1. Introduction
It is a well-known fact that during phacoemulsification a fundamental change occurs not only in the state of the lens, but the operation also affects the integrity of the eye as a whole. From this point of view, the most studied part of the eye is the corneal endothelium and the corneal curvature. The former one can be significantly damaged due to high flow rates and ultrasound energy, or the insufficient use of viscoelastic material [
1,
2], while the curvature of the cornea is primarily influenced by the size of the wound, its location, its relation to the limbus, and the mechanical effects suffered during surgery [
3,
4]. Experienced surgeons also know how to perform phacoemulsification with as little damage to the iris as possible. Since 1975 it was reported in several publications that posterior vitreous detachment (PVD) occurs much more often after cataract extraction [
5,
6,
7], and the chance of retinal detachment increases several folds after phacoemulsification, especially in highly myopic eyes [
6,
7,
8]. On the other hand, the vitreolenticular compartment or the behavior of anterior hyaloid membrane (AHM) after phacoemulsification has only recently been studied. The behavior of Berger’s space (BS), first described by Wieger [
9], during phacoemulsification was reported first by Tassignon and Dhubhghaill in 2016 [
10]. Real-time intraoperative optical coherence tomography (iOCT) [
10,
11,
12,
13,
14] and anterior segment optical coherence tomography (AS-OCT) [
11,
12,
14,
15,
16,
17,
18] was used to visualize the posterior capsule, the AHM, and the possible change of the intervening BS during surgery [
10,
11,
12,
13,
14]. It has now become clear that phacoemulsification has a significant impact on BS [
10,
11,
12,
13,
14,
15,
16,
17,
18]. After phacoemulsification the preoperatively existing BS in most cases enlarges after surgery, or the preoperatively attached AHM detaches from the posterior capsule in certain cases [
12,
14,
15,
16,
17,
18]. It has been proven that the age of the patient, the axial length of the eye, presence of lens materials in BS (LM-BS), weakness of zonular fibers, and corneal power affect the behavior of AHM [
12,
13,
14,
15,
16,
18]. Data are available on how certain surgical parameters of phacoemulsification such as total surgical time, cumulative dissipated energy (CDE), ultrasonic time, mean longitudinal power, total aspiration time, fluid usage, infusion pressure, aspiration flow rate, bottle height and vacuum influence the development of BS [
12,
14,
15,
17,
19]. However, some surgical parameters that can be assumed to have an effect on the state of the vitreolenticular compartment were not investigated. In the present study, in addition to those listed above, we investigated the significance of nuclear sclerosis grade, hydrodissection, capsulorhexis size and capsular polish, the difficulty of nucleus rotation and cortical irrigation-aspiration. Previously LM-BS and certain anatomical parameters were correlated, as well as the relationship between LM-BS and some surgical parameters of phacoemulsification were studied [
10,
11,
12,
13,
14].
Until now, the vitreolenticular interface was observed for a maximum term of 3 months. To the best of our knowledge, this is the first one-year-long prospective report documenting the attachment or detachment of AHM after and related to intraoperative parameters during phacoemulsification. AHM detachment will be referred to as anterior vitreous detachment or AVD further on.
2. Materials and Methods
In this prospective non-randomized interventional study, we assessed the evolution of the retrolental space before and after cataract surgery using swept source AS-OCT (SS-AS-OCT). The study was conducted with approval from the local ethics committee. The study was conducted in accordance with the Declarations of Helsinki.
2.1. Patients
Between 2021 July and 2023 July, we examined 82 eyes of 82 patients: preoperatively, on 1-day, 1-month, 3-months, 1-year follow-up. At each check-up, visual acuity, intraocular pressure, pupil dilation and slit-lamp examination with fundus examination were performed. All patients had preoperative Lens Opacity Classification System III (LOCSIII) grade 1–6 nuclear sclerosis, diagnosed through slit-lamp examination. Preoperatively and on each follow-up SS-AS-OCT in pupil dilation were performed with the Anterion (Heidelberg Engineering, Heidelberg, Germany). Eyes with corneal, iris, or lens malformations, phacodonesis, poorly dilated pupils, or eyes previously undergone any eye surgery were excluded from the study. Out of the initial 100 eyes, in 1 case posterior capsular rupture occurred, and 17 patients did not come for regular check-ups, these eyes were also excluded from the study.
