1. Introduction
Virus-induced gene silencing (VIGS) is an RNA-mediated reverse genetics technology with wide applications for analyzing gene function (1,2). VIGS is typically used to downregulate endogenous genes by exploiting plant post-transcriptional gene silencing processes. The first VIGS vector was adapted from a tobacco mosaic virus (TMV) (3), although most applications for members of the Poaceae use a barley stripe mosaic virus (BSMV)-derived vector (4). For example, VIGS has been successfully used in wheat (5), barley (6), and maize (7). The related use of BSMV-derived vectors for gene overexpression (VOX) also has been reported for wheat (8) and other members of the Poaceae. However, previous VIGS studies on hexaploid (Avena sativa) and diploid (A. strigosa) oats were not successful (9), and we are not aware of reports on the use of VIGS for A. fatua.
The widespread and intensive use of herbicides has been selected for resistant weed populations in numerous cropping systems, posing a significant threat to global food security by reducing crop yields and increasing production costs. Of all resistant species, multiple herbicide-resistant (MHR) Avena fatua L. (wild oat) populations have a disproportionate economic impact due to their worldwide presence and competition in small grain crops (10). MHR weeds with non-target site resistance (NTSR) mechanisms can express resistance to herbicides without previous exposure and are thus particularly troubling (11).We recently described MHR inbred lines of the pernicious weed Avena fatua (wild oat) that are resistant to at least 10 members of HRAC Mode of Action Groups (12) 0, 1, 2, 15, and 22 (13,14).
In addition to several disparate NTSR mechanisms, resistance co-segregates with constitutively elevated expression of genes with roles in stress response, xenobiotic metabolism, heat shock response, disease resistance, and transcriptional regulation (15). Additional comparisons of soluble proteins revealed that MHR plants contain constitutively elevated levels of proteins with functions in core biosynthetic pathways (glycolysis, Calvin cycle, C-2 glycolate pathway), stress response pathways (photorespiration, mannose-binding lectin, protein kinase), and stress-related transcription factors. Phosphoproteome and redox proteome analyses show that numerous transcription factors and stress-related proteins are preferentially subjected to constitutive post-translational modifications (PTMs) in MHR plants (16).
The functions of these candidate genes and their potential linkage with MHR merit further investigation. We propose that the use of VIGS and VOX can provide valuable insights into the molecular mechanisms of MHR, an idea first proposed for resistant species by Macgregor in 2020 (17). In Alopecurus myosuroides (blackgrass), loss and gain of function of the glutathione S-transferase AmGSTF1 successfully changed the MHR response to fenoxaprop (18). The current work demonstrates the successful knockdown of the A. fatua phytoene desaturase (PDS) and overexpression of a green fluorescence protein (GFP) gene in A. fatua and A. sativa using a BSMV-mediated system as a first step in manipulating MHR phenotypes.
2. Materials and Methods
2.1. Plant Material and Growth Conditions
Inbred MHR and herbicide susceptible (HS1) A. fatua lines were derived from field-selected populations as previously described (15,19), and a second inbred HS line (HS2) was derived from the nondormant A. fatua line SH430 used in seed dormancy research (20,21). Plants were grown in 10.2 cm square pots under a 16-hr photoperiod of natural sunlight supplemented with mercury vapor lamps (165 μmol m-2 sec-1) at 25 ± 4 C. Growth medium was greenhouse soil mix [1:1:1 (by vol) Bozeman silt loam:Sunshine mix #12 (Sun Gro Horticulture, Inc., Bellevue, WA):perlite], plants were fertilized weekly with 100 ppm of Jack’s Classic 20-20-20 All Purpose fertilizer, and watered as needed. Plants used for inoculations were tested at Zadoks (21) stages 10 to 12 (1 ½ leaf to 2 1/2 leaf stage, respectively).
2.2. Preparations of BSMV-RNAs
The pα, pβ, and pγ plasmid DNAs were linearized with restriction enzymes MluI, SpeI, and MluI, respectively. The pγPDS and pγGFP were linearized with BssHII. Each reaction was conducted in a 20 μl volume containing 2.5 μg plasmid DNA, 6 units of enzyme, 2 μl 10X enzyme buffer, and nuclease-free H2O. All enzymes were from New England, Biolabs (Rowley, MA, USA). The reactions with MluI or SpeI were incubated at 37°C for 90 mins and then inactivated at 80°C for 20 mins. Reactions with BssHII were incubated at 50°C for 3 hours and then heat-inactivated at 65 °C for 20 mins. The completion of linearization was checked side-by-side with the corresponding uncut plasmid via electrophoresis on 1% w/v agarose gel.
Linearized plasmid DNAs were treated with 40 units (1μl) RNase inhibitor (New England, Biolabs Inc) per 20 μl linearization reaction. In-vitro transcription was performed in a 20 μl reaction containing 2 μl T7 10X buffer, 1 μl of 20 U/μl T7 RNA polymerase (Sigma Aldrich), 2 μl cap / rNTP mix, 7 μl linearized plasmid, and 8 μl nuclease-free H2O. The reaction was incubated at 37°C for 2 hours. Transcription reactions were examined on a 1% w/v agarose gel by sampling 1 μl of each reaction every 2 hours while incubation continued. We prepared 20 μl of each α, β and γ/PDS/GFP RNAs for 20 plants.
2.3. Inoculation Methods
We used the leaf-rub inoculation as described by Huang (2017) (22) to introduce RNAs of α, β, and γ/PDS/GFP (BSMV:00; BSMV:PDS and BSMV:GFP) into plants. In vitro-transcribed RNAs were combined in a 1:1:1 ratio, which amounts to 60 μl of total transcript for each of the 20 plants to be inoculated. We then added 440 μl FES buffer and mixed thoroughly on ice. Plants were inoculated with 23 μl RNA, which was rubbed on the second leaf between the index finger and thumb three times starting at the base and extending to the tip of the leaf while holding the plant stem steady with the other hand.
