1. Introduction
Colloidal quantum dots (CQDs) are of great interest for optoelectronics including light emitting devices [
1], energy harvesting photovoltaics [
2], solar concentrators [
3], and photodetectors [
4]. However, when subjected to elevated temperatures that can occur during manufacturing, testing, or while in use, CQDs and their device performance can degrade. One mode of degradation is thermal sintering, during which neighboring CQDs fuse together. Sintering occurs at elevated temperatures as the activation barrier to ion diffusion is overcome and interfaces can reconstruct to reduce the high surface energy of the CQDs. Consequently, quantum confinement is lost during sintering, causing broadening of the absorption spectrum and harmful changes to the electrical properties of the films.
A common solution to this problem is to grow a shell on the surface of the CQDs to prevent neighboring core materials from fusing together. Such a core-shell structure has been most successful for light emitting and display technologies. However, the shell is typically designed to confine the charge carriers rather than to promote charge separation. This effect of the shell impedes carrier extraction, making the approach impractical for photovoltaic and photodetector devices. Therefore, a method other than a shell is needed for the practical development of CQD-based, charge-collecting devices.
In bulk semiconductor devices, metal oxides and nitrides have been used to protect the integrity of the active elements and interconnects from metal ion diffusion at elevated process temperatures and during operation [
5]. Such diffusion barriers may also prevent sintering between neighboring colloidal quantum dots without preventing charge transport, if such a diffusion barrier could be grown within the CQD thin film. Low-temperature atomic layer deposition (ALD) has been used to fill the spaces in CQD films with metal oxides, leading to improved charge carrier transport and providing protection against oxidation for air-stable PbX (X=S,Se) [
6,
7] and InP CQDs [
8]. However, the impact of in-filled ALD metal oxides on the thermal stability of CQD solids has not yet been reported.
Here, metal oxides as barriers were prepared by low-temperature ALD on conductive HgTe CQD solid films. Zinc oxide (ZnO), titanium dioxide (TiO2), and alumina (Al2O3) were readily available and evaluated as barrier materials against thermally induced sintering. The optical and electronic properties were evaluated to determine the effectiveness of the respective barrier material against sintering before and after subjecting the ALD-coated HgTe CQD thin films to elevated temperatures.
