Submitted:
16 July 2024
Posted:
17 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
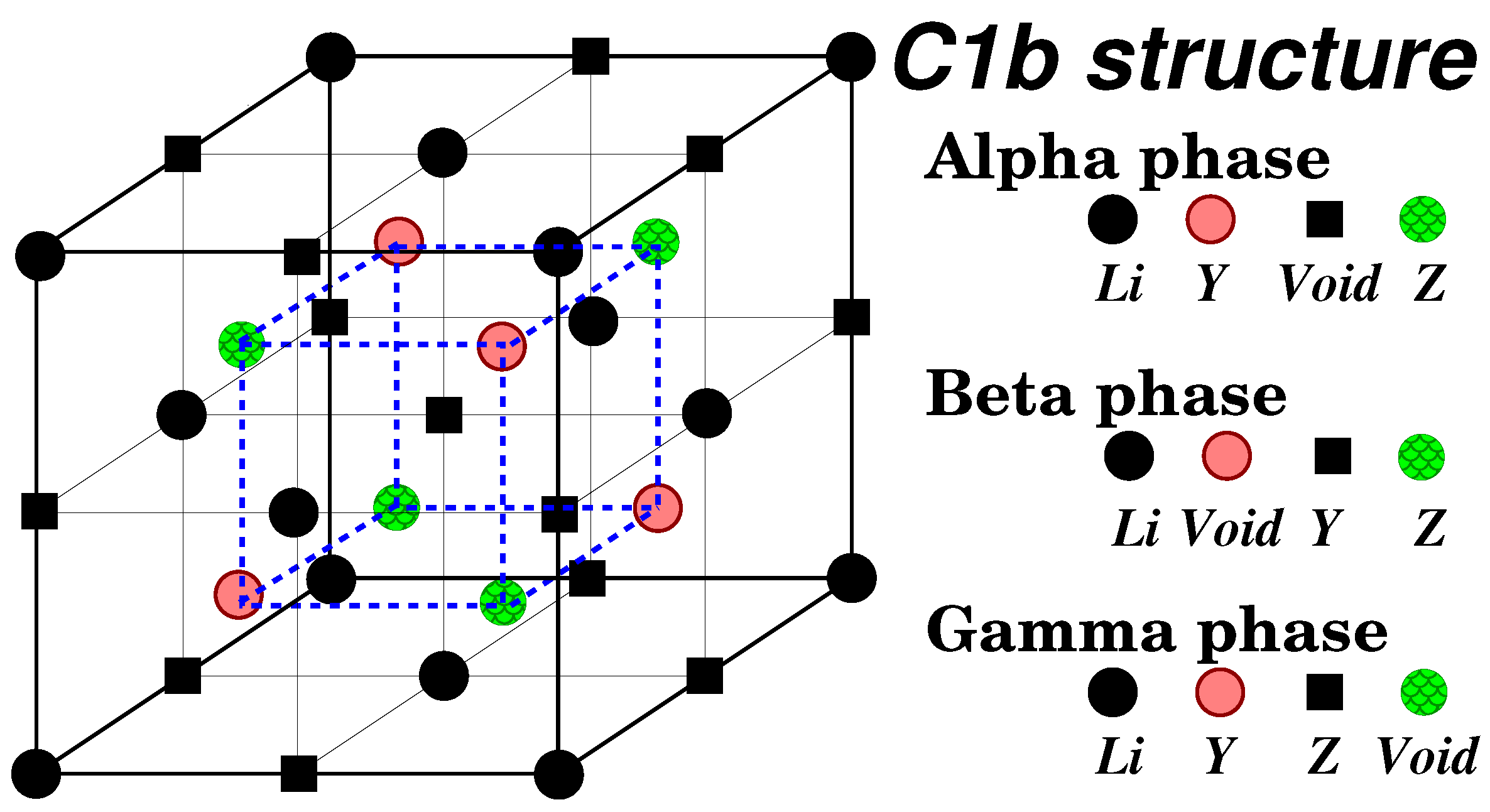
- Semi-Heusler compounds, such as NiMnSb, follow the chemical formula. Here, X and Y represent transition metal atoms or lanthanides, while Z is a metalloid. The lattice structure of semi-Heusler compounds is denoted as "".
- Full-Heusler compounds, such as Co2MnSi, have the chemical formula , with X, Y, and Z atoms similar to those in semi-Heuslers. These compounds crystallize in the "" lattice structure.