2.4. Green Fluorescence Imaging
GFP fluorescence images were captured with a confocal light microscope (Zeiss Axioscope 7) and photographed with the ZEISS Axiocam camera portfolio and ZEISS ZEN 2 core imaging software. Leaf samples showing visible viral symptoms at 14 dpi with BSMV:GFP and BSMV:PDS were viewed under a microscope using bright light and fluorescence.
2.5. Quantitative RT-PCR Analysis
Total RNA was isolated from leaf tissues of BSMV:PDS and BSMV:00 inoculated plants using the RNeasy plant mini kit (Qiagen, Valencia, CA, USA) and treated with RNase-Free DNase (Qiagen, Valencia, CA, USA) to remove DNA based on the manufacturer’s instructions. The concentration of total RNA was assessed using 260/280 ABS measurements on a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, DE, USA). RNA integrity was checked using a 1% agarose gel at 120 volts for 30 mins of 1 μl RNA, 4 μl of water, and 1 μl loading buffer (98% formamide, 10 mM EDTA, 0.25% [w/v] bromophenol blue, and 0.2% [w/v] xylene cyanole) and stained with GelRed (Bio-Rad, Hercules, CA, USA).
Quantitative real-time PCR (qRT-PCR) was performed on a CFX 96 real-time PCR detection system (Bio-Rad, Hercules, CA, USA). The reaction contained 2 μl of a mixture of forward and reverse primers (1 pmol/μl), 10.25 μl of iScript One-Step SYBR Premix (Bio-Rad, Hercules, CA, USA), 2 μl of RNA (37.5 ng/μl), and 5.75 μl of RNase-free water. PDS gene expression was assayed using primers flanking the inserted region of the gene in the γPDS vector (