Result and Discussion
Thin films of HgTe CQDs were coated onto sapphire substrates and in-filled by ALD with different metal oxides. Infrared-transparent sapphire was selected as the substrate to facilitate FTIR absorption measurements before and after ALD and high-temperature baking. The amount of the metal oxide deposited was controlled by the number of cycles and dwell time of the precursor gases.
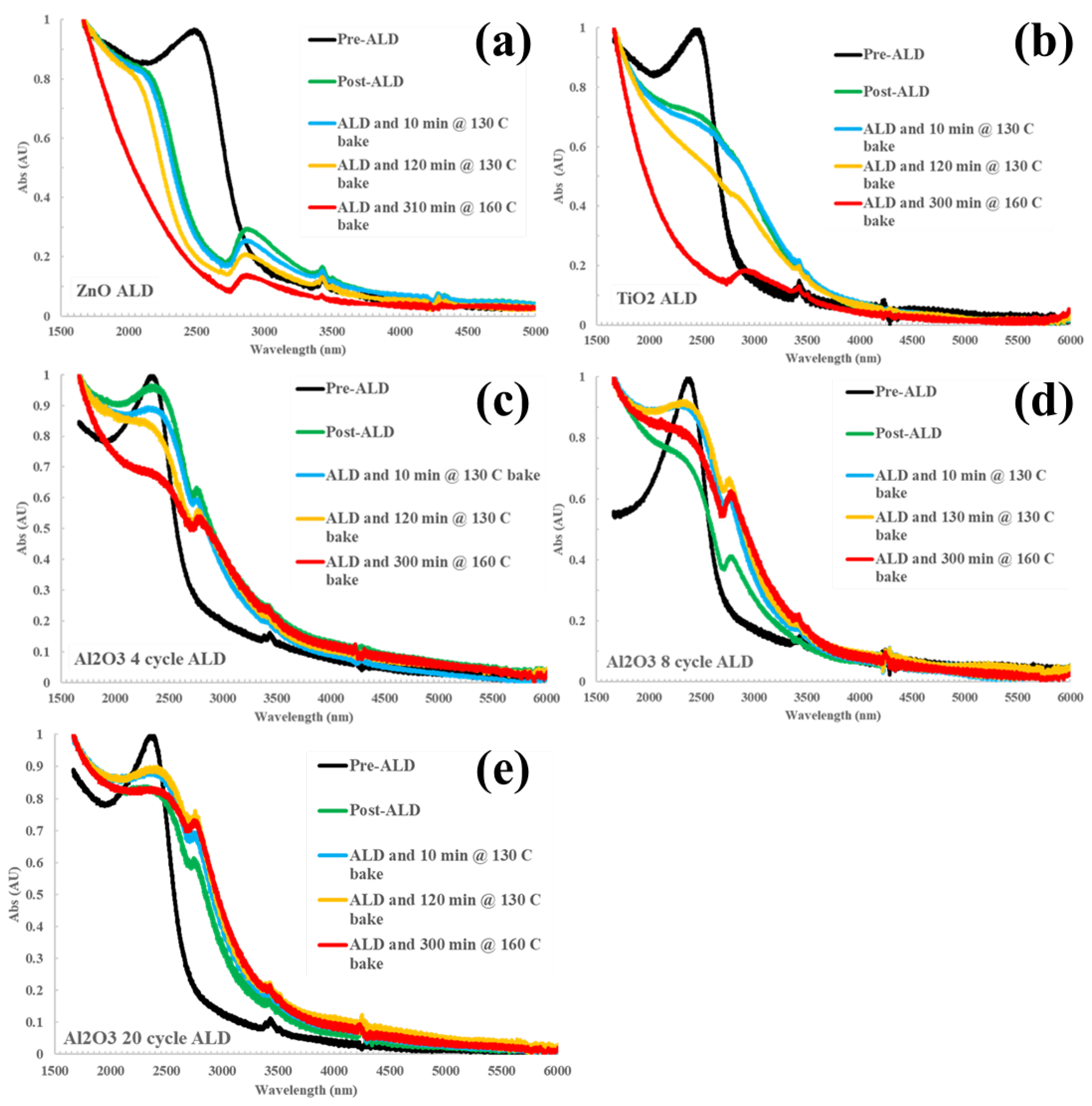
Figure 1 shows the evolution of the HgTe CQD infrared absorption spectra as a function of ALD deposition and baking conditions for zinc oxide (ZnO), titanium dioxide (TiO
2), and alumina (Al
2O
3). Both ZnO and TiO
2 failed to preserve the exciton absorption feature following ALD such that these materials were deemed to be incompatible with HgTe CQDs as a barrier against thermal sintering. In the case of ZnO (
Figure 1a), the exciton absorption feature of the HgTe CQDs blue-shifted immediately upon ALD treatment. The spectral shape was maintained with ZnO coating until the baking was increased to 160
oC for 5 hours, at which point the exciton absorption feature was completely quenched. For TiO
2 (
Figure 1b), the exciton feature broadened and red-shifted following ALD, then failed to mitigate sintering when baked above 130
oC as evidenced by the loss of the exciton feature. The additional peaks found between 2700 and 3000 nm can be assigned to coordinated hydroxyl group residuals at the surface of the ALD deposited oxides, varying in position depending on coordination number and species with respect to Al, Zn, or Ti [
11,
12,
13].
In contrast, Al
2O
3 both preserved the exciton feature overall—suffering a marginal broadening and red-shift—and provided significantly improved thermal stability against sintering compared to ZnO and TiO
2.
Figure 1c-e show the evolution of the absorption spectrum with increasing temperature and increasing number of cycles (4, 8, and 20, respectively) of the alumina on the HgTe CQD. A minor redshift was observed between the control and post-ALD/pre-bake steps that cannot be attributed to thermal effects. Between 8 cycles (
Figure 1d) and up to 20 cycles (
Figure 1e) of ALD were sufficient to retain the exciton feature of the absorption spectrum after baking at 130
oC for 2 hours and 160
oC for 5 hours. This demonstration is evidence that ALD can be used to fabricate barriers that can provide thermal stability against sintering. Further development of alumina—as well as the investigation of other materials such as metal chalcogenides that are commonly used with infrared semiconductors—may lead to the robust design of CQD-based optoelectronics, especially photodetectors.