2.2. Surgical Procedure
The operations were performed by a single experienced surgeon under topical anesthesia. A 2.7mm clear corneal incision was placed in the temporal 180-0 degrees, followed by uneventful phacoemulsification and implantation of a one-piece IOL into the capsular bag. Megatron S4 phaco system (Geuder AG, Heidelberg, Germany) was used. Stop-and-chop technique was performed with the following fluidic parameters: flow rate 25 mL/min, maximal vacuum 300 mmHg, maximal ultrasound energy 70%, linear cold flash mode, infusion bottle height 70 cm. During irrigation-aspiration of the cortex material, the following parameters were used: flow rate 23 mL/min, maximal vacuum 540 mmHg, infusion bottle height 70 cm. The posterior capsule was polished as necessary in each case. During polishing, the following parameters were used: flow rate 10 mL/min, maximal vacuum 30 mmHg, infusion bottle height 70 cm.
2.3. Intraoperative Data Collection
The following data were recorded: capsulorhexis size (with an accuracy of 0.5 mm), number of hydrodissection, difficulty of nucleus rotation, CDE, ultrasonic time, total surgical time, difficulty of irrigation/aspiration, intensity of capsular polish, weakness of zonular fibers, LM-BS (yes, no), fluid usage (with an accuracy of 20 ml). Semi-quantitatively described nucleus rotation, irrigation/aspiration, capsular polish, and weakness of zonular fibers were scored on a scale of 1 to 3 or 1 to 4 for further statistical analysis (
Table 1.).
2.4. Image Acquisition
Image acquisition was performed in accordance with the method description created by our own in the previous article [
18]. Preoperatively, we performed intraocular lens power calculation with the Cataract App of Anterion device, and we also captured eyes using the Metrics App. The Metrics App automatic mode creates multiple radial B-scans up to 16.5 mm in length and 14.0 mm depth using 1300 nm wavelength. The images taken clearly depict the cornea, anterior chamber, angle of the chamber, iris, and lens, but the clear visibility of retrolental space in phakic eyes is limited. The presence or absence of AHM was checked in the Imaging App. Turning off the Imaging App automatic mode, in manual settings, by pushing the device closer to the eyes it was possible to take deep and clear images of retrolental space. By choosing the most appropriate dense pattern in Imaging App manual mode, we made sure if the hyperreflective line visible in the Metrics mode is not just a vitreous fiber, but actually the AHM. Postoperatively, we only used the Metrics App automatic mode in all the follow-ups, the AHM is clearly visible behind the narrow IOL.
