum L.) are important sources of vitamins and mineral nutrients in the human diet [
1,
2]. In Brazil, green peppers are among the ten most economically important vegetables, which is predominantly sold
in natura [
3]. In the semi-arid region of Brazil, green peppers are widely cultivated, however, water scarcity generally promotes a decrease in fruit productivity, which can also affect their nutritional quality [
4].
Water deficit negatively affects the production and quality of vegetables such as tomatoes and peppers, especially during flowering, fruiting and fruit maturation [
5,
6]. In some situations, water stress can increase the concentration of soluble solids, titratable acidity and vitamin C, but decreases others quality parameters, such as fruit size, firmness and mineral elements content [
6]. The water deficit decreases fruit yield, translocation of photoassimilates and content of minerals such as calcium, potassium, magnesium and iron to the fruits [
5]. The decrease in mineral nutrients in fruits also occurs due to the decrease in the diffusion process and mass flow that contribute to the largest portion of the amount of nutrients that reach the roots, and subsequently their redistribution to the fruits [
7].
The foliar application of zinc, as zinc oxide nanoparticles (ZnONPs) in some plant species, including fruit vegetables, can contribute to mitigates the negative effects of environmental stresses such as water deficit and saline stress on the plants and improve some aspects of the physical-chemical quality of fruits [
8,
9,
10,
11,
12,
13]. Due to their high specific surface, the main mode of action of ZnONPs would be through the stimulating effect on plant metabolism, through their interaction with biomolecules, promoting an increase in enzymatic activity and the synthesis of substances that act to mitigates oxidative stress in plants [
14,
15].
ZnONPs can also act as an efficient source of zinc (Zn) for plants, with slower release than conventional sources, such as zinc sulfate [
8,
16]. In plants, zinc is involved in the synthesis of several hormones, such as gibberellin, auxin, cytokinin and abscisic acid [
17]. In addition, Zn to participate on the synthesis of chlorophyll, the development of chloroplasts and the integrity of the cell membrane, providing protection, stability and structure [
17,
18]. Furthermore, zinc plays a crucial role in fruiting, seed growth and fruit maturation, increasing resistance to environmental stress, acting as a co-enzyme in enzymatic processes related to protein synthesis, glucose metabolism and DNA sinthesys, being also essential for the production of auxins, which regulate root growth, cell expansion and the beginning of flowering [
19,
20,
21].
Under water stress, several plant species can be favored by the presence of plant growth-promoting bacteria (PGPB) in the rhizosphere region [
22,
23,
24,
25,
26]. Indirectly, PGPB can alleviate the effects of oxidative stress caused by drought, through the stimulation of enzymes from the antioxidant system such as catalase, superoxide dismutase and peroxidase [
27,
28,
29,
30]. The plant inoculation with
B. amyloliquefaciens improved the survival of pepper plants under different stress conditions, resulting in a higher seedling growth rate and better physicochemical characteristics [
31]. In the study conducted by [
32], it was noted that the application of
B. amyloliquefaciens to corn resulted in an increase in nutrient absorption and stimulated plant growth mechanisms, reflected in higher concentrations of amino acids, such as tryptophan, isoleucine, alanine, valine, tyrosine, and sugars, such as fructose and glucose. Similarly, for blueberry plants the inoculating the soil with different strains of
B. amyloliquefaciens and B. subtilis increased the foliar chlorophyll content, nutrient absorption and increases the levels of soluble solids, vitamin C and anthocyanins in the fruits [
33].
In the present study, two main hypotheses were considered. The first hypothesis is that water deficit alters the physical-chemical quality and mineral composition of green pepper fruits. The second hypothesis is that zinc oxide nanoparticles (ZnONPs) applied via leaves, associated or not with PGPB could mitigate the negative effects of water deficit on physicochemical attributes of quality and mineral composition of green pepper fruits, and that ZnONPs would be more efficient than the conventional source of zinc (ZnSO4.7H2O).
The objective of this work was to evaluate the effect of applying zinc oxide nanoparticles, associated or not with PGPB, on some physical-chemical parameters and mineral composition of green pepper fruits, from plants grown under two irrigation levels.
2. Materials and Methods
2.1. Characterization of the Experimental Area
The present research was carried out under field conditions, from December 2022 to April 2023, in the Experimental Farm area of the Center for Agro-food Science and Technology at the Federal University of Campina Grande (UFCG), Pombal-PB Campus. The Experimental Farm is located in the municipality of São Domingos-PB. According to the Köppen classification, the predominant climate is type Aw, hot and humid. Annual rainfall is around 800 mm and the temperature range is always below 5°C. The Experimental Farm is located at an altitude of 190 m, at the following coordinates: Latitude 6° 50' 4'' South, Longitude 37° 53' 9'' West. According to Gaussen's classification, the Mediterranean type bioclimate, also known as medium-dry northeastern, prevails, with a dry season that lasts between 4 and 6 months.
The preparation of the area consisted of plowing and harrowing with a plow harrow. After this stage, soil sampling was carried out in the area, in the 0 – 20 cm layer for its chemical and physical characterization (
Table 1) according to the methodology described in Embrapa [
34].
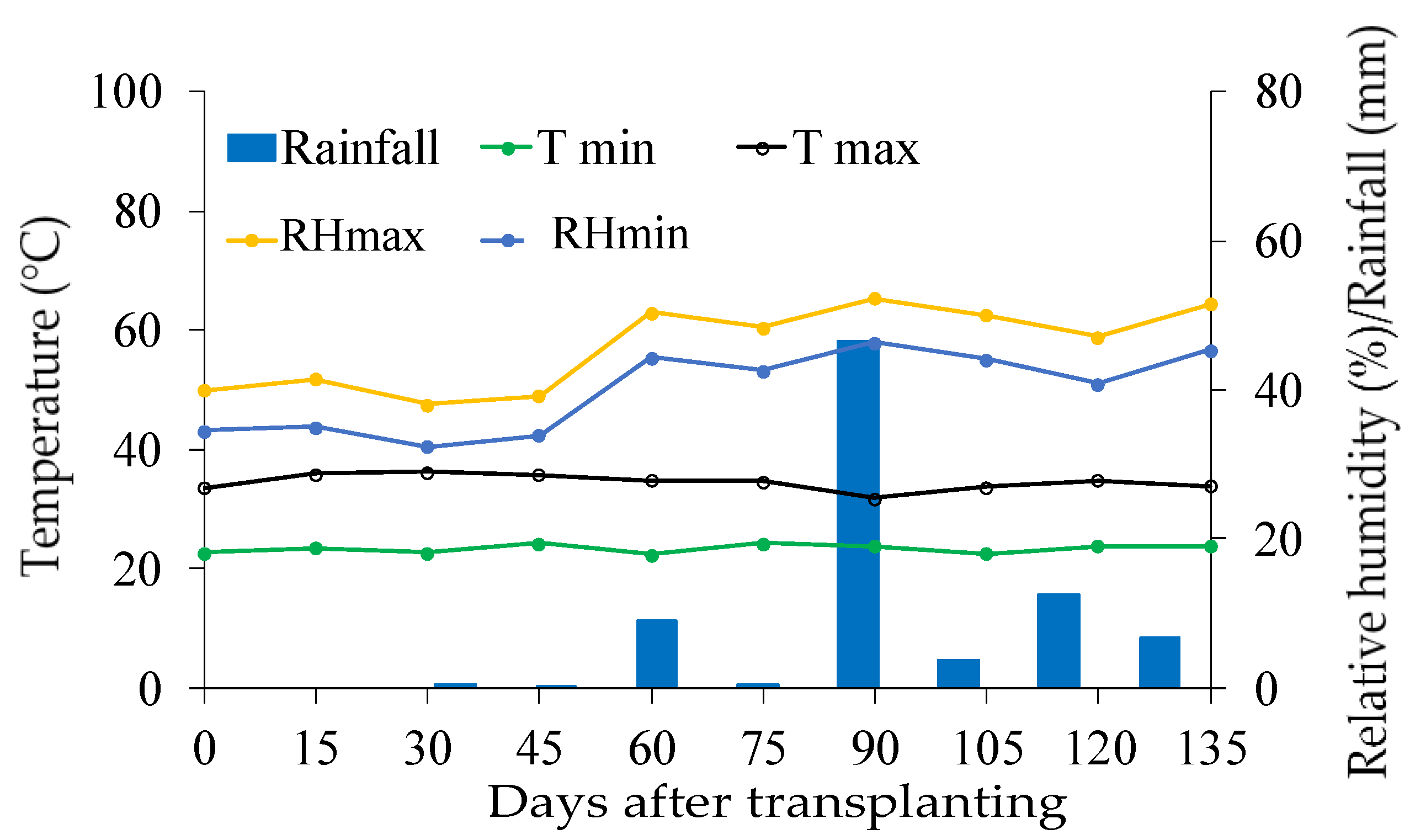
During the experiment period, climatological data on temperature, relative humidity and precipitation (
Figure 1) were collected through the Agritempo website [
35].