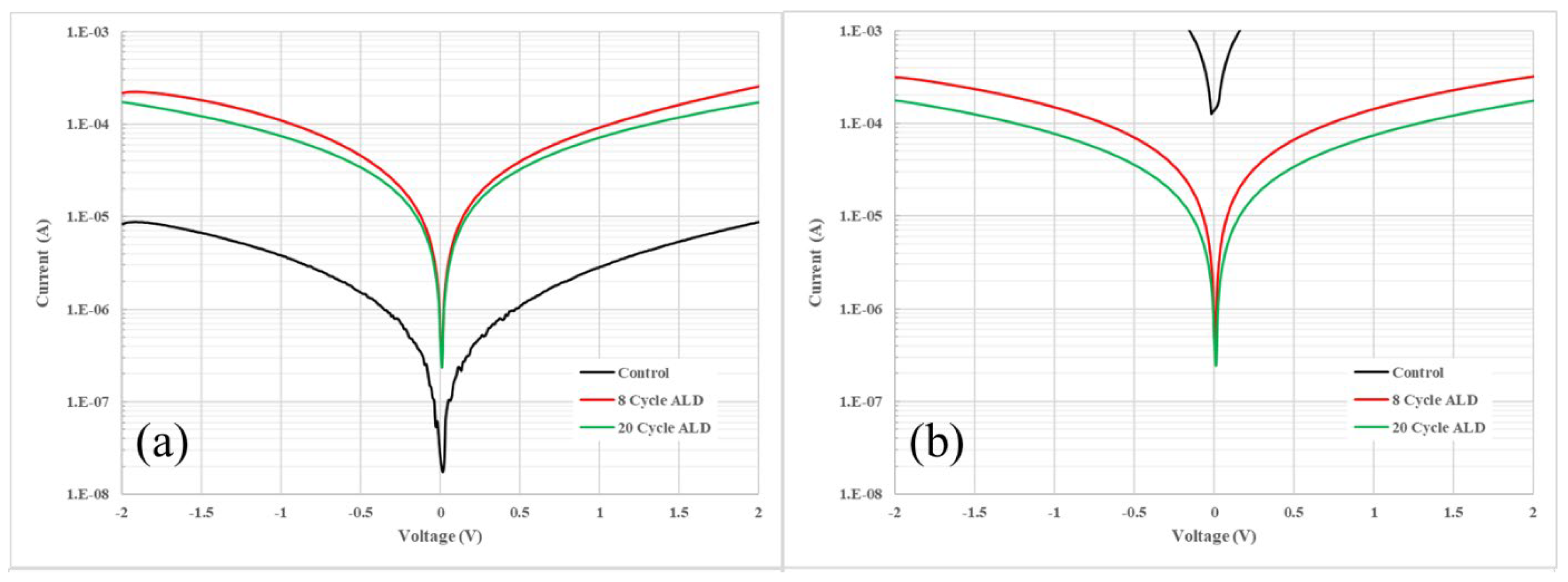
Having established the feasibility of ALD as a method for preserving the integrity of the HgTe CQDs, the impact of ALD on the electrical properties of these thin films was investigated. First, simple photoconductor measurements, as shown in
Figure 2, were used to assess the impact of ALD in-filling on the conductance of HgTe CQD films. Thin films of HgTe CQDs were deposited on gold interdigitated electrodes and then coated with either no alumina (control sample), 8 cycles, or 20 cycles of alumina by ALD. The photoconductors were characterized before and after baking at 130
oC for 2 hours. Comparing current levels before baking (
Figure 2a) and after baking (
Figure 2b), it was apparent that both 8 and 20 cycle alumina ALD preserved the conducting properties of the HgTe CQD film while un-passivated films degraded, as indicated by the significant increase in current after baking.
