1. Introduction
Rapid global population growth exerts pressure on agricultural production for food and feed [
1]. It is estimated that by 2050, food production needs to increase by 70% compared to 2009 and the demand for proteins of animal origin will have increased by 74% [
2]. The agricultural industry is currently using 37% of Earth’s land mass for food production. This causes deforestation and as a result it inflicts a loss of biodiversity and increased greenhouse gas emissions [
3]. Alone livestock production is responsible for 14% (excluding land-use) of the world’s greenhouse gas emissions. These emissions originate from manure management, ruminant waste on pastures, ruminant emissions, and fertiliser production [
3]. In addition, results suggest that the agricultural industry is responsible for 92% of the global water consumption [
3].
Moreover, one third of food is lost from primary production to consumption, which has both economic and environmental consequences [
4]. In addition, food loss and waste account for 8% of the global greenhouse gas emissions [
3]. The loss occurs at several stages of the food supply chain. Already during the production of grain, fish, and fruit, food is discarded. Damaged, spilled, and uneaten food will also be lost during processing, packaging, and consumption [
3]. These problems necessitate proper handling of waste and by-products while providing with a sustainable high quality protein source for food and feed.
Insects are an interesting alternative solution for the above-mentioned agricultural related challenges, as it enables the conversion of waste- and by-products from the agricultural industry into biomass that can potentially be used for commercial feed for livestock animals, as a potential food source for humans [
5,
6], and as fertiliser producers [
7].
Currently most of the insects being produced are used as pet feed, fish feed, and aquaculture companies. However, there is a growing interest worldwide for insects as food and feed. The most prominent insects for insect production in Europe are the black soldier fly (
Hermetia illucens), yellow mealworm (
Tenebrio molitor), lesser mealworm (
Alphitobius diaperinus) and the house fly (
Musca domestica) [
8]. However, current EU legislation imposes challenges for the implementation of insects as food and feed and currently only a few insect species are allowed for human consumption and as feed for livestock [
9]. Moreover, only certain feeds are currently allowed as growth substrate in insect production, with current bans on slurry or manure and unprocessed foodstuffs containing meat or fish [
10]. These principles illustrate the demand for a greater focus on the commercialisation of insect production.
Insects are an effective commercial food- and feed product because of the high feed conversion rate in comparison to livestock [
11,
12]. In addition, insects can be valuable in the valorisation of waste- and by-products, such as used grains, household waste, and manure [
9]. Specific substrates appear to be more efficient than others. For example,
H. illucens larvae fed on grape pomace and spinach showed higher mortality, while a diet of pumpkin and red cabbage showed high bio-conversion rates. The differences are likely due to different nutritional values and digestibility of the substrates [
13]. Manure substrates have been found to be effective feed for
M. domestica and
H. illucens, and other insects have been postulated to be effective in utilising manure as feed [
6].
Microorganisms play critical role in the fitness of insects [
14]. Some bacterial species exists as either endosymbionts or as ectosymbionts and can be mutualistic, commensalistic or pathogenic [
15]. Microorganisms can be acquired in various ways, such as vertically from mother to offspring [
16], or horizontally transmitted through substrates [
17,
18]. Furthermore, free-living bacteria can be acquired throughout the life cycle, and can either become obligate or facultative endosymbionts. These, especially obligate endosymbionts, are entirely dependent on the transmission between generations [
15]. Vertically transmitted microbes have shown to be conducive to increased fitness through the influence on factors like reproductive ability or metabolic performance [
14,
19]. They are also involved in inhibition of pathogens and biofouling, and in oxygen- and nutrient acquisition [
14]. Therefore, microorganisms appear to be crucial for many aspects of the insect’s overall fitness during their life cycle.