Table 1). The housekeeping gene alpha glucan phosphorylase (GLUP) was used as a reference gene. The PDS silencing level is presented as the fold change of 2
-∆∆Ct relative to the abundance of the gene in BSMV:00 inoculated oats.
2.6. Statistical Analysis
qRT-PCR data are presented as the means of absolute quantification number (Ct) ± standard deviation (SD) of three biological replicates. The data were subjected to t-tests using comparisons of mean PDS RNA abundance from BSMV:PDS- to BSMV:00-inoculated plants. Statistical differences between mean values were determined using standard errors in Microsoft Excel.
3. Results
3.1. Construction of Gamma (γ) Vectors for BSMV-Mediated Assays
The DNAs corresponding to the three sub-genomes of BSMV were cloned into three plasmids designated as pα, pβ and p
γ, comprising respectively the tripartite genomes (α, β and
γ) of BSMV (23). Two constructs, each with a 215-bp or a 300-bp fragment of the
A. fatua PDS gene (
Supplementary file 1) were inserted into the pγ-PCR cloning vector (24), (
Figure 1A), and termed pγPDS. The GFP reporter gene was used to evaluate BSMV-mediated gene overexpression. The GFP coding sequence plus a 110-bp ribosome binding site (RBS) at the 5’end was inserted in an inverted orientation after the stop codon of the BSMV γ genome in the pγ-PCR cloning vector
(Figure 1B, Supplementary file 2) and termed pγGFP.
3.2. BSMV-Mediated Gene Silencing in Oats
An essential requirement for using a virus-mediated assay in a plant is that the virus must infect the plant. To test if BSMV can infect oats and induce gene silencing, we rub inoculated the RNAs of the virus onto the second leaves of 20 plants from each of