2.2. Treatments and Experimental Design
The experiment was carried out in a randomized block design, in a split-plot scheme, where the plots were two irrigation levels (50 and 100% of crop evapotranspiration - Etc) and the subplots were four water deficit irrigation mitigation treatments (DHA) composed from nanoparticles of zinc oxide (NPsZnO), zinc sulfate (ZnSO4) and bioinoculants (PGPB) (T1 = ZnSO4, T2 = foliar NPsZnO, T3 = foliar NPsZnO + Bio, T4 = ZnSO4 via soil + PGPB or via soil), and check treatment, with four blocks, totaling 40 experimental plots.
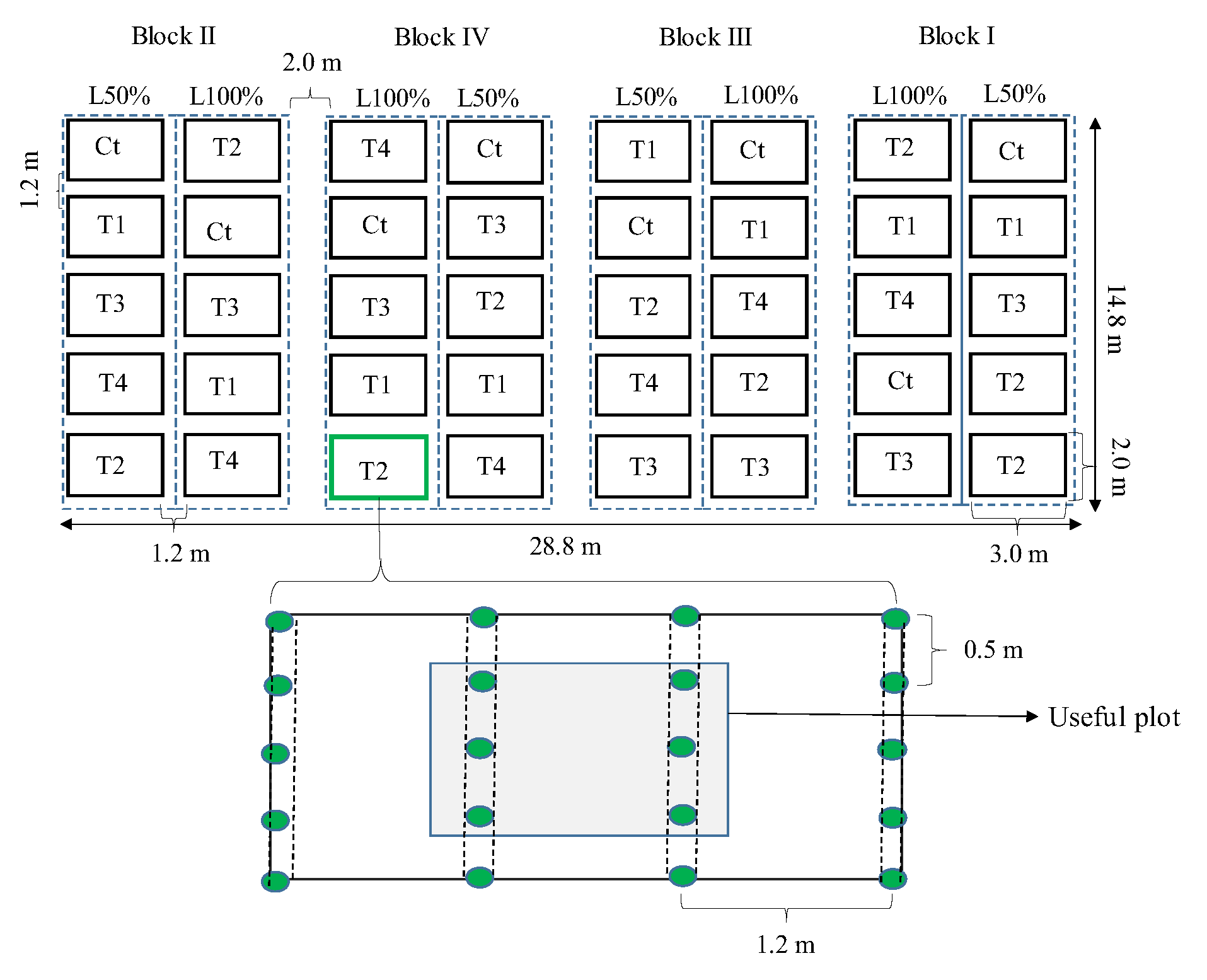
The total area of the experiment corresponded to 22.8 m in length by 14.8 m in width (Appendix A1). The plants were grown at a spacing of 1.2 m between rows and 0.5 m between plants in the row. Each subplot consisted of four 3.0 m long rows in which 20 plants were grown. The useful plot consisted of an area measuring 3.0 x 2.0 m in the central part of the plot, where 6 plants were evaluated. When installing the experiment, there was a space of 1.2 m between subplots and 2.0 m between blocks (Appendix A1).
2.3. Seedlings Transplanting and Soil Fertilization
Pepper seedlings (
Capsicum annuum L.) cv. ‘Kolima’ were produced in expanded polystyrene trays (Isopor®), filled with coconut fiber as a substrate. The transplant was carried out when the seedlings had 4 to 6 true leaves. The seedlings were transplanted in ridges measuring 0.4 m wide and 0.30 m high. Based on the soil analysis (
Table 1), planting and supplementary fertilization were carried out, carried out according to the recommendations of the Fertilization Recommendation Manual for the State of Pernambuco [
36]. For planting fertilization, the formula NPK 10-10-10 (10%N, 10% P
2O
5 and 10% K
2O) was applied at a dose of 150 g per row, in a single dose and incorporated into the soil when lifting the planting rows. Nitrogen and potassium fertilizations were carried out via fertigation, according to the technical recommendations for the crop, with the first application being made with 39.2 kg ha
−1 of urea and 15.8 kg ha
-1 of KCl carried out at 15 days after transplanting (DAT), the second with 39.2 kg ha
−1 of urea and 15.8 kg ha
−1 of KCl at 30 days after transplantation, the third with 39.2 kg ha
−1 of urea and 15.8 kg ha
−1 of KCl at 45 DAT and the fourth with 39.2 kg ha
−1 of urea and 15.8 kg ha
−1 of KCl at 60 DAT.
2.4. Application of Treatments
As ZnONPs, the product nano-zinc oxide (NZnO, P.A.) from the Sigma-Aldrich® brand was used, which has 97% urity, particle size smaller than 100 nm and specific surface area of 10.8 m2g−1. NPsZnO suspensions and ZnSO47H2O (P.A) solution (referred in the text only ZnSO4) were prepared at concentrations of 0.125 g L−1 (100 mg Zn L−1) and 4.54 g L-1 (1,000 mg Zn L−1), respectively. The treatments referring to bioinoculation (PGPB) consisted of the combination of two commercial products, one containing Bacillus subtilis BV-09 at a dosage of 1.0 x 108 CFU mL−1 and the other containing B. amyloliquefaciens at a dosage of 1.0 x 109 CFU mL−1. Each product was applied at a rate of 3 L ha−1, totaling 6 L ha−1. At the time of application, 45 mL of each PGPB source were mixed with 10 L of water.
The foliar application of the suspensions ZnONPs or ZnSO4 solution for each treatment (T1, T2 and T3) was carried out with the aid of a knapsack sprayer with a capacity of 20 liters, with an average of 35 mL applied per plant. Neutral detergent it was used as adjuvant in the proportion of 1 mL L−1 in all foliar treatments. Regarding soil application, the treatments were applied close to the root zone of the plants using a beaker. For the T4 treatment, 50 mL of the ZnSO4 solution was applied per plant, corresponding to a dose of 2.42 kg of ZnSO4 per hectare. The mixture of bioinoculants was applied around 30 mL per plant. The treatments were applied 25 days after transplanting the seedlings, after homogenizing the plants in the field plots.
2.5. Irrigation Levels Application
After transplanting, the plants were irrigated by drip, with drippers spaced 0.20 m apart and with a nominal flow of 1.6 L h−1. After emergence and standardization of the number and size of plants in the subplots, the plants were irrigated following different levels of water depth, which ran 32 days after transplanting.
The total irrigation required (TIR) was calculated using the following expression:
On what:
TIR - corresponds to the initial total depth of water applied in mm;
FC – Soil moisture corresponding to field capacity, %;
WP – Soil moisture corresponding to wilting point, %;
Z - Effective depth of the pepper root system (30 cm);
BD – bulk density, g cm−3;
f – water availability factor (0.5).
The water irrigation uniformity test was carried out according to the Christiansen Uniformity Coefficient (CUC) evaluation methodology, proposed by Christiansen [
37].
Control of the volume of water supplied to each irrigation level was carried out daily, at a standardized time, according to the ratio of the flow rate of the drippers to the time to reach the reference evapotranspiration proportions (ETo). As the time interval for each volume of the respective irrigation level was reached, the drip tapes corresponding to each irrigation level were successively disconnected. The irrigation depth corresponding to 100% of ETc was obtained by calculating ETc, according to the expression from Jessen [
38].
On what:
ETc - Crop evapotranspiration, mm day −1;
ETo - Reference evaporation, mm day −1;
Kc - Crop coefficient (dimensionless).
The Kc values adopted for each crop depending on their phenological phases, and the daily ETo values were obtained according to the FAO Penman-Monteith model [
39]. During the experiment, climatological data were obtained from the automatic meteorological station in the municipality of São Gonçalo, Paraíba, as it is the closest to the experiment site, through the Agritempo platform [
35].