Bacteria are predominantly present in the digestive system, where they play a role in key aspects of their host’s metabolism—primarily aiding in the acquisition of nutrients such as nitrogen, vitamins, and sterols [
15,
20]. For example, in honeybees (
Apis mellifera), the bacteria
Gilliamella apicola helps the breakdown of pollen. Additionally, the bacteria
Ishikawaella capsulata aids in growth and reproduction in the Japanese common plataspid stinkbug (
Megacopta punctatissima) and is also found to contain pathways for many essential and non-essential amino acids [
21]. Larvae of
H. illucens have also indicated dependence of microorganisms in the environment, since substrate-associated microorganisms were able to influence the survival, weight, and proportion of pre-pupae in
H. illucens [
22]. Furthermore, microorganisms in insects have found to improve resistance to environmental disturbance, enhance longevity, and shorten larval development time [
23]. Moreover, specific microorganisms play specific roles for hosts. The bacteria
Candidatus ishikawealla is important for the bean platasid’s (
Megacopta cribraria) development to the adult stage. For aphids, it has been shown that the bacteria
Buchnera aphidicola assists in reaching nutritional demands, and that the survival and fecundity of aphids are affected negatively without the presence of this specific bacteria [
15]. For the house fly (
Musca domestica) it has been determined that different bacteria are present at different life stages. For example, the bacteria strain
Weissella dominated colonisation of newly hatched larvae, and at the second larval stage the dominant strains were
Stenotrophomonas,
Bacillus, and
Lactococcus. This suggests that the bacteria have different impacts at different life stages [
24]. A specific example of this impact is the bacteria
Klebsilla oxytoca’s ability to restrict further oviposition at sites with recently laid eggs for the female flies [
24].
Besides in larval intestines, bacteria are also present on the surface of eggs. Mazza et al. (2020) isolated 9 different bacteria present on eggs of
H. illucens. Specific combinations of the bacteria present could either negatively or positively impact the weight gain and conversion rates in their larval stage [
25]. It is therefore not surprising that several papers have suggested bacteria to play a key role in commercial insect production [
9,
26].
Eggs are especially sensitive to pathogens and the host can, via microorganisms, inhibit pathogens [
14]. Microorganisms within the water flea (
Daphnia magna) eggs and in the surrounding environment have shown to result in positive effects on the reproductive ability and development of
D. magna. A sterile environment for the eggs has shown to decrease these parameters [
14].
M. domestica is an organism which can be used to determine the effects of vertically transmitted and substrate-associated microorganisms, and in addition, the larvae are a promising applicant as an alternative food- and feed-source as it feeds on waste- and by-products, which in turn, creates usable biomass for commercial production [
27]. This study aims to quantify the influence of the egg- and substrate-associated microorganisms on
M. domestica larvae’s performance. This will be investigated through autoclaved substrate (a combination of wheat bran (
Triticum aestivum), alfalfa (
Medicago sativa) and water) and surface-disinfected eggs using sodium hypochlorite solution. Tests were performed to examine the effects on the performance of
M. domestica larvae through the following parameters: development of biomass, final biomass, and survival rate.
4. Discussion
Microorganisms can affect insect fitness in various ways [
20]. The presence of microorganisms on eggs and in the environment can impact different factors like development, biomass, and survival rate [
15,
22,
23,
33]. However, few studies exist on the impact of microbes on the production of
M. domestica for food- and feed production. It was expected that disinfection of eggs, autoclaving of substrate, or both would lead to a decreased development of biomass, reduced final biomass, and impaired survival rate for larvae of
M. domestica compared to non-disinfected and -autoclaved conditions (control).
For all treatments a significant difference in development of biomass between days were found within each treatment. For disinfected eggs and/or autoclaved substrate a decreased final biomass for the larvae was found. Furthermore, the two treatments with only autoclaved substrate, and both disinfected eggs and autoclaved substrate led to a decreased survival rate.
4.1. The Effect of Disinfected Eggs
It was expected that disinfected eggs would lead to decreased development of biomass, reduced biomass, and impaired survival. This current study found a significant difference between disinfected- and non-disinfected eggs between days in the development of biomass and a significant difference in final biomass, but no significant difference in survival rate.
