Submitted:
17 July 2024
Posted:
18 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Case Reports
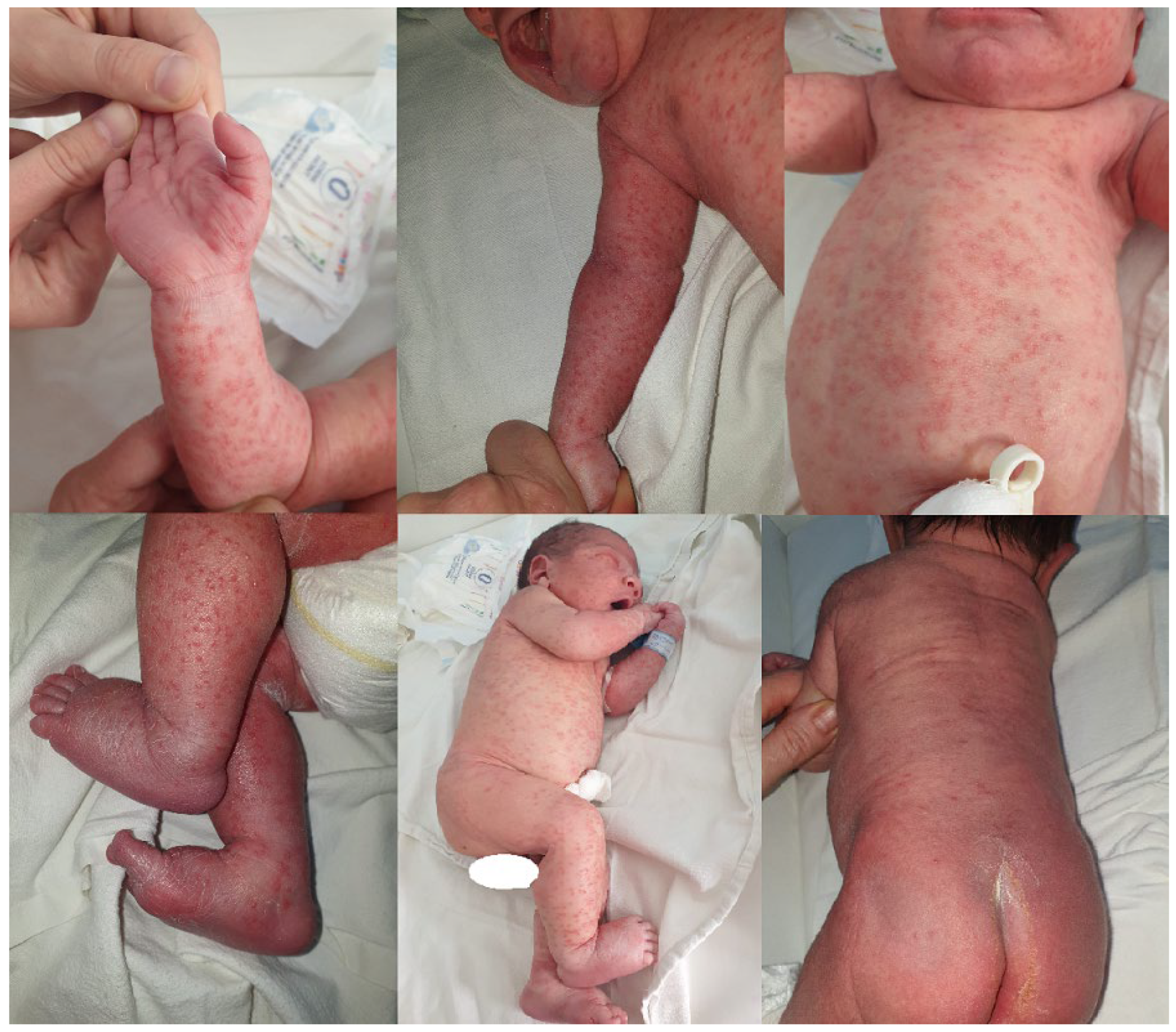
2.1. Case Report 1
2.2. Case Report 2
| Patient 1 | Patient 2 | |
| Gestational age | 39 weeks | 28 weeks |
| Birth weight | 3600 g | 1250 g |
| Maternal history | Vaginal discharge and genital itching one week before delivery | 132 hours of membrane rupture, intravenous ampicillin for 5 days, associated with ceftriaxone at birth |
| Maternal vaginal cultures | No pathogen growth at admission in our unit (2nd day after delivery) | No pathogen growth 5 days before delivery, C. albicans was isolated in cultures sampled at birth |
| Onset | At birth, with characteristic rash | The 4th day, respiratory distress syndrome |
| Antibiotic therapy (newborn) | Penicillin plus amikacin, 3 days after admission, intravenous | Penicillin plus amikacin, 2 days intravenous, empiric therapyColistin plus amikacin for 10 days, starting DOL1 5, intravenous |
| Clinical course | No other signs or symptoms suggestive of sepsis, rash entirely resolved by DOL1 13 | Re-initiated respiratory support (after initial treatment for respiratory distress syndrome due to surfactant deficiency with Bubble CPAP2 and surfactant), gradually decreased pressure and oxygen concentration, weaned on HHHFNC3 on DOL1 15, free low flow oxygen starting DOL1 27, oxygen-independent at DOL1 41 |
| Complications | None | Hepatic and renal involvementGrade I retinopathy of prematurity |
| Discharge | DOL1 24 | DOL1 58 |
| Follow up | Normal growth and development at the age of 2 | Recently discharged, follow-up scheduled |
| Patient 1 | Patient 2 | ||||||||||||
| 12 h | Day 1 | Day 3 | Day 12 | Day 17 | 12 h | Day 1 | Day 2 | Day 4 | Day 5 | Day 10 | Day 15 | Day 20 | |
| Blood count and differential | |||||||||||||
| Hemoglobin (g/dL) | 14 | 11.8 | 11.4 | 11,2 | - | 16.1 | 18.1 | 16.7 | 17.9 | 17.8 | 14.7 | 13.5 | 11.9 |
| Platelets (103/µL) | 434 | 412 | 389 | 354 | - | - | 374 | - | - | 331 | 356 | - | - |
| Leucocytes (/µL) | 17,440 | 14,430 | 11,280 | 9,760 | - | - | 23,300 | - | - | 21,240 | 15,050 | - | - |
| Neutrophils (/µL) | 10,580 | 7,540 | 6,820 | 5,730 | - | - | 15,090 | - | - | 14,350 | 9,030 | - | - |
| Bands (%) | - | 5 | 6 | 1 | - | - | 3.4 | - | - | 6.5 | 2 | - | - |
| I/T1 ratio | - | 0.09 | 0.09 | 0.017 | - | - | 0.05 | - | - | 0.088 | 0.032 | - | - |
| Monocytes (%) | 1.4 | 12 | 12.5 | 4.1 | - | - | 8.4 | - | - | 9.5 | 5 | - | - |
| Inflammatory markers | |||||||||||||
| C-RP2 (mg/L) | 19.38 | 8.3 | 7.4 | 1,6 | 3,1 | 2.1 | 1.3 | <1 | 41.4 | 36.6 | 14.3 | 12.5 | 2.9 |
| Biochemistry | |||||||||||||
| Blood glucose (mg/dL) | 63.1 | 74 | 85 | - | - | 59 | 77 | 57 | 123 | 49 | 87 | 89 | 78 |
| AST3 (U/L) | 38.4 | 34 | 29 | 27 | 15 | 29 | 29 | 114 | 107 | 87 | 20 | 27 | 28 |
| ALT4 (U/L) | 13.3 | 16 | 18 | 15 | 14 | <7 | <7 | 14 | 21 | 22 | 9 | 8 | 9 |
| Creatinine (mg/dL) | 1.01 | 0.61 | 0,52 | 0.54 | 0.45 | 0.52 | 0.85 | 0.97 | 1.15 | 1.25 | 0.91 | 0.75 | 0.54 |
| BUN5 (mg/dL) | 30.5 | 12 | 13 | 15 | 24 | 24 | 63 | 83 | 79 | 74 | 37 | 26 | 19 |
| Total bilirubin (mg/dL) | 3.4 | 4.3 | 5.5 | 1.2 | 0.17 | 2.3 | 4.6 | 6.8 | 6.9 | 6.9 | 1.7 | - | - |