- Inverse Heuslers are similar to full-Heuslers, but the valence of X is smaller than that of Y. Their lattice structure is known as "" or "."
2. Computational Details
3. Results and Discussion
3.1. Structural Properties
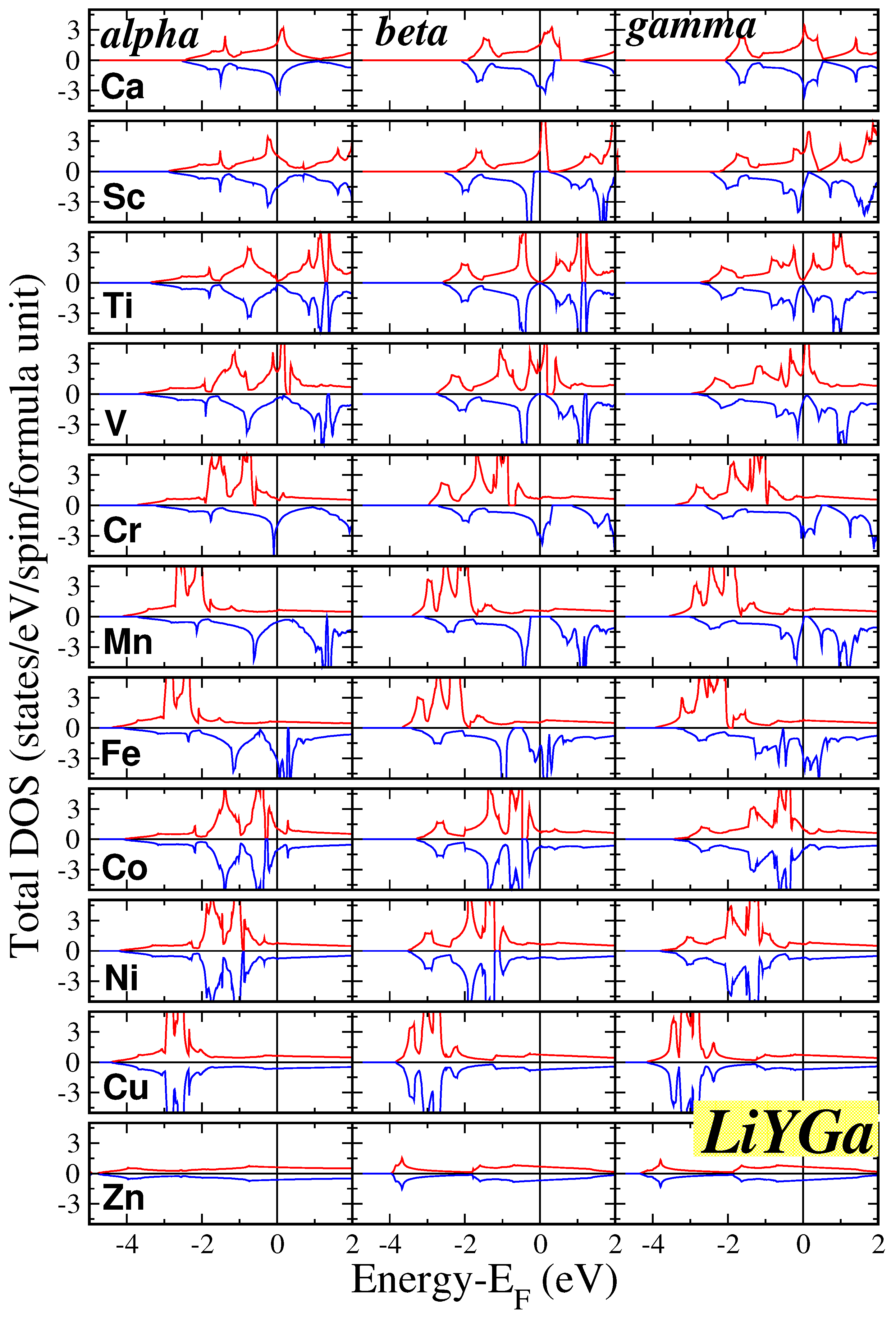
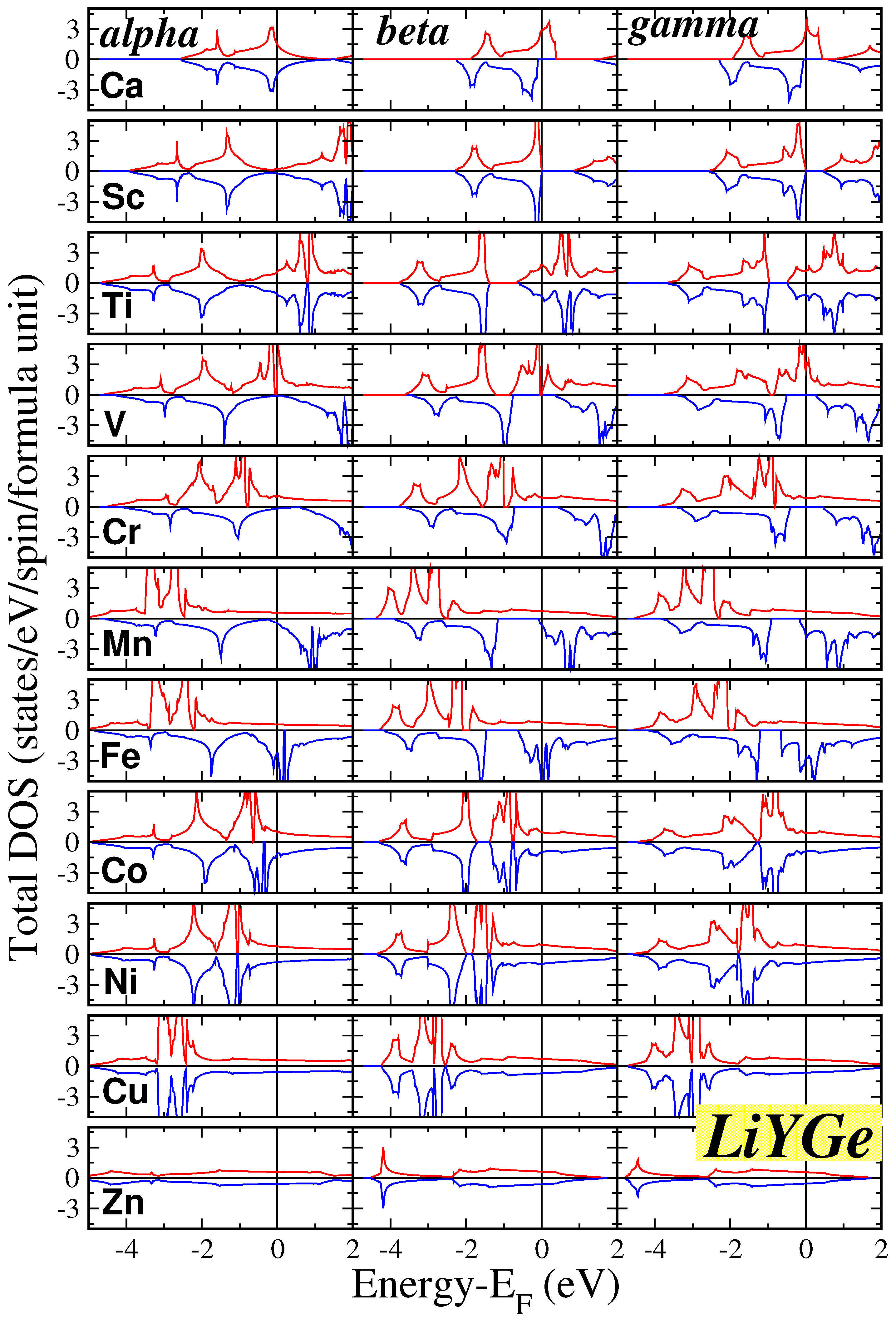
3.2. Electronic Properties
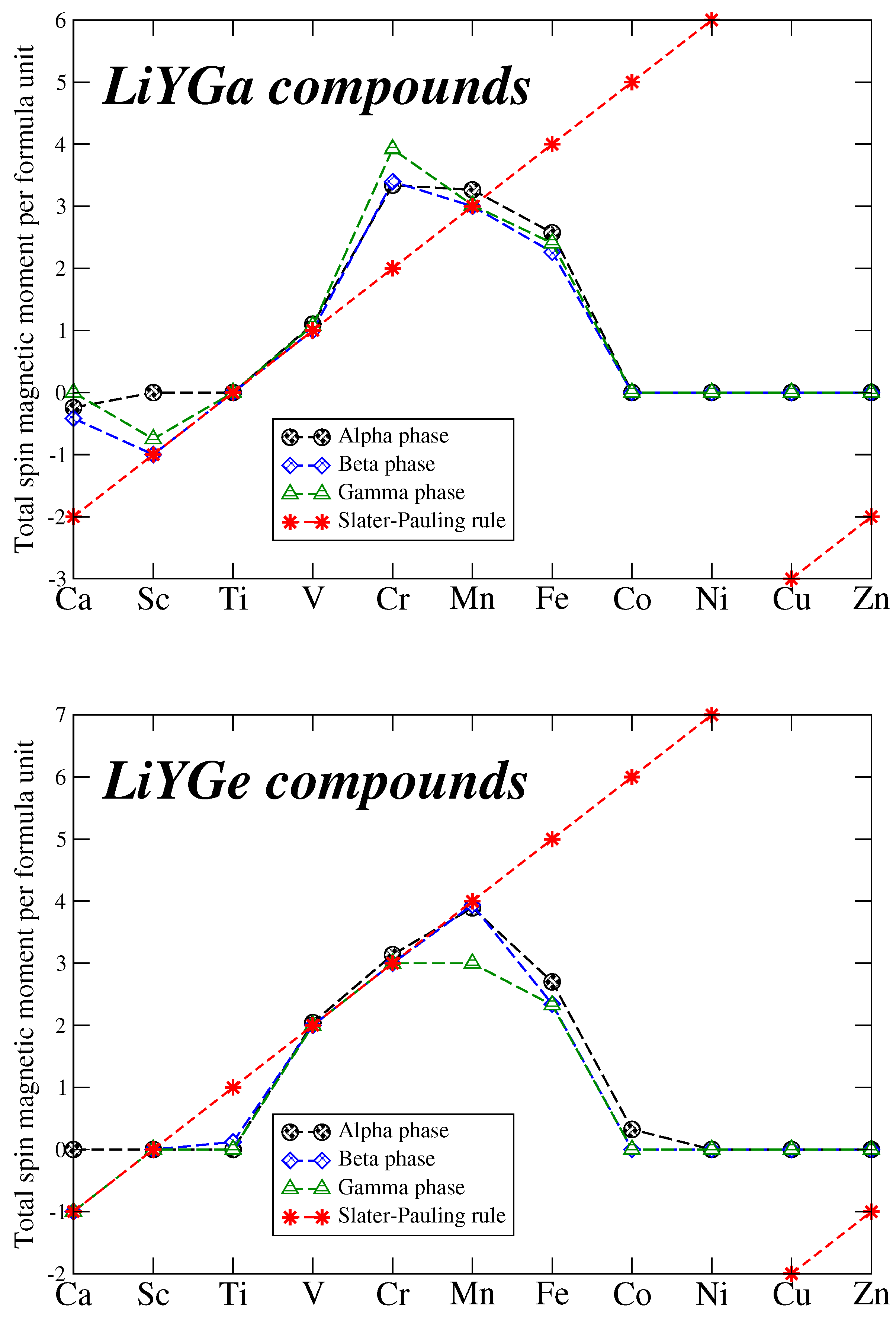
3.3. Magnetic Properties
4. Summary and Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DOS | Density of States |
| f.u. | formula unit |
| FPLO | Full-potential nonorthogonal local-orbital minimum- basis band structure approach |
| GGA | Generalized gradient approximation |
| PBE | Perdew Burke Ernzerhof |
References
- Heusler, F. Über magnetische manganlegierungen. Verh. Dtsch. Phys. Ges. 1903, 12, 219. [Google Scholar]
- Heusler, F.; Take, E. The nature of the Heusler alloys. Phys. Z. 1912, 13, 897. [Google Scholar] [CrossRef]
- Webster, P.J.; Ziebeck, K.R.A. Alloys and Compounds of d-Elements with Main Group Elements. Part 2. In Landolt-Börnstein, New Series, Group III, vol 19c; Editor Wijn, H.R.J.; Springer: Berlin. 1988; pp. 75–184.
- Ziebeck, K.R.A.; Neumann, K.-U. Magnetic Properties of Metals. In Landolt-Börnstein, New Series, Group III, vol 32/c. Editor Wijn, H.R.J.; Springer: Berlin. 2001; pp. 64–414.