Schreven et al. (2021) examined the implications a sterile egg surface had on the performance, which includes final biomass and survival rate, of
H. illucens. Contrary to the current study, they found no significant effect of sterile eggs on larval survival and biomass. They argue that the microbiota present on eggs were so few compared to the substrate-associated microorganisms, that they had no effect on the overall microbiota composition nor the larval performance [
22]. However, Mazza et al. (2019) examined the specific egg-associated bacteria present on the eggs of
H. illucens. They found that the bacteria were able to enhance biomass and influence accumulation of crude fat and crude protein depending on the specific bacteria present on the eggs [
25]. Similarly, Lam et al. (2009) found bacteria present on eggs of
M. domestica contribute as a nutrient reserve [
33], which may explain the reduced final biomass observed in this study.
Development of biomass for disinfected eggs did not seem to be influenced in this current study. While significance between days were observed within treatments, the effect size for disinfected eggs and control were similar (GES: 0.768 and 0.739, respectively), indicating the major factor for variance is time for both treatments.
This is not in accordance with a different study by Lam et al. (2009) which uncovered that the presence of bacterial colonies on eggs were associated with suppression of fungal growth in their substrate and showed the removal of said bacteria increased fungal growth which inadvertently affected
M. domestica adult fly emergence negatively [
34]. While this current study did not investigate emergence, the presence of fungicidal bacteria could be affecting the development of larvae, however, these findings are not sustained in this study, but instead might still be influencing the observed difference in final biomass. As mentioned, this study found no significant difference for the survival rate between the disinfected- and the non-disinfected eggs. However, this is not in accordance with the study by Lam et al. (2009), who observed decreased survival for the sterilised egg treatment [
34]. This could be explained by the fact that insects contain egg-associated microbiota which assist with the suppression of fungi in the substrate and protecting against pathogens [
14,
34]. Therefore, it could be speculated that the removal of microbiota from the egg-surface would reduce survival rate. This study does not find a correlation between survival and egg-associated microbiota.
4.2. The Effect of Autoclaved Substrate
It was expected that autoclaved substrate would lead to a decreased development of biomass, final biomass, and survival rate. This current study found a significant difference between autoclaved- and non-autoclaved substrate in between days in the development of biomass, and a significant decreased final biomass and survival rate.
Similarly, Greenwood et al. (2021) observed that the microorganisms in the substrate significantly affect final biomass, and fat- and protein content of
H. illucens larvae [
35]. This is in accordance with previous studies, which showed microorganisms from the substrate are connected to gut microbial composition, as 66% of all microorganisms in the
H. illucens larvae’s gut originate from the substrate [
36]. These concepts give insight into why larvae are affected negatively when microorganisms are removed from the substrate in this current study. Therefore, the difference in development of biomass, final biomass, and survival rate observed, is not unexpected, considering the removal of essential microorganisms.
The decrease in biomass and survival could also be due to decreased availability of nutrients as a result of the Maillard reaction, which might be occurring during autoclaving. This leads to a decrease in the concentration of amino acids, but also a reduced digestibility of amino acids [
37]. Another study by Hefnawy (2011) showed that autoclaving lentils (
Lens culinaris) led to a decrease in different nutrients like sucrose and raffinose [
38]. Therefore, it is possible that the autoclaved substrate in the current study lacks important nutrients which may affect both microorganisms and the larvae. These principles help explain the observed reduction in final biomass and decreased survival rate. Moreover, it might explain the impaired development of biomass, which is supported by the lower effect size when comparing to the control treatment (GES: 0.36 and 0.739, respectively). This suggests other factors than time are influencing the variance observed throughout the development. Somroo et al. (2019) also determined the positive effects of microorganisms on
H. illucens larvae. With soybean curd residues as substrate for the larvae and the presence of
Lactobacillus buchneri, parameters such as final biomass, fat- and protein content were increased compared to the absence of
L. buchneri [
39]. Overall, other studies and this current study show how important the substrate’s microorganisms are for larval performance.