| Conjugated bilirubin (mg/dL) | 0.24 | 0.31 | 0,35 | - | - | 0.28 | 0.31 | 0.26 | 0.35 | 0.33 | 0.6 | - | - |
| Blood gases and electrolytes | |||||||||||||
| Blood gases (arterial) | - | Normal | Normal | - | - | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal |
| Electrolytes | - | Normal | - | - | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal | |
| Imaging | |||||||||||||
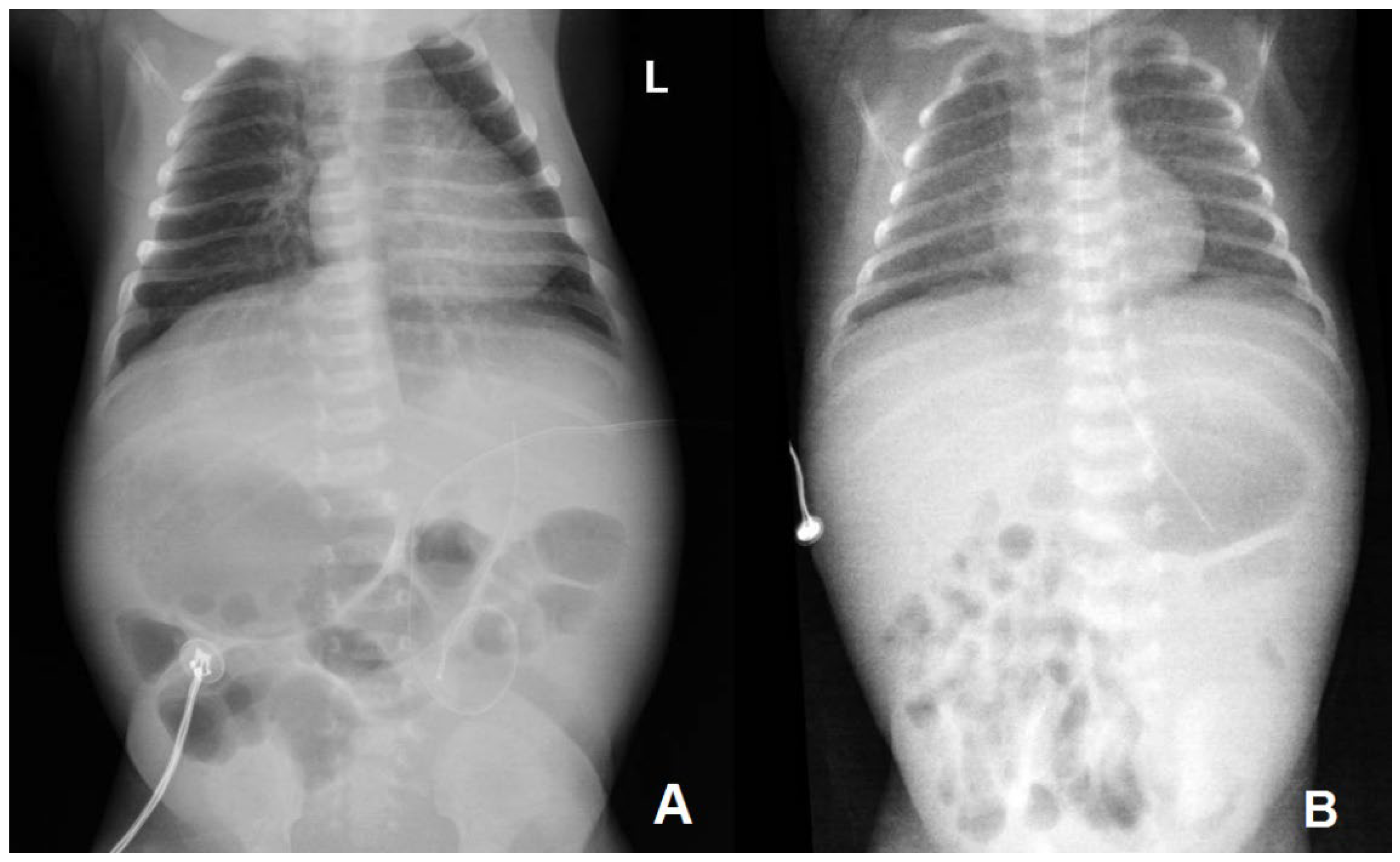
| Thoraco-abdominal radiography | No lung or abdominal involvement | Suggestive of respiratory distress syndrome due to surfactant deficiency on DOL6 0; pronounced reticular, micronodular bilateral lung interstitium on DOL6 5 | |||||||||||
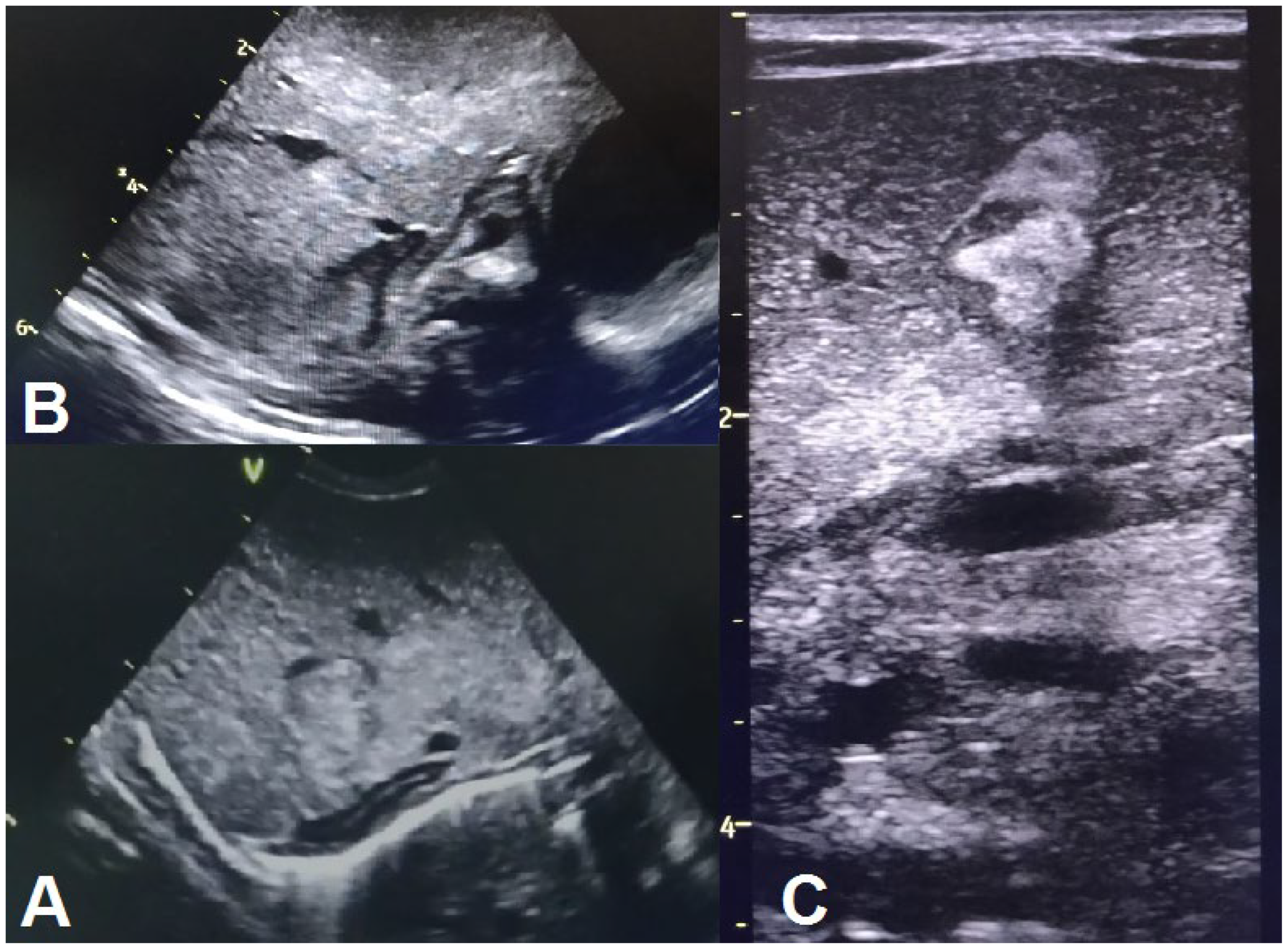
| Abdominal ultrasound | Suspected neuroblastoma on DOL6 1; abdominal situs inversus on DOL6 2; no abdominal parenchymal involvement on DOL6 2 and 10 | Gross, inhomogenous, patchy echogenic areas disseminated, almost throughout the entire liver on DOL6 9; fine granular echogenic areas limited to the fourth hepatic segment by DOL6 14; normal hepatic ultrasound structure on DOL6 45 | |||||||||||
| Head ultrasound | Normal at admission and on follow up | Normal for the gestational or corrected age at DOL6 3, 7, and at discharge | |||||||||||
| Doppler echocardiography | Small PDA7, PFO8, right-sided aortic arch (DOL6 1) | Small PDA7, PFO8 on DOL6 4 and 14 | |||||||||||
| Microbiology | |||||||||||||
| Blood culture | Positive for C. albicans at admission, no growth at 14 days | Positive for C. albicans on DOL6 0 and 11, negative on DOL6 18 | |||||||||||
| Nasal swab culture | No pathogen identified | No pathogen identified on DOL6 4 and 18 | |||||||||||
| Gastric aspirate | No pathogen identified | C. albicans isolated on DOL6 0, and 4, no pathogen growth on DOL6 18 | |||||||||||
| Pharyngeal culture | No pathogen identified | C. albicans isolated on DOL6 4, no pathogen growth on DOL6 18 | |||||||||||
| Umbilical line tip culture | C. albicans (in situ for 2 days) | C. albicans (in situ for 5 days) | |||||||||||
3. Discussion
3.1. General Aspects - Epidemiology, Etiology
3.2. Physiopathology
3.3. Clinical Aspects
3.4. Diagnosis
3.5. Treatment
3.6. Clinical Course and Complications
3.7. Prevention
4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, L.; Johnson, H.L; Cousens, S.; Perin, J.; Scott, S.; Lawn, J.E.; Rudan, I.; Campbell, H.; Cibulskis, R.; Li, M.: Mathers, C.; Black, R.E.; Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151-61. [CrossRef]
- Odabasi, I.O.; Bulbul, A. Neonatal Sepsis. Sisli Etfal Hastan Tip Bul. 2020;54(2):142-158. [CrossRef]
- Cohen-Wolkowiez, M.; Moran, C.; Benjamin, D.K.; Cotten, C.M.; Clark, R.H.; Benjamin, D.K. Jr; Smith, P.B. Early and late onset sepsis in late preterm infants. Pediatr Infect Dis J. 2009;28(12):1052-6. [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Sánchez, P.J.; Faix, R.G.; Poindexter, B.B.; Van Meurs, K.P.; Bizzarro, M.J.; Goldberg, R.N.; Frantz, I.D. 3rd; Hale, E.C.; Shankaran, S.; Kennedy, K.; Carlo, W.A.; Watterberg, K.L.; Bell, E.F.; Walsh, M.C.; Schibler, K.; Laptook, A.R.; Shane, A.L.; Schrag, S.J.; Das, A.; Higgins, R.D.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Early onset neonatal sepsis: The burden of group B Streptococcal and E. coli disease continues. Pediatrics. 2011;127(5):817-26. [CrossRef]
