Submitted:
16 July 2024
Posted:
17 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
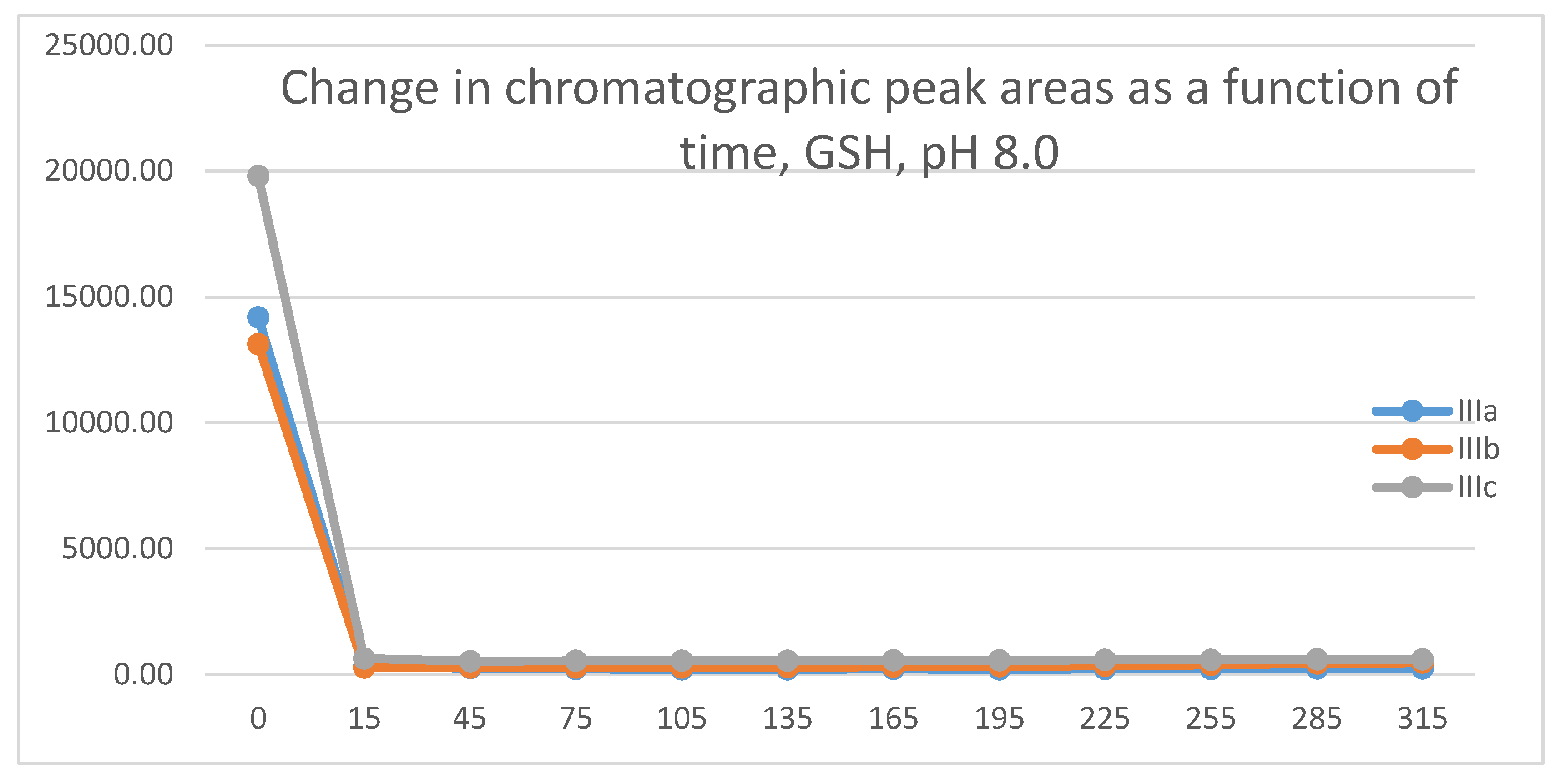
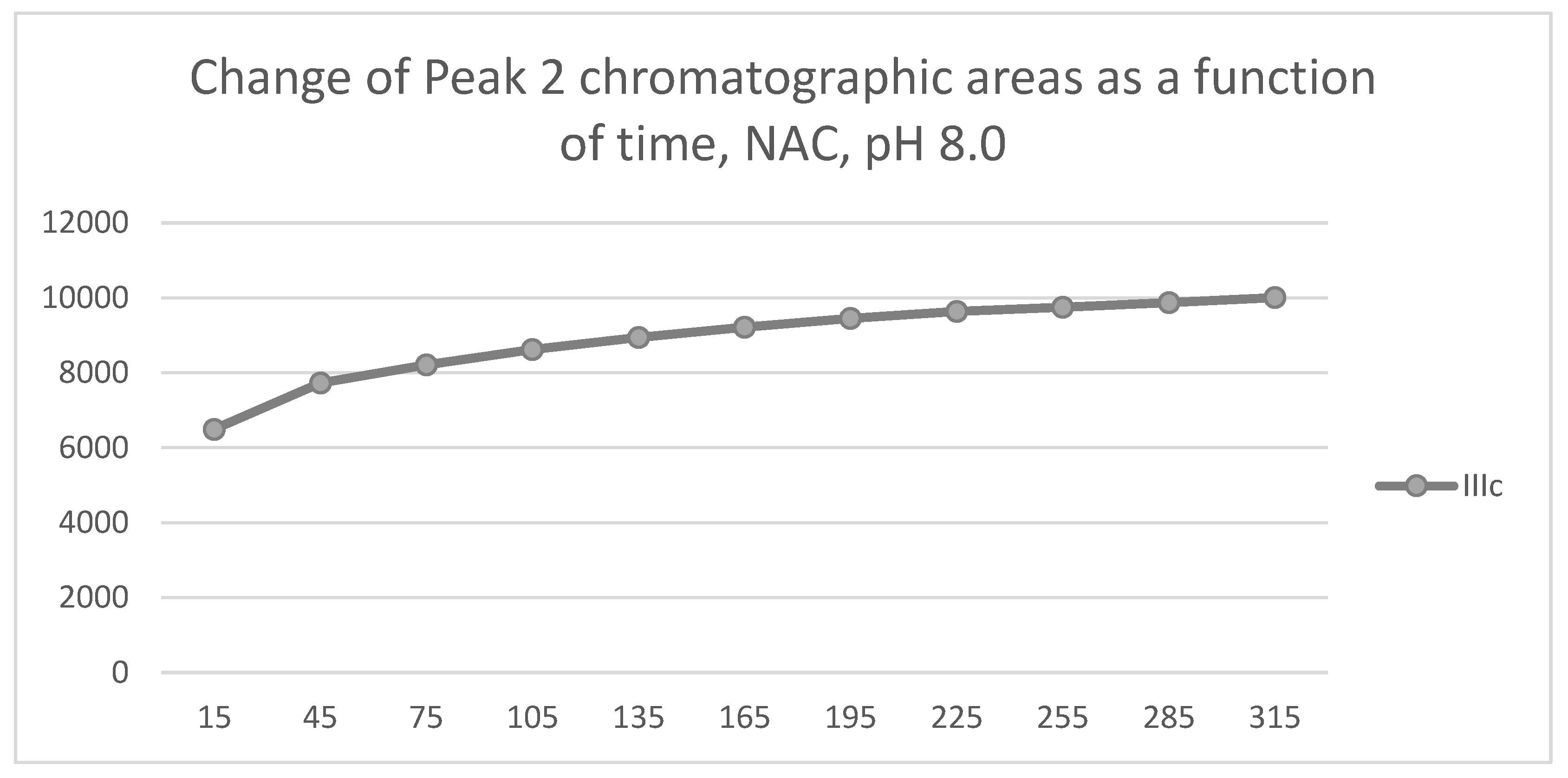
2.1. Reactions under Slightly Basic (pH 8.0) Conditions
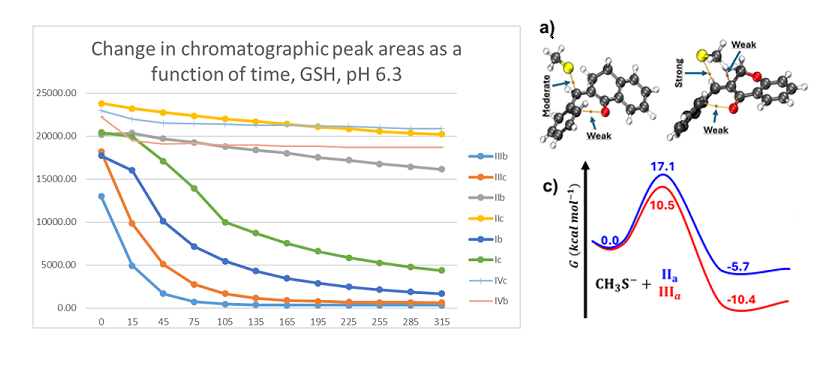
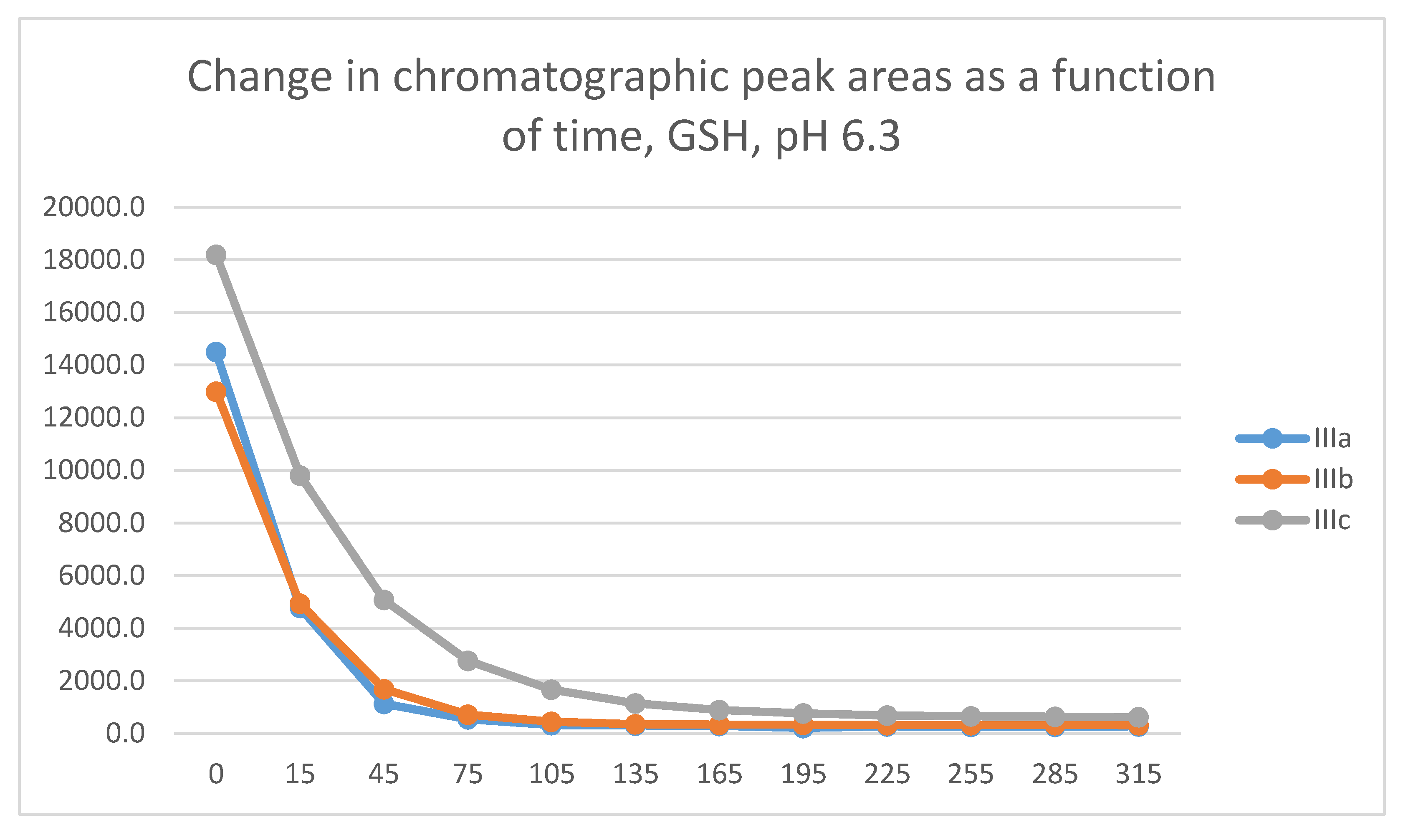
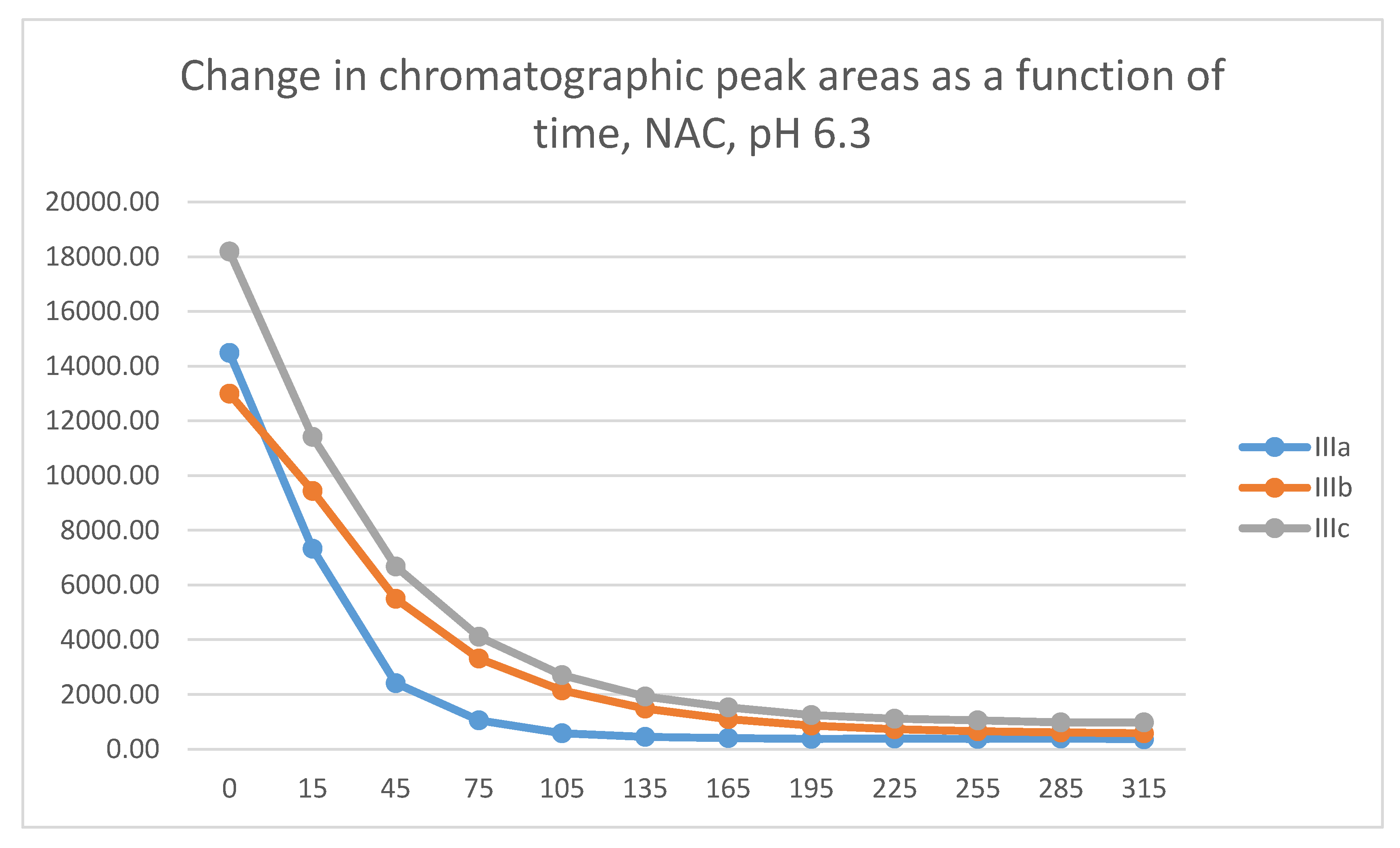
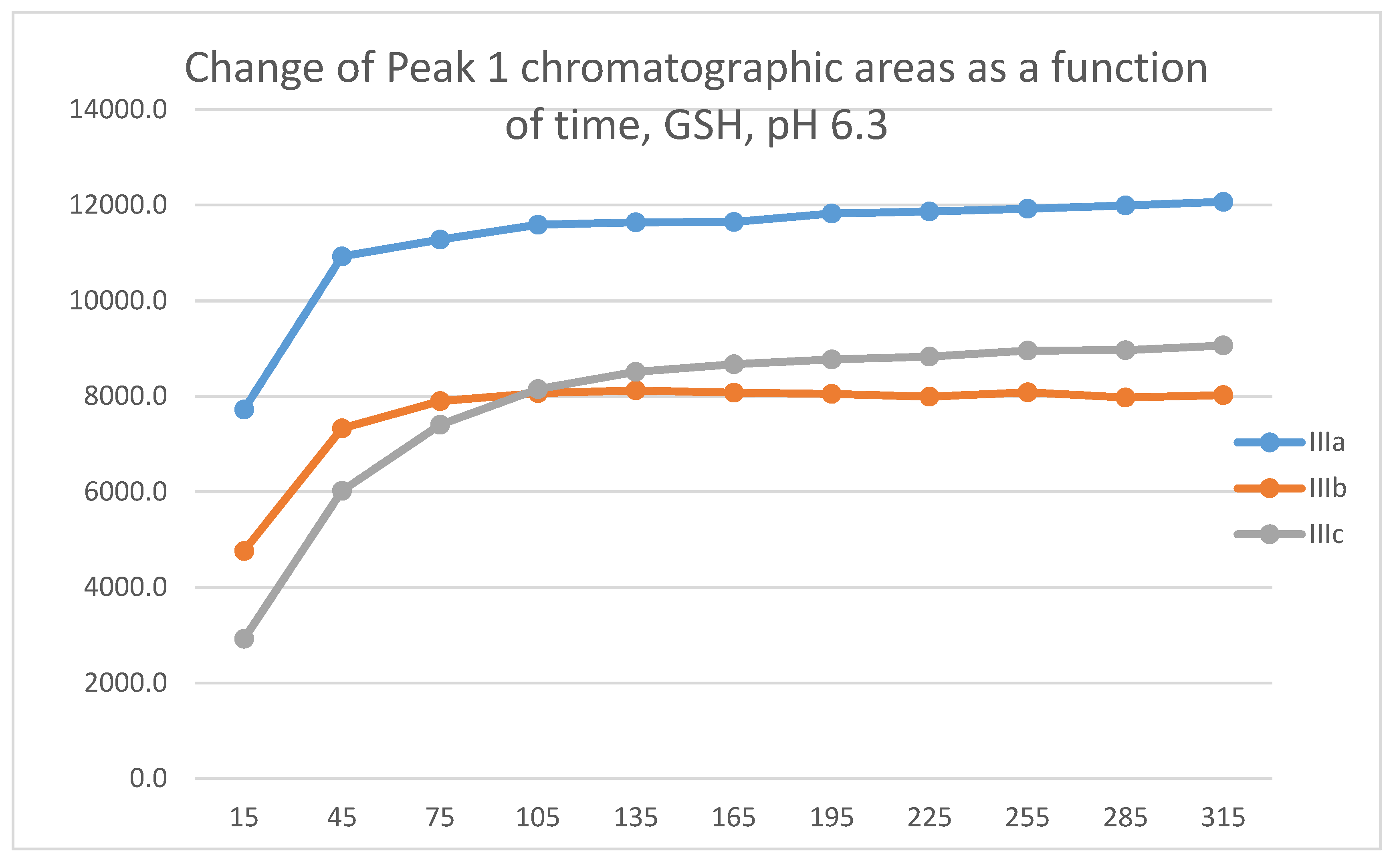
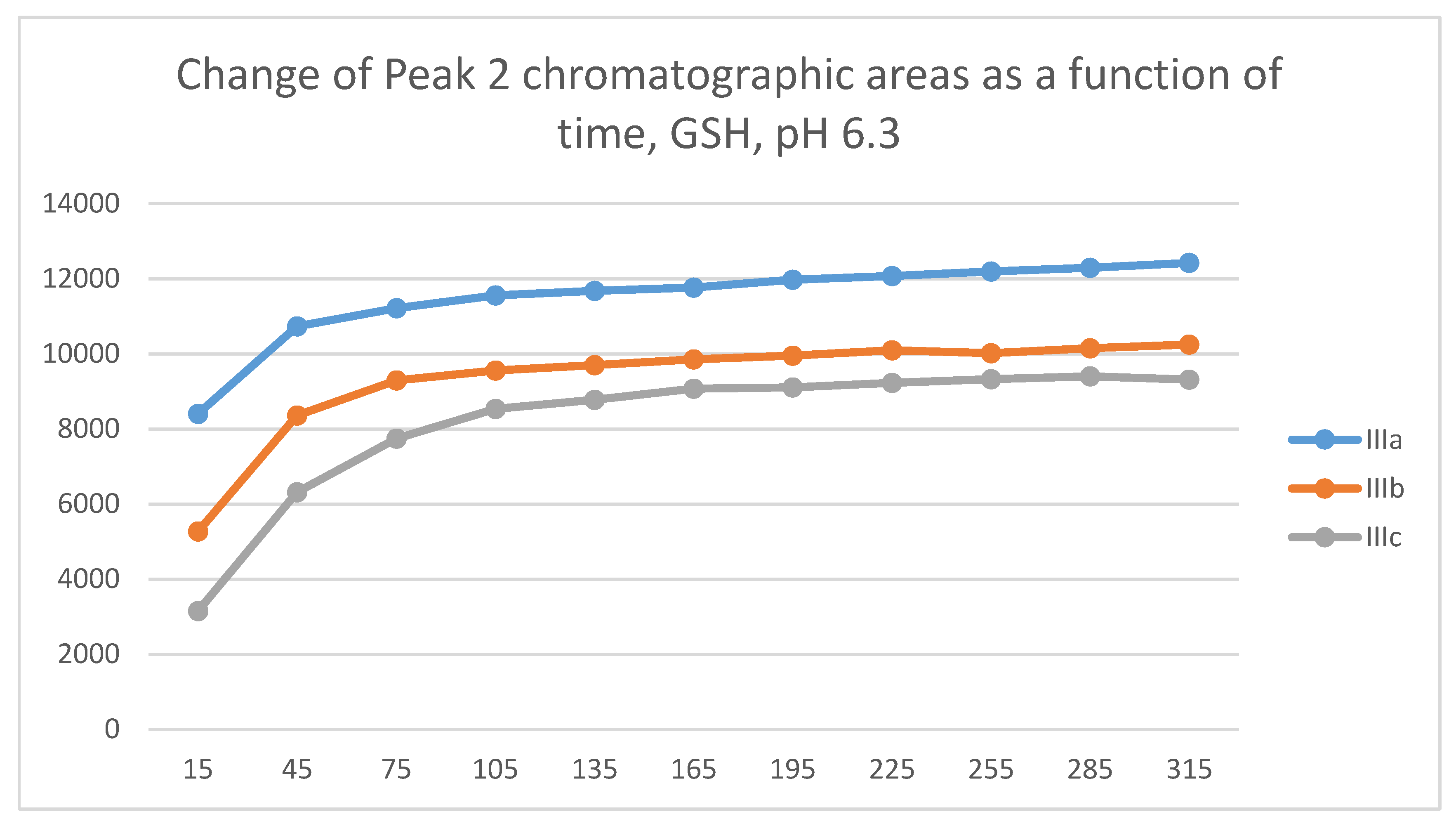
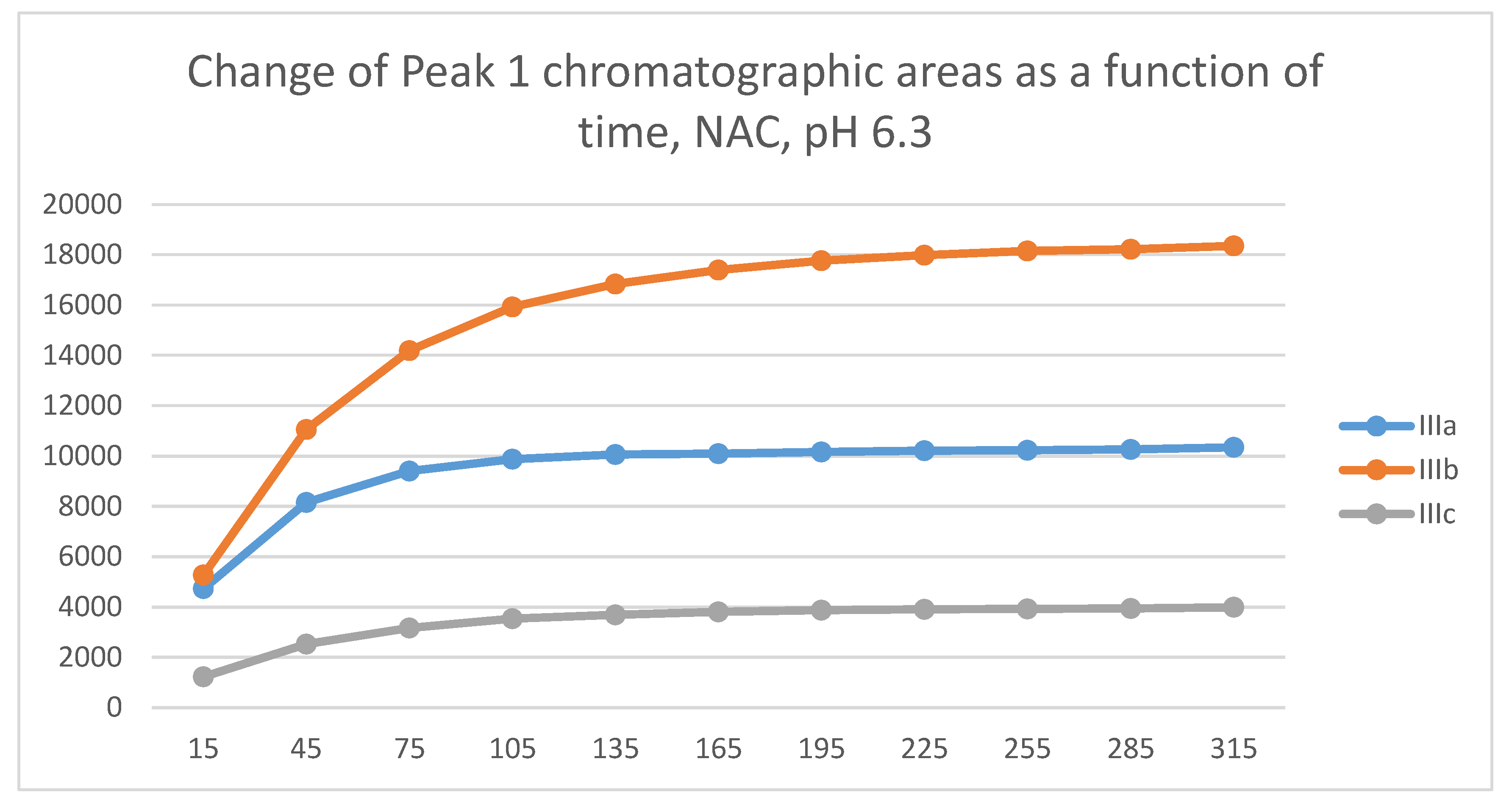
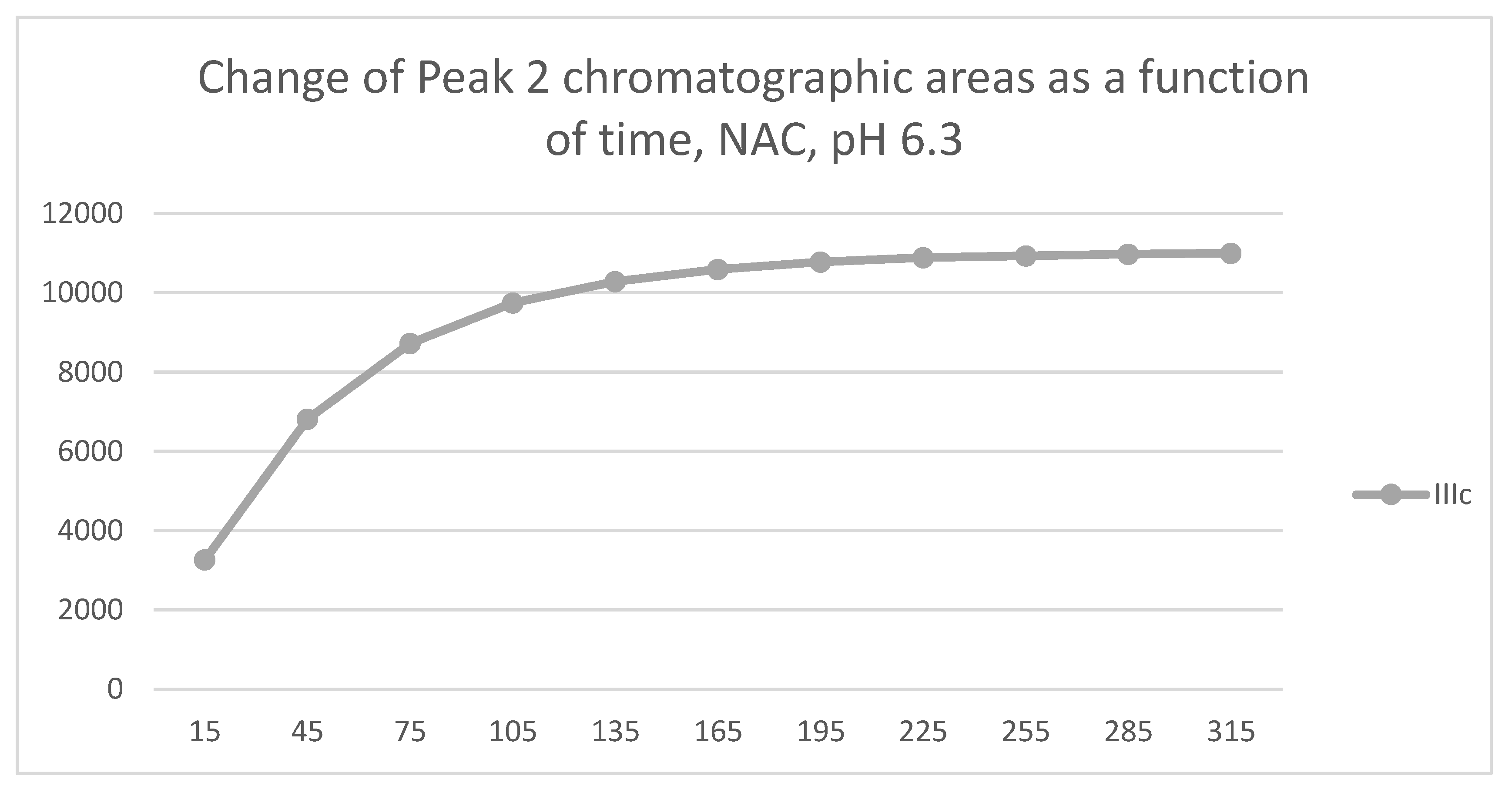
2.2. Reactions under Slightly Acidic (pH 6.3) Conditions
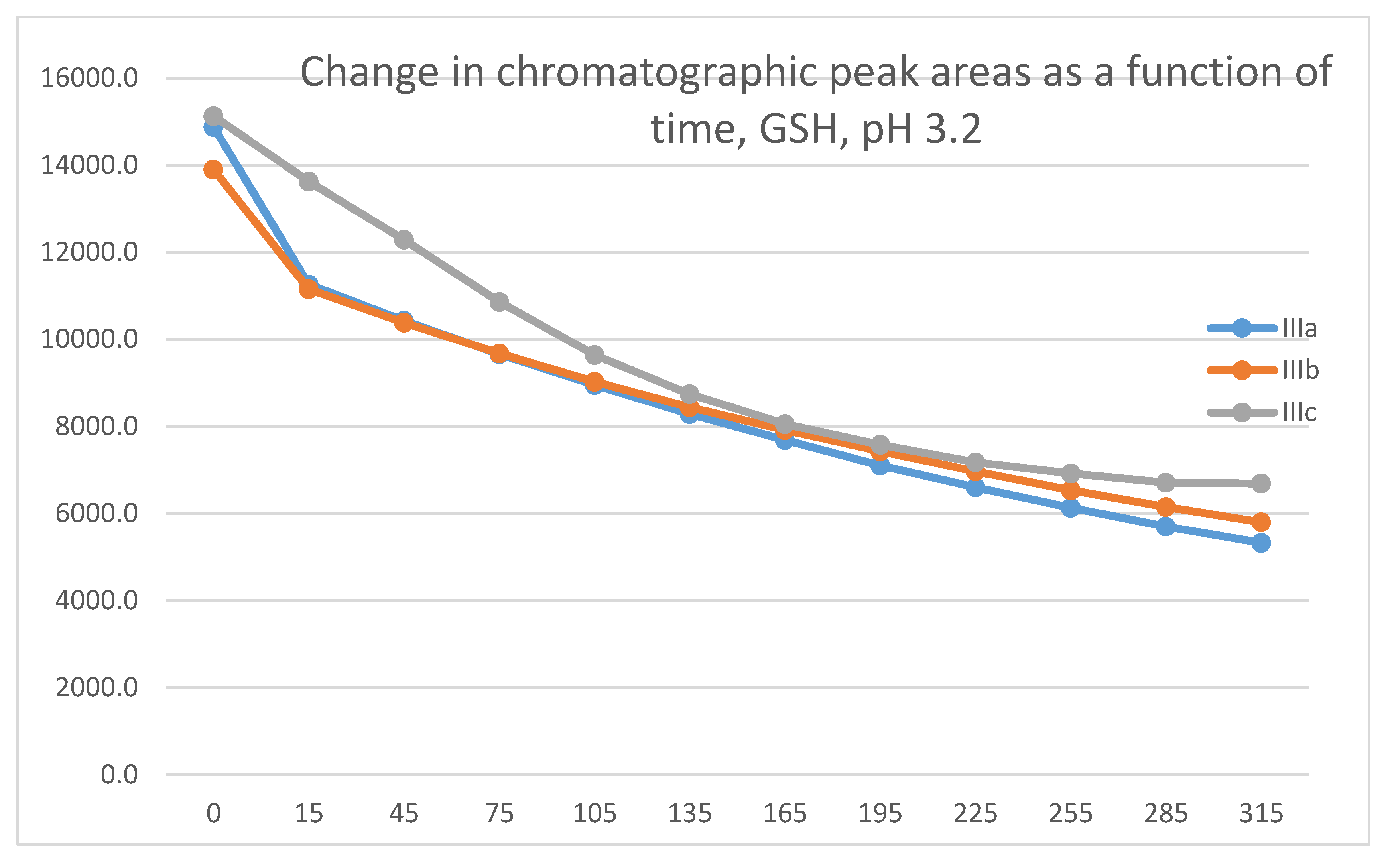
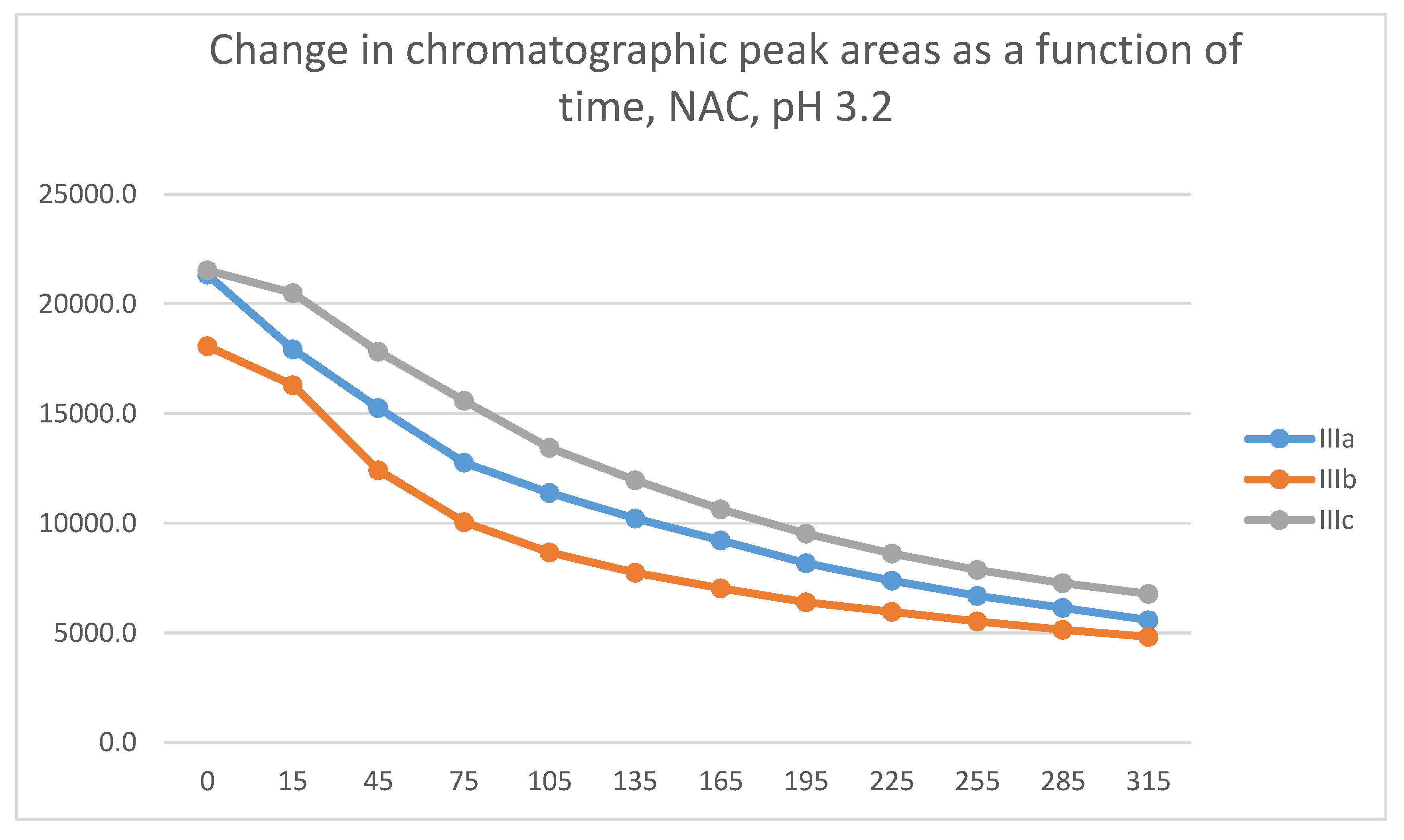
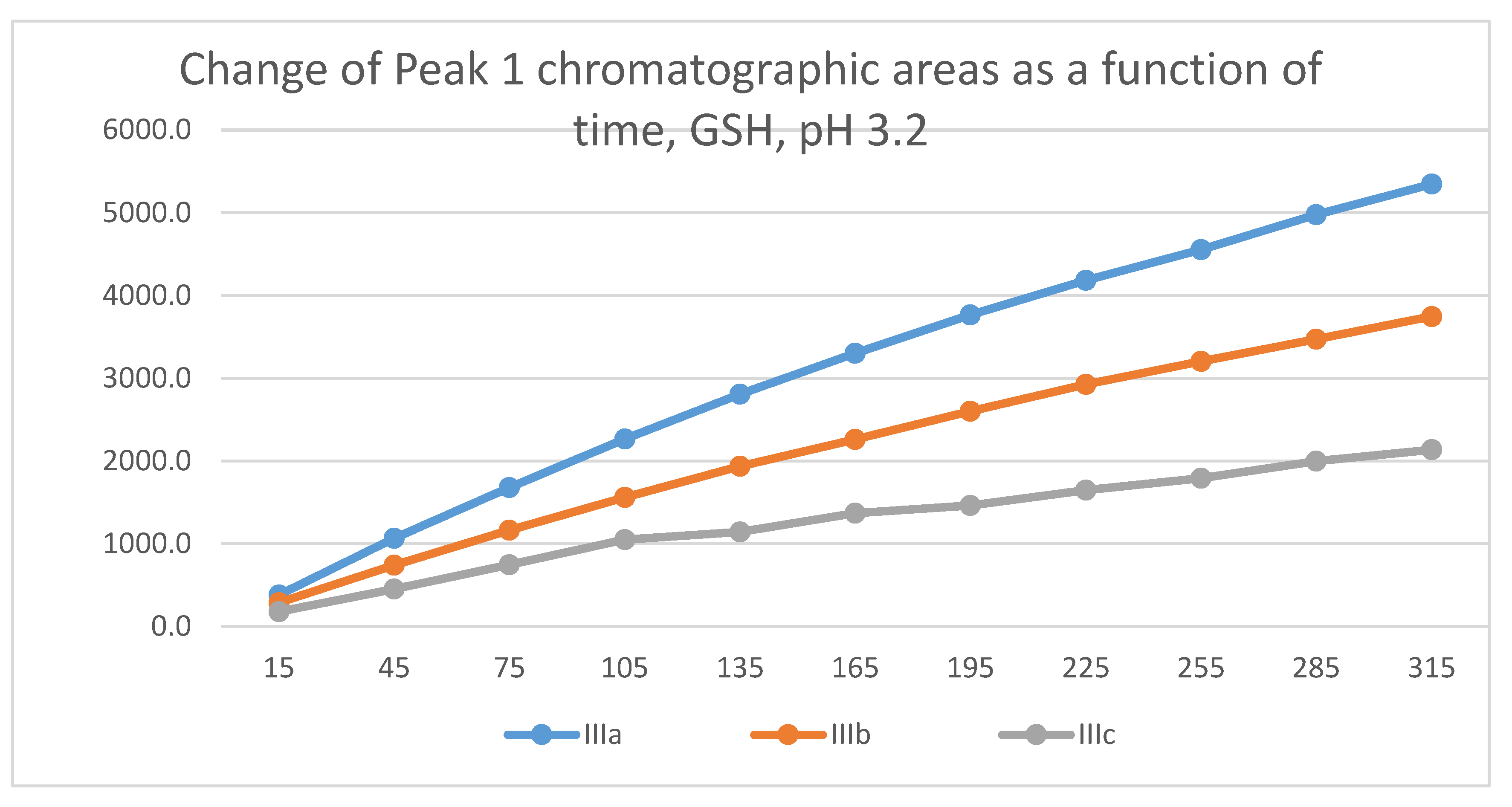
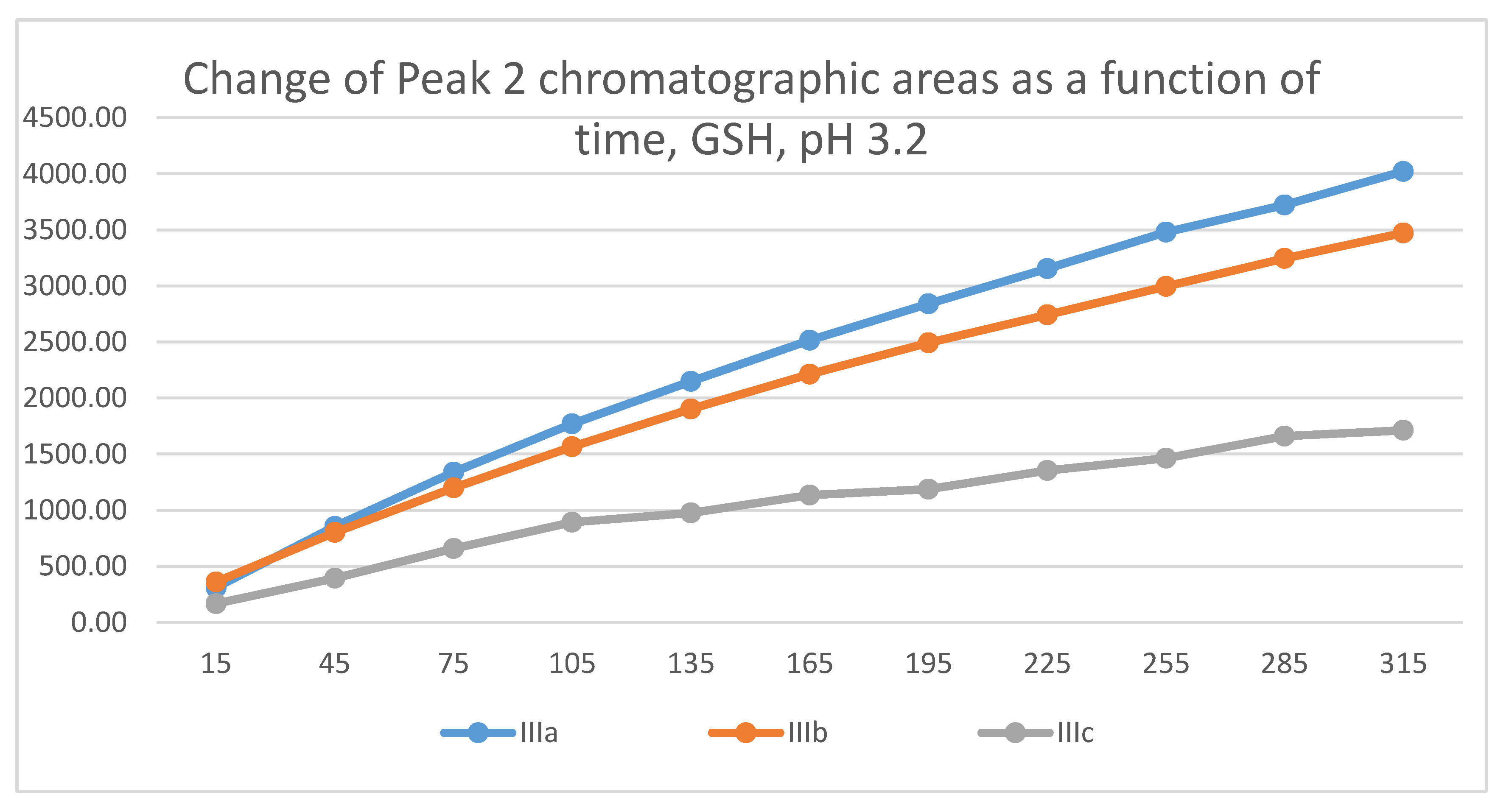
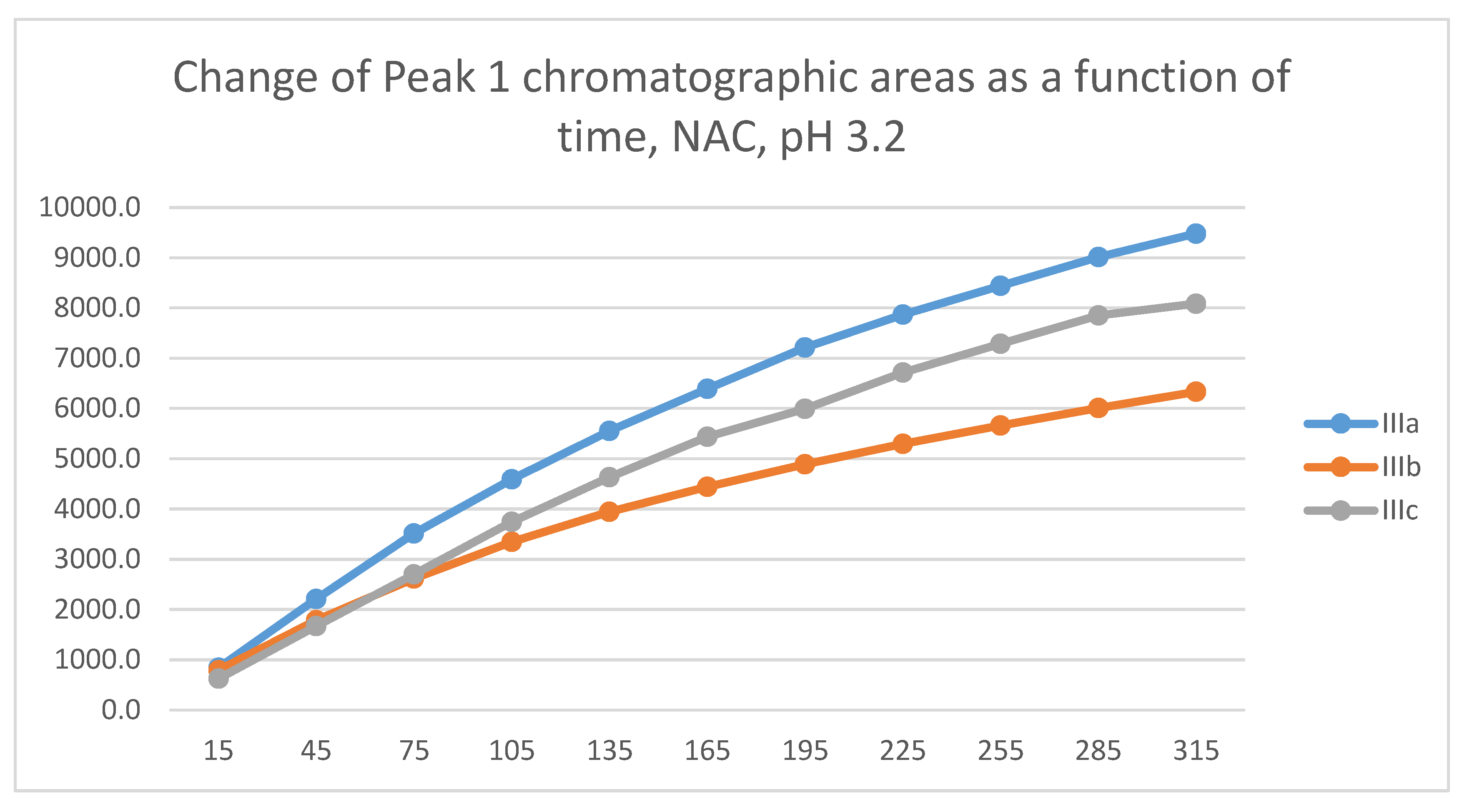
2.3. Reactions under Acidic (pH 3.2) Conditions
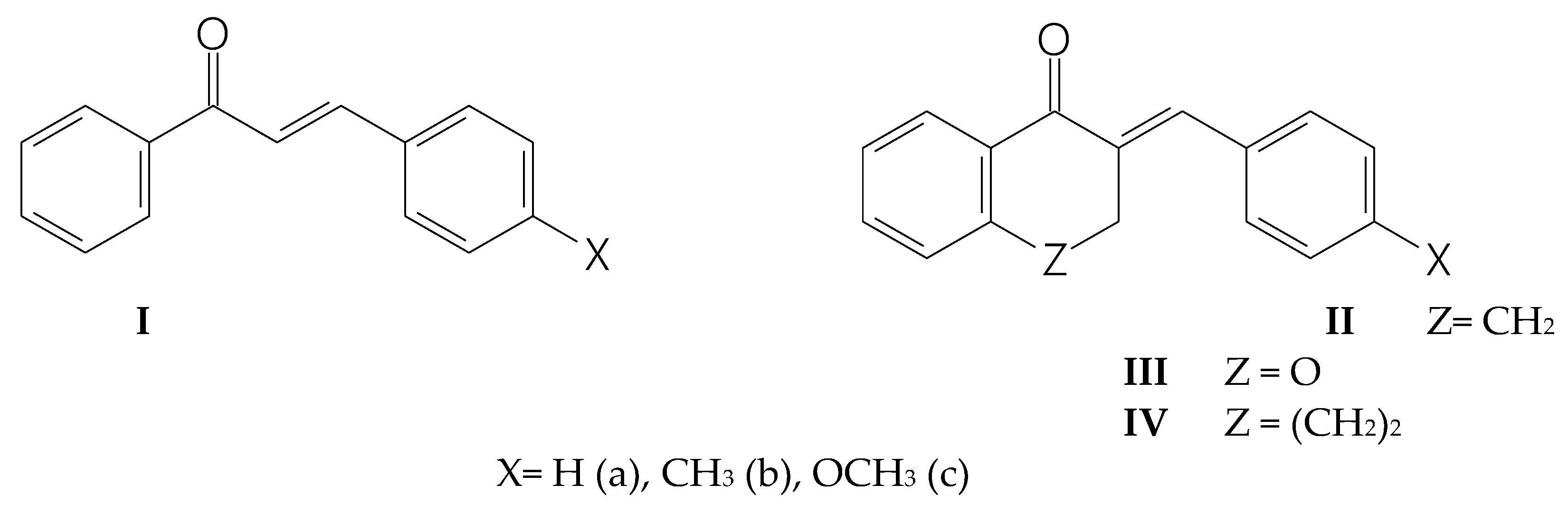
2.4. Molecular Modeling Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Solutions
4.3. RP-HPLC-UV Measurements
4.4. HPLC-MS Measurements
4.5. Molecular Modeling Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Part XX. Kenari, F.; Pintér, Z.; Molnár S.; Borges, I.D.; Camargo, A.J.; Napolitano, H.B.; Perjési, P. (E)-2-Benzylidenecyclanones: Part XIX. Reaction of (E)-2-(4’-X-benzylidene)-1-tetralones with cellular thiols. Comparison of thiol reactivities of open-chain chalcones and their six- and seven-membered cyclic analogs. Int. J. Mol. Sci. (under review).