4.3. The Effect of Disinfected Eggs and Autoclaved Substrate
It was expected that disinfection of eggs and autoclaving substrate would lead to a decreased development of biomass, final biomass, and survival rate. This current study found a significant difference between disinfected eggs and autoclaved substrate in development of biomass with a low effect size when comparing to control (GES: 0.07 and 0.739, respectively), indicating other factors than time are influencing the development. A significant difference for final biomass and survival rate were found when comparing with the control. The reasons for these findings might be the accumulation of all the previously listed effects of reduced nutrient digestibility and loss of beneficial microbiota.
4.4. Perspectives
It is not certain that the disinfected eggs and the autoclaved substrate were uncontaminated. Agar plates (see
Appendix B) revealed that the autoclaved substrate had less colonies compared to the non-autoclaved. However, there were no visible colonies on the disinfected eggs. A beneficial addition to this study would be a 16S ribosomal RNA analysis to identify the quantity of different species of bacteria present in substrate and on eggs. Moreover, while the measure of final biomass was the day after peak biomass, it might have been more accurate to investigate by instar-stages instead, as this would assure all larvae have reached same stage in their life cycle.
Furthermore, some of the samples with autoclaved substrate were infected by fungus. This might be the result of an absence of fungicidal bacteria present on eggs [
34]. The survival in samples with fungus were zero which might show the importance of fungicidal bacteria. Doing 16S ribosomal RNA analysis could uncover if these species of bacteria were absent. This study illustrates the importance of microorganism’s influence on
M. domestica larvae’s biomass and survival. In addition, it is important to evaluate both the number of bacteria and species present when rearing
M. domestica for optimisation of biomass and survival when establishing commercial production of
M. domestica larvae for the purpose of maximising outcome.
Future studies are necessary to understand the full scope of the specific bacteria which are relevant for aforementioned parameters. Moreover, it could be interesting to investigate which physical conditions (pH, temperature, humidity etc.) are optimal for enhancing biomass and survival rate for production. Also, to investigate which substrates are most optimal in increasing development of biomass, final biomass, and the survival rate to maximize the outcome. Finally, it is crucial to uncover specifically which microorganisms are present on the eggs and in the substrate, and if possible, to discover the implications of each bacteria on the host.
Author Contributions
Conceptualization, R.M.D, P.A.S., S.B, A.T.M, C.P., and T.M.S.; methodology, R. M. D. and P.A.S; software, R.M.D. and P.A.S.; validation, R.M.D, P.A.S., S.B, A.T.M, C.P; formal analysis, R.M.D. and P.A.S.; investigation, R.M.D. and P.A.S.; resources, S.B, C.P; data curation, R.M.D. and P.A.S.; writing—original draft preparation, R.M.D. and P.A.S.; writing—review and editing, R.M.D, P.A.S., S.B, A.T.M, C.P., and T.M.S.; visualization, R.M.D. and P.A.S.; supervision, S.B., A.T.M, C.P., and T. M. S.; project administration, S.B; funding acquisition, S.B, C.P. All authors have read and agreed to the published version of the manuscript.