The daily supply of irrigation depth was carried out through the irrigation time considering the characteristics of the cultivation system and the irrigation system according to the following expression:
Where:
Ti – Irrigation time, h;
Eto – Reference evaporation, mm day−1;
Kc – Crop coefficient, dimensionless;
A– Area occupied by one plant, m2;
Ea - Application efficiency (0.90);
n - Number of emitters per plant; and
q - Emitter flow rate, L h−1.
2.6. Cultivation Practices and Phytosanitary Control
To avoid any interference from phytosanitary chemicals on the action of products containing PGPB, weed and pests control was carried out mechanically, manually and, or with the use of hand hoes. During experimental period, not dieses were detected. In addition, horizontal staking was carried out using wooden stakes measuring an average of 1.60 m in length. The stakes were spread across the lines, being placed every 5 meters. The ribbons were tied to the poles before the fruiting period to prevent the plants from tipping over due to the weight of the fruits.
2.7. Variables Evaluated
2.7.1. Physicochemical Analysis
At the time of the first fruit harvest, which occurred 70 days after transplanting, physical attributes of color, ascorbic acid (viamin C), pH, soluble solids (SS), titratable acidity (TA) and SS/TA ratio was evaluated.
The physical attributes of fruit color were measured using a Konica Minolta CR-400™ digital colorimeter, using the CIELAB system, which defines a three-dimensional color space with three axes in rectangular coordinates (L*a*b*). These coordinates represent lightness (L*), tones from red to green (*a positive to -a* negative) and tones from yellow to blue (*b positive to -b* negative). Furthermore, it defines cylindrical coordinates (L*, C*, H°), and the a* and b* values were converted into Hue angle (H°), representing the intensity of the color, and Chroma (C*), indicating the purity of the color, according to [
40]. The analysis was carried out outside, with one reading per fruit for each experimental plot.
The pH was measured using a digital benchtop pH meter, with direct readings taken from the homogenized pulp, according to [
41]. The determination of vitamin C was carried out using the Tillman method. The results of the analyzes were expressed in mg per 100 g of the sample [
42]. Titratable acidity was determined following the methodology recommended by the [
42]. The results were expressed in g of citric acid per 100 g of the sample. Soluble solids content was determined directly in the homogenized pulp using a digital refractometer (model PR-100, Palette, Atago Co., LTD., Japan). The results were expressed in °Brix [
42]. The SS/TA ratio was calculated as the ratio between soluble solids and titratable acidity (SS/TA).
2.7.2. Mineral Nutrient Content
Six commercial fruits were separated from each subplot to take a sample consisting of 100g of fresh fruit mass. These samples were dried in a forced air oven (65-70 °C) until constant weight, to later obtain the dry mass and water content. Subsequently, this material was crushed in a Wiley-type mill and then sulfuric digestion, according to the methodology described in [
43]. Digestion was obtained in a Kjeldahl microdigester block, using samples of 0.2g of dry plant material in 3 mL of concentrated sulfuric acid and 1 mL of hydrogen peroxide, with an initial temperature of 180°C and a final temperature of 360°C. In the sulfuric digestion stratum, the levels of the macronutrients calcium (Ca), potassium (K), phosphorus (P) and magnesium (Mg) and the micronutrients boron (B), manganese (Mn), iron (Fe) and zinc were determined by using of the ICP-OES (Inductively Coupled Plasma Optical Emission Spectrometry) technique. The element contents obtained in terms of dry mass content were converted to fresh mass contents, correcting the values by the water content of the fruits.
2.7.3. Overall quality Index
A general fruit quality index (FQI) was generated with physical-chemical variables, physical color variables and macro and micronutrient content in the fruits. Initially, all values of the studied variables (X) were converted into percentages (Xi%) using the following expression:
Where qi represents the value of a quality attribute X originating from treatment t and Xc represents the average value of attribute x obtained in the control treatment (check).
FQI values were obtained according to [
44], by using the geometric mean of the physical-chemical attributes and the mineral nutrient contents evaluated, according to the following expression:
3.8. Statistical Analyzes
The data were previously subjected to the normality test using the Shapiro-Wilk test [
45]. The data obtained were subjected to analysis of variance, followed by the comparison of means using the Tukey test (p ≤ 0.05). These statistical analyzes were performed using R statistical software R [
46].
3. Results
3.1. Physicochemical and Physical Color Parameters
Irrigation depths and water deficit mitigation treatments (DHA) did not influence fruit quality parameters pH, SS, TA, SS/TA, C* and Hue angle (
Table 2). In most of the variables evaluated, the average values obtained on the 50% Etc irrigation level were quite similar to those obtained on the 100% Etc, with variations of less than 5%. Among the DHA treatments, the T4 treatment (ZnSO
4 via leaf) provided an increase of 10.45% in the C* value and the T3 treatment (ZnSO
4 via soil+PGPB) increased the SS/TA ratio by 17.79%, both under the 100% ETc.
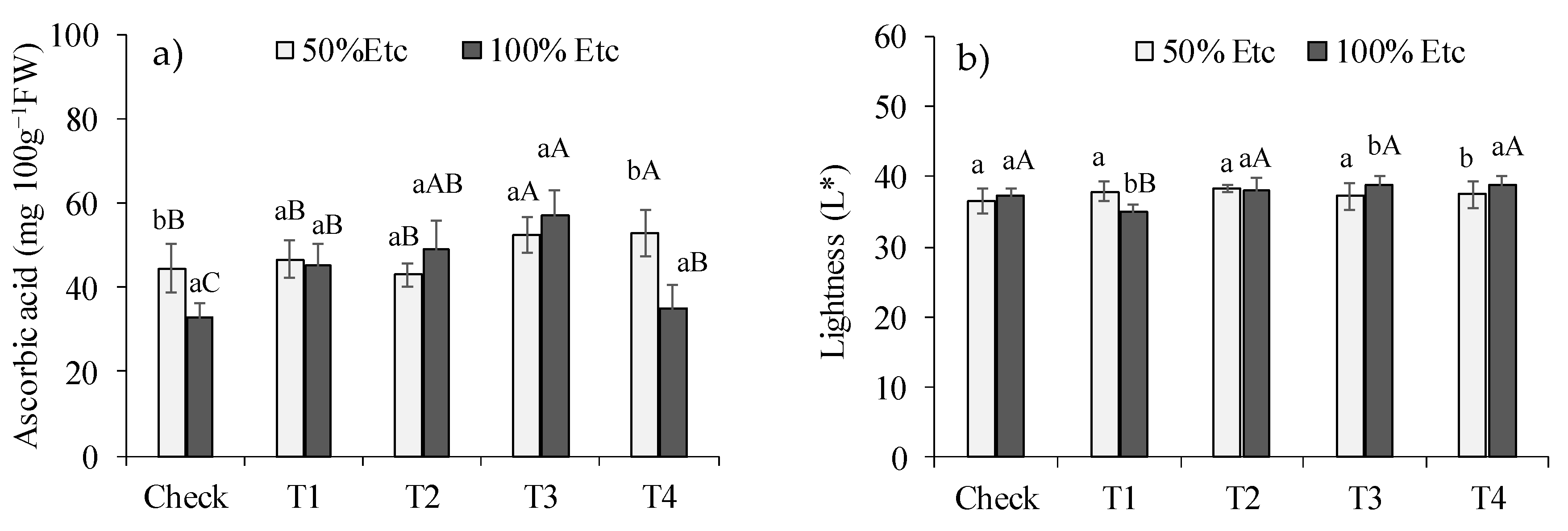
The interaction of irrigation depth and DHA treatments influenced the ascorbic acid content (
Figure 2a) and color luminosity (L*) (
Figure 2b). The 50% Etc irrigation level reduced the ascorbic acid content by approximately 30.4% in the control treatment and 33.8% in the T4 treatment (ZnSO
4 via leaf). Under the 50% Etc irrigation level, the T3 treatment (ZnSO
4 via soil+PGPB) promoted a 40.8% increase in the ascorbic acid content, in relation to the control, while the T1, T2 and T4 treatments had similar performance to the control. Under the 100% Etc irrigation level, the T3 treatment increased the ascorbic acid content by 24.3% in relation to the control, while in the other treatments, the values of this variable were similar. The 50% Etc irrigation level increased the L* value in treatment T1 (NPZnO via leaf) and decreased it in treatments T3 and T4. Under water restriction, DHA treatments did not change the L* value, while under 100%ETc, the T1 treatment decreased the value of this parameter.
The interaction of irrigation levels and DHA treatments influenced the ascorbic acid content (
Figure 2a) and color luminosity (L*) (
Figure 2b). The 50% Etc Irrigation level increased the ascorbic acid content by approximately 43.8% in the control treatment and 51.0% in the T4 treatment (ZnSO
4 via leaf). Under the level of 50% Etc, treatments T3 (ZnSO
4 via soil+PGPB) and T4 (ZnSO
4 via leaf) promoted an increase of 18.1% in ascorbic acid content, in relation to the control, while treatments T1, T2 had similar performance to the control. Under the 100% Etc irrigation level, the T3 treatment increased the ascorbic acid content by 72.7% in relation to the control. For this variable, treatments T1, T2 and T4 were also superior to the control, but T2 was similar to the treatment T3. The 50% Etc irrigation level increased the L* value in treatment T1 (NPZnO via leaf) and decreased it in treatments T3 and T4. Under water restriction, DHA treatments did not change the L* value, while under 100%ETc, the T1 treatment decreased the value of this variable.