- Rozmer, Z.; Perjési, P. Naturally occurring chalcones and their biological activities. Phytochem. Rev. 2016, 15, 87–120. [Google Scholar] [CrossRef]
- Banoth, R. K.; Thatikonda, A. A review on natural chalcones an update. Int. J. Pharm. Sci. Res. 2020, 11, 546–555. [Google Scholar]
- Constantinescu, T.; Mihis, A.G. Two important anticancer mechanisms of natural and synthetic chalcones. Int. J. Mol. Sci. 2022, 23, 11595. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, G.; Bhanu, D.; Aruchamy, B.; Ramani, P.; Pandurangan, N.; Bobba, K. N. Oh, E.J.; Chung, H. Y.; Gangadaran, P.; Ahn, B.-C. Chalcone: A promising bioactive scaffold in medicinal chemistry. Pharmaceuticals 2022, 15, 1250. [Google Scholar] [CrossRef] [PubMed]
- Leite, F. F.; de Sousa, N. F.; de Oliveira, B. H. M.; Duarte, G. D.; Ferreira, M. D. L.; Scotti, M. T.; Filho, J. M. B.; Rodrigues, L. C.; de Moura, R. O.; Mendonça-Jr, F. J. B..; Scotti, L. Anticancer activity of chalcones and its derivatives: Review and in silico studies. Molecules 2023, 28, 4009. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, M.A.; Rizk, S.A.; Fahim, A.M. Synthesis, reactions and application of chalcones: a systematic review. Org. Biomol. Chem. 2023, 21, 5317–5346. [Google Scholar] [CrossRef] [PubMed]