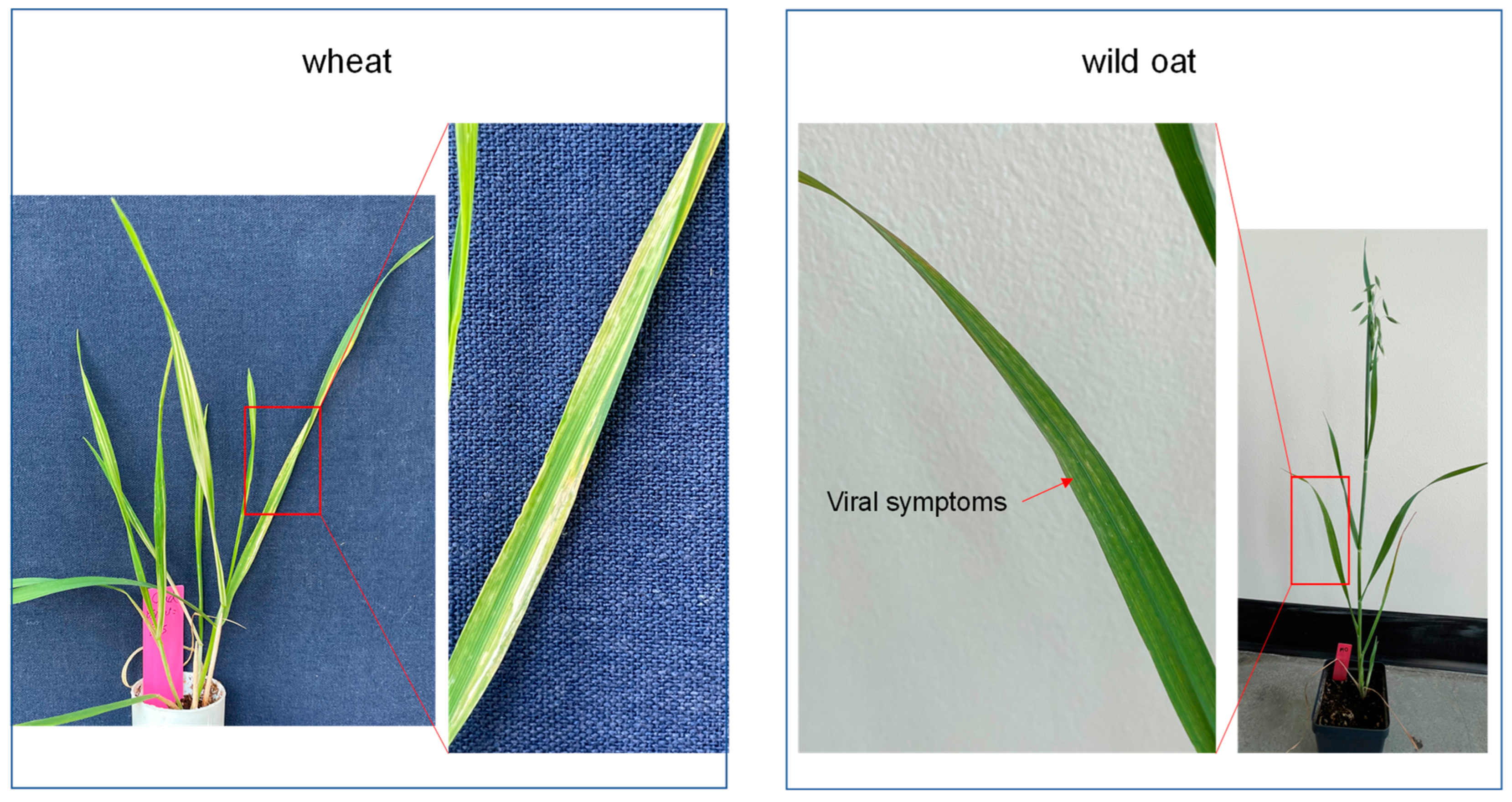
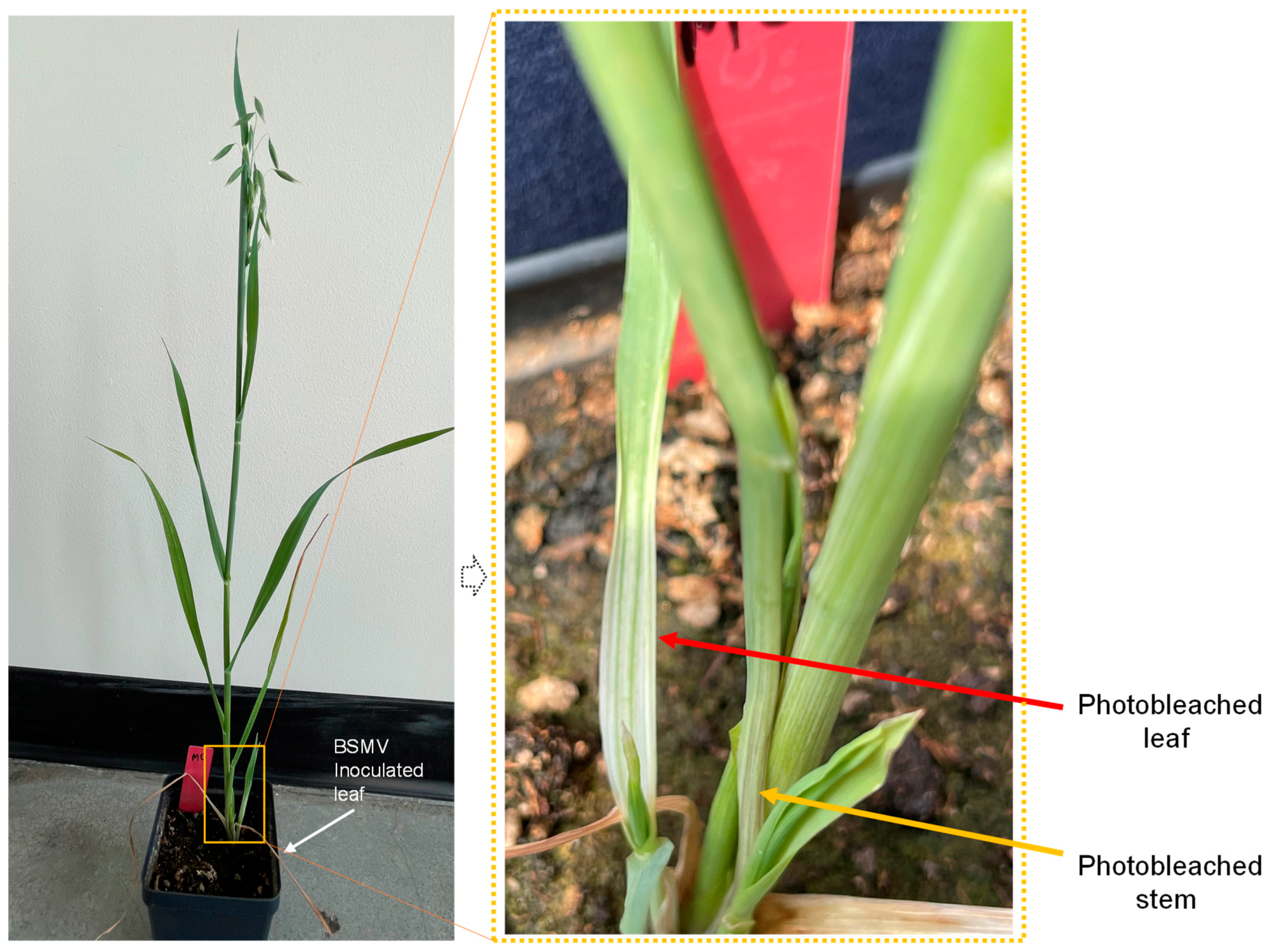
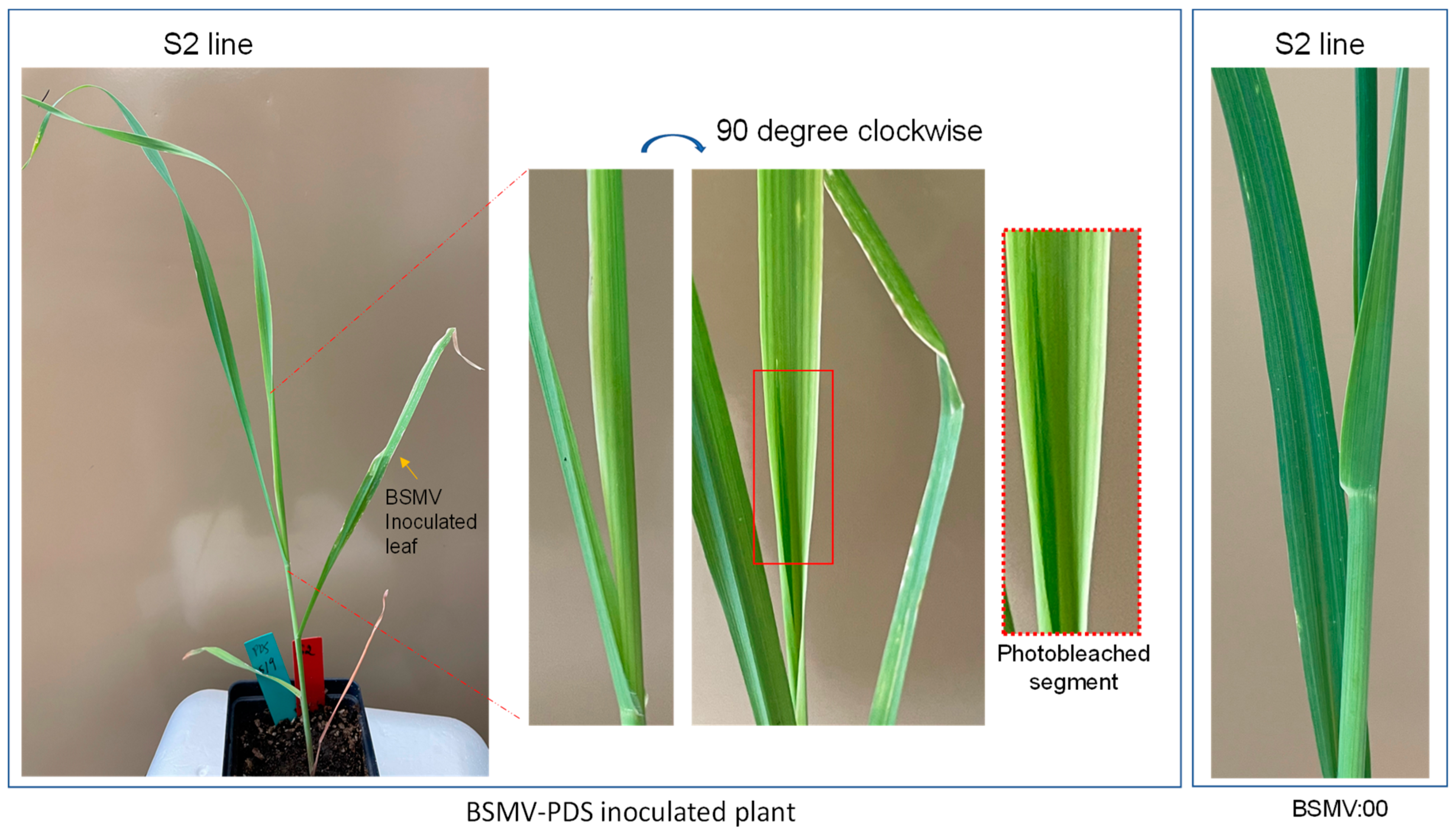
A. fatua and A. sativa at the 2-leaf stage. Five wheat plants were included as a positive control to confirm the quality of the experiments since we routinely use BSMV-mediated gene silencing assays for wheat functional analysis (5,25). For the silencing assay, we used PDS as a reporter gene because silencing of this gene gave a photobleaching phenotype. In-vitro synthesized RNA of α, β, and γPDS was mixed in a 1:1:1 ratio (BSMV:PDS) for inoculation, and an α, β, and γ mixture containing only the BSMV genome (BSMV: 00) was used as a control. Streak patterns were visible on BSMV-inoculated oat plants at 14 days post-inoculation (dpi) (
Figure 2), suggesting BSMV could replicate in both
A. sativa and
A. fatua. However, there were significant differences in the time required and severity of viral symptoms between wheat and oat plants. Mosaic viral symptoms were evident within 9 dpi for wheat, but only after 14 dpi for oats. Further, symptoms were more severe on wheat than on oat plants (
Figure 2). The efficiency rate of BSMV inoculation for
A. sativa and
A. fatua was around 50%, while the rate for wheat was 100% (
Table 2).
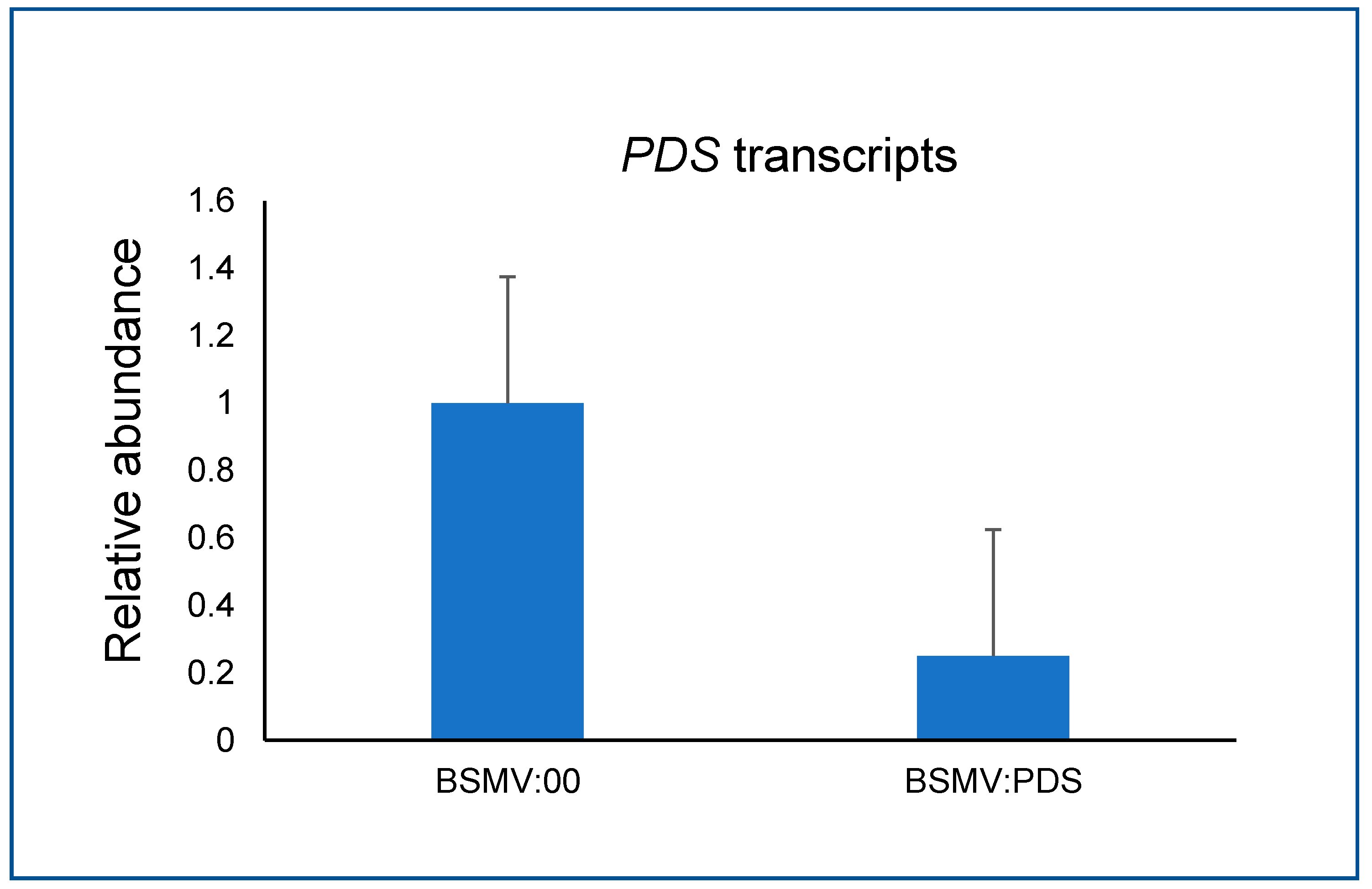
Three photobleached segments of
A. fatua plants were sampled and tested for the PDS transcript abundance. The corresponding segments from three BSMV:00 inoculated