- Graf, T.; Felser, C.; Parkin, S.S.P. Simple rules for the understanding of Heusler compounds. Progress in Solid State Chemistry 2011, 39, 1. [Google Scholar] [CrossRef]
- Galanakis, I.; Dederichs, P.H.; Papanikolaou, N. Origin and Properties of the Gap in the Half-Ferromagnetic Heusler Alloys. Phys. Rev. B 2002, 66, 134428. [Google Scholar] [CrossRef]
- Galanakis, I.; Dederichs, P.H.; Papanikolaou, N. Slater-Pauling Behavior and Origin of the Half-Metallicity of the Full-Heusler Alloys. Phys. Rev. B 2002, 66, 174429. [Google Scholar] [CrossRef]
- Skaftouros, S.; Özdoğan, K.; Şaşıoğlu, E.; Galanakis, I. Generalized Slater-Pauling rule for the inverse Heusler compounds. Phys. Rev. B 2013, 87, 024420. [Google Scholar] [CrossRef]
- Özdoğan, K.; Şaşıoğlu, E.; . Galanakis, I. Slater-Pauling behavior in LiMgPdSn-type multifunctional quaternary Heusler materials: Half-metallicity, spin-gapless and magnetic semiconductors. J. Appl. Phys. 2013, 113, 193903. [Google Scholar] [CrossRef]
- Katsnelson, M.I.; Irkhin, V. Yu; Chioncel, L.; Lichtenstein, A.I.; de Groot, R.A. Half-metallic ferromagnets: From band structure to many-body effects. Rev. Mod. Phys. 2008, 80, 315. [Google Scholar] [CrossRef]
- Hirohata, A.; Takanashi, K. Perspectives of Heusler compounds. J. Phys. D: Appl. Phys. 2014, 47, 193001. [Google Scholar] [CrossRef]
- Half-metallic Alloys. Fundamentals and Applications. In Lectures Notes in Physics, vol. 676. Editors Galanakis, I.; Dederichs, P.H. Springer: Berlin Heidelberg. 2005.
- Spintronics. From Materials to Devices. Editors Felser, C.; Fecher, G.H. Spintronics. From Materials to Devices. Editors Felser, C.; Fecher, G.H., Springer. 2013.
- Half-metallic Materials and Their Properties. In Series on Materials for Engineering, vol. 2. Editors Fong, C.Y.; Pask, J.E.; Yang, L.H. Imperial College Press. 2013.
- Heusler Alloys. Properties, Growth, Applications. In Springer Series in Materials Science, vol. 222. Editors Felser, C.; Hirohata, A. Springer International Publishing. 2018.
- Galanakis, I.; Özdoğan, K.; Şaşıoğlu, E. Spin-filter and spin-gapless semiconductors: The case of Heusler compounds. AIP Adv. 2016, 6, 055606. [Google Scholar] [CrossRef]
- Gillessen, M.; Dronskowski, R. A combinatorial study of full Heusler alloys by first-principles computational methods. J. Comput. Chem. 2009, 30, 1290. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, M.; Dronskowski, R. A combinatorial study of inverse Heusler alloys by first-principles computational methods. J. Comput. Chem. 2010, 31, 612. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Hegde, V.I.; Munira, K.; Xie, Y.; Keshavarz, S.; Mildebrath, D.T.; Wolverton, C.; Ghosh, A.W.; Butler, W.H. Computational investigation of half-Heusler compounds for spintronics applications. Phys. Rev. B 2017, 95, 024411. [Google Scholar] [CrossRef]