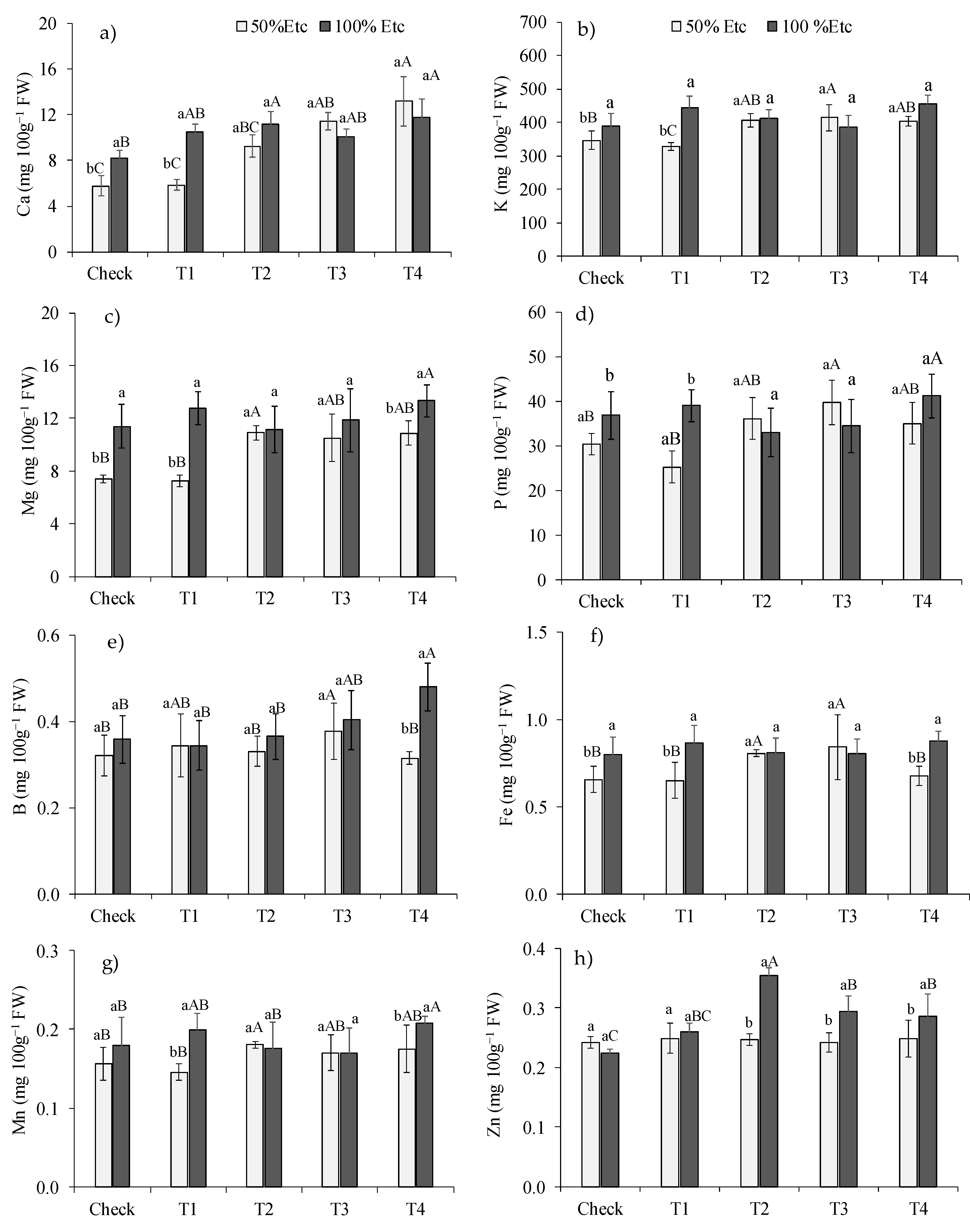
3.2. Mineral Nutrient Contents
The effect of DHA treatments on mineral nutrient content in green pepper fruits was dependent on irrigation depths (p<0.05). The water deficit (50%Etc) reduced the levels of Ca (
Figure 3a), K (
Figure 3b) and P (
Figure 3d) in the DHA control and T1 treatments, but did not change the levels of these nutrients in the other treatments. The 50%Etc Irrigation level reduced the Mg (
Figure 3c) and Fe (
Figure 3f) contents in the control treatments, T1 and T4, the B contents (
Figure 3e) in the T4 treatment and the Fe contents in the control treatments, T1 and T4. The water deficit reduced the levels of Mn (
Figure 3g) and Zn (
Figure 3h) in treatments T1 and T4.
Within each irrigation level, the most prominent DHA treatments were T2 and T3. Under the 50% Etc irrigation level, the T2 treatment increased the contents of K, Mg, Fe and Mn, the T3 treatment increased the contents of Ca, K, Mg, P, B and Fe and the T4 treatment increased the Ca contents in G. Under 100% Etc water supply, the T2 treatment increased the Ca and Zn contents and the T4 treatment increased the Ca and Mn contents. Compared to the control treatment, the most notable increases occurred for Ca (69%) with the T4 treatment, under the level of 50%Etc and for zinc (57%), with the T2 treatment, under 100%Etc.
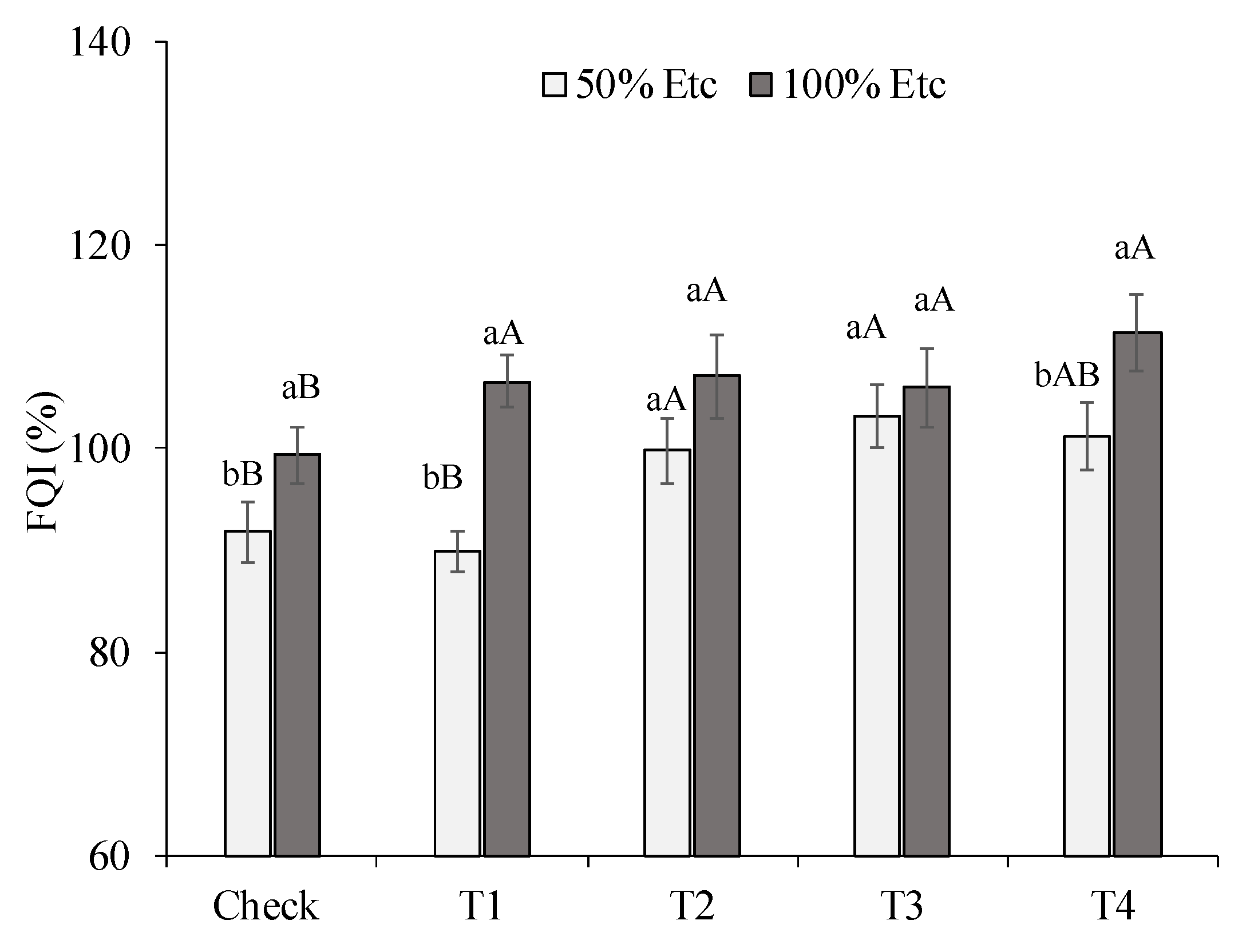
There was a significant interaction between irrigation depths and DHA treatments, for the general fruit quality index (FQI) (
Figure 3). The water deficit decreased the FQI in the control, T1 and T4 treatments, while in the other DHA treatments, there was no difference between the irrigation levels for this variable. At the irrigation level of 50%Etc, treatments T2 and T3 increased the FQI value by 8 and 12%, respectively, in relation to the in FQI in relation to the control, however, this treatment was similar to the T1, T2 and T3 treatments.