- Turhan, E.E.; Gürsoy, T.; Ovalı, F. Factors which affect mortality in neonatal sepsis. Turk Pediatri Ars. 2015;50(3):170-5. [CrossRef]
- Johansson Gudjónsdóttir, M.; Elfvin, A.; Hentz, E.; Adlerberth, I.; Tessin, I.; Trollfors, B. Changes in incidence and etiology of early-onset neonatal infections 1997-2017 - a retrospective cohort study in western Sweden. BMC Pediatr. 2019;19(1):490. [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin Microbiol Rev. 2007;20(1):133-63. [CrossRef]
- Benjamin, D.K. Jr.; Stoll, B.J.; Gantz, M.G.; Walsh, M.C.; Sánchez, P.J.; Das, A.; Shankaran, S.; Higgins, R.D.; Auten, K.J.; Miller, N.A.; Walsh, T.J.; Laptook, A.R.; Carlo, W.A.; Kennedy, K.A.; Finer, N.N.; Duar,a S.; Schibler, K.; Chapman, R.L.; Van Meurs, K.P.; Frantz, I.D. 3rd.; Phelps, D.L.; Poindexter, B.B.; Bell, E.F.; O'Shea, T.M.; Watterberg, K.L.; Goldberg, R.N.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal candidiasis: Epidemiology, risk factors, and clinical judgment. Pediatrics. 2010;126(4):e865-73. [CrossRef]
- Al-Taiar, A.; Hammoud, M.S.; Thalib, L.; Isaacs, D. Pattern and etiology of culture-proven early-onset neonatal sepsis: A five-year prospective study. Int J Infect Dis. 2011;15(9):e631-4. [CrossRef]
- Georgescu, T.A.; Lisievici, A.C.; Munteanu, O.; Furtunescu, F.L.; Bratu, O.G.; Berceanu, C.; Bohîlţea, R.E. Congenital systemic candidiasis: A comprehensive literature review and meta-analysis of 44 cases. Rom J Morphol Embryol. 2020;61(3):673-680. [CrossRef]
- Benirschke, K.; Raphael, S.I. Candida albicans infection of the amniotic sac. Am J Obstet Gynecol. 1958;75(1):200-2. [CrossRef]
- Barton, M.; Shen, A.; O'Brien, K.; Robinson, J.L.; Davies, H.D.; Simpson, K.; Asztalos, E.; Langley, J.; Le Saux, N.; Sauve, R.; Synnes, A.; Tan, B.; de Repentigny, L.; Rubin, E.; Hui, C.; Kovacs, L.; Yau, Y.C.; Richardson, S.E; Paediatric Investigators Collaborative Network on Infections in Canada (PICNIC). Early-Onset Invasive Candidiasis in Extremely Low Birth Weight Infants: Perinatal Acquisition Predicts Poor Outcome. Clin Infect Dis. 2017;64(7):921-927. [CrossRef]
- Kaufman, D.A. Challenging issues in neonatal candidiasis. Curr Med Res Opin. 2010;26(7):1769-78. [CrossRef]
- Chitnis, A.S.; Magill, S.S.; Edwards, J.R.; Chiller, T.M.; Fridkin, S.K.; Lessa, F.C.; Trends in Candida central line-associated bloodstream infections among NICUs, 1999-2009. Pediatrics. 2012;130(1):e46-52. [CrossRef]
- Kaufman, D.A. "Getting to Zero": Preventing invasive Candida infections and eliminating infection-related mortality and morbidity in extremely preterm infants. Early Hum Dev. 2012;88 Suppl 2:S45-9. [CrossRef]
- Kaufman, D.A; Springe,r SC. Fungal Infections in Preterm Infants. https://emedicine.medscape.com/article/980487-overview?st=fpf&scode=msp&socialSite=google&form=fpf&icd=login_success_gg_mismatch_fpf, Updated: Dec 27, 2020; accessed on 10 July 2024.
- Feng, Y.; Lu, H.; Whiteway, M.; Jiang, Y. Understanding fluconazole tolerance in Candida albicans: Implications for effective treatment of candidiasis and combating invasive fungal infections. J Glob Antimicrob Resist. 2023;35:314-321. [CrossRef]