- Dimmock, J.R.; Kandepu, N.M.; Nazarali, A.J.; Kowalchuk, T.P.; Motaganahalli, N.; Quail, J.W.; Mykytiuk, P.A.; Audette, G.F.; Prasad, L.; Perjési, P.; et al. Conformational and quantitative structure−activity relationship study of cytotoxic 2-arylidenebenzocycloalkanones. J. Med. Chem. 1999, 42, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Dimmock, J.R.; Zello, G.A.; Oloo, E.O.; Quail, J.W.; Kraatz, H.-B.; Perjési, P.; Aradi, F.; Takács-Novák, K.; Allen, T.M.; Santos, C.L.; et al. Correlations between cytotoxicity and topography of some 2-arylidenebenzocycloalkanones determined by X-ray crystallography. J. Med. Chem. 2002, 45, 3103–3111. [Google Scholar] [CrossRef]
- Perjési, P.; Das, U.; De Clercq, E.; Balzarini, J.; Kawase, M.; Sakagami, H.; Stables, J.P.; Loránd, T.; Rozmer, Z.; Dimmock, J.R. Design, synthesis and antiproliferative activity of some 3-benzylidene-2,3-dihydro-1-benzopyran-4-ones which display selective toxicity for malignant cells. Eur. J. Med. Chem. 2008, 43, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Folmer, F.; Blasius, R.; Morceau, F.; Tabudravu, J.; Dicato, M.; Jaspars, M.; Diederich, M. Inhibition of TNFα-induced activation of nuclear factor ΚB by Kava (Piper Methysticum) derivatives. Biochem. Pharmacol. 2006, 71, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Laphanuwat, P.; Kongpetch, S.; Senggunprai, L.; Prawan, A.; Kukongviriyapan, V. Licochalcone A induces cholangiocarcinoma cell death via suppression of Nrf2 and NF-ΚB signaling pathways. Asian Pac. J. Cancer Prev. 2022, 23, 115–123. [Google Scholar] [CrossRef]