4. Discussion
4.1. Physicochemical Attributes and Color Parameters
Among the physical-chemical attributes (ascorbic acid content, pH, titratable acidity -TA, soluble solids -SS and SS/TA ratio) and physical color parameters (L*, C* and Hue angle), only the acid content ascorbic acid and the L* parameter were affected by water deficit and DHA treatments. Water deficit increased the ascorbic acid content in mango fruits [
47]. Ascorbic acid plays crucial roles in plant metabolism, such as protecting cells against oxidative damage and acting as a cofactor for enzymes involved in the production of flavonoids and phytohormones [
51]. The increase in ascorbic acid content under water stress, therefore, is a plant response to adjust to the stress condition, playing an important role in detoxifying reactive forms of oxygen (ROS) and thus avoiding damage to the plant's general metabolism [
49].
Contrary to the results obtained in the present study, an increase in the levels of SS, TA and the SS/TA ratio and changes in the color parameters C* and Hue angle were expected in fruits whose plants were subjected to water deficit, such as observed in other works [
52,
53]. The increase in SS content, TA titratable acidity and color parameters in fruits under water deficit is generally attributed to the increase in the synthesis of metabolites, and increased activity of hexokinase enzymes, due to the senescence process [
54] and the loss of water by the fruit [
53,
55]. However, in eggplant, water restriction (50%ETc) decreased ascorbic acid levels, and the C* parameters and Hue angle [
56]. According to these authors, this decrease was probably due to the decrease in the photosynthetic rate and consequently in photoassimilates and pigments. In the present study, under water restriction, the triggering processes to increase the levels of SS, TA and the SS/TA ratio and changes in the C* parameters and Hue angle were probably counterbalanced by the decrease in the production of photoassimilates, with the decrease in the photosynthetic rate. In this sense, an increase or decrease observed in the levels of metabolites (SS, organic acids, ascorbic acid and carotenoids) in tomato fruits grown under water deficit (60% Etc) was dependent on the stage of fruit maturation (green or ripe) and the metabolites considered [
48].
The DHA treatments affected only the ascorbic acid levels and slightly, the fruit luminosity (L*). As already highlighted, ascorbic acid levels were the attribute most sensitive to water deficit. For this attribute, the similarity of the values obtained between the irrigation regimes (50% Etc and 100%Ect) in treatments T1 (foliar ZnSO
4), T2 (foliar NPZnO) and T3 (foliar NPZnO + PGPB) indicates that Zn applied as ZnSO4 or ZnONPs possibly attenuates the negative effects of water deficit on ascorbic acid biosynthesis, which was enhanced by inoculation of plants with BPCP. ZnONPs at a dose of 500 mg L
−1 increased the antioxidant activity in peppers in the germination phase [
8] and ascorbic acid content in bell pepper fruits applied at a dose of 30 mgL
−1 via the leaves [
12]. The application of Zn as ZnSO
4 also increased SS contents in red pepper [
29]. In plants, Zn performs important functions such as activating enzymes that protect against oxidative stress (such as superoxide dismutase), synthesizing tryptophan, which is a precursor of IAA (indole acetic acid), acting on cell division and integrity of the cell membrane [
7,
18,
21]. In turn, the synergistic effect of PGPB with Zn may be due to the increase in the biological activity of the soil and/or production of plant growth-stimulating substances in the rhizosphere which, such as organic acids, hormones, enzymes, which can favor the photosynthetic rate and photoassimilates [
25,
26,
57].
4.2. Mineral Nutrient Content
Among the minerals evaluated, green pepper exhibited higher levels of K, P, Mg and Ca, in that order, followed by Fe, B, Zn and Mn. Depending on the ADH treatments, the levels of these minerals were considerably reduced by the water deficit. Water deficit (80% Etc) reduced the contents of K and Ca in pepper [
58], decreased the contents of Mg, K, Cu and Zn in tomato under 50% ETc [
59] and Ca and B in strawberry under 70% Etc [
60]. The process of nutrient accumulation in fruits depends on factors such as the genetic potential of the species, supply via soil or leaves and nutrient mobility in the phloem [
7]. Nutrient supply via soil depends on many factors, including soil moisture [
60]. Mass flow and diffusion are the two main mechanisms that deliver mineral nutrients to plant roots [
7]. The decrease in mineral nutrient content in pepper fruits was possibly due to the lower amount of these nutrients in contact with the plant's roots, since Ca, Mg, K, Fe, Mn, B and Zn reach the roots, mainly by mass flow, while K and P, by diffusion [
7,
61].
Under water deficit, treatments T2 (NPZnO via leaf +PGPB) and T3 (ZnSO
4 via soil+PGPB) had a greater influence on the mineral composition of pepper fruits, since they increased the levels of K, Ca, Mg, P, Fe, B and Mn. On the other hand, when there was no water restriction, these treatments (except for Ca and Zn) did not have the same performance in relation to the control, that is, only the T4 treatment (ZnSO
4 via leaf) increased the Mn and B contents. Highlights it is clear that treatments T2, T3 and T4 increased Zn levels to the level of 100%ETc, but this did not occur under water deficit. Foliar application of ZnONP increased the contents of K, Ca, P, Mg and Zn in pepper fruits [
12]. The role of Zn in cell division and the growth of fine roots contributes to greater absorption of water and nutrients by the pepper plants and their subsequent translocation to the fruits [
62]. The presence of PGPB in treatments T2 and T3 enhanced the beneficial effect of zinc on the accumulation of nutrients in pepper fruits. The role of beneficial microorganisms such as
Bacillus subtilis and
B. amyloliquefaciens in mitigating water stress in various crops appears to be due to their direct effects on plants and indirectly on the soil [
24,
25,
26]. These bacteria possibly contributed to increasing the absorption of water and nutrients by peppers, due to the increase in the photosynthetic rate with better adaptation of metabolism and osmotic adjustment under water deficit [
63,
64] and/or, due to increase of solubility of low soluble forms of nutrients in the soil as well as by increasing the biological activity of the soil [
26].
The FQI index as a measure of general fruit quality revealed, in a summarized way, the negative effect of water deficit on the quality of pepper fruits and the role of Zn as attenuator, both in the form of ZnONPs and in the form of ZnSO4, as well as PGPB. This effect was more pronounced in treatments T2 and T3, in which no significant differences were observed between the irrigation levels tested. On the other hand, the superiority of treatments T2, T3 and T4 for the FQI index revealed the stimulating effect of Zn and BPCP, even under adequate irrigation (100%ETc).
Finally, it is worth highlighting that the first hypothesis considered in this work was confirmed for the ascorbic acid content and the mineral element content. The second hypothesis can be considered confirmed based on the mineral nutrient content and the general quality index (FQI) and considering that the dose of Zn supplied via ZnONsP was 10 times lower than the dose of Zn via ZnSO
47H
2O, thus proving, the highest efficiency of ZnONPs. Although water deficit increased the ascorbic acid content in fruits, lack of water decreased the overall quality of fruits, especially in terms of mineral nutrients, especially when considering the role of these nutrients in human nutrition [
65].
5. Conclusions
Water deficit increased ascorbic acid levels in green pepper fruits, without altering soluble solids levels, color parameters (C* and Hue angle) and titratable acidity, but decreased mineral nutrient levels. The application of Zn via foliar, in the form of zinc oxide nanoparticles (ZnONPs) or zinc sulfate (ZnSO4), mitigated the negative effects of water deficit on the quality of pepper fruits. The ZnONPs source was more efficient than the ZnSO4 source. The water deficit alleviating effect of both zinc sources was enhanced when the plants were inoculated with Bacillus subtilis and B. amyloliquefaciens.
The findings of the present work, together with other research, are important in terms of new technological perspectives for adapting plants to water deficit, whether in a semi-arid climate or in climates that are already suffering from the effects of climate changes.
Author Contributions
Conceptualization, J.L.A.R..; methodology, J.L.A.R. and J.Z.L.; software, T.I.S.; validation, R.H.C.R.A., T.I.S. and G.L.; formal analysis, T.I.S..; investigation, B.L.R.M. and K.N.F.; resources, J.L.A.R.; data curation, K.N.F, W.I.S. and B.L.R.M..; writing—original draft preparation, J.L.A.R..; writing—review and editing, G.S.L.; visualization, F.B.N. and F.V.S.S and L.C.S.; supervision, F.B.N. and F.J.S.P.; project administration, J.L.A.R.; funding acquisition, J.L.A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FAPESQ (Foundation to support research in the state of Paraíba), grant number 3071/2021-Fapesq/PB Edital Universal 09/2021 SEECT/Fapesq.
Data Availability Statement
The data used to support the findings of this study are included within the article.
Acknowledgments
We sincerely thank the Department of Soil Science at the Federal University of Lavras for laboratory support for fruit analysis.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Figure A1.