An order-of-magnitude increase in the conductance following ALD coating was also notable in
Figure 2a before subjecting the films to baking. An increase in the conductance of the CQD film could follow from higher carrier mobility, higher doping density, or both due to ALD in-filling. To investigate what contributed to this change, field effect transistor (FET) measurements were made on HgTe CQD thin films before and after ALD. Samples consisted of HgTe CQD films coated on Au-Au interdigitated electrodes (source and drain) patterned on a heavy n-type doped silicon substrate (gate) with a 300 nm SiO
2 dielectric layer. Measurements were made across multiple FET devices.
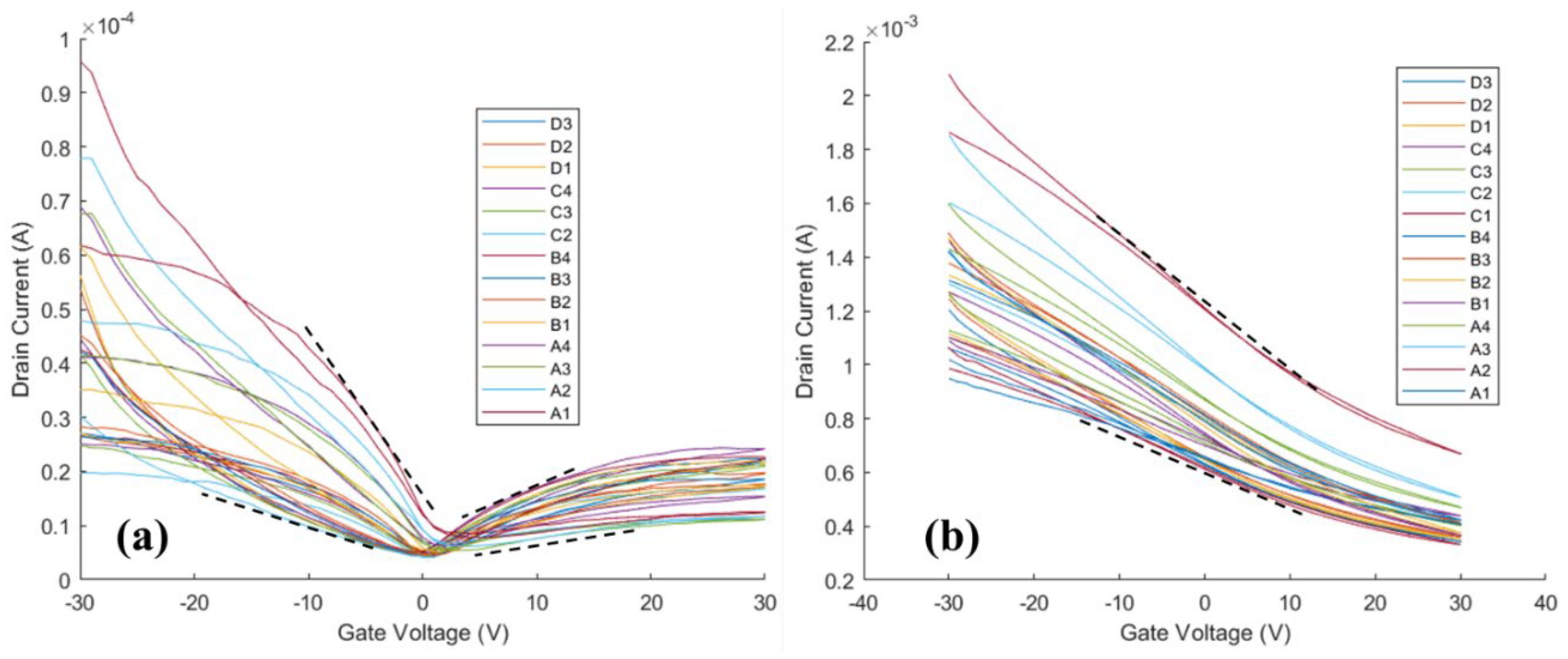
The transfer curves, shown in
Figure 3, were measured before (
Figure 3a) and after (
Figure 3b) alumina ALD and analyzed to understand the impact of ALD on the mobility and doping the HgTe CQD thin films. Before ALD (
Figure 3a), the average minima of conductance were at gate voltages (V
g) between 0 V and +5 V, which suggests near-intrinsic to light p-type doping, respectively, of the thin films. After ALD (
Figure 3b), the minima of conductance were greater than +30 V and not observable on the scale of the measurement, suggesting a shift to heavy p-type doping of the thin films following ALD coating.