- de Freitas Silva, M.; Pruccoli, L.; Morroni, F.; Sita, G.; Seghetti, F.; Viegas, C.; Tarozzi, A. The Keap1/Nrf2-ARE pathway as a pharmacological target for chalcones. Molecules 2018, 23, 1803. [Google Scholar] [CrossRef] [PubMed]
- Egbujor, M.C.; Saha, S.; Buttari, B.; Profumo, E.; Saso, L. Activation of Nrf2 signaling pathway by natural and synthetic chalcones: A therapeutic road map for oxidative stress. Expert Rev. Clin. Pharmacol. 2021, 14, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: a privileged structure in medicinal chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.; Muratov, E.; Pereira, M.; Peixoto, J.; Rosseto, L.; Cravo, P.; Andrade, C.; Neves, B. Chalcone derivatives: Promising starting points for drug design. Molecules 2017, 22, 1210. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, T.; Mihis, A.G. Two important anticancer mechanisms of natural and synthetic chalcones. Int. J. Mol. Sci. 2022, 23, 11595. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, M.A.; Rizk, S.A.; Fahim, A.M. Synthesis, reactions and application of chalcones: a systematic review. Org. Biomol. Chem. 2023, 21, 5317–5346. [Google Scholar] [CrossRef] [PubMed]
- Kozurkova, M.; Tomeckova, V. Interaction of Chalcone Derivatives with Important Biomacromolecules. In Chalcones and Their Synthetic Analogs; Nova Science Publishers, Inc, 2020; Chapter 4, 95-133.