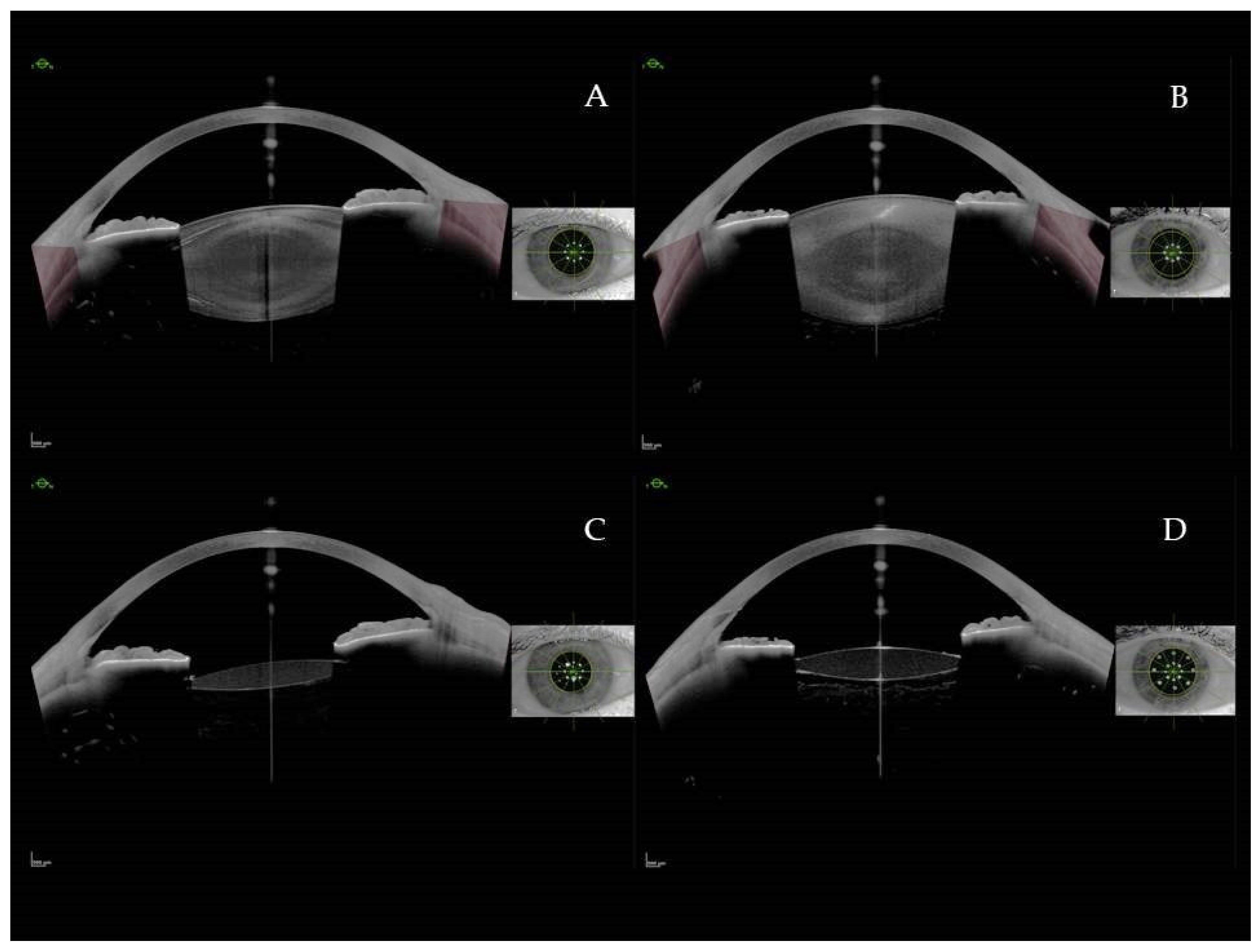
Figure 1.
AS-SS-OCT images: Preoperative (A) and postoperative (C) image without visible anterior hyaloid detachment (AHD). Preoperative (B) and postoperative (D) image with AHD.
Figure 1.
AS-SS-OCT images: Preoperative (A) and postoperative (C) image without visible anterior hyaloid detachment (AHD). Preoperative (B) and postoperative (D) image with AHD.
2.5. Statistical Analysis
In the case of quantitative variables, the valid N, mean, standard deviation, minimum, 25th percentile, median, 75th percentile and maximum values were given, while in the case of qualitative variables, the number of cases and the percentage.
For qualitative variables, the Fisher test (less than 5 observed values) or the Pearson Chi-Square test (more than 5 observed values) was used. In the case of qualitative variables where the variable can be considered ordinal, the linear association was also examined (linear-by-linear association).
The Kolmogorov-Smirnov Test was used to examine the deviation of the variables from their normal distribution. Since the distribution of all variables, except for the age variable, differed from the normal distribution, non-parametric versions of the tests were applied. Independent t-test (two groups) or One-Way Anova test (> two groups) was used to test the differences between individual groups. In the case of multiple comparisons, Bonferroni correction was used to modify the p-value.
The limit of statistical significance was p<0.05.
3. Results
82. eyes of 82 patients were included in the analysis: 21 (25.6%) men and 61 (74.4%) women, mean age: 70.9 years. The average AL was 23.43 mm. AHM conditions for the different follow-up times are summarized in
Table 2.
Distribution of values of capsulorhexis size, number of hydrodissections, difficulty of nucleus rotation, difficulty of irrigation/aspiration, intensity of capsular polish, weakness of zonular fibers, LM-BS (yes, no) are shown in
Table 3.
The results were discussed in relation to the situation of the AHM. The significant correlations were discussed separately for the follow-up dates, and the LM-BS is also described in a separate paragraph in relation to the intraoperative surgical parameters.
Postoperative 1-day follow-up: A significant linear trend was observed between postoperative 1-day AVD and the increasing degree of zonular weakness (p=0.023). The CDE was significantly higher in the postop 1-day AVD group (t-test, p=0.005).