- Năstase, L., Rădulescu, L., Luminos, M.L., Merisescu, M.M., Jugulete, G., Stoicescu, S.M., Severe Modifications of Biological Markers in Late Neonatal Sepsis in a Very Low Birth Weight Due to Candida lusitaniae, Rev. Chim., 2019, 70(2): 393-397. Corpus ID: 214480005. [CrossRef]
- Nouri-Merchaoui, S.; Mahdhaoui, N.; Fekih, M.; Adouani, M.; Zakhama, R.; Methlouthi, J.; Ghith, A.; Seboui, H. Candidose congénitale systémique, forme rare de candidose néonatale : À propos d'une observation chez un nouveau-né prématuré [Systemic congenital candidiasis, a rare condition in neonates: Case report in a premature infant]. Arch Pediatr. 2011;18(3):303-7. French. [CrossRef]
- Fernández-Ruiz, M.; Mosqueda-Peña, R.; Pérez-Ayala, A.; Blázquez-Gamero, D. Congenital cutaneous candidiasis associated with maternal peripartum candidemia. Rev Iberoam Micol. 2020;37(2):68-71. [CrossRef]
- Hammoud, M.S.; Al-Taiar, A.; Fouad, M.; Raina, A.; Khan, Z. Persistent candidemia in neonatal care units: Risk factors and clinical significance. Int J Infect Dis. 2013;17(8):e624-8. [CrossRef]
- Wang, S.M.; Hsu, C.H.; Chang, J.H. Congenital candidiasis. Pediatr Neonatol. 2008;49(3):94-6. [CrossRef]
- Aruna, C.; Seetharam, K. Congenital candidiasis. Indian Dermatol Online J. 2014;5(Suppl 1):S44-7. [CrossRef]
- Shope, C.; Ritter, A.; Karlin, S.; Lee, L.W.; Cotton, C.H. Congenital Cutaneous Candidiasis in Preterm Infants. Neoreviews. 2023;24(3):e175-e180. [CrossRef]
- Pradeepkumar, V.K.; Rajadurai, V.S.; Tan, K.W. Congenital candidiasis: Varied presentations. J Perinatol. 1998;18(4):311-6. PMID: 9730205. [PubMed]
- Ferreras-Antolín, L.; Sharland, M.; Warri,s A. Management of Invasive Fungal Disease in Neonates and Children. Pediatr Infect Dis J. 2019;38(6S Suppl 1):S2-S6. [CrossRef]
- Sanni, U.A.; Lawal, T.O.; Na'uzo, A.M.; Audu, L.I. Invasive Fungal Infection Presenting as Early-Onset Neonatal Sepsis: A Case Report from Northern Nigeria. Journal of Clinical Neonatology. 2023; 12(1):p 38-41. [CrossRef]
- Jung, Y.J. Early- and late-onset candidemia in very low birth weight infants in the Korean neonatal network, 2013-2017. Pediatr Neonatol. 2024;S1875-9572(24)00084-6. [CrossRef]
- Shane, A.L.; Sánchez, P.J.; Stoll, B.J. Neonatal sepsis. Lancet. 2017;390(10104):1770-1780. [CrossRef]
- Melville, C.; Kempley, S.; Graham, J.; Berry, C.L. Early onset systemic Candida infection in extremely preterm neonates. Eur J Pediatr. 1996;155(10):904-6. [CrossRef]
- Aldana-Valenzuela, C.; Morales-Marquec, M.; Castellanos-Martínez, J.; Deanda-Gómez, M. Congenital candidiasis: A rare and unpredictable disease. J Perinatol. 2005;25(10):680-2. [CrossRef]
- Sousa. R.A.; Martins Oliveira Diniz, L.; Lapa Marinho, F.E.; Gonçalves Rezende, L.; Machado Carelos, E.; de castro Romanelli R.M. Risk factors for candidemia in neonates: Systematic review and meta-analysis Journal of Neonatal Nursing. 2022; 28:83–92. [CrossRef]
- Trofa, D.; Gácser, A.; Nosanchuk, J.D. Candida parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev. 2008;21(4):606-25. [CrossRef]
- Daniel, K.; Greenberg, R.G.; Boutzoukas, A.; Katakam, L. Updated Perspectives on the Diagnosis and Management of Neonatal Invasive Candidiasis. Research and Reports in Neonatology. 2023;13:45-63. [CrossRef]