- Kenari, F.; Molnár, S.; Perjési, P. Reaction of chalcones with cellular thiols. The effect of the 4-substitution of chalcones and protonation state of the thiols on the addition process. Diastereoselective thiol addition. Molecules 2021, 26, 4332. [Google Scholar] [CrossRef] [PubMed]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef] [PubMed]
- [22].Armstrong, R. N. Structure, catalytic mechanism, and evolution of the glutathione transferases. Chem. Res. Toxicol. 1997, 10, 2-18.
- Kupcewicz, B.; Balcerowska-Czerniak, G.; Malecka, M.; Paneth, P.; Krajewska, U.; Rozalski, M. Bioorg. Med. Chem. Lett. 2013, 23, 4102–4106. [CrossRef] [PubMed]
- Rohani, N.; Hao, L.; Alexis, M.; Joughin, B.; Krismer, K.; Moufarrej, M.; Soltis, A.; Lauffenburger, D.; Yaffe, M.B.; Burge, C.B..; Bhatia, S.N.; Gertler, F.B. Acidification of tumor at stromal boundaries drives transcriptome alterations associated with aggressive phenotypes. Cancer Res. 2019, 79, 1952–1966. [Google Scholar] [CrossRef] [PubMed]
- Kenari, F.; Molnár, S.; Borges, I.D.; Napolitano, H.B.; Perjési, P. (E)-2-Benzylidenecyclanones: Part XVIII. Study the possible link between glutathione reactivity and cancer cell cytotoxic effects of some cyclic chalcone analogs a comparison of the reactivity of the open-chain and the seven-membered homologs. Int. J. Mol. Sci, 2023; 24, 8557. [Google Scholar]
- LoPachin, R.M.; Gavin, T.; DeCaprio, A.; Barber, D.S. Application of the hard and soft, acids and bases (HSAB) theory to toxicant–target interactions. Chem. Res. Toxicol. 2012, 25, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Perjési, P.; Linnanto, J.; Kolehmainen, E.; Ősz, E.; Virtanen E., E. E-2-Benzylidenebenzocycloalkanones. IV. Studies on transmission of substituent effects on 13C NMR chemical shifts of E-2-(X-benzylidene)-1-tetralones, and -benzosuberones. Comparison with the 13C NMR data of chalcones and E-2-(X-benzylidene)-1-indanones. J. Mol. Struct. 2005, 740, 81–89. [Google Scholar] [CrossRef]
- Xue-Ming, C.; Zhi-Tang, H.; Qi-Yu, Z. Topochemical photodimerization of (E)-3-benzylidene-4-chromanone derivatives from b-type structures directed by halogen groups. Tetrahedron 2011, 67, 9093e9098. [Google Scholar]