Experimental design used in the research showing the distribution of blocks, combination of irrigation levels with treatments from subplots, delimitation of experimental plots and useful plot, as well as spacing between blocks, plots, planting rows and plants.
Figure A1.
Experimental design used in the research showing the distribution of blocks, combination of irrigation levels with treatments from subplots, delimitation of experimental plots and useful plot, as well as spacing between blocks, plots, planting rows and plants.
Figure A2.
Partial view of the experimental area showing the installation of the drip irrigation system (a) and the and plants in the fruit production phase (b).
Figure A2.
Partial view of the experimental area showing the installation of the drip irrigation system (a) and the and plants in the fruit production phase (b).
Figure A3.
Harvest (a), sanitization process and classification (b) of green pepper fruits before carrying out physical-chemical analyzes.
Figure A3.
Harvest (a), sanitization process and classification (b) of green pepper fruits before carrying out physical-chemical analyzes.
References
- Kabir, M.Y.; Nambeesau, S.U.; Bautista, J.; Diasz-Perez, J.C. Effect of irrigation level on plant growth, physiology and fruit yield and quality in bell pepper (Capsicum annuum L.). Sci. Hort. 2021, 281, 109902. [Google Scholar] [CrossRef]
- Bhardwaj, R.L.; Parashar, A.; Parewa, H.P.; Vyas, L. An alarming decline in the nutritional quality of foods: the biggest challenge for future generations’ health. Foods 2024, 13, 877. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.M.; Azevedo, B.M.; Vasconcelos, D.V.; Dantas, M.S.M.; Viana, T.V.A. Management of water deficit in the irrigated production of the green pepper. Rev. Ciênc. Agron. 2023, 54, e20228414. [Google Scholar] [CrossRef]
- Mačkić, K.; Bajić, I.; Pejić, B.; Vlajić, S.; Adamović, B.; Popov, O.; Simić, D. Yield and water use efficiency of drip irrigation of pepper. Water 2023, 15, 2891. [Google Scholar] [CrossRef]
- Marouelli, W.A.; Silva, L.C.W. Irrigation in pepper cultivation. Brasília, DF: Ministério da Agricultura, Pecuária e Abastecimento, 2012. (Technical Circular Embrapa Hortaliças, 101), 2012.
- Alordzinu, K. E.; Appiah, S. A.; AL Aasmi, A.; Darko, R. O.; Li, J.; Lan, Y.; Adjibolosoo, D.; Lian, C.; Wang, H.; Qiao, S. Evaluating the influence of deficit irrigation on fruit yield and quality indices of tomatoes grown in sandy loam and silty loam soils. Water, 2022, 14, e1753. [Google Scholar] [CrossRef]
- Marschner, H. Mineral nutrition of higher plants. 2 ed. London: Academic Press 2012, p. 889.
- García-López, J.I.; Niño-Medina, G.; Olivares-Sáenz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Vázquez-Alvarado, R.; Rodríguez-Salinas, P.A.; Zavala-García, F. Foliar application of zinc oxide nanoparticles and zinc sulfate boosts the content of bioactive compounds in habanero peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef]
- Uresti-Porras, J.-G.; Cabrera-De-La Fuente, M.; Benavidez-Mendoza, A.; Olivares-Sáenz, E.; Cabrera, R.I.; Juárez-Maldonado, A. Effect of graft and nano ZnO on nutraceutical and mineral content in bell pepper. Plants 2021, 10, 2793. [Google Scholar] [CrossRef]
- Semida, W.M.; Abdelkhalik, A.; Mohamed, G.F.; Abd El-Mageed, T.A.; Abd El-Mageed, S.A.; Rady, M.M.; Ali, E.F. Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.). Plants, 2021; 10, e421. [Google Scholar] [CrossRef]
- Rasouli, F.; Asadi, M.; Hassanpouraghdam, M.B.; Aazami, M.A.; Ebrahimzadeh, A.; Kakaei, K.; Dokoupil, L.; Mlcek, J. Foliar application of ZnO-NPS influences chlorophyll fluorescence and antioxidants pool in Capsicum annum l. under salinity. Horticulturae 2022, 8, 908. [Google Scholar] [CrossRef]
- Durán Zuazo, V.H.; Franco, T.D.; Cárceles, R.B.; Gálvez, R.B.; Cermeño, S.P.; Cuadros, T.S.; García, T.I.F. Mango fruit quality improvements in response to water stress: implications for adaptation under environmental constraints. Hort. Sci. 2021, 48, 1–11. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Ahmad, A.; Battaglia, M.L.; Bilal, H.M.; Alhammad, B.A.; Khan, N. Zinc oxide nanoparticles: A unique saline stress mitigator with the potential to increase future crop production. South African J. Bot. 2023, 59, 208–218. [Google Scholar] [CrossRef]
- Gholinezhad, E. Effect of drought stress and Fe nanofertilizer on seed yield, morphological traits, essential oil percentage and yield of dill (Anethum graveolens L). J. Essen. Oil Bearing Plants 2017, 20, 1006–1017. [Google Scholar] [CrossRef]
- Li, J.; Zafar, S.; Javaid, A.; Perveen, S.; Hasnain, Z.; Ihtisham, M.; Abbas, A.; Usman, M.; El-Sappah, A.H.; Abbas, M. Zinc nanoparticles (ZnNPs): High-Fidelity amelioration in turnip (Brassica rapa L.) production under drought stress. Sustainability 2023, 15, 6512. [Google Scholar] [CrossRef]
- Azan, M.; Bhatti, H.N.; Khan, A.K.; Zafar, L.; Iqbal, M. Zinc oxide nano-fertilizer application (foliar and soil) effect on the growth, photosynthetic pigments and antioxidant system of maize cultivar. Biocat. Agric Biotech. 2022, 42, 102343. [Google Scholar] [CrossRef]
- Baybordi, A. Zinc in soils and crop nutrition. Parivar Press: Tehran, Iran, 2006; p. 179.
- Elsheery, N.I.; Helaly, M.N.; El-Hoseiny, H.M.; Alam-Eldein, S.M. Zinc oxide and silicone nanoparticles to improve the resistance mechanism and annual productivity of salt-stressed mango trees. Agronomy 2020, 10, e558. [Google Scholar] [CrossRef]
- Ybaez, Q. E.; Sanchez, P. B.; Badayos, R. B. Synthesis and characterization of nano zinc oxide foliar fertilizer and its influence on yield and postharvest quality of tomato. Philippine Agric. Scientist 2020, 103, 55–65. [Google Scholar]
- Ganguly, R.; Sarkar, A.; Dasgupta, D.; Acharya, K.; Keswani, C.; Popova, V.; Minkina, T.; Maksimov, A. Y.; Chakraborty, N. Unravelling the efficient applications of zinc and selenium for mitigation of abiotic stresses in plants. Agriculture 2022, 12, pe1551. [Google Scholar] [CrossRef]
- Ahmed, N.; Zhang, B.; Chachar, Z.; Li, J.; Xiao, G.; Wang, Q.; Hayat, F.; Deng, L.; Narejo, M.; Bozdar, B.; Tu, P. Micronutrients and their effects on horticultural crop quality, productivity and sustainability. Sci. Hort. 2024, 323, 112512. [Google Scholar] [CrossRef]
- Pereira, S.I.A.; Abreu, D.; Moreira, H.; Veja, A.; Castro, P.M.L. Plant growth-promoting rhizobacteria (PGPR) improve the growth and nutrient use efficiency in maize (Zea mays L.) under water deficit conditions. Heliyon 2020, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.S.; Amby, D. B.; Hegelund, J.N.; Fimognari, L.; Großkinsky, D.-K.; Westergaard, J.C. Müller, R.; Moelbak, L.; Liu, F.; Roitsch., T. Bacillus licheniformis FMCH001 increases water use efficiency via growth stimulation in both normal and drought conditions. Front. Plant Sci. 2020, 11, 297. [Google Scholar] [CrossRef]
- Ramakrishna, W.; Rathore, P.; Kumari, R.; Yadav, R. Brown gold of marginal soil: Plant growth-promoting bacteria to overcome plant abiotic stress for agriculture, biofuels and carbon sequestration. Sci. Total Environment 2020, 711, 135062. [Google Scholar] [CrossRef]
- Kiran, S.; Furtana, G.B.; Ellialtioglu, Ş.Ş. Physiological and biochemical assay of drought stress responses in eggplant (Solanum melongena L.) inoculated with commercial inoculant of Azotobacter chroococum and Azotobacter vinelandii. Sci. Hort. 2022, 305, 111394. [Google Scholar] [CrossRef]
- Araujo, J.L.; Mesquita Alves, J.; Rocha, R.H.C.; Santos, J.Z.L.; Santos Barbosa, R.; Costa, F.M.N.; Lima, G.S.; Freitas, L.N.; Lima, A.S.; Nogueira, A.E.P.; Silva, A.A.; Santos, L.C.; Bezerra Neto, F.; Sá, V.F. Beneficial microorganisms affect soil microbiological activity and corn yield under deficit irrigation. Agriculture 2023, 13, 1169. [Google Scholar] [CrossRef]
- Chiappero, J.; Cappellari, L.R.; Alderete, L.G.; Palermo, T.B.; Banchio, E. Plant growth promoting rhizobacteria improve the antioxidant status in Mentha piperita grown under drought stress leading to an enhancement of plant growth and total phenolic content. Ind. Crops Prod. 2019, 139, 111553. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, L.; Hao, R.; Bai, X.; Wang, Y.; Yu, X. Drought-tolerant plant growth-promoting rhizobacteria isolated from jujube (Ziziphus jujuba) and their potential to enhance drought tolerance. Plant Soil 2020, 452, 423–440. [Google Scholar] [CrossRef]
- Ahmet, T.; Hayrettin, K.; Özmen, N. Response of red pepper (Capsicum annuum L.) to foliar applications of Zinc. Comm. Soil Sci. Plant Anal. 2021, 52, 11, 1256–1263. [Google Scholar] [CrossRef]
- Lin, Y.; Watts, D.B.; Kloepper, J.K.; Feng, Y.; Torbert, H.A. Influence of plant growth-promoting rhizobacteria on corn growth under drought stress. Comm. Soil Sci. Plant Anal. 2020, 51, 250–264. [Google Scholar] [CrossRef]
- Kazerooni, E.A.; Maharachchikumbura, S.S.N.; Adhikari, A.; Al-Sadi, A.M.; Kang, S.M.; Kim, L.R.; Lee, I.J. Rhizospheric Bacillus amyloliquefaciens Protects Capsicum annuum cv. Geumsugangsan from multiple abiotic stresses via multifarious plant growth-promoting attributes. Front. Plant Sci. 2021, 25, 669693. [Google Scholar] [CrossRef] [PubMed]
- Vinci, G.; Cozzolino, V.; Mazzei, P.; Monda, H. Effects of Bacillus amyloliquefaciens and different phosphorus sources on maize plants as revealed by NMR and GC-MS based metabolomics. Plant Soil 2018, 429, 437–450. [Google Scholar] [CrossRef]
- Yu, Y.-Y.; Xu, J.-D.; Huang, T.-X.; Zhong, J.; Yu, H.; Qiu, J.-P.; Guo, J.-H. Combination of beneficial bacteria improves blueberry production and soil quality. Food Sci. Nutr. 2020, 8, 5776–5784. [Google Scholar] [CrossRef]
- EMBRAPA. Empresa Brasileira de Pesquisa Agropecuária. Centro Nacional de Pesquisa em Solos. Manual of soil analysis methods. 2.ed. Rio de Janeiro. 2011. 225p.