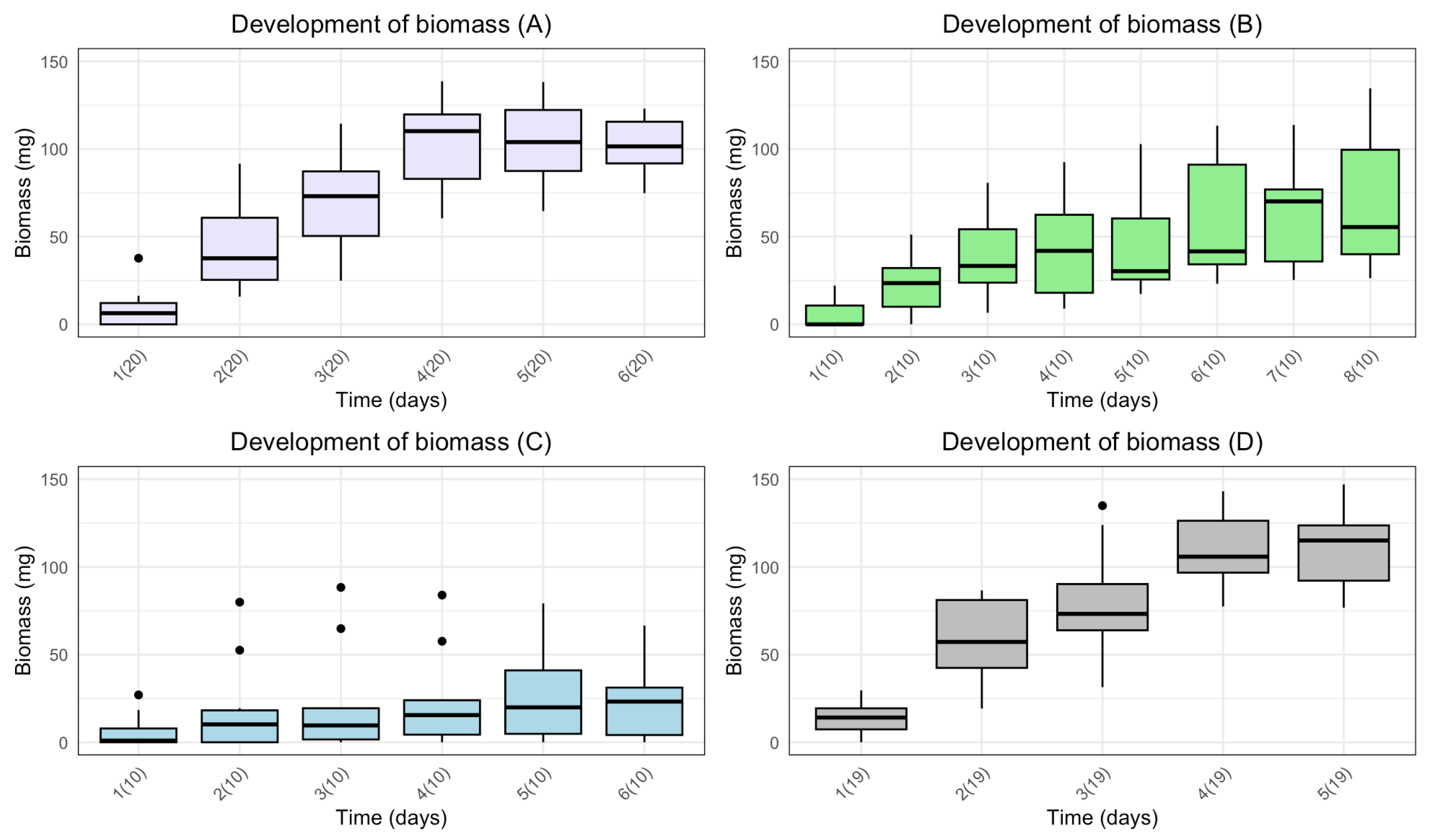
Figure 1.
Boxplots with the medians (25%-75% quantiles) of the biomass of larvae after the first five larvae were present in one sample for treatment A (disinfected eggs), B (autoclaved substrate), C (disinfected eggs and autoclaved substrate) and D (control). The x-axis shows time (days) with sample size in parentheses, and the y-axis shows the biomass (mg).
Figure 1.
Boxplots with the medians (25%-75% quantiles) of the biomass of larvae after the first five larvae were present in one sample for treatment A (disinfected eggs), B (autoclaved substrate), C (disinfected eggs and autoclaved substrate) and D (control). The x-axis shows time (days) with sample size in parentheses, and the y-axis shows the biomass (mg).