- Weimer, K.E.D.; Smith, P.B.; Puia-Dumitrescu, M.; Aleem, S. Invasive fungal infections in neonates: A review. Pediatr Res. 2022;91(2):404-412. [CrossRef]
- Simonsen, K.A.; Anderson-Berry, A.L.; Delair, S.F.; Davies, H.D. Early-onset neonatal sepsis. Clin Microbiol Rev. 2014;27(1):21-47. [CrossRef]
- Messina, A.; Mariani, A.; Brandolisio, R.; Tavella, E.; Germano, C.; Lipari, G.; Leo, L.; Masturzo, B.; Manzoni, P. Candidiasis in Pregnancy: Relevant Aspects of the Pathology for the Mother and the Fetus and Therapeutic Strategies. Trop Med Infect Dis. 2024;9(5):114. [CrossRef]
- Waguespack-LaBiche, J., Chen, S.H.; Yen. A. Disseminated congenital candidiasis in a premature infant. Arch Dermatol. 1999;135(5):510-2. [CrossRef]
- Krallis, N.; Tzioras, S.; Giapros, V.; Leveidiotou, S.; Paschopoulos, M.; Stefanou, D.; Andronikou, S. Congenital candidiasis caused by different Candida species in a dizygotic pregnancy. Pediatr Infect Dis J. 2006;25(10):958-9. [CrossRef]
- Arai, H.; Goto, R.; Matsuda, T.; Saito, S.; Hirano, H.; Sanada, H.; Sato, A.; Takada, G. Case of congenital infection with Candida glabrata in one infant in a set of twins. Pediatr Int. 2002;44(4):449-50. [CrossRef]
- Pineda, C.; Kaushik, A.; Kest, H.; Wickes, B.; Zauk, A. Maternal sepsis, chorioamnionitis, and congenital Candida kefyr infection in premature twins. Pediatr Infect Dis J. 2012;31(3):320-2. [CrossRef]
- Nichols, A.; Khong, T.Y.; Crowther, C.A. Candida tropicalis chorioamnionitis. Am J Obstet Gynecol. 1995;172(3):1045-7. [CrossRef]
- Chen, W.Y.; Chen, S.J.; Tsai, S.F.; Tsao, P.C.; Tang, R.B.; Soong, W.J. Congenital Systemic Fungus Infection in Twin Prematurity-A Case Report and Literature Review. AJP Rep. 2015;5(1):e46-50. [CrossRef]
- Ruiz-Cabrera, J.R.; Meléndrez-Vásquez, D.; Moreno, D.M.; Prieto-Jure. R. Congenital cutaneous candidiasis in a premature neonate: A case report. Clin Case Rep. 2022;10(5):e05773. [CrossRef]
- Kumar, M.; Saadaoui, M.; Al Khodor. S. Infections and Pregnancy: Effects on Maternal and Child Health. Front Cell Infect Microbiol. 2022;12:873253. [CrossRef]
- Maki, Y.; Fujisaki, M.; Sato, Y.; Sameshima, H. Candida Chorioamnionitis Leads to Preterm Birth and Adverse Fetal-Neonatal Outcome. Infect Dis Obstet Gynecol. 2017;2017:9060138. [CrossRef]
- Disha, T.; Haque, F. Prevalence and Risk Factors of Vulvovaginal Candidosis during Pregnancy: A Review. Infect Dis Obstet Gynecol. 2022;2022:6195712. [CrossRef]
- Shazniza Shaaya, E.; Halim, S.A.A.; Leong, K.W.; Ku, K.B.P.; Lim, P.S.; Tan, G.C.; Wong, Y.P. Candida Chorioamnionitis in Mothers with Gestational Diabetes Mellitus: A Report of Two Cases. Int J Environ Res Public Health. 2021;18(14):7450. [CrossRef]
- Salusti-Simpson, M.; Marghoob, N.; Greene, L.; Morley. K. Congenital cutaneous candidiasis in a full-term neonate. Pediatr Dermatol. 2022;39(6):952-954. Epub 2022 Jul 25. [CrossRef]
- Pammi, M. Candida infections in neonates: Epidemiology, clinical manifestations, and diagnosis. Last updated: May 22, 2024. https://www.uptodate.com/contents/candida-infections-in-neonates-epidemiology-clinical-manifestations-and-diagnosis.