- Valkonen, A.; Laihia, K.; Kolehmainen, E.; Kauppinen, R.; Perjési, P. Structural studies of seven homoisoflavonoids, six thiohomoisoflavonoids, and four structurally related compounds. Struct. Chem. [CrossRef]
- Chen, J.; Jiang, X.; Carroll, S. L.; Huang, J.; Wang, J. Theoretical and experimental investigation of thermodynamics and kinetics of thiol-Michael addition reactions: A case study of reversible fluorescent probes for glutathione imaging in single cells. Org. Letters 2015, 17, 5978–5981. [Google Scholar] [CrossRef]
- Kupcewicz, B.; Balcerowska-Czerniak, G.; Malecka, M.; Paneth, P.; Krajewska, U.; Rozalski, M. Bioorg. Med. Chem. Lett. 2013, 23, 4102–4106. [CrossRef] [PubMed]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian 16 Revision C. 01. 2016; Gaussian Inc. Wallingford CT 2016, 421. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, non-covalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Zhang, G.; Musgrave, C.B. Comparison of DFT methods for molecular orbital eigenvalue calculations. J. Phys. Chem. A. 2007, 111, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Weiner, P.K.; Langridge, R.; Blaney, J.M.; Schaefer, R.; Kollman, P.A. Electrostatic potential molecular surfaces. Proc. Natl. Acad. Sci. U.S.A. 1982, 79, 3754–3758. [Google Scholar] [CrossRef]
- Náray-Szabó, G.; Ferenczy, G.G. Molecular electrostatics. Chem. Rev. 1995, 95, 829–847. [Google Scholar] [CrossRef]
- Fukui, K. The role of frontier orbitals in chemical reactions (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1982, 21, 801–809. [Google Scholar] [CrossRef]
- Fukui, K. Role of frontier orbitals in chemical reactions. Science 1982, 218, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Sanches-Neto, F.; Coutinho, N.; Aquilanti, V.; Silva, W.; Carvalho-Silva, V. Mechanism and kinetics of the degradation of nitazoxanide and hydroxychloroquine drugs by hydroxyl radicals: theoretical approach to ecotoxicity. J. Braz. Chem. Soc. 2023, 34, 1119–1129. [Google Scholar] [CrossRef]
- Sanches-Neto, F.O.; Coutinho, N.D.; Palazzetti, F.; Carvalho-Silva, V.H. Temperature dependence of rate constants for the H(D) + CH4 reaction in gas and aqueous phase: Deformed transition-state theory study including quantum tunneling and diffusion effects. Struct. Chem. 2020, 31, 609–617. [Google Scholar] [CrossRef]