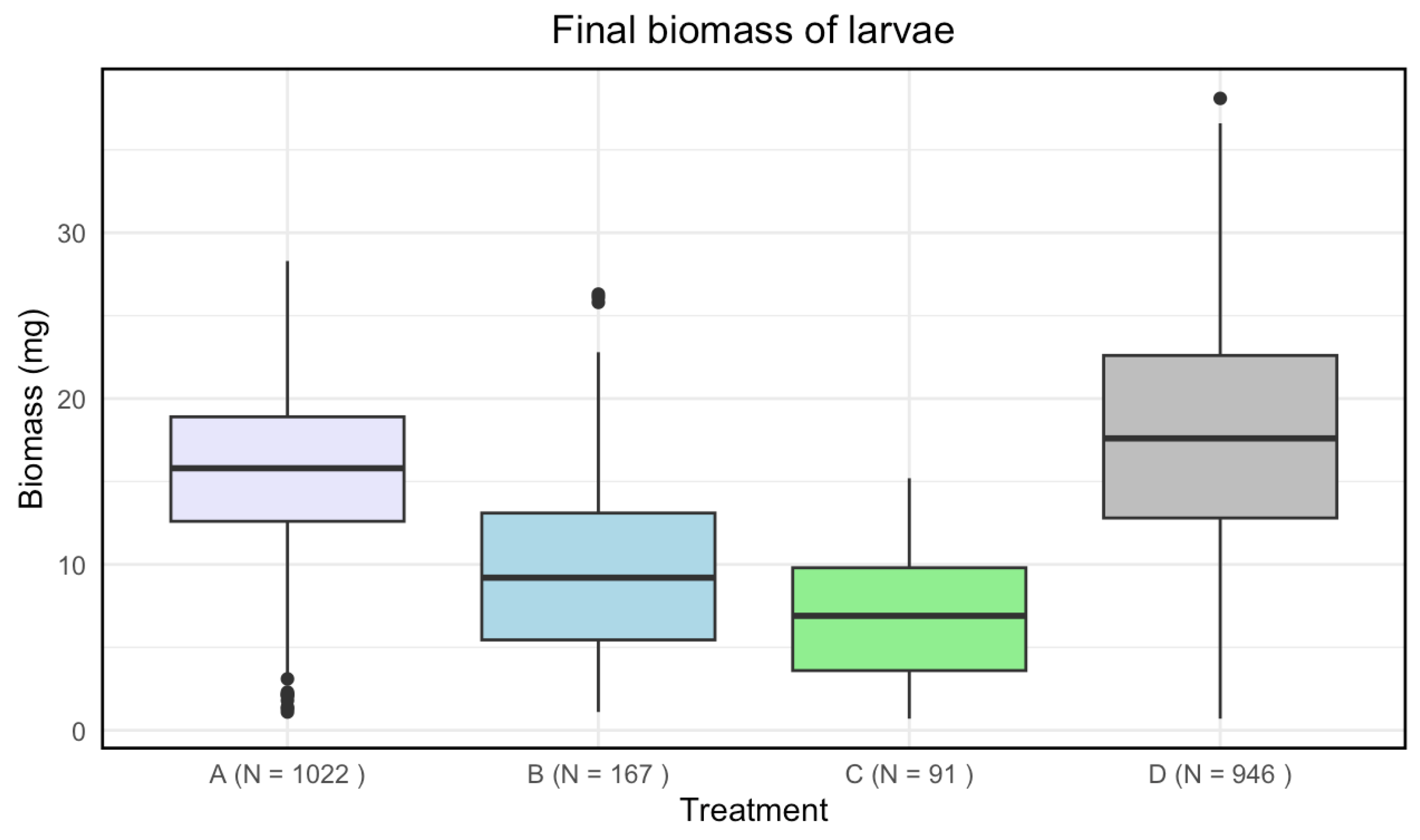
Figure 2.