A. fatua plants were used to reference the PDS transcript abundance level. The qRT-PCR revealed a significant reduction of PDS in the silenced segments, about 25% of the transcript level in the PDS silenced segments related to the non-silenced control (
Figure 5).
Our results demonstrate that BSMV can infect two species of oats and induce their silencing machinery as an antiviral defense response. To our knowledge, this is the first report of BSMV-based VIGS in either species of oats.
3.3. BSMV-Mediated Gene Over-Expression in Oats
Having demonstrated that BSMV can replicate and activate silencing machinery in
A. fatua, we further tested whether this system could be used to over-express a gene of interest. Similar to the silencing assay described above,
in-vitro synthesized RNA of α, β, and γGFP was mixed in a 1:1:1 ratio as BSMV:GFP and used to inoculate 20
A. sativa and
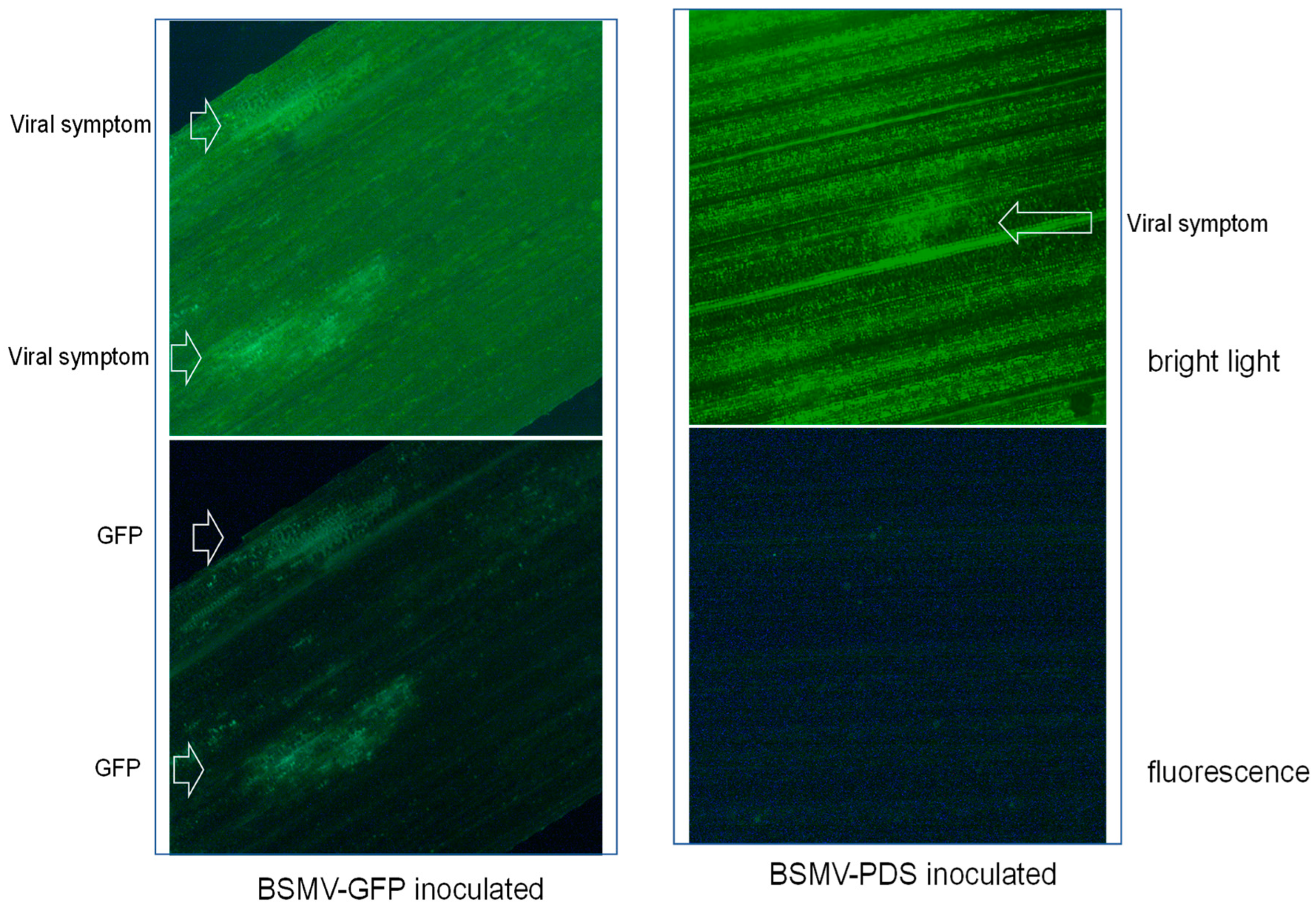
A. fatua plants at the 2-leaf stage. At 14 dpi, visible virus symptoms were observed, and symptomatic sections of leaves that emerged after inoculation were viewed under a fluorescence microscope in two different light settings. Under bright light, patches of viral symptoms were distinguishable as lighter green on leaves from plants treated with BSMV:GFP or BSMV:PDS (
Figure 5). When the leaf sections were viewed under fluorescence with a green filter, green fluorescence signals were clearly visualized and overlapped with the patches of viral symptoms on leaves from the BSMV:GFP treated plants, but no/zero signals from the BSMV:PDS inoculated plants (
Figure 6). This result demonstrated that BSMV could be used to overexpress a gene of interest in