- Suárez, J.A.G.; Calumby, R.J.N.; Silva, D.P.; Barbosa, V.T.; Maranhão, F.C.A.; Moreira, I.F.; Melhem, M.S.C.; Moreira, R.T.F. Neonatal innate immunity response in invasive candidiasis. Braz J Biol. 2024;84:e275155. [CrossRef]
- Michalski, C.; Kan, B.; Lavoie, P.M. Antifungal Immunological Defenses in Newborns. Front Immunol. 2017;8:281. [CrossRef]
- Mahieu, L.M.; Van Gasse, N.; Wildemeersch, D.; Jansens, H.; Ieven, M. Number of sites of perinatal Candida colonization and neutropenia are associated with nosocomial candidemia in the neonatal intensive care unit patient. Pediatr Crit Care Med. 2010;11(2):240-5. [CrossRef]
- Roqué, H.; Abdelhak, Y.; Young, B.K. Intra amniotic candidiasis. Case report and meta-analysis of 54 cases. J Perinat Med. 1999;27(4):253-62. [CrossRef]
- Jagtap, S.A.; Saple, P.P.; Dhaliat, S.B. Congenital cutaneous candidiasis: A rare and unpredictable disease. Indian J Dermatol. 2011;56(1):92-3. [CrossRef]
- Blomberg, L.; Backman, K.; Kirjavainen, P.V.; Karvonen, A.M.; Harju, M.; Keski-Nisula, L. Vulvovaginal yeast infections, gestational diabetes and pregnancy outcome. BMC Pregnancy Childbirth. 2023;23(1):70. [CrossRef]
- Rasti, S.; Asadi, M.A.; Taghriri, A.; Behrashi, M.; Mousavie, G. Vaginal candidiasis complications on pregnant women. Jundishapur J Microbiol. 2014;7(2):e10078. [CrossRef]
- Drummond, R.A.; Lionakis, M.S. Candidiasis of the Central Nervous System in Neonates and Children with Primary Immunodeficiencies. Curr Fungal Infect Rep. 2018;12(2):92-97. [CrossRef]
- Bider, D.; Ben-Rafael, Z.; Barkai, G.; Mashiach, S. Intrauterine fetal death apparently due to Candida chorioamnionitis. Arch Gynecol Obstet. 1989;244(3):175-7. [CrossRef]
- Guzel, A.B.; Ilkit, M.; Burgut, R.; Urunsak, I.F.; Ozgunen, F.T. An evaluation of risk factors in pregnant women with Candida vaginitis and the diagnostic value of simultaneous vaginal and rectal sampling. Mycopathologia. 2011;172(1):25-36. [CrossRef]
- Roberts, C.L.; Rickard, K.; Kotsiou, G.; Morris, J.M. Treatment of asymptomatic vaginal candidiasis in pregnancy to prevent preterm birth: An open-label pilot randomized controlled trial. BMC Pregnancy Childbirth. 2011;11:18. [CrossRef]
- Stronati, M.; Decembrino, L. Neonatal invasive candidiasis. Minerva Pediatr. 2006;58(6):537-49. PMID: 17093376. [PubMed]
- Torres-Alvarez, B.; Hernandez-Blanco, D.; Ehnis-Perez, A.; Castanedo-Cazares, J.P. Cutaneous congenital candidiasis in a full-term newborn from an asymptomatic mother. Dermatol Online J. 2013;19(7):18967. PMID: 24010513. [PubMed]
- Gonçalves, B.; Ferreira, C.; Alves, C.T.; Henriques, M.; Azeredo, J.; Silva, S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42(6):905-27. [CrossRef]
- He, Y.; Tang, R.; Deng, J.; Cai, T.; He, P.; Wu, J.; Cao, Y. Effects of oestrogen on vulvovaginal candidosis. Mycoses. 2022;65(1):4-12. [CrossRef]
- Kumar, S.; Vasant, B.; Mathur, A.; De, M. A study of neonatal sepsis due to Candida species. Bombay Hospital Journal. 2011;53: 524-528.
- Saiman, L.; Ludington, E.; Pfaller, M.; Rangel-Frausto, S.; Wiblin, R.T.; Dawson, J.; Blumberg, H.M.; Patterson, J.E.; Rinaldi, M.; Edwards, J.E.; Wenzel, R.P.; Jarvis, W. Risk factors for candidemia in Neonatal Intensive Care Unit patients. The National Epidemiology of Mycosis Survey study group. Pediatr Infect Dis J. 2000;19(4):319-24. [CrossRef]
- Prinsloo, B.; Weldhagen, G.F.; Blaine, R.W. Candida famata central nervous system infection. S Afr Med J. 2003;93(8):601-2. PMID: 14531119. [PubMed]
- Manzoni, P.; Farina, D.; Galletto, P.; Leonessa, M.; Priolo, C.; Arisio, R.; Gomirato, G. Type and number of sites colonized by fungi and risk of progression to invasive fungal infection in preterm neonates in neonatal intensive care unit. J Perinat Med. 2007;35(3):220-6. [CrossRef]
- Darmstadt, G.L.; Dinulos, J.G.; Miller, Z. Congenital cutaneous candidiasis: Clinical presentation, pathogenesis, and management guidelines. Pediatrics. 2000;105(2):438-44. [CrossRef]
- Arsenault, A.B.; Bliss, J.M. Neonatal Candidiasis: New Insights into an Old Problem at a Unique Host-Pathogen Interface. Curr Fungal Infect Rep. 2015;9(4):246-252. [CrossRef]
- Zhao, H.H.; Zhe-Zhou, Lu L.; Zhao, Y.Z.; Yang, L.J.; Ding, Y.X. Congenital candidiasis in a full-term infant: A case report. J Int Med Res. 2023;51(4):3000605231158015. [CrossRef]
- Ito, F.; Okubo, T.; Yasuo, T.; Mori, T.; Iwasa, K.; Iwasaku, K.; Kitawaki, J. Premature delivery due to intrauterine Candida infection that caused neonatal congenital cutaneous candidiasis: A case report. J Obstet Gynaecol Res. 2013;39(1):341-3. [CrossRef]
- Ganer Herman, H.; Mevorach Zussman. N.; Krajden Haratz, K.; Bar, J.; Sagiv, R. Candida glabrata Chorioamnionitis following in vitro Fertilization: Review of the Literature. Gynecol Obstet Invest. 2015;80(3):145-7. [CrossRef]
- Obermair, H.M.; Bhagwanani, G.; Caldas, R.; Doyle, H.; Smoleniec, J.; Adno, A. Candida chorioamnionitis associated with late stillbirth: A case report. Case Rep Womens Health. 2020;27:e00239. [CrossRef]
- Diana, A.; Epiney, M.; Ecoffey, M.; Pfister, R.E. "White dots on the placenta and red dots on the baby": Congential cutaneous candidiasis--a rare disease of the neonate. Acta Paediatr. 2004;93(7):996-9. [CrossRef]
- Blanc, W.A. Pathways of fetal and early neonatal infection. Viral placentitis, bacterial and fungal chorioamnionitis. J Pediatr. 1961;59:473-96. [CrossRef]
- Jin, Y.; Endo, A.; Shimada, M.; Minato, M.; Takada, M.; Takahashi, S.; Harada, K. Congenital systemic candidiasis. Pediatr Infect Dis J. 1995;14(9):818-20.