Boxplots with the medians (25%-75% quantiles) of the final biomass of larvae for treatment A (disinfected eggs), B (autoclaved substrate), C (disinfected eggs and autoclaved substrate) and D (control). The x-axis shows treatments and sample size (N), and the y-axis shows the biomass (mg).
Figure 2.
Boxplots with the medians (25%-75% quantiles) of the final biomass of larvae for treatment A (disinfected eggs), B (autoclaved substrate), C (disinfected eggs and autoclaved substrate) and D (control). The x-axis shows treatments and sample size (N), and the y-axis shows the biomass (mg).
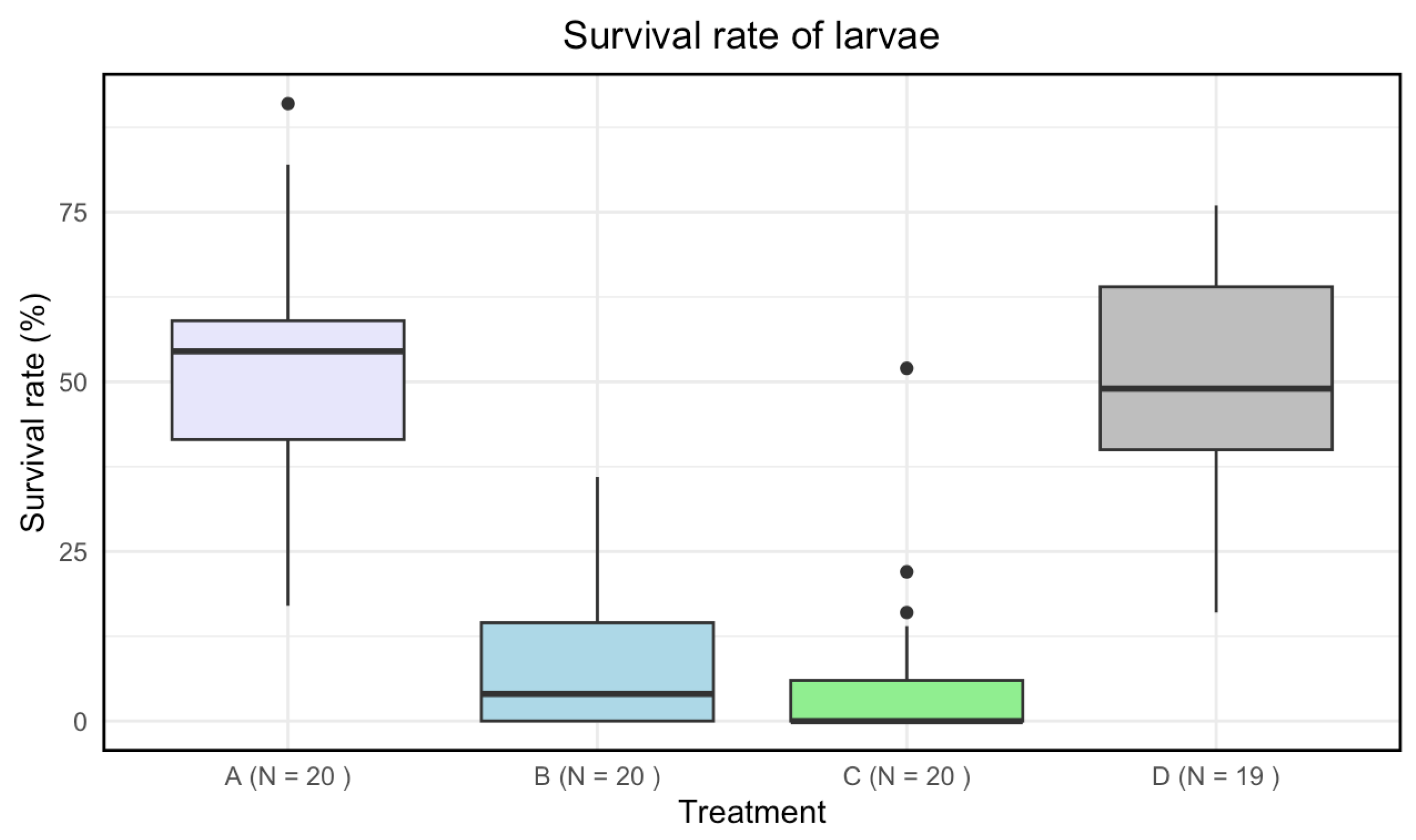
Figure 3.