A. sativa and
A. fatua.
3.4. Progress and Timeline of BSMV-Mediated Assays
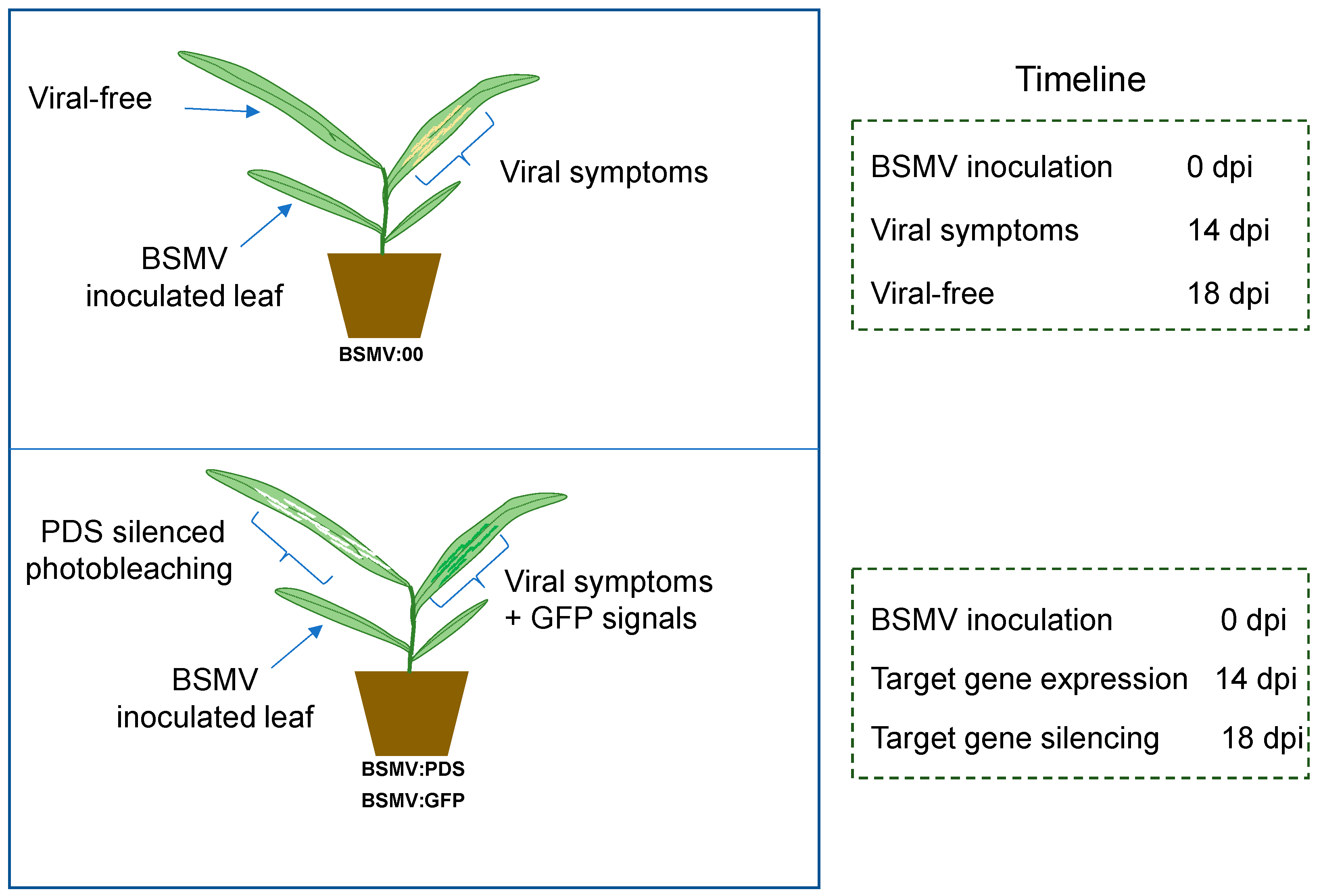
We inoculated BSMV by rubbing RNAs into the second leaves of
Avena plants. We conclude that the virus started replicating in the living cells of the inoculated leaves, but in general, the viral loads were not high enough to show symptoms. Only leaves younger than the inoculated ones had viral symptoms starting at 14 dpi (
Figure 2 and
Figure 7).