- Sanches-Neto, F.O.; Ramos, B.; Lastre-Acosta, A.M.; Teixeira, A.C.S.C.; Carvalho-Silva, V.H. Aqueous picloram degradation by hydroxyl radicals: Unveiling mechanism, kinetics, and ecotoxicity through experimental and theoretical approaches. Chemosphere 2021, 278, 130401. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W. Atoms in molecules. Acc. Chem. Res. 1985, 18, 9–15. [Google Scholar] [CrossRef]
- Matta, C.F.; Boyd, R. J. (2007). The Quantum Theory of Atoms in Molecules: From Solid State to DNA and Drug Design (C. F. Matta and R. J. Boyd, Eds.). John Wiley & Sons. 2007.
- de Almeida, L.R.; Carvalho, Jr., P.S.; Napolitano, H.B.; Oliveira, S.S.; Camargo, A.J.; Figueredo, A.S.; de Aquino, G.L.B.; Carvalho-Silva, V.H. Contribution of directional dihydrogen interactions in the supramolecular assembly of single crystals: Quantum chemical and structural investigation of C17H17N3O2 azine. Cryst. Growth Des. 2017, 17, 10. 5145–5153. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Laidler, K.J.; Eyring, H.; Glasstone, S.; Laidler, K.J.; Eyring, H. The Theory of Rate Processes: The Kinetics of Chemical Reactions, Viscosity, Diffusion and Electrochemical Phenomena (Inc. McGraw-Hill Book Company, Ed.). McGraw-Hill. 1941.
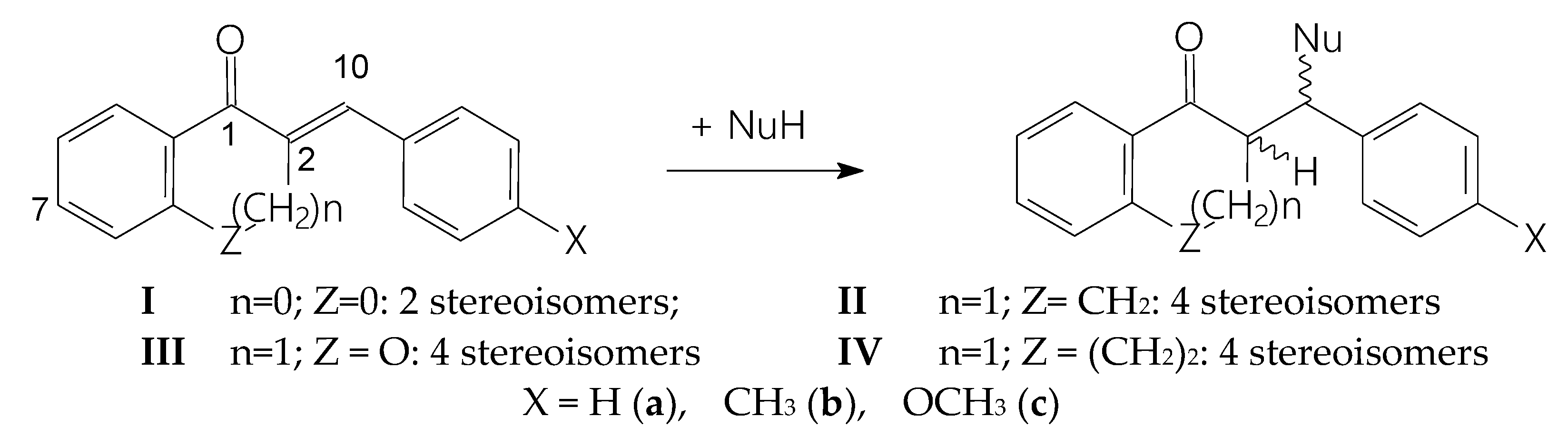
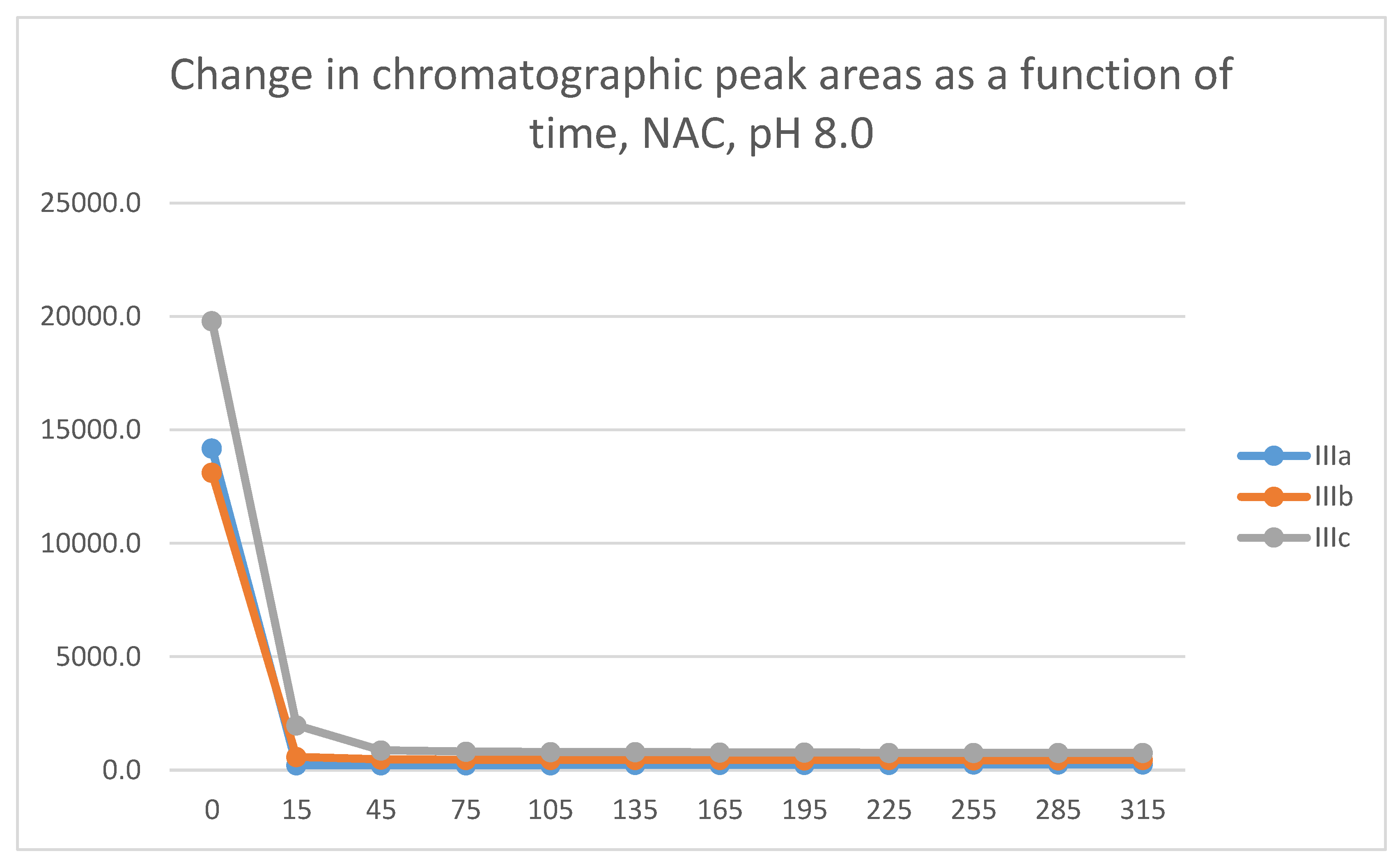
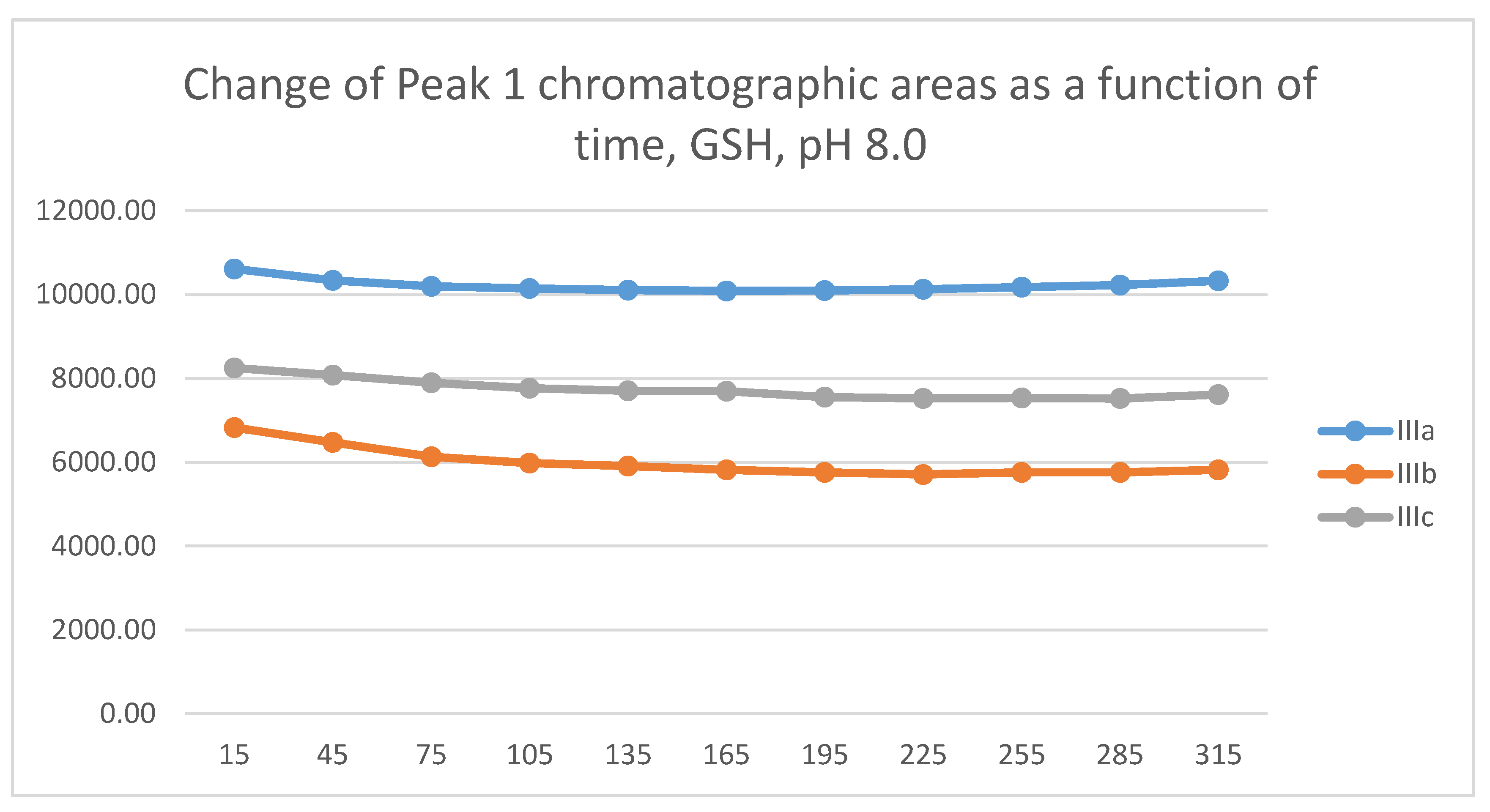
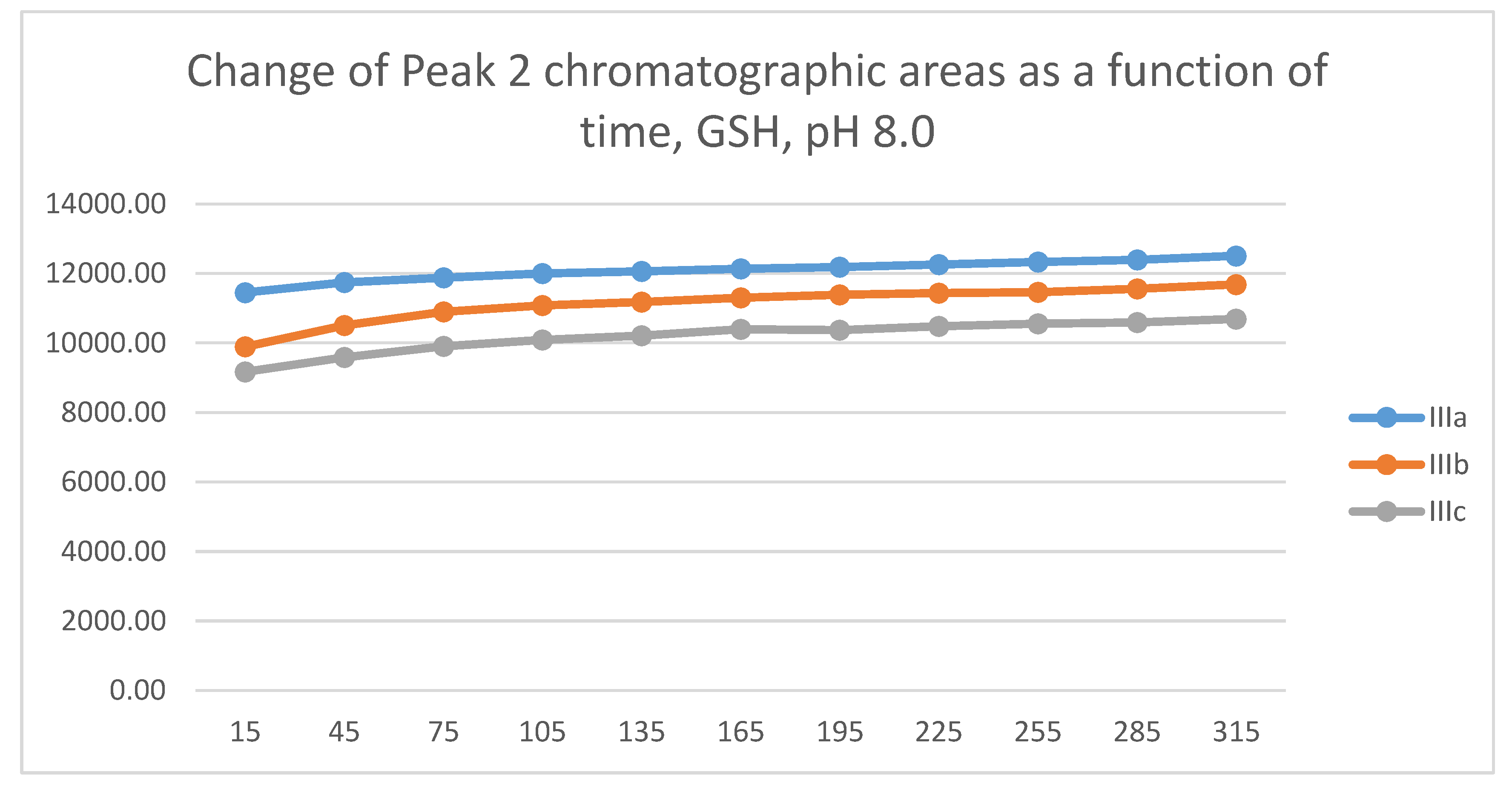
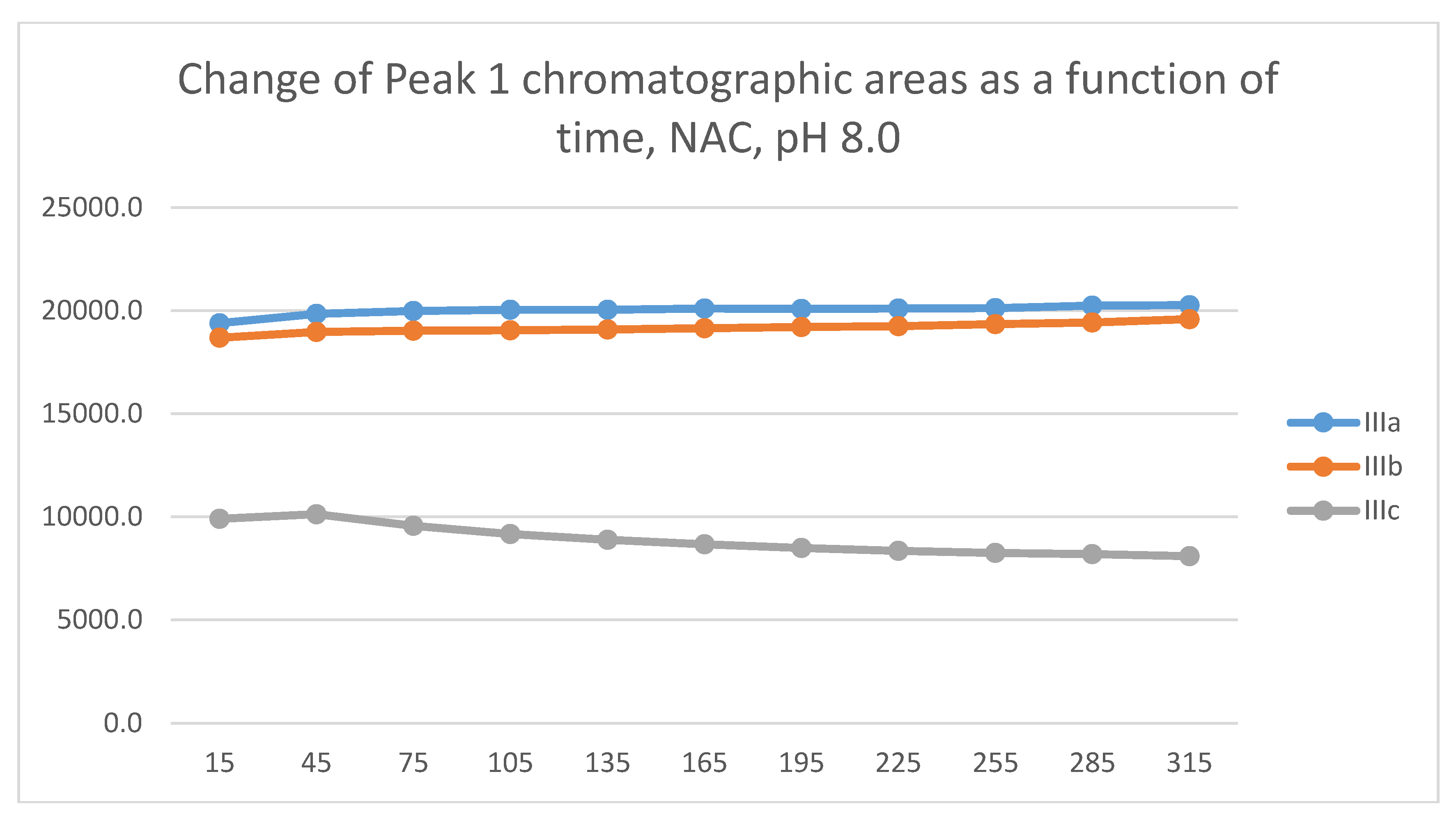
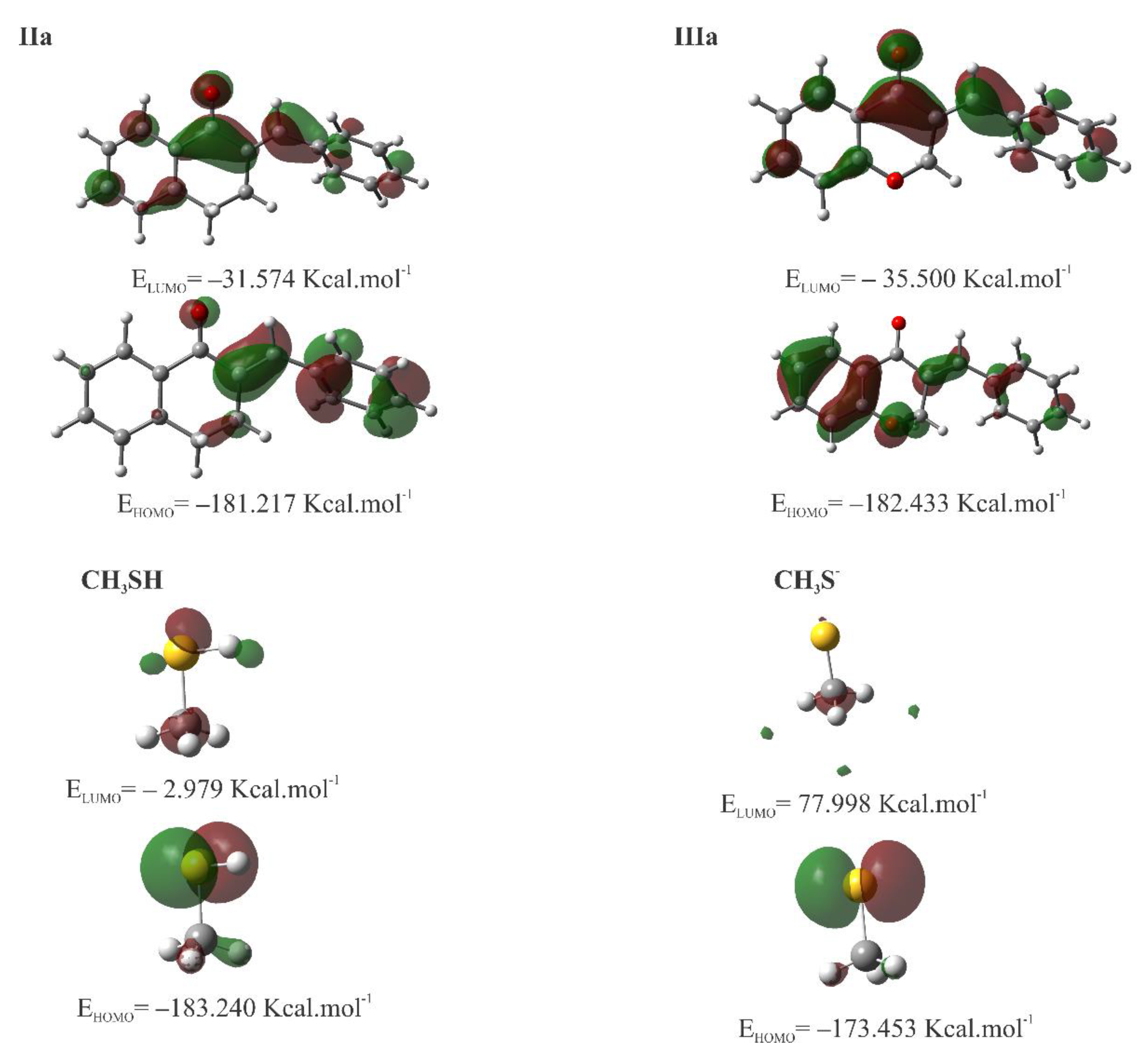
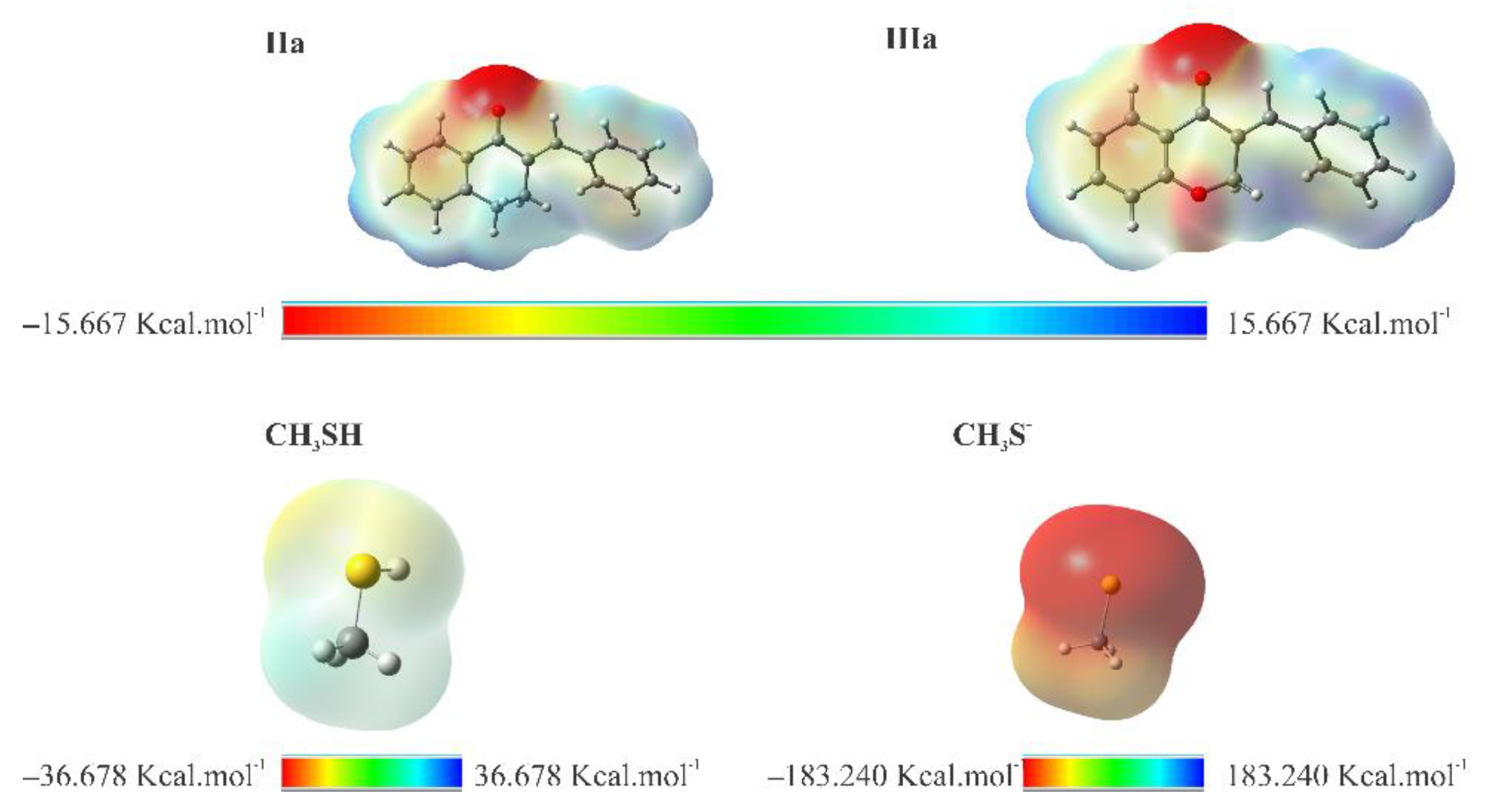
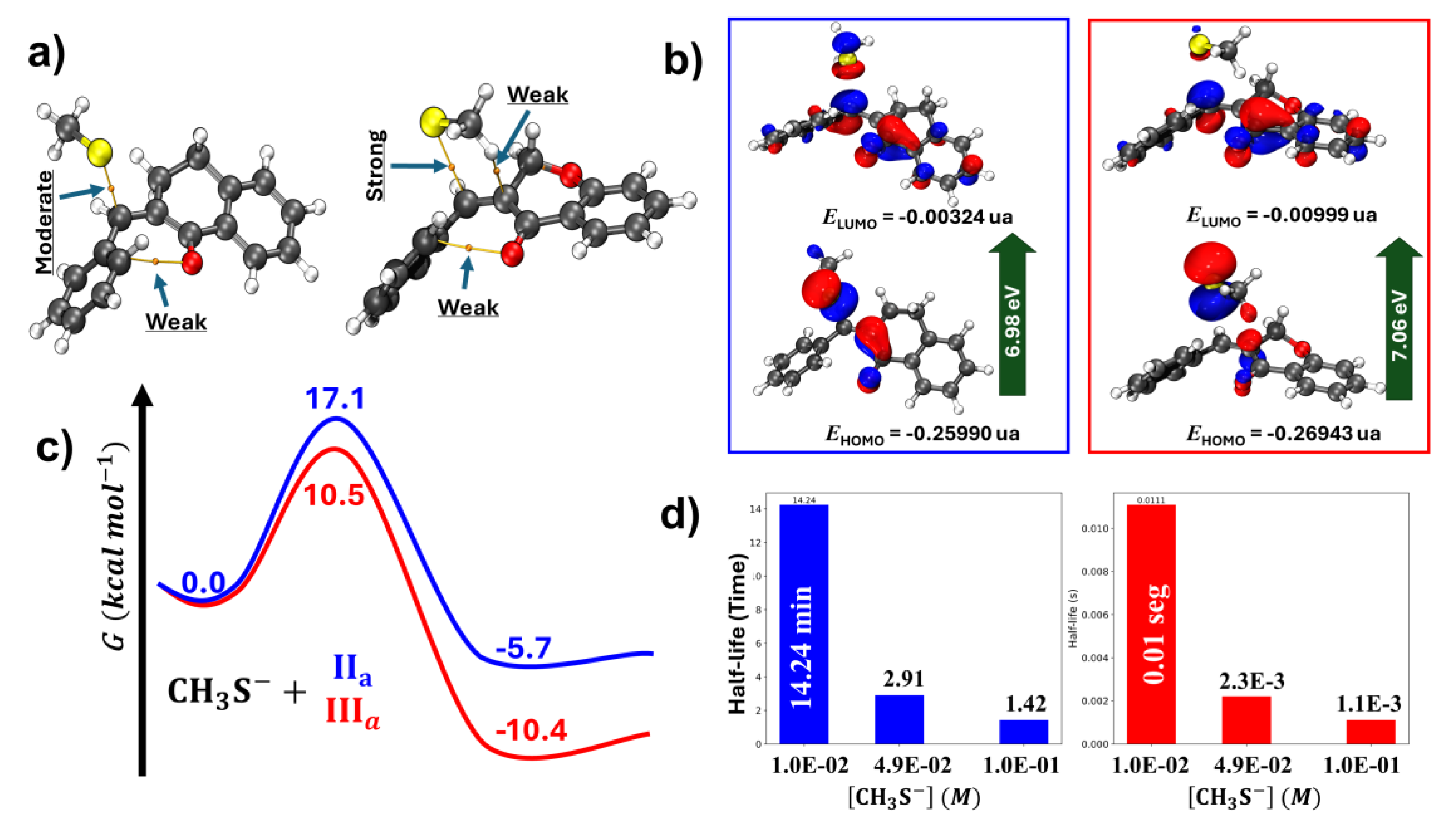
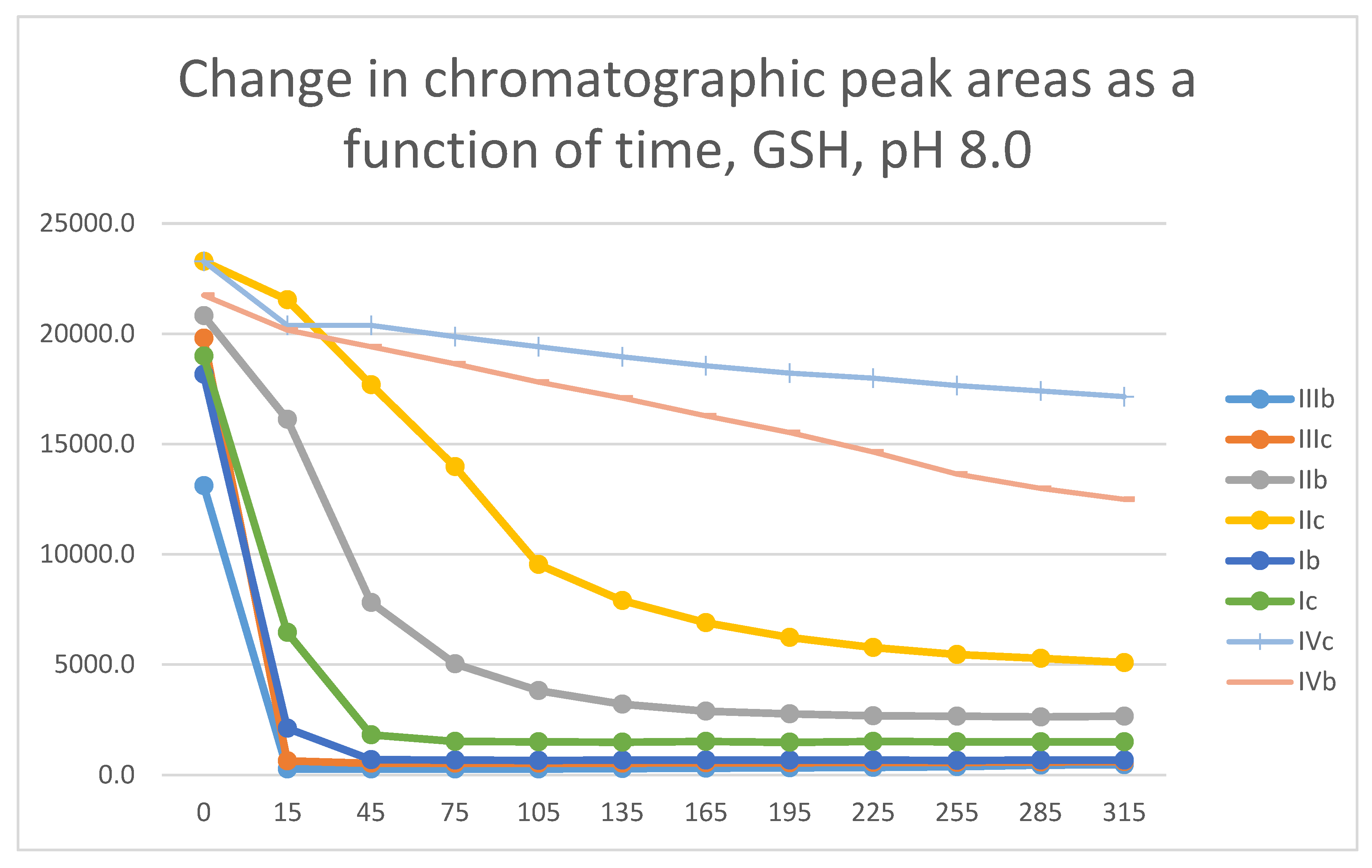
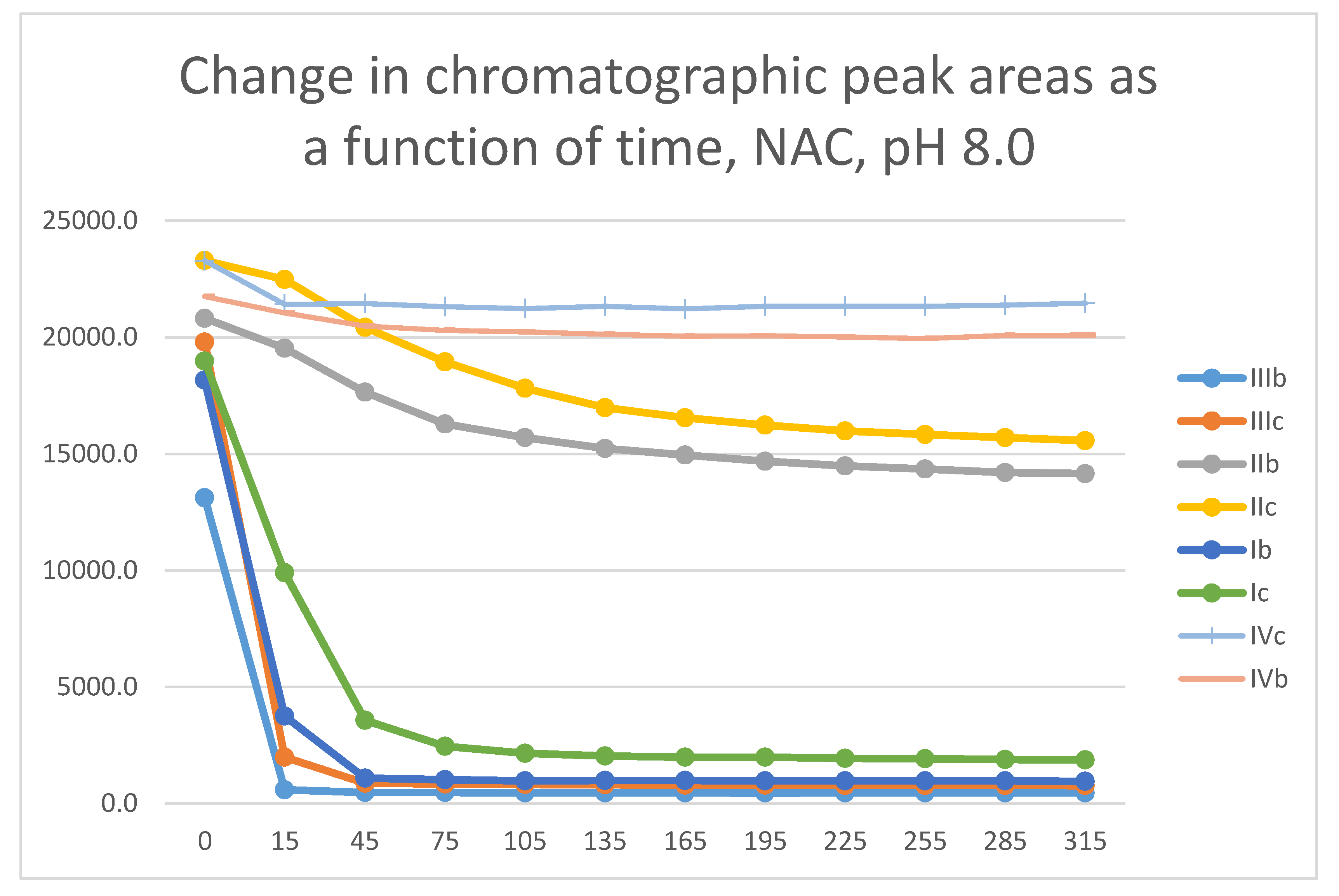
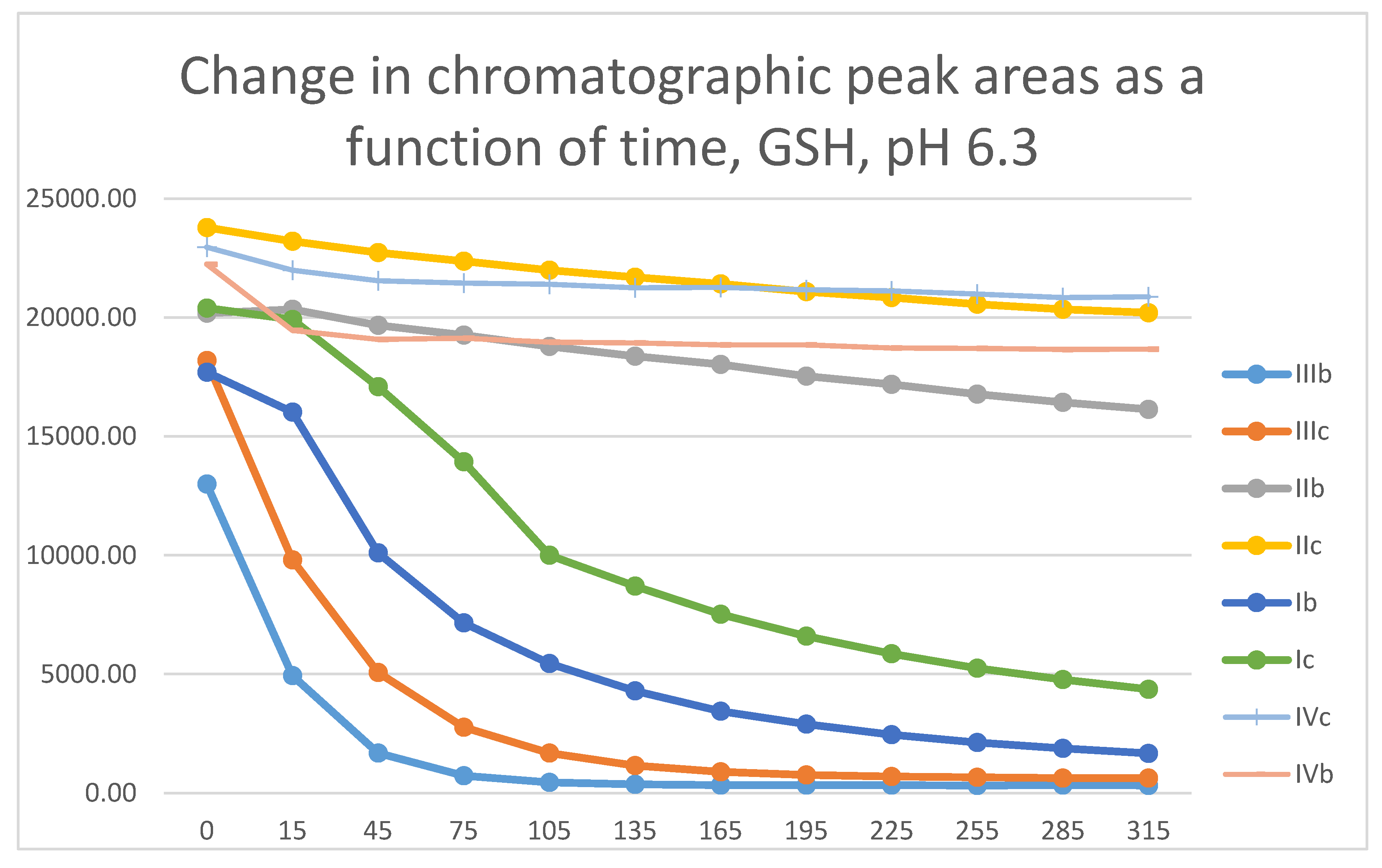
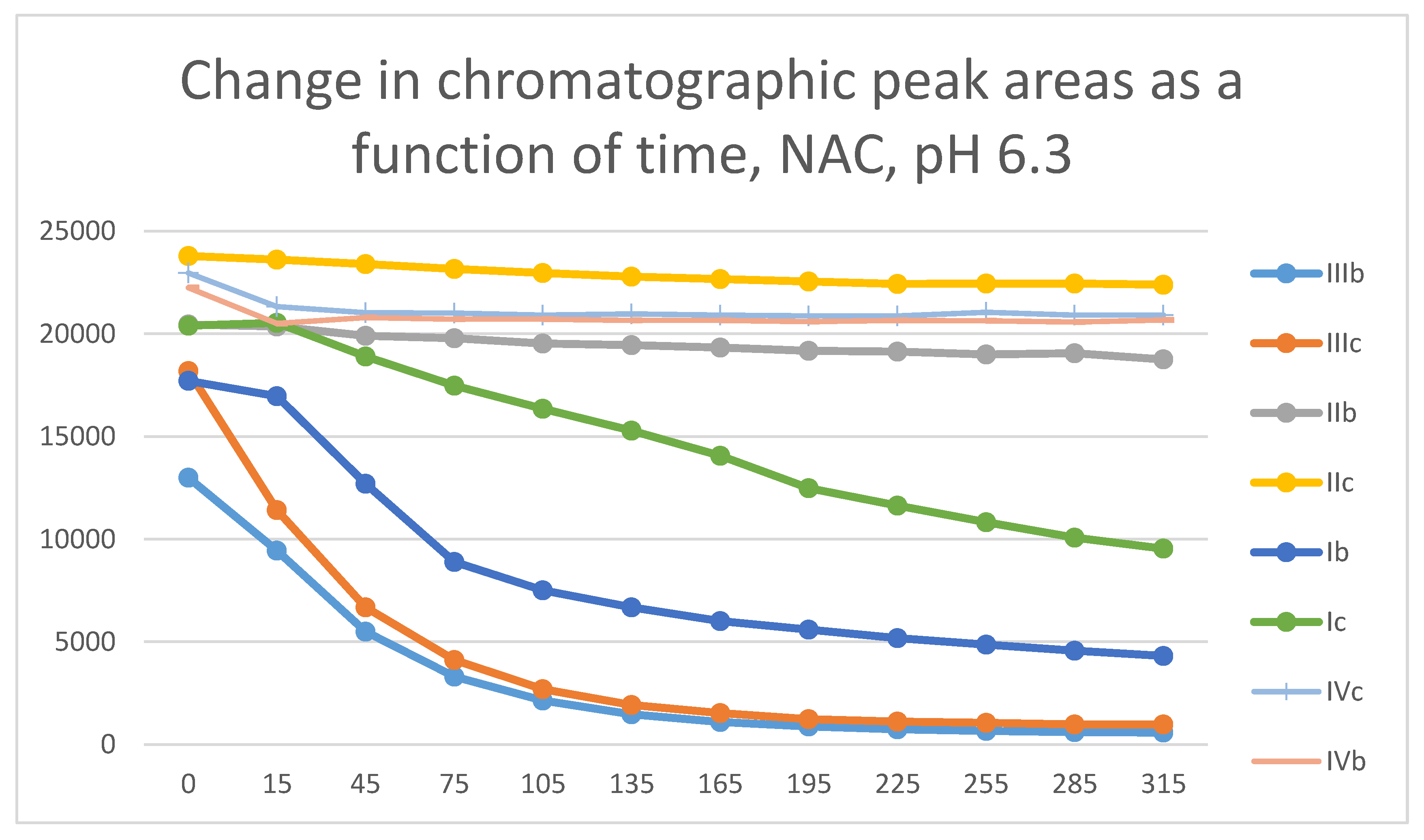
| pH3 | Com- pound |
tR (E)-isomer |
Area Ratio4 A315/A0 | tR (Z)-isomer |
tR2 GSH-1 |
Area GSH-1 |
tR2 GSH-2 |
Area GSH-2 |
|---|---|---|---|---|---|---|---|---|
| 3.2 | IIIa | 16.6 | 0.36 | N.D.5 | 12.9 | 5346 | 13.2 | 4021 |
| 3.2 | IIIb | 17.1 | 0.42 | N.D.5 | 14.6 | 3748 | 14.8 | 3472 |
| 3.2 | IIIc | 16.6 | 0.44 | N.D.5 | 12.9 | 2136 | 13.4 | 1712 |
| 6.3 | IIIa | 16.6 | 0.02 | N.D.5 | 12.3 | 12073 | 12.7 | 12421 |
| 6.3 | IIIb | 17.1 | 0.03 | N.D.5 | 14.5 | 8028 | 14.7 | 10248 |
| 6.3 | IIIc | 16.6 | 0.03 | N.D.5 | 13.0 | 9064 | 13.5 | 9315 |
| 8.0 | IIIa | 16.6 | 0.02 | N.D.5 | 12.3 | 10330 | 12.7 | 12509 |