- AGRITEMPO. Agrometeorological Monitoring System: Meteorological stations for the state of PB. Available on:https://www.agritempo.gov.br/agritempo/jsp/Estacao/index.jsp?siglaUF=PB.Accessed on: Nov. 2023.
- Cavalcante, F.J.A. Fertilization recommendations for the State of Pernambuco (Brazil). 2nd approximation. Recife: Instituto Agronômico de Pernambuco - IPA 2008, 212p.
- Christiansen, J.E. Irrigation by sprinkling. Berkeley: University of California, 1942.
- Jessen, M.E. Water consumption by agriculture plants. Kozlowski, T.T. (Ed.) Water deficits and plant growth, Academic Press, New York & London, 1968. 22p.
- Allen, R. G.; Pereira, L. S.; Raes, D.; Smith, M. Crop evapotranspiration-Guidelines for computing crop water requirements. Fao: Rome, 300, D05109. FAO Irrigation and drainage paper 56., 1998.
- Pinheiro, J.M.S. Post-harvest technology for preserving bananas of the cultivar Tropical. Janaúba: Universidade Estadual de Montes Claros- Thesis (Master's degree) 2009. 59p.
- IAL- Instituto Adolfo Lutz. Normas analíticas do Instituto Adolfo Lutz. Physicochemical methods for food analysis 4.ed, 2008. 1.020p.
- AOAC – Association of Official Analytical Chemists. Official methods of analysis of the association of official analytical chemists. Gaithersburg, Maryland, 2012.
-
Tedesco, M.J.; Milan, P.A.; Ernani, P.R. Analysis of soil, plants and other materials. 2. ed. Porto Alegre: Universidade Federal do Rio Grande do Sul, Departamento de Solos, 1995. 118 p. (UFRGS. Boletim Técnico, 5).
- Funsueb, S.; Thanavanich, C.; Theanjumpol, P.; Kittiwachana, S. Development of new fruit quality indices through aggregation of fruit quality parameters and their predictions using near-infrared spectroscopy. Postharvest Biol. Tech. 2023, 204, 112438. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika Trust 1965, 52, 591–609. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. Viena: Austria, 2023.
- Agbemafle, R.; Owusu-Sekyere, J.D.; Bart-Plange, A. Effect of deficit irrigation and storage on the nutritional composition of tomato (Lycopersicon esculentum Mill. cv. Pectomech). Croatian J. Food Technol Biotechnol Nutr. 2015, 10, 59–65. [Google Scholar]
- Medyouni, I.; Zouaoui, R.; Rubio, E.; Serino, S.; Ahmed, H.B; Bertin, N. Effects of water deficit on leaves and fruit quality during the development period in tomato plant. Food. Sc.i Nutr. 2021, 9, 1949–1960. [Google Scholar] [CrossRef] [PubMed]
- Al-Selwey, W.A.; Alsadon, A.A.; Al-Doss, A.A.; Solieman, T.H.; Dewir, Y.F.; Ibrahim, A.A. Effect of deficit irrigation on total yield, fruit physical characteristics, and nutritional value of four drought tolerant tomato (Solanum lycopersicum L.) genotypes. J. Agr. Sci. Tech. 2021, 23, 1105–1118. [Google Scholar]
- Nurzy´nska-Wierdak, R.; Buczkowska, H.; Sałata, A. Do AMF and Irrigation Regimes Affect Sweet Pepper Fruit Quality under Open Field Conditions? Agronomy 2021, 11, 2349. [Google Scholar] [CrossRef]
- Toumi, I.; Zarrouk, O.; Ghrab, M.; Nagaz, K. Improving peach fruit quality traits using deficit irrigation strategies in southern Tunisia arid area. Plants 2022, 11, 1656. [Google Scholar] [CrossRef]
- Silva, T.I.; Sousa, S.V.; Cruz, R.R.P.; Costa, F.B.; Silva, L.A.; Sales, J.N.B.; Brito, M.E.B.; Ribeiro, W.S.R.; Soares, L.A.A.; Soares-Filho, W.S. Fruit quality of ‘Tahiti’ lime grafted onto different rootstocks grown under salt stress. Arid Land Res. Man. 2024, 38, 447–463. [Google Scholar] [CrossRef]
- Mira-García, A.B.; Conejero, W.; Vera, J.; Ruiz-Sánchez, M.C. Effect of water stress and shading on lime yield and quality. Plants 2023, 12, 503. [Google Scholar] [CrossRef]
- Dai, N.; Schaffer, A.; Petreikov, M.; Shahak, Y.; Giller, Y.; Ratner, K. Overexpression of Arabidopsis hexokinase in tomato plants inhibits growth, reduces photosynthesis, and induces rapid senescence. Plant Cell 1999, 11, 1253–1266. [Google Scholar] [CrossRef]
- Cui, J.; Shao, G.; Lu, J.; Keabetswe, L.; Hoogenboom, G. Yield, quality and drought sensitivity of tomato to water deficit during different growth stages. Sci. Agric. 2020, 77, e20180390. [Google Scholar] [CrossRef]
- Martins, B.L.R.; Araujo, R.H.C.R.; Rocha, J.L.A.; Silva, T.I.; Alves, K.A.; Almeida, E.S.; Ferreira, K.N. Zinc oxide nanoparticles and bioinoculants on the postharvest quality of eggplant subjected to water deficit. Rev. Bras. Eng. Agric. Amb. 2024, 28, e279019. [Google Scholar] [CrossRef]
- Farooq, A.; Javad, S.; Jabeen, K.; Shah, A. A.; Ahmad, A.; Shah, A. N.; Alyemeni, M. N.; Walid F. A., M.; Abbas, A. Effect of calcium oxide, zinc oxide nanoparticles and their combined treatments on growth and yield attributes of Solanum lycopersicum L. J. King Saud Univ-Sci. 2023, 35, e102647. [Google Scholar] [CrossRef]
- Albuquerque, F.S.; Silva, E.F.F.; Bezerra Neto, E.; Souza, A.E.R; Santos, A.N. Nutrientes minerais em pimentão fertirrigado sob lâminas de irrigação e doses de potássio. Hort. Bras. 2012, 30, 681–687. [Google Scholar] [CrossRef]
- Djidonou, D.; Simonne, A.H.; Koch, K.E.; Brecht, J.K.; Zhao, X. Nutritional quality of field-grown tomato fruit as affected by grafting with interspecific hybrid rootstocks. Hort. Sci. 2016, 51, 1618–1624. [Google Scholar] [CrossRef]
- Perin, E.C.; Messoas, R.S.; Gallo, V.; Bdrdwsko, J.M.; Sduza, E.R.; Avola, L.D.; Bamberg, A.L.; Rdmbaldo, C.V. Mineral content and antioxidant compounds in strawberry fruit submitted to drough stress. Food Sci. Technol. 2019, 39, 245–254. [Google Scholar] [CrossRef]
- Silva, E.C.; Nogueira, R.J.; Silva, M.A.; Albuquerque, M.B. Drought stress and plant nutrition. Plant stress. Global Sci. Books 2011, 5, 32–41. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Zinc oxide nanoparticles alleviate drought-induced alterations in sorghum performance, nutrient acquisition, and grain fortification. Sci. Total Environ. 2019, 688, 926–934. [Google Scholar] [CrossRef]
- Lu, H.; Qi, X.; Rahman, S.; Qiao, D.; Li, P.; Han, Y.; Zhao, Z. Rice physiological response with Bacillus subtilis and saccharomyces cerevisiae inoculation into soil under reclaimed water–fresh water combined irrigation. Water 2021, 13, e773. [Google Scholar] [CrossRef]
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef]
- Mahouachi, J.; Marrero-Díaz, E. Plant growth and fruit nutrient changes in carica papaya l. genotypes subjected to regulated deficit irrigation. Life 2022, 12, 1831. [Google Scholar] [CrossRef]
Figure 1.