1-month follow-up: A significant linear trend was observed between the presence of AVD at 1-month follow-up and high-grade nuclear sclerosis (p=0.011), respectively with the increase in intraoperative zonular weakness (p=0.001). The CDE (t-test, p=0.001), and higher amount of fluid used (t-test, p=0.012) was significantly higher in postop 1-month AVD group. A significant correlation was observed between the 1-month AVD presence and the LM-BS intraoperatively (p=0.026).
3-month follow-up: A significant linear trend was observed between the visible AVD at 3-month follow-up and high-grade nuclear sclerosis (p=0.014), and a significant linear trend was also observed with increasing zonular weakness (p=0.004). Correlation was seen between AVD and separately higher CDE (t-test, 0.001), high total surgical time (t-test, 0.028) and high amount of fluid used (t-test, 0.021).
1-year follow-up: A significant linear trend was observed between AVD at 1-year follow-up and high-grade nuclear sclerosis (p=0.015), and a significant linear trend was also observed with increasing zonular weakness (p=0.024). Higher CDE (t-test, 0.002), and high amount of fluid used (t-test, 0.014) correlated to 1-year AVD.
Capsulorhexis size, number of hydrodissections, difficulty of nucleus rotation, difficulty of irrigation/aspiration, intensity of capsular polish, and ultrasonic time did not show a significant correlation with AVD at any time point.
Intraoperative parameters and the BS
A significant linear trend was observed in the group of LM-BS as the nuclear sclerosis increases (p=0.004), respectively as the polish value increases (p=0.012), and as the zonular weakness value increases (p<0.001).
In the LM-BS group the CDE (ANOVA, p=0.025), the total surgical time (ANOVA, p<0.001), and the used fluidd were also significantly higher (ANOVA, p<0.001). Capsulorhexis size, number of hydrodissections, difficulty of nucleus rotation, difficulty of irrigation/aspiration, and ultrasonic time did not show a significant correlation with the presence of LM-BS at any time point.
4. Discussion
In 1986, Weidle described a method of visualizing the BS in vivo by injecting an ophthalmic viscosurgical device to protect the AHM during posterior capsulotomy [
20]. Tassignon et al., who routinely performed bag-in-the-lens implantation, developed a technique for sparing the AHM during posterior capsulorhexis [
21]. In 2016, Tassignon and Dhubhghaill used iOCT to visualize the AHM and the changes of BS during phacoemulsification, and they hypothesized that the BS is a true anatomical space with a true anatomical purpose what needs to be protected [
10]. In 2017, Masuda et al. suggested the use of irrigation dynamic pressure-assisted hydrodissection technique which prevents high-pressure hydrodissection-related complications, such as capsular block syndrome and tears in the AHM during cataract surgery [
22]. Nowadays, Scarfone et al. proved that the high infusion pressure used during phacoemulsification has a detrimental effect on the AHM barrier which can prevent using active fluidics and active sentry [
17]. These few examples above also show that it is worthwhile to investigate as broadly as possible which parameters of phacoemulsification predispose to AVD. It is also worthwhile to be aware of the coexistent anatomical features with disintegration of the vitreolenticular compartment.
In our study, a significant linear trend was observed between the visible AVD and zonular weakness (p≤0.024) at all follow-up times. High CDE correlated to AVD also at all follow-up times (t-test, p≤0.005). Similarly, AVD was observed at 1-month, 3-month, and 1-year follow-up in cases of high-grade nuclear sclerosis (p≤0.044) and high fluid usage (p≤0.021). On the other hand, the impact of capsulorhexis size, number of hydrodissections, CDE, total surgical time, difficulty of irrigation/aspiration, and intensity of capsular polish showed no significant association with AVD. The effect of various phacoemulsification parameters on postoperative AVD have been investigated by several authors [
12,
14,
15,
16,
17]. These previous study results are summarized in
Table 4.
Our own experience in agreement with the relevant literature suggest that zonular weakness [
12], higher than average ultrasound use [
12,
14,
15], and high-pressure infusion [
15,
17], higher CDE [
14] predispose to AVD. Regarding to high-grade nuclear sclerosis and high fluid usage - previous comparisons did not find a significant correlation with AHM detachment [
14,
16]; however, we observed both parameters to be significantly correlated with AHM detachment for most of the follow-up times. This issue might require further investigation.