| 8.0 | IIIb | 17.0 | 0.03 | N.D.5 | 14.4 | 5820 | 14.5 | 11685 |
| 8.0 | IIIc | 16.6 | 0.03 | N.D.5 | 13.2 | 7615 | 13.6 | 10692 |
| pH3 | Com- pound |
tR (E)-isomer |
Area Ratio4 A315/A0 |
tR2 (Z)-isomer |
tR NAC-1 |
Area2 NAC-1 |
tR NAC-2 |
Area2 NAC-2 |
|---|---|---|---|---|---|---|---|---|
| 3.2 | IIIa | 16.7 | 0.262 | N.D.5 | 15.1 | 9483 | N.D. | N.D. |
| 3.2 | IIIb | 17.2 | 0.267 | N.D.5 | 15.9 | 6333 | N.D. | N.D. |
| 3.2 | IIIc | 16.7 | 0.315 | N.D.5 | 15.2 | 8088 | N.D. | N.D. |
| 6.3 | IIIa | 16.6 | 0.025 | N.D.5 | 15.1 | 10347 | N.D. | N.D. |
| 6.3 | IIIb | 17.1 | 0.044 | N.D.5 | 15.9 | 18353 | N.D. | N.D. |
| 6.3 | IIIc | 16.6 | 0.054 | N.D.5 | 15.0 | 3987 | 15.2 | 11003 |
| 8.0 | IIIa | 16.6 | 0.017 | N.D.5 | 15.0 | 20271 | N.D. | N.D. |
| 8.0 | IIIb | 17.1 | 0.035 | N.D.5 | 15.9 | 19590 | N.D. | N.D. |
| 8.0 | IIIc | 16.7 | 0.038 | N.D.5 | 15.1 | 8099 | 15.2 | 10006 |
| Descriptor | Ia kcal.mol-1 |
IIa kcal.mol-1 |
IIIa kcal.mol-1 |
IVa kcal.mol-1 |
CH3SH kcal.mol-1 |
CH3S- kcal.mol-1 |
|---|---|---|---|---|---|---|
| EHOMO | -183.24 | -181.22 | -182.43 | -180.38 | -183.24 | -173.45 |
| ELUMO | -35.98 | -31.57 | -28.44 | -28.44 | -2.98 | 77.80 |
| ΔEHOMO-LUMO | 147.26 | 149.65 | 153.99 | 151.94 | 180.26 | 251.27 |
| Chemical Potential (μ) | -109.61 | -104.83 | -105.44 | -104.405 | -93.11 | 47.73 |
| Chemical Hardness (η) | 147.26 | 149.65 | 153.99 | 151.94 | -180.26 | -251.27 |
| Electrophilicity Index (ω) | 40.79 | 35.96 | 36.10 | 35.87 | -24.05 | 4.53 |
| IIa | IIIa | ||||||
|---|---|---|---|---|---|---|---|
| Atom | f- | f+ | Δf | Atom | f- | f+ | Δf |
| C1 | 0.011 | 0.098 | 0.087 | C4 | 0.004 | 0.093 | 0.089 |
| O(=C) | 0.053 | 0.114 | 0.060 | O(=C) | 0.039 | 0.111 | 0.072 |
| C7 | 0.029 | 0.060 | 0.032 | C3 | 0.013 | 0.038 | 0.025 |
| C10 | 0.062 | 0.082 | 0.020 | C3a | 0.043 | 0.086 | 0.043 |
| CompoundReference | δC1*(C4**) | δC2*(C3**) | δC10*(C3a**) | Δ(δ(C10/*C3a**)-δ(C2*/C3**)) |
|---|---|---|---|---|
| Ia27 | 190.5 | 122.1 | 144.8 | 22.78 |
| IIa27 | 187.9 | 135.4 | 136.6 | 1.2 |
| IIb27 | 187.9 | 134.7 | 136.8 | 2.1 |
| IIc27 | 187.8 | 133.5 | 136.6 | 3.1 |
| IIIa28 | 182.4 | 131.8 | 137.8 | 6.0 |
| IIIb29 | 182.2 | 130.1 | 137.5 | 7.4 |
| IIIc29 | 182.1 | 128.9 | 137.2 | 3.3 |
| IVa27 | 198.0 | 137.8 | 138.0 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