Climatological data on maximum (T max) and minimum (T min) air, maximum (RHmax) and minimum (RHmin) air relative humidity and rainfall during the experimental period in the experiment area.
Figure 1.
Climatological data on maximum (T max) and minimum (T min) air, maximum (RHmax) and minimum (RHmin) air relative humidity and rainfall during the experimental period in the experiment area.
Figure 2.
Ascorbic acid content (a) and luminosity (*L) of color (b) in green pepper fruits, as a function of irrigation depths and water deficit alleviating treatments. Control - no product application, T1 - ZnSO4 foliar, T2 - NPZnO foliar, T3 - NPZnO foliar + PGPB, T4 - ZnSO4 soil + PGPB. Columns with the same lowercase letters do not differ for water stress, and bars with the same uppercase letters do not differ for DHA treatments by Tukey’s test (p ≤ 0.05). Vertical bars represent the standard error.
Figure 2.
Ascorbic acid content (a) and luminosity (*L) of color (b) in green pepper fruits, as a function of irrigation depths and water deficit alleviating treatments. Control - no product application, T1 - ZnSO4 foliar, T2 - NPZnO foliar, T3 - NPZnO foliar + PGPB, T4 - ZnSO4 soil + PGPB. Columns with the same lowercase letters do not differ for water stress, and bars with the same uppercase letters do not differ for DHA treatments by Tukey’s test (p ≤ 0.05). Vertical bars represent the standard error.
Figure 3.
Contents of calcium (a), potassium (b), magnesium (c), phosphorus (d), boron (e), iron (f), manganese (g) and zinc (h) in green pepper fruits, in function of irrigation depths and treatments to alleviate water deficit. Control - no product application, T1 - ZnSO4 foliar, T2 - NPZnO foliar, T3 - NPZnO foliar + PGPB, T4 - ZnSO4 soil + PGPB. Columns with the same lowercase letters do not differ for water stress, and bars with the same uppercase letters do not differ for DHA treatments by Tukey’s test (p ≤ 0.05). Vertical bars represent the standard error.
Figure 3.
Contents of calcium (a), potassium (b), magnesium (c), phosphorus (d), boron (e), iron (f), manganese (g) and zinc (h) in green pepper fruits, in function of irrigation depths and treatments to alleviate water deficit. Control - no product application, T1 - ZnSO4 foliar, T2 - NPZnO foliar, T3 - NPZnO foliar + PGPB, T4 - ZnSO4 soil + PGPB. Columns with the same lowercase letters do not differ for water stress, and bars with the same uppercase letters do not differ for DHA treatments by Tukey’s test (p ≤ 0.05). Vertical bars represent the standard error.
Figure 4.
General fruit quality index (FQI) of green pepper, as a function of irrigation depths and water deficit alleviating treatments. Control - no product application, T1 - ZnSO4 foliar, T2 - NPZnO foliar, T3 - NPZnO foliar + PGPB, T4 - ZnSO4 soil + PGPB. Columns with the same lowercase letters do not differ for water stress, and bars with the same uppercase letters do not differ for combinations involving zinc oxide nanoparticles (NPZnO) or bioinoculants (PGPB) by Tukey’s test (p ≤ 0.05). Vertical bars represent the standard error.
Figure 4.
General fruit quality index (FQI) of green pepper, as a function of irrigation depths and water deficit alleviating treatments. Control - no product application, T1 - ZnSO4 foliar, T2 - NPZnO foliar, T3 - NPZnO foliar + PGPB, T4 - ZnSO4 soil + PGPB. Columns with the same lowercase letters do not differ for water stress, and bars with the same uppercase letters do not differ for combinations involving zinc oxide nanoparticles (NPZnO) or bioinoculants (PGPB) by Tukey’s test (p ≤ 0.05). Vertical bars represent the standard error.
Table 1.
Chemical and physical attributes of the soil used in the experiment.
Table 1.
Chemical and physical attributes of the soil used in the experiment.
| Chemical |
Value |
Physical |
Value |
| pH (CaCl2) |
6.20 |
Sand (g kg−1) |
444 |
| P (mg kg−1) |
291 |
Silt (g kg−1) |
353 |
| K+ (cmolc dm−3) |
1.19 |
Clay (g kg−1) |
203 |
| Na+ (cmolc dm−3) |
0.54 |
BD (g cm−3) |
1.36 |
| Ca2+ (cmolc dm−3) |
5.80 |
PD (g cm−3) |
2.59 |
| Mg2+ (cmolc dm−3) |
3.40 |
TP (m3 m−3) |
0.47 |
| Zn2+ (cmolc dm−3) |
1.69 |
FC (%) |
12.87 |
| H + Al (cmolc dm−3) |
2.30 |
PWP (%) |
5.29 |
| OM (g kg−1) |
6.40 |
AWC (%) |
7.58 |
| V (%) |
83.0 |
|
|
| CEC (%) |
4.10 |
- |
- |
Table 2.
pH values, soluble solids (SS), titratable acidity (TA), SS/TA ratio, chromaticity (C*), Hue angle (°Hue) of green peppers fruits as a function of irrigation levels and deficit irrigation treatments mitigating (DHA).
Table 2.
pH values, soluble solids (SS), titratable acidity (TA), SS/TA ratio, chromaticity (C*), Hue angle (°Hue) of green peppers fruits as a function of irrigation levels and deficit irrigation treatments mitigating (DHA).
| Irrigation depth (%) |
Treatment DHA |
pH |
SS (°Brix) |
TA (g per 100g) |
| |
Check |
5.443±0.464 |
4.858±0.175 |
0.109±0.018 |
| |
T1 (NPZnO via leaf) |
5.432±0.265 |
4.975±0.325 |
0.109±0.001 |
| 50% Etc |
T2 (NPZnO via leaf )+PGPB |
5.370±0.204 |
4.767±0.639 |
0.130±0.018 |
| |
T3 (ZnSO4 via soil+PGPB) |
5.443±0.252 |
4.792±0.382 |
0.119±0.022 |
| |
T4 (ZnSO4 via leaf) |
5.437±0.388 |
5.289±0.615 |
0.103±0.011 |
| |
Mean |
5.425 |
4.936 |
0.114 |
| |
Check |
5.392±0.292 |
5.233±0.183 |
0.119±0.013 |
| |
T1 (NPZnO via leaf) |
5.438±0.263 |
4.992±0.185 |
0.114±0.011 |
| 100% Etc |
T2 (NPZnO via leaf )+PGPB |
5.350±0.479 |
5.054±0.042 |
0.109±0.001 |
| |
T3 (ZnSO4 via soil)+PGPB |
5.282±0.342 |
5.183±0.745 |
0.114±0.021 |
| |
T4 (ZnSO4 via leaf) |
5.246±0.138 |
5.042±0.203 |
0.125±0.021 |
| |
Mean |
5.341 |
5.101 |
0.116 |
| |
|
SS/TA |
C* |
Hue |
| |
Check |
42.792±4.477 |
20.581±0.910 |
50.673±4.500 |
| |
T1 (NPZnO via leaf) |
45.820±2.991 |
20.809±1.900 |
53.451±5.700 |
| 50% Etc |
T2 (NPZnO via leaf )+PGPB |
37.420±4.065 |
21.514±0.842 |
50.670±3.060 |
| |
T3 (ZnSO4 via soil)+PGPB |
42.878±2.473 |
21.276±2.196 |
50.859±6.110 |
| |
T4 (ZnSO4 via leaf) |
46.971±5.969 |
21.161±1.872 |
50.692±4.560 |
| |
Mean |
43.176 |
21.068 |
51.269 |
| |
Check |
44.246±5.600 |
20.607±2.472 |
50.311±1.222 |
| |
T1 (NPZnO via leaf) |
44.029±3.802 |
21.164±1.079 |
50.755±5.330 |
| 100% Etc |
T2 (NPZnO via leaf )+PGPB |
46.549±3.840 |
21.911±2.09] |
50.959±4.060 |
| |
T3 (ZnSO4 via soil)+PGPB |
53.822±2.628 |
22.170±0.629 |
49.863±1.485 |
| |
T4 (ZnSO4 via leaf) |
41.123±6.286 |
23.012±2.622 |
50.230±2.891 |
| |
Mean |
45.954 |
21.773 |
50.424 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).