- Paul, A.A.; Hoffman, K.L.; Hagan, J.L.; Sampath, V.; Petrosino, J.F.; Pammi, M. Fungal cutaneous microbiome and host determinants in preterm and term neonates. Pediatr Res. 2020;88(2):225-233. [CrossRef]
- Miras, I.; Vierge, E.; García, M.; Arruza, L.; Criado, E.; Ramos, J.T.; Martínez-Orgado, J. Congenital Cutaneous Candidiasis With Systemic Dissemination in a Preterm Infant. Pediatr Infect Dis J. 2021;40(6):e230-e233. [CrossRef]
- Colantonio, S.; Hedin, E.; Li, H.O.; Gavigan, G. Management of congenital cutaneous candidiasis in a healthy term baby: A case report. SAGE Open Med Case Rep. 2019;7:2050313X19876707. [CrossRef]
- Almeida Santos, L.; Beceiro, J.; Hernandez, R.; Salas, S.; Escriba, R.; Garcia Frias, E.; Perez Rodriguez, J.; Quero, J. Congenital cutaneous candidiasis: Report of four cases and review of the literature. Eur J Pediatr. 1991;150(5):336-8. [CrossRef]
- Kucinskiene, V.; Sutkute, A.; Valiukeviciene, S. Cutaneous fungal infection in a neonatal intensive care unit patient: A case report and literature review. Pediatr Dermatol. 2014;31(3):267-70. [CrossRef]
- Korting, H.C.; Patzak, U.; Schaller, M.; Maibach, H.I. A model of human cutaneous candidosis based on reconstructed human epidermis for the light and electron microscopic study of pathogenesis and treatment. J Infect. 1998;36(3):259-67. [CrossRef]
- Scheffler, E.; Miller, G.G.; Classen, D.A. Zygomycotic infection of the neonatal upper extremity. J Pediatr Surg. 2003;38(7):E16-7. [CrossRef]
- Smolinski, K.N.; Shah, S.S.; Honig, P.J.; Yan, A.C. Neonatal cutaneous fungal infections. Curr Opin Pediatr. 2005;17(4):486-93. [CrossRef]
- Smith, P.B.; Steinbach, W.J.; Benjamin, D.K. Jr.; Neonatal candidiasis. Infect Dis Clin North Am. 2005;19(3):603-15. [CrossRef]
- Ng, P.C.; Siu, Y.K.; Lewindon, P.J.; Wong, W.; Cheung, K.L.; Dawkins, R. Congenital Candida pneumonia in a preterm infant. J Paediatr Child Health. 1994;30(6):552-4. [CrossRef]
- Carmo, K.B.; Evans, N.; Isaacs, D. Congenital candidiasis presenting as septic shock without rash. Arch Dis Child. 2007;92(7):627-8. [CrossRef]
- Tezer, H.; Canpolat, F.E.; Dilmen, U. Invasive fungal infections during the neonatal period: Diagnosis, treatment and prophylaxis. Expert Opin Pharmacother. 2012;13(2):193-205. [CrossRef]
- Barone, S.R.; Krilov, L.R. Neonatal candidal meningitis in a full-term infant with congenital cutaneous candidiasis. Clin Pediatr (Phila). 1995;34(4):217-9. [CrossRef]
- Baradkar, V.P.; Taklikar, S.M. Meningitis caused by Candida albicans in a premature neonate. J Pediatr Neurosci, 2007,2(2):90–91. [CrossRef]
- Liu, S.H.; Mitchell, H.; Nasser Al-Rawahi, G. Epidemiology and associated risk factors for candidemia in a Canadian tertiary paediatric hospital: An 11-year review. J Assoc Med Microbiol Infect Dis Can. 2023;8(1):29-39. [CrossRef]
- Kopanou Taliaka P, Tsantes AG, Konstantinidi A, Houhoula D, Tsante KA, Vaiopoulos AG, Piovani D, Nikolopoulos GK, Bonovas S, Iacovidou N, Tsantes AE, Sokou R. Risk Factors, Diagnosis, and Treatment of Neonatal Fungal Liver Abscess: A Systematic Review of the Literature. Life (Basel). 2023 Jan 6;13(1):167. [CrossRef] [PubMed] [PubMed Central]
- Meizoso, T.; Rivera, T.; Fernández-Aceñero, M.J.; Mestre, M.J.; Garrido, M.; Garaulet, C. Intrauterine candidiasis: Report of four cases. Arch Gynecol Obstet. 2008;278(2):173-6. [CrossRef]
- Qureshi, F.; Jacques, S.M.; Bendon, R.W.; Faye-Peterson, O.M.; Heifetz, S.A.; Redline, R.; Sander, C.M. Candida funisitis: A clinicopathologic study of 32 cases. Pediatr Dev Pathol. 1998;1(2):118-24. [CrossRef]
- Aliaga, S.; Clark, R.H.; Laughon, M.; Walsh, T.J.; Hope, W.W.; Benjamin, D.K.; Kaufman, D.; Arrieta, A.; Benjamin, D.K. Jr.; Smith, P.B. Changes in the incidence of candidiasis in neonatal intensive care units. Pediatrics. 2014;133(2):236-42. [CrossRef]
- Ellepola, A.N.; Morrison, C.J. Laboratory diagnosis of invasive candidiasis. J Microbiol. 2005;43 Spec No:65-84. [PubMed]
- Clancy, C.J.; Nguyen, M.H. Finding the "missing 50%" of invasive candidiasis: How nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56(9):1284-92. [CrossRef]
- Fleiss, N.; Schwabenbauer, K.; Randis, T.M.; Polin, R.A. What's new in the management of neonatal early-onset sepsis? Arch Dis Child Fetal Neonatal Ed. 2023;108(1):10-14. [CrossRef]
- Celik, I.H.; Hanna, M.; Canpolat, F.E.; Pammi, M. Diagnosis of neonatal sepsis: The past, present and future. Pediatr Res. 2022;91(2):337-350. [CrossRef]
- Schelonka, R.L.; Moser, S.A. Time to positive culture results in neonatal Candida septicemia. J Pediatr. 2003;142(5):564-5. [CrossRef]
- Oeser, C.; Pond, M.; Butcher, P.; Bedford Russell, A.; Henneke, P.; Laing, K.; Planche, T.; Heath, P.T.; Harris, K. PCR for the detection of pathogens in neonatal early onset sepsis. PLoS ONE. 2020;15(1):e0226817. [CrossRef]
- Greenberg, R.G.; Benjamin, D.K. Jr. Neonatal candidiasis: Diagnosis, prevention, and treatment. J Infect. 2014;69 Suppl 1(0 1):S19-22. [CrossRef]
- Stein, A.; Soukup, D.; Rath, P.M.; Felderhoff-Müser, U. Diagnostic Accuracy of Multiplex Polymerase Chain Reaction in Early Onset Neonatal Sepsis. Children (Basel). 2023;10(11):1809. [CrossRef]
- Aittakorpi, A.; Kuusela, P.; Koukila-Kähkölä, P.; Vaara, M.; Petrou, M.; Gant, V.; Mäki, M. Accurate and rapid identification of Candida spp. frequently associated with fungemia by using PCR and the microarray-based Prove-it Sepsis assay. J Clin Microbiol. 2012;50(11):3635-40. [CrossRef]