With respect to carrier transport, the carrier mobilities were estimated by taking the slopes indicated by the dashed lines in
Figure 3 and calculating the mobility (µ
FET) according to the expression
where L is the channel length (gap), W is the channel width, V
SD is the source-drain bias, C
i is the capacitance per unit area for 300 nm SiO
2 insulator, I
SD is the source-drain current, and V
g is the gate voltage [
15]. Before ALD (
Figure 3a), carrier mobilities were 5×10
-3 up to 4×10
-2 cm
2/Vs for holes and 10
-3 up to 10
-2 cm
2/Vs for electrons. However, after ALD (
Figure 3b) the FET was dominated by hole currents with hole mobilities from 0.2 to 0.4 cm
2/Vs—a 10-times increase in hole mobility following alumina ALD. Both the increase in the doping and the increase in the carrier mobility contribute to the increased conductance of the HgTe CQD thin films following ALD.
From these observations, it is reasonable to conclude that alumina ALD coatings will consistently increase the p-type doping and carrier mobilities of HgTe CQD thin films. Compared to HgTe CQDs, prior works on Pb chalcogenides have higher carrier mobilities because of ALD in-filling, attributing the improvements to trap filling for alumina-coated Pb chalcogenides [
7]. However, with respect to doping, Pb chalcogenides consistently showed less p-type and more n-type doping—that is, the opposite direction to the changes observed here for HgTe CQDs. The changes in doping for Pb chalcogenides have been attributed to acceptor state passivation by alumina ALD, thus reducing the p-type doping density [
7]. By analogy then, alumina ALD on HgTe CQDs could have the effect of passivating hole traps, increasing acceptor states, and/or decreasing donor states. Alternatively, changes in the net surface dipole of the CQDs following from reactions with the ALD precursors during deposition may also explain the doping shifts of these materials [
14]. From the perspective of the CQDs, these effects are functionally equivalent, causing the Fermi level to shift relative to the conduction and valence levels and shifting the doping of the system.
To better understand the possible impact of ALD on doping and mobilities of HgTe CQDs, n-type HgTe CQDs were synthesized as reported by Yang et al. and studied by FET measurements [
9]. The n-type doping was achieved by transferring the HgTe CQDs from non-polar octane into polar N,N-dimethylformamide (DMF) in the presence of excess mercury chloride (HgCl
2). The HgTe CQDs were cleaned to remove excess polar ligands and re-dissolved in DMF. Thin-film backside-gated FETs were made using n-type HgTe CQDs, coated from DMF, to characterize their doping and mobility before and after ALD in-filling. From the previous discussion, it was reasonable to expect that the n-type HgTe CQDs would become increasingly p-type and the carrier mobilities would improve with ALD in-filling.