- Sanvito, S.; Oses, C.; Xue, J.; Tiwari, A.; Zic, M.; Archer, T.; Tozman, P.; Venkatesan, M.; Coey, M.; Curtarolo, S. Accelerated discovery of new magnets in the Heusler alloy family. Sci. Adv. 2017, 3, e1602241. [Google Scholar] [CrossRef] [PubMed]
- Faleev, S.V.; Ferrante, Y.; Jeong, J.; Samant, M.G.; Jones, B.; Parkin, S.S.P. Unified explanation of chemical ordering, the Slater-Pauling rule, and half-metallicity in full Heusler compounds. Phys. Rev. B 2017, 95, 045140. [Google Scholar] [CrossRef]
- Faleev, S.V.; Ferrante, Y.; Jeong, J.; Samant, M.G.; Jones, B.; Parkin, S.S.P. Heusler compounds with perpendicular magnetic anisotropy and large tunneling magnetoresistance. Phys. Rev. Mater. 2017, 1, 024402. [Google Scholar] [CrossRef]
- Galanakis, I.; Özdoğan, K.; Şaşıoğlu, E. High-TC fully compensated ferrimagnetic semiconductors as spin-filter materials: the case of CrVXAl (X = Ti, Zr, Hf) Heusler compounds. J. Phys.: Condens. Matter 2014, 26, 086003. [Google Scholar] [PubMed]
- Venkateswara, Y.; Gupta, S.; Samatham, S.S.; Varma, M.R.; Enamullah, *!!! REPLACE !!!*; Suresh, K.G.; Alam, A. Competing magnetic and spin-gapless semiconducting behavior in fully compensated ferrimagnetic CrVTiAl: Theory and experiment. Phys. Rev. B 2018, 97, 054407. [Google Scholar] [CrossRef]
- Damewood, L.; Busemeyer, B.; Shaughnessy, M.; Fong, C.Y.; Yang, L.H.; Felser, C. Stabilizing and increasing the magnetic moment of half-metals: The role of Li in half-Heusler LiMnZ (Z=N,P,Si). Phys. Rev. B 2015, 91, 064409. [Google Scholar] [CrossRef]
- Dehghan, A.; Davatolhagh, S. d0-d half-Heusler alloys: A potential class of advanced spintronic materials. J. All. Comp. 2019, 773, 132. [Google Scholar] [CrossRef]
- Dehghan, A.; Davatolhagh, S. First principles study of d0-d half-Heusler alloys containing group-IV, -V, and -VI sp atoms as prospective half-metals for real spintronic applications. Mat. Chem. Phys. 2021, 273, 125064. [Google Scholar] [CrossRef]
- Galanakis, I. Slater–Pauling Behavior in Half-Metallic Heusler Compounds. Nanomaterials 2023, 13, 2010. [Google Scholar] [CrossRef]
- Javari, A.R.; Davatolhagh, S.; Dehghan, A. Half-metallic p0-d half-Heusler alloys with stable structure in ferromagnetic state. J. Phys. Chem. Solids 2022, 166, 110702. [Google Scholar] [CrossRef]
- Koepernik, K.; Eschrig, H. Full-potential nonorthogonal local-orbital minimum-basis band-structure scheme. Phys. Rev. B 1999, 59, 1743. [Google Scholar] [CrossRef]
- Kopernik, K. Full Potential Local Orbital Minimum Basis Bandstructure Scheme User’s Manual (http://www.fplo.de/download/doc.pdf).
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
| Compound | Lattice constant a in Å | Energy difference in eV | Most stable |
Least stable |
||||
| Li | -phase | -phase | -phase | phase | phase | |||
| LiCaGa | 6.72 | 7.00 | 7.14 | -0.13 | -0.86 | -0.73 | ||
| LiScGa | 6.13 | 6.42 | 6.52 | -0.17 | -1.04 | -0.87 | ||