- Innings, A.; Ullberg, M.; Johansson, A.; Rubin, C.J.; Noreus, N.; Isaksson, M.; Herrmann, B. Multiplex real-time PCR targeting the RNase P RNA gene for detection and identification of Candida species in blood. J Clin Microbiol. 2007;45(3):874-80. Erratum in: J Clin Microbiol. 2007;45(6):2104. [CrossRef] [PubMed] [PubMed Central]
- Kosmeri, C.; Giapros, V.; Serbis, A.; Baltogianni, M. Application of Advanced Molecular Methods to Study Early-Onset Neonatal Sepsis. Int J Mol Sci. 2024;25(4):2258. [CrossRef]
- Shabaan, A.E.; Elbaz, L.M.; El-Emshaty, W.M.; Shouman, B. Role of serum (1,3)-β-d-glucan assay in early diagnosis of invasive fungal infections in a neonatal intensive care unit. J Pediatr (Rio J). 2018;94(5):559-565. [CrossRef]
- Warris, A.; Lehrnbecher, T. Progress in the Diagnosis of Invasive Fungal Disease in Children. Curr Fungal Infect Rep. 2017;11(2):35-44. [CrossRef]
- Manzoni, P.; Mostert, M.; Galletto, P.; Gastaldo, L.; Gallo, E.; Agriesti, G.; Farina, D. Is thrombocytopenia suggestive of organism-specific response in neonatal sepsis? Pediatr Int. 2009;51(2):206-10. [CrossRef]
- Wolach, B.; Bogger-Goren, S.; Whyte, R. Perinatal hematological profile of newborn infants with candida antenatal infections. Biol Neonate. 1991;59(1):5-12. [CrossRef]
- Tasneem, F.; Hossain, M.M.; Mahmud, S, Ahmed, S.S.;. Clinical profile of fungal sepsis in new born: A tertiary centre experience from Bangladesh. J Pediatr Neonatal Care. 2020;10(6):170-173. [CrossRef]
- Ratridewi, I.; Amalia, K.; Huwae, T.E.; Putera, M.A.; Sulistijono, E. Systemic Candidosis Diagnostic Test with Candida Score and Monocyte Count in Premature Infants with Late-Onset Sepsis: Research in Low Resources Country // Russian Journal of Infection and Immunity. 2023; 13(1): 133-140. [CrossRef]
- Noyola, D.E.; Fernandez, M.; Moylett, E.H.; Baker, C.J. Ophthalmologic, visceral, and cardiac involvement in neonates with candidemia. Clin Infect Dis. 2001;32(7):1018-23. [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; Zaoutis, T.E.; Sobel, J.D. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1-50. [CrossRef]
- Ruhnke, M.; Rickerts, V.; Cornely, O.A.; Buchheidt, D.; Glöckner, A.; Heinz, W.; Höhl, R.; Horré, R.; Karthaus, M.; Kujath, P.; Willinger, B.; Presterl, E.; Rath, P.; Ritter, J.; Glasmacher, A.; Lass-Flörl, C.; Groll, A.H; German Speaking Mycological Society; Paul-Ehrlich-Society for Chemotherapy. Diagnosis and therapy of Candida infections: Joint recommendations of the German Speaking Mycological Society and the Paul-Ehrlich-Society for Chemotherapy. Mycoses. 2011;54(4):279-310. [CrossRef]
- Hope, W.W.; Castagnola, E.; Groll, A.H.; Roilides, E.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; Bassetti, M.; Bille, J.; Cornely, O.A.; Cuenca-Estrella, M.; Donnelly, J.P.; Garbino, J.; Herbrecht, R.; Jensen, H.E.; Kullberg, B.J.; Lass-Flörl, C.; Lortholary, O.; Meersseman, W.; Petrikkos, G.; Richardson, M.D.; Verweij, P.E.; Viscoli, C.; Ullmann, A.J; ESCMID Fungal Infection Study Group. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect. 2012;18 Suppl 7:38-52. [CrossRef]
- Vasileiou, E.; Apsemidou, A.; Vyzantiadis, T.A.; Tragiannidis, A. Invasive candidiasis and candidemia in pediatric and neonatal patients: A review of current guidelines. Curr Med Mycol. 2018;4(3):28-33. [CrossRef]
- Robbins, N.; Caplan, T.; Cowen, L.E. Molecular Evolution of Antifungal Drug Resistance. Annu Rev Microbiol. 2017;71:753-775. [CrossRef]
- Gharaghani, M.; Taghipour, S.; Halvaeezadeh, M.; Mahmoudabadi, A.Z. Candiduria; a review article with specific data from Iran. Turk J Urol. 2018;44(6):445-452. [CrossRef]
- Wade, K.C.; Benjamin, D.K. Jr.; Kaufman, D.A.; Ward, R.M.; Smith, P.B.; Jayaraman, B.; Adamson, P.C.; Gastonguay, M,R.; Barrett, J.S. Fluconazole dosing for the prevention or treatment of invasive candidiasis in young infants. Pediatr Infect Dis J. 2009;28(8):717-23. [CrossRef]
- Lu, H.; Shrivastava, M.; Whiteway, M.; Jiang, Y. Candida albicans targets that potentially synergize with fluconazole. Crit Rev Microbiol. 2021;47(3):323-337. [CrossRef]
- Berman, J.; Krysan, D.J. Drug resistance and tolerance in fungi. Nat Rev Microbiol. 2020;18(6):319-331. Erratum in: Nat Rev Microbiol. 2020;18(9):539. Erratum in: Nat Rev Microbiol. 2020;18(9):539. doi: 10.1038/s41579-020-0415-y. [CrossRef]
- Rosenberg, A.; Ene, I.V.; Bibi, M.; Zakin, S.; Segal, E.S.; Ziv, N.; Dahan, A.M.; Colombo, A.L.; Bennett, R.J.; Berman, J. Antifungal tolerance is a subpopulation effect distinct from resistance and is associated with persistent candidemia. Nat Commun. 2018;9(1):2470. [CrossRef]
- Benjamin, D.K. Jr.; Stoll, B.J.; Fanaroff, A.A.; McDonald, S.A.; Oh, W.; Higgins, R.D.; Duara, S.; Poole, K.; Laptook, A.; Goldberg, R; National Institute of Child Health and Human Development Neonatal Research Network. Neonatal candidiasis among extremely low birth weight infants: Risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006;117(1):84-92. [CrossRef]
- de Haan, T.R.; Beckers, L.; de Jonge, R.C.; Spanjaard, L.; van Toledo, L.; Pajkrt, D.; van Wassenaer-Leemhuis, A.G.; van der Lee, J.H. Neonatal gram negative and Candida sepsis survival and neurodevelopmental outcome at the corrected age of 24 months. PLoS ONE. 2013;8(3):e59214. [CrossRef]
- Mendling, W.S.H. Antimykotische Therapie der Vaginalen Hefepilz-Kolonisation von Schwangeren zur Verhutung von Kandidamykosen beim Neugeborenen. AMWF 2008;15:S1.
- Mantadakis, E.; Pana, Z.D.; Zaoutis, T. Candidemia in children: Epidemiology, prevention and management. Mycoses. 2018;61(9):614-622. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