Further, we observed that the AHM detachments related to the intraoperative risk factors do not change during the one-year follow-up period.
The previously mentioned LM-BS concept is used in the literature when the lens material is visualized with different methods during cataract surgery: this may be the modern iOCT, but the LM-BS also has a characteristic image with the operating microscope. [
10,
11,
12,
13,
14]. LM-BS was also assessed postoperatively by AS-OCT [
11,
14]. Our findings agree with Anisimova [
11] and Lin [
14]: phacoemulsification can result in zonular dehiscence, AHM detachment and/or Wieger’s ligament injury, which can lead to lens material entering the BS.
In our study we found other risk factors for LM-BS in addition to those mentioned above. A significant linear trend was observed in the group of LM-BS as the grade of nuclear sclerosis, the intensity of capsular polish, and the zonular weakness value increases. In the LM-BS group the CDE, the total surgical time, and the used fluid were also significantly higher.
5. Conclusions
In summary, this is the first prospective report documenting the impact of surgical parameters of phacoemulsification on behavior of AHM over a 1-year period. Using SS-AS-OCT the effect of capsulorhexis size, number of hydrodissections, difficulty of nucleus rotation, nuclear sclerosis grade, CDE, ultrasonic time, total surgical time, difficulty of irrigation/aspiration, intensity of capsular polish, weakness of zonular fibers, presence of LM-BS, and fluid usage were investigated related to the behavior of AHM. Relation between LM-BS and certain eye parameters as well as relation between LM-BS and certain surgical parameters of phacoemulsification were also studied. A significant linear trend was observed between the visible AHM detachment and zonular weakness at all follow-up times. High CDE correlated to AHM detachment group also at all follow-up times. Similarly, AHM detachment was observed at 1-month, 3-month, and 1-year follow-up in cases of high-grade nuclear sclerosis and high fluid usage. Zonular weakness, high-grade nuclear sclerosis, extensive capsular polish, high CDE, high total surgical time, and high fluid usage seems to be risk factors the presence of LM-BS.
Author Contributions
Conceptualization, Á.E. and P.V.; methodology, P.V.; software, Á.E.; validation, Á.E. and P.V; formal analysis, Á.E.; investigation, P.V.; resources, P.V.; data curation, Á.E.; writing—original draft preparation, P.V.; writing—review and editing, Á.E.; visualization, Á.E.; supervision, P.V.; project administration, Á.E.; funding acquisition, P.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Péterfy Sándor Hospital (protocol code 18/2023, date of approval 4 September 2023).
Informed Consent Statement
Participation in this study was voluntary and written informed consent was obtained from all participants.
Data Availability Statement
Data will be available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Binder, P.S.; Sternberg, H.; Wickman, M.G.; Worthen, D.M. Corneal endothelial damage associated with phacoemulsification. Am. J. Ophthalmol. 1976, 82, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Mayali, H.; Baser, E.F.; Kurt, E.; Ilker, S.S. Corneal endothelial damage in phacoemulsification using an anterior chamber maintainer compared with using an ophthalmic viscosurgical device. J. Cataract Refract. Surg. 2021, 47, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Langenbucher, A.; Szentmáry, N.; Cayless, A.; Casaza, M.; Weisensee, J.; Hoffmann, P.; Wendelstein, J. Surgically Induced Astigmatism after Cataract Surgery - A Vector Analysis. Curr. Eye Res. 2022, 47, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.N.; Goel, R.; Kumar, S. Factors affecting surgically induced astigmatism in manual small-incision cataract surgery. Indian J. Ophthalmol. 2022, 70, 3779–3784. [Google Scholar] [CrossRef] [PubMed]
- Friedman, Z.; Neumann, E. Posterior vitreous detachment after cataract extraction in nonmyopic eyes and the resulting retinal lesions. Br. J. Ophthalmol. 1975, 59, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Yoshida, M.; Hayashi, K.; Tsubota, K. Progression of posterior vitreous detachment after cataract surgery. Eye (Lond). 2022, 36, 1872–1877. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.H.; Steel, D.H.W. Retinal detachment following cataract phacoemulsification-a review of the literature. Eye (Lond). 2020, 34, 616–631. [Google Scholar] [CrossRef] [PubMed]
- Neuhann, I.M.; Neuhann, T.F.; Heimann, H.; Schmickler, S.; Gerl, R.H.; Foerster, M.H. Retinal detachment after phacoemulsification in high myopia: analysis of 2356 cases. J. Cataract Refract. Surg. 2008, 34, 1644–1657. [Google Scholar] [CrossRef]
- Wieger, G. Über den Canalis Petiti und ein ‘Ligamentum hyaloideocapsulare’; thesis. Université de Strasbourg, Strasbourg, 1883.