The principle of BSMV-mediated gene overexpression is to use the virus to introduce and increase the target gene transcripts during the infection and genome replication processes. Therefore, only cells containing virus will have the target gene transcripts and proteins, as seen by GFP signals that overlapped with viral lesions symptoms on BSMV:GFP inoculated oats (
Figure 7). In contrast, BSMV-mediated gene silencing is a host anti-viral defense activated by the presence of double-stranded RNAs during BSMV replication, and only occurs after the virus enters host cells and successfully replicates to levels detectable by the host. In these studies, Avena plants showed viral symptoms on the second leaf but not on the 3rd and subsequent leaves, indicating that host anti-viral gene silencing and degradation had been activated. For the same reason, the photobleaching phenotype due to the PDS gene silencing was only detected on newly emerged leaves of plants with viral symptoms at 18 dpi. These results correspond to those seen in virus-free leaves on plants inoculated with BSMV:00 (
Figure 7).
4. Discussion
4.1. Challenges of the BSMV-Mediated Assays
Our results demonstrate that BSMV can be used to silence, knock down, or overexpress genes of interest in two Avena species. We conclude that assay success depends on two prerequisites: good viral inoculation and stabilization of the inserted fragment. Both aspects can result in target-gene silencing and over-expression, but only if successful virus replication occurs in live cells of the host plant. A key requirement is to balance the level of cell wounding to allow the viral RNAs to enter without excess leaf damage. Finding the appropriate pressure for rubbing inoculation takes practice, and achieving the balance of wounding the cells and recovery is relatively easier on young leaves. Therefore, we recommend inoculating at least 10 plants for each treatment at a very early growth stage, as early as the 2-leaf stage.
Loss of foreign DNA or the instability of inserted fragments can be a general problem of using virus-mediated assays (26). There is also a minimum requirement of 120 bp for insert size to achieve sufficient silencing (27,28). For BSMV, a 120- to 500-bp insert size is recommended to avoid the loss of the fragment by the virus within the first 10 replications (29,30).
Our results showed that BSMV could produce viral symptoms in oats, but the symptoms were mild and delayed as compared to wheat (
Figure 2), suggesting that the wheat cellular machinery is more suitable for BSMV replication and movement than that of oats. The results also indicated to us that intermediate transcript levels of the inserted gene were produced by the virus in oats, leading to lower levels of short dsRNAs produced by the initial host silencing machinery. This issue could potentially be overcome by increasing the amount of BSMV RNAs for inoculation to increase the initial BSMV viral loads and thus achieve higher target gene transcripts.
4.2. Advantages and Disadvantages
Based on the results shown here, we conclude that levels of the inserted fragment were increased at 14 dpi, but then decreased due to dsRNA-mediated degradation at 18 dpi (Figs. 2, 3 7). A potential advantage of BSMV-mediated assays is that the same host endogenous gene could be overexpressed and silenced with one inoculation of the same plant if the full-length gene is inserted in the orientation shown in
Figure 1B. However, the instability of genes >500 bp increases significantly, so that the virus would lose the inserted DNA within 10 replications (29,30). We conclude that BSMV-mediated gene manipulation is not an ideal choice for genes >500 bp.
Research on weed evolution, population genetics, and resistance mechanisms to one or more herbicides has been limited because of a lack of genetic resources and tools. However, this situation has improved in recent years as resources for weedy species are made available. For example, more than 30 weed genomes have now been sequenced, stable transformation systems are being developed, and one application of VIGS-mediated gene silencing has been reported (31). In our laboratories, current work focuses on the creation of recombinant inbred lines and resulting quantitative trait locus (QTL) mapping for MHR (32). Establishing VIGS- and VOX-mediated gene manipulation protocols for A. fatua, a serious worldwide weed, can now be included in these advances.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. File S1: Avena fatua PDS sequence and region used for gene silencing; File S2: BSMV gamma vector plus GFP and RBS sequence.
Author Contributions
Conceptualization, L.H. and W.D.; methodology, E.N.A., A.B., B.K., L.H.; validation, L.H., E.N.A.; formal analysis, E.N.A..; investigation, E.N.A. and L.H..; resources, W.D.; B.K..; data curation, E.N.A.; and L.H.; writing—original draft preparation, L.H..; E.N.A.; and W.D.; writing—review and editing, E.N.A.; W.D.; B.K.; and L.H..; visualization, E.N.A.; and L.H..; supervision, L.H..; project administration, L.H..; and W.D. funding acquisition, L.H.; and W.D. All authors have read and agreed to the published version of the manuscript.”
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Montana’s Agricultural Experiment Station (MAES) for support for the research.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results”.
References
- Rhaeva K, Kumar A. Chapter 37 - Application of virus-induced gene silencing in crops: mechanism, assessment of risk factors and ecological advantages. In: Gaur RK, Sharma P, Czosnek H, editors. Geminivirus: Detection, Diagnosis and Management [Internet]. Academic Press; 2022 [cited 2024 Jul 15]. p. 589–97.
- Zulfiqar S, Farooq MA, Zhao T, Wang P, Tabusam J, Wang Y, et al. Virus-Induced Gene Silencing (VIGS): A powerful tool for crop improvement and its advancement towards Epigenetics. Int J Mol Sci. 2023 Mar 15;24(6):5608. [CrossRef]
- Kumagai MH, Donson J, della-Cioppa G, Harvey D, Hanley K, Grill LK. Cytoplasmic inhibition of carotenoid biosynthesis with virus-derived RNA. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1679–83. [CrossRef]
- MACFARLANE, SA. Tobraviruses—plant pathogens and tools for biotechnology. Mol Plant Pathol. 2010 Mar 3;11(4):577–83. [CrossRef]
- Nyamesorto B, Zhang H, Rouse M, Wang M, Chen X, Huang L. A transcriptomic-guided strategy used in identification of a wheat rust pathogen target and modification of the target enhanced host resistance to rust pathogens. Front Plant Sci. 2022;13:962973.