Boxplots with the medians (25%-75% quantiles) of the survival rate of larvae for treatment A (disinfected eggs), B (autoclaved substrate), C (disinfected eggs and autoclaved substrate) and D (control). The x-axis shows treatments and sample size (N), and the y-axis shows the survival rate (%).
Figure 3.
Boxplots with the medians (25%-75% quantiles) of the survival rate of larvae for treatment A (disinfected eggs), B (autoclaved substrate), C (disinfected eggs and autoclaved substrate) and D (control). The x-axis shows treatments and sample size (N), and the y-axis shows the survival rate (%).
Table 1.
Concentrations of sodium hypochlorite ranged from lowest to highest concentration.
Table 1.
Concentrations of sodium hypochlorite ranged from lowest to highest concentration.
| Sodium Hypochlorite |
|---|
| 0% w/w |
| 0.006% w/w |
| 0.0075% w/w |
| 0.01% w/w |
| 0.015% w/w |
| 0.03% w/w |
Table 2.
Repeated measures ANOVA including p-value, F-value and generalised eta squared (GES) for treatment A (disinfected eggs), B (autoclaved substrate), C (disinfected eggs and autoclaved substrate) and D (control). Significant p-values are indicated with asterisk (*) when p<0.05.
Table 2.
Repeated measures ANOVA including p-value, F-value and generalised eta squared (GES) for treatment A (disinfected eggs), B (autoclaved substrate), C (disinfected eggs and autoclaved substrate) and D (control). Significant p-values are indicated with asterisk (*) when p<0.05.
| Treatment |
p-Value |
F-Value |
GES |
| A |
* |
159.253 |
0.768 |
| B |
* |
14.481 |
0.36 |
| C |
0.028 * |
4.375 |
0.07 |
| D |
* |
186.309 |
0.739 |
Table 3.
A Kruskal-Wallis indicated a significant effect of all treatments. A Dunn’s test was then performed between treatments, A (disinfected eggs), B (autoclaved substrate), C (disinfected eggs and autoclaved substrate) and D (control). Significant adjusted p-values are indicated with asterisk (*) when p < 0.05.
Table 3.
A Kruskal-Wallis indicated a significant effect of all treatments. A Dunn’s test was then performed between treatments, A (disinfected eggs), B (autoclaved substrate), C (disinfected eggs and autoclaved substrate) and D (control). Significant adjusted p-values are indicated with asterisk (*) when p < 0.05.
| Comparison |
Adjusted p-Value |
| A-B |
* |
| A-C |
* |
| B-C |
* |
| A-D |
* |
| B-D |
* |
| C-D |
* |
Table 4.
A Kruskal-Wallis indicated a significant effect of all treatments. A Dunn’s test was then performed between treatments, A (disinfected eggs), B (autoclaved substrate), C (disinfected eggs and autoclaved substrate) and D (control). Significant adjusted p-values are indicated with asterisk (*) when p < 0.05 for both tests.
Table 4.
A Kruskal-Wallis indicated a significant effect of all treatments. A Dunn’s test was then performed between treatments, A (disinfected eggs), B (autoclaved substrate), C (disinfected eggs and autoclaved substrate) and D (control). Significant adjusted p-values are indicated with asterisk (*) when p < 0.05 for both tests.
| Comparison |
Adjusted p-Value |
| A-B |
* |
| A-C |
* |
| B-C |
1 |
| A-D |
1 |
| B-D |
* |
| C-D |
* |