- Tassignon, M.-J.; Dhubhghaill, S.N. Real-time intraoperative optical coherence tomography imaging confirms older concepts about the Berger space. Ophthalmic. Res. 2016, 56, 222–226. [Google Scholar] [CrossRef]
- Anisimova, N.S.; Arbisser, L.B.; Shilova, N.F.; Melnik, M.A.; Belodedova, A.V; Knyazer, B.; Malyugin, B.E. Anterior vitreous detachment: Risk factor for intraoperative complications during phacoemulsification. J. Cataract Refract. Surg. 2020, 46, 55–62. [Google Scholar] [CrossRef]
- Lin, W.L.; Geng, W.J.; Ji, M.; Li, P.F.; Luo, L.W.; Guan, H.J. Effect of phacoemulsification on Berger space. Zhonghua Yan. Ke. Za. Zhi. 2022, 58, 506–512. [Google Scholar] [CrossRef]
- Vael, A.; Os, L.V.; Melis, K.; Tassignon, M.-J. Evaluation of the vitreolenticular interface with intraoperative OCT. J. Cataract Refract. Surg. 2022, 48, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Luo, J.; Li, P.; Ji, M.; Guan, H. Anterior vitreous detachment and retrolental material during cataract surgery: incidence and risk factors, with pathological evidence. J. Cataract Refract. Surg. 2023, 49, 578–583. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, J.; Chang, S.; Kanclerz, P.; Khoramnia, R.; Deng, M.; Wang, X. Incidence and risk factors for Berger’s space development after uneventful cataract surgery: Evidence from swept-source optical coherence tomography. J. Clin. Med. 2022, 11, 3580. [Google Scholar] [CrossRef]
- Mori, H.; Ueno, Y.; Fukuda, S.; Oshika, T. Detection of anterior hyaloid membrane detachment using deep-range anterior segment optical coherence tomography. J. Clin. Med. 2022, 11, 3057. [Google Scholar] [CrossRef] [PubMed]
- Scarfone, H.A.; Rodriguez, E.C.; Rufiner, M.G.; Riera, J.J.; Fanego, S.E.; Charles, M.; Albano, R.J. Vitreous-lens interface changes after cataract surgery using active fluidics and active sentry with high and low infusion pressure settings. J. Cataract Refract. Surg. 2024, 50, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Elekes, Á.; Németh, G.; Lauter, D.; Edelmayer, M.; Rupnik, Zs.; Vámosi, P. Examination of the Vitreolenticular Interface in Relation to Uneventful Phacoemulsification over One-Year Postoperative Period. J. Clin. Med. 2024, 13, 3219. [Google Scholar] [CrossRef]
- Vasavada, V.; Srivastava, S.; Vasavada, V.; Vasavada, S.; Vasavada, A.R.; Sudhalkar, A.; Bilgic, A. Impact of fluid parameters during phacoemulsification on the anterior vitreous face behavior: Experimental study. Indian J. Ophthalmol. 2019, 67, 1634–1637. [Google Scholar] [CrossRef]
- Weidle, E.G.; Lisch, W.; Thiel, H.J. Management of the opacified posterior lens capsule: an excision technique for membranous changes. Ophthalmic Surg. 1986, 17, 635–640. [Google Scholar] [CrossRef]
- Tassignon, M.; Gobin, L.; Mathysen, D.; van Looveren, J.; De Groot, V. Clinical outcomes of cataract surgery after bag-in-the-lens intraocular lens implantation following ISO standard 11979-7:2006. J. Cataract Refract. Surg. 2011, 37, 2120–2129. [Google Scholar] [CrossRef]
- Masuda, Y.; Iwaki, H.; Kato, N.; Takahashi, G.; Oki, K.; Tsuneoka, H. Irrigation dynamic pressure-assisted hydrodissection during cataract surgery. Clin. Ophthalmol. 2017, 11, 323–328. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).