| LiTiGa | 5.80 | 6.06 | 6.17 | -0.28 | -1.00 | -0.72 | ||
| LiVGa | 5.69 | 5.93 | 6.03 | -0.30 | -0.67 | -0.37 | ||
| LiCrGa | 5.84 | 6.08 | 6.17 | -0.35 | -0.36 | -0.01 | ||
| LiMnGa | 5.78 | 5.95 | 5.99 | -0.31 | -0.28 | 0.03 | ||
| LiFeGa | 5.66 | 5.81 | 5.89 | -0.42 | -0.06 | 0.36 | ||
| LiCoGa | 5.44 | 5.63 | 5.66 | -0.66 | -0.04 | 0.63 | ||
| LiNiGa | 5.55 | 5.69 | 5.71 | -0.55 | 0.19 | 0.74 | ||
| LiCuGa | 5.75 | 5.87 | 5.87 | -0.32 | 0.29 | 0.61 | ||
| LiZnGa | 5.97 | 6.06 | 6.05 | -0.32 | 0.09 | 0.41 | ||
| LiCaGe | 6.49 | 6.86 | 6.94 | -0.08 | -1.10 | -1.03 | ||
| LiScGe | 5.98 | 6.31 | 6.38 | -0.41 | -1.24 | -0.83 | ||
| LiTiGe | 5.72 | 6.01 | 6.10 | -0.48 | -0.94 | -0.46 | ||
| LiVGe | 5.69 | 5.96 | 5.98 | -0.61 | -0.76 | -0.15 | ||
| LiCrGe | 5.72 | 5.94 | 5.95 | -0.59 | -0.51 | 0.08 | ||
| LiMnGe | 5.76 | 5.99 | 5.96 | -0.49 | -0.30 | 0.19 | ||
| LiFeGe | 5.59 | 5.75 | 5.76 | -0.62 | -0.04 | 0.58 | ||
| LiCoGe | 5.42 | 5.59 | 5.58 | -0.93 | 0.06 | 0.98 | ||
| LiNiGe | 5.49 | 5.65 | 5.62 | -0.68 | 0.25 | 0.93 | ||
| LiCuGe | 5.67 | 5.81 | 5.77 | -0.47 | 0.31 | 0.78 | ||
| LiZnGe | 5.88 | 6.00 | 5.96 | -0.58 | 0.06 | 0.64 | ||
| Compound | phase | ||||||
| LiCaGa | -0.062 | -0.046 | -0.134 | -0.242 | 6 | -2 | |
| -0.068 | -0.066 | -0.282 | -0.417 | 6 | -2 | ||
| LiCaGe | -0.006 | -0.091 | -0.903 | -1.000 | 7 | -1 | |
| 0.008 | -0.176 | -0.832 | -1.000 | 7 | -1 | ||
| LiScGa | -0.076 | -0.458 | -0.466 | -1.000 | 7 | -1 | |
| -0.089 | -0.388 | -0.271 | -0.748 | 7 | -1 | ||
| LiTiGe | 0.008 | 0.135 | -0.022 | 0.121 | 9 | 1 | |
| LiVGa | 0.006 | 1.332 | -0.237 | 1.101 | 9 | 1 | |
| -0.066 | 1.288 | -0.222 | 1.000 | 9 | 1 | ||
| -0.001 | 1.311 | -0.232 | 1.078 | 9 | 1 | ||
| LiVGe | 0.066 | 2.404 | -0.426 | 2.044 | 10 | 2 | |
| 0.023 | 2.438 | -0.461 | 2.000 | 10 | 2 | ||
| 0.104 | 2.362 | -0.466 | 2.000 | 10 | 2 | ||
| LiCrGa | -0.073 | 3.835 | - 0.422 | 3.340 | 10 | 2 | |
| -0.190 | 3.933 | -0.348 | 3.396 | 10 | 2 | ||
| -0.002 | 4.203 | -0.280 | 3.921 | 10 | 2 | ||
| LiCrGe | -0.013 | 3.747 | - 0.596 | 3.138 | 11 | 3 | |
| -0.030 | 3.697 | -0.667 | 3.000 | 11 | 3 | ||
| 0.076 | 3.636 | -0.712 | 3.000 | 11 | 3 | ||
| LiMnGa | -0.204 | 3.979 | -0.511 | 3.264 | 11 | 3 | |
| -0.227 | 3.830 | -0.603 | 3.000 | 11 | 3 | ||
| -0.125 | 3.768 | -0.625 | 3.018 | 11 | 3 | ||
| LiMnGe | -0.028 | 4.264 | -0.343 | 3.892 | 12 | 4 | |
| -0.034 | 4.290 | -0.317 | 3.940 | 12 | 4 | ||
| 0.089 | 4.054 | -0.427 | 3.717 | 12 | 4 | ||
| LiFeGa | -0.160 | 2.986 | -0.254 | 2.572 | 12 | 4 | |
| -0.154 | 2.748 | -0.333 | 2.261 | 12 | 4 | ||
| -0.093 | 2.785 | -0.287 | 2.406 | 12 | 4 | ||
| LiFeGe | -0.088 | 2.978 | -0.189 | 2.701 | 13 | 5 | |
| -0.055 | 2.640 | -0.244 | 2.342 | 13 | 5 | ||
| 0.024 | 2.604 | -0.254 | 2.326 | 13 | 5 | ||
| LiCoGe | -0.018 | 0.395 | -0.053 | 0.324 | 14 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