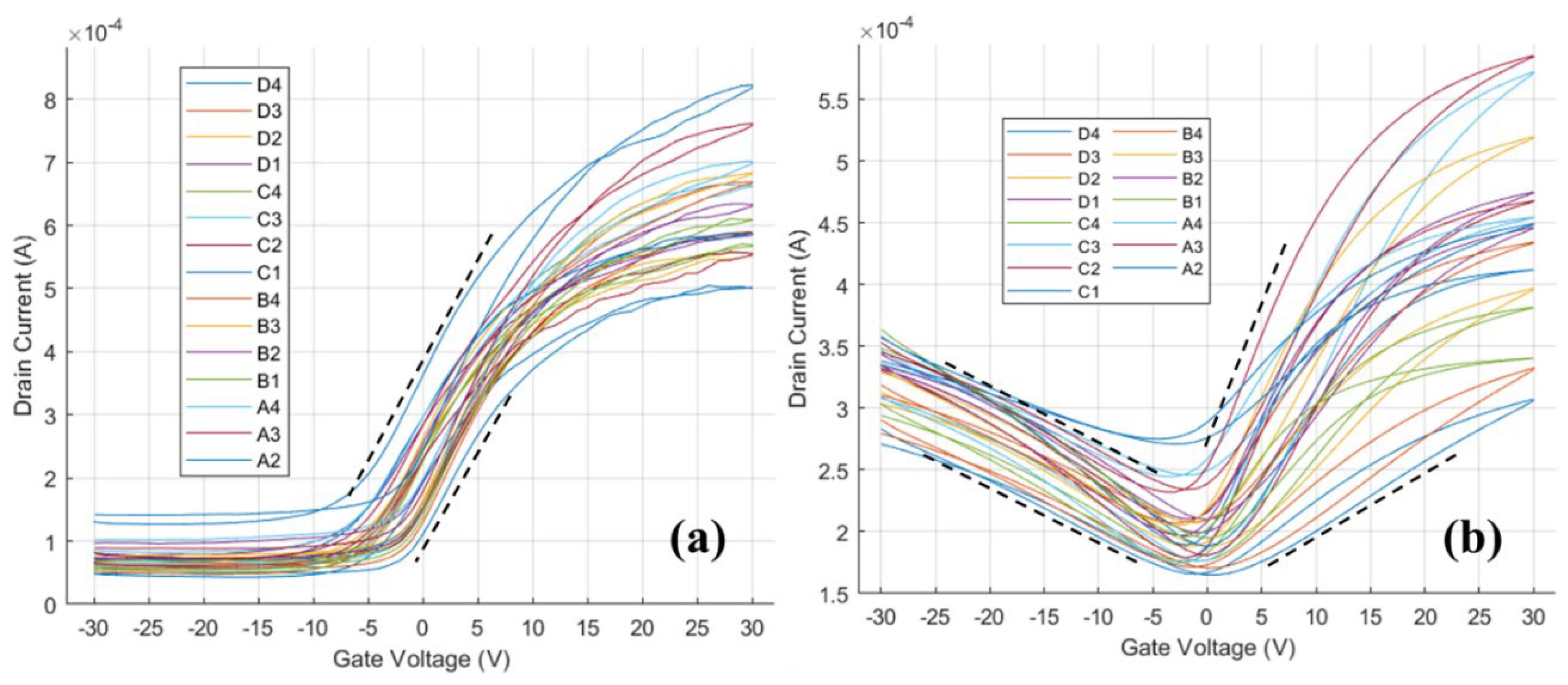
The transfer curve for the n-type HgTe CQD film without and with ALD is shown in
Figure 4. In this case, before ALD (
Figure 4a) the HgTe CQDs show only electron transport with carrier mobility of 0.3 to 0.5 cm
2/Vs and minima of conductance at V
g less than -5 V, which suggests lightly n-type doped films. Following ALD (
Figure 4b), the minima of conductance shifted toward V
g = 0 V and hole currents were now measurable at negative gate voltages. Electron and hole mobilities following ALD were both between 0.1 to 0.3 cm
2/Vs and comparable in magnitude to the electron carrier mobility before ALD. The shift in the conductance minima and the increase in the hole conductance were both consistent with decreasing n-type character of the film following alumina ALD in-filling.
As in the previous case of p-type HgTe CQDs shown in
Figure 3, ALD in-filling tended to decrease the n-type doping and increase the p-type doping of the HgTe CQD thin films. Contrary to the previous case however, the carrier mobilities were not improved, as evidenced by the comparable electron mobilities before and after ALD for the n-type HgTe CQDs. Furthermore, when p-type HgTe CQDs were prepared by phase exchange to polar DMF in a deficit of HgCl2 and then coated with alumina by ALD, similar results were observed (
Supplementary Figure S3) with hole mobility effectively constant before and after alumina ALD. The impact of alumina ALD on carrier mobility was much less significant when HgTe CQDs were phase transferred to DMF. These observations may be explained by the differences in the processing of each of the HgTe CQD thin films investigated here.