- Qin D, Liu G, Liu R, Wang C, Xu F, Xu Q, et al. Positional cloning identified HvTUBULIN8 as the candidate gene for round lateral spikelet (RLS) in barley (Hordeum vulgare L.). Theor Appl Genet. 2023 Jan;136(1):7. [CrossRef]
- Han X, Zhang D, Hao H, Luo Y, Zhu Z, Kuai B. Transcriptomic analysis of three differentially senescing maize (Zea mays L.) inbred lines upon heat stress. International Journal of Molecular Sciences. 2023 Jan;24(12):9782. [CrossRef]
- Cheuk A, Houde M. A rapid and efficient method for uniform gene expression using the barley stripe mosaic virus. Plant Methods. 2017;13:24. [CrossRef]
- Pacak A, Geisler K, Jørgensen B, Barciszewska-Pacak M, Nilsson L, Nielsen TH, et al. Investigations of barley stripe mosaic virus as a gene silencing vector in barley roots and in Brachypodium distachyon and oat. Plant Methods. 2010 Nov 30;6:26. [CrossRef]
- Heap, I. Global perspective of herbicide-resistant weeds. Pest Manag Sci. 2014 Sep;70(9):1306–15. [CrossRef]
- Dyer, WE. Stress-induced evolution of herbicide resistance and related pleiotropic effects. Pest Manag Sci. 2018 Aug;74(8):1759–68. [CrossRef]
- Beffa R, Menne H, Köcher H. Herbicide Resistance Action Committee (HRAC): Herbicide classification, resistance evolution, survey, and resistance mitigation activities. In: Modern Crop Protection Compounds [Internet]. John Wiley & Sons, Ltd; 2019 [cited 2024 Jul 15]. p. 5–32.
- Keith BK, Lehnhoff EA, Burns EE, Menalled FD, Dyer WE. Characterisation of Avena fatua populations with resistance to multiple herbicides. Marshall J, editor. Weed Research. 2015 Dec;55(6):621–30. [CrossRef]
- Lehnhoff EA, Keith BK, Dyer WE, Peterson RK, Menalled F. Multiple herbicide resistance in wild oat and impacts on physiology, germinability, and seed production. Agronomy Journal. 2013 May;105(3):854–62. [CrossRef]
- Keith BK, Burns EE, Bothner B, Carey CC, Mazurie AJ, Hilmer JK, et al. Intensive herbicide use has selected for constitutively elevated levels of stress-responsive mRNAs and proteins in multiple herbicide-resistant Avena fatua L. Pest Manag Sci. 2017 Nov;73(11):2267–81. [CrossRef]
- Burns EE, Keith BK, Refai MY, Bothner B, Dyer WE. Constitutive redox and phosphoproteome changes in multiple herbicide resistant Avena fatua L. are similar to those of systemic acquired resistance and systemic acquired acclimation. J Plant Physiol. 2018 Jan;220:105–14. [CrossRef]
- MacGregor, DR. What makes a weed a weed? How virus-mediated reverse genetics can help to explore the genetics of weediness. outlook pest man. 2020 Oct 1;31(5):224–9. [CrossRef]
- Mellado-Sánchez M, McDiarmid F, Cardoso V, Kanyuka K, MacGregor DR. Virus-mediated transient expression techniques enable gene function studies in black-grass. Plant Physiol. 2020 Jun;183(2):455–9. [CrossRef]
- Burns EE, Keith BK, Refai MY, Bothner B, Dyer WE. Proteomic and biochemical assays of glutathione-related proteins in susceptible and multiple herbicide resistant Avena fatua L. Pesticide Biochemistry and Physiology. 2017 Aug 1;140:69–78. [CrossRef]
- Naylor JM, Jana S. Genetic adaptation for seed dormancy in Avena fatua. Can J Bot. 1976 Feb 1;54(3–4):306–12. [CrossRef]
- Johnson RR, Cranston HJ, Chaverra ME, Dyer WE. Characterization of cDNA clones for differentially expressed genes in embryos of dormant and nondormant Avena fatua L. caryopses. Plant Mol Biol. 1995 Apr 1;28(1):113–22. [CrossRef]
- Huang F, Spangler JR, Huang AY. In vivo cloning of up to 16 kb plasmids in E. coli is as simple as PCR. PLoS One. 2017 Aug 24;12(8):e0183974. [CrossRef]
- Holzberg S, Brosio P, Gross C, Pogue GP. Barley stripe mosaic virus-induced gene silencing in a monocot plant. Plant J. 2002 May;30(3):315–27. [CrossRef]
- Wang X, Zhang H, Nyamesorto B, Luo Y, Mu X, Wang F, et al. A new mode of NPR1 action via an NB-ARC–NPR1 fusion protein negatively regulates the defence response in wheat to stem rust pathogen. New Phytologist [Internet]. 2020 Nov [cited 2022 Apr 29];228(3):959–72. [CrossRef]
- Wang X, Zhang H, Nyamesorto B, Luo Y, Mu X, Wang F, et al. A new mode of NPR1 action via an NB-ARC–NPR1 fusion protein negatively regulates the defence response in wheat to stem rust pathogen. New Phytologist. 2020;228(3):959–72. [CrossRef]
- Pond SLK, Murrell B, Poon AFY. Evolution of viral genomes - interplay between selection, recombination and other forces.
- Scofield SR, Huang L, Brandt AS, Gill BS. Development of a virus-induced gene-silencing system for hexaploid wheat and its use in functional analysis of the Lr21-mediated leaf rust resistance pathway. Plant Physiology. 2005 Aug 1;138(4):2165–73. [CrossRef]
- Bruun-Rasmussen M, Madsen CT, Jessing S, Albrechtsen M. Stability of barley stripe mosaic virus-induced gene silencing in barley. Mol Plant Microbe Interact. 2007 Nov;20(11):1323–31. [CrossRef]
- Scofield SR, Nelson RS. Resources for Virus-Induced Gene Silencing in the Grasses. Plant Physiology. 2009 Jan 6;149(1):152–7. [CrossRef]
- Scofield, SR. Loss of Foreign DNA inserts from barley stripe mosaic virus vectors - potential consequences for use in functional genomics studies. International Journal of Plant Biology. 2023 Dec;14(4):1100–4.
- Montgomery J, Morran S, MacGregor DR, McElroy JS, Neve P, Neto C, et al. Current status of community resources and priorities for weed genomics research. Genome Biol. 2024 May 27;25(1):139. [CrossRef]
- Cook J, Wright L, Fiedler J, Keith BK, Dyer WE. Quantitative trait locus analysis of multiple herbicide resistance in Avena fatua. submitted.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).