Introduction
Despite the official end of the coronavirus disease 2019 (COVID-19) pandemic, cardiologists still face the long-term consequences of severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) infection. The possibility of acute coronavirus myocarditis, including virus-positive, has long been shown [1]. Much less is known about the incidence and mechanisms of post-COVID myocarditis.
Post-COVID syndrome presents as the irreversible tissue damage 12 weeks after diagnosis with varying degrees of dysfunction and symptoms [2]. Various mechanisms of such damage have been suggested for the heart: from low-intensity virus-negative inflammation to active viral myocarditis and immune-mediated myocardial damage [3]. The realisation of these mechanisms in the long-term is poorly studied. The incidence of chronic myocarditis among patients with cardiovascular post-COVID complications has been estimated at 0.4-28.9% [4], but these data do not support the evidence from endomyocardial biopsy (EMB).
Reports of morphologically proven post-COVID myocarditis remain a unique case. Thus, lymphocytic myocarditis was diagnosed in EMB 8 weeks after acute coronavirus infection; PCR for cardiotropic viruses and SARS-CoV-2 was negative [5]. The longest time of SARS-CoV-2 persistence in post-COVID myocarditis was one month [6]. Our data indicate the possibility of myocarditis with viral persistence in long-term after COVID-19 [7]. Evidence of the etiologic role of coronavirus is also detection of its proteins in the myocardium.
Another mechanism of chronic post-COVID myocarditis is the high autoimmunity (anti-heart antibodies production), that gives a background for the successful use of corticosteroids. It has been previously shown that high expression of toll-like receptors (TLR) type 4 is a marker of autoimmune myocarditis and a predictor of good response to immunosuppressive therapy (IST), [
7]. However, the clinical and morphologic features of post-COVID myocarditis, including expression of TLR, in comparison with non-COVID myocarditis have not been studied so far. There are also no comparative studies of therapy efficacy in this groups of patients.
Purpose: to compare morphological activity, toll-like receptors expression and response to the immunosuppressive therapy in patients with non-COVID and post-COVID myocarditis.
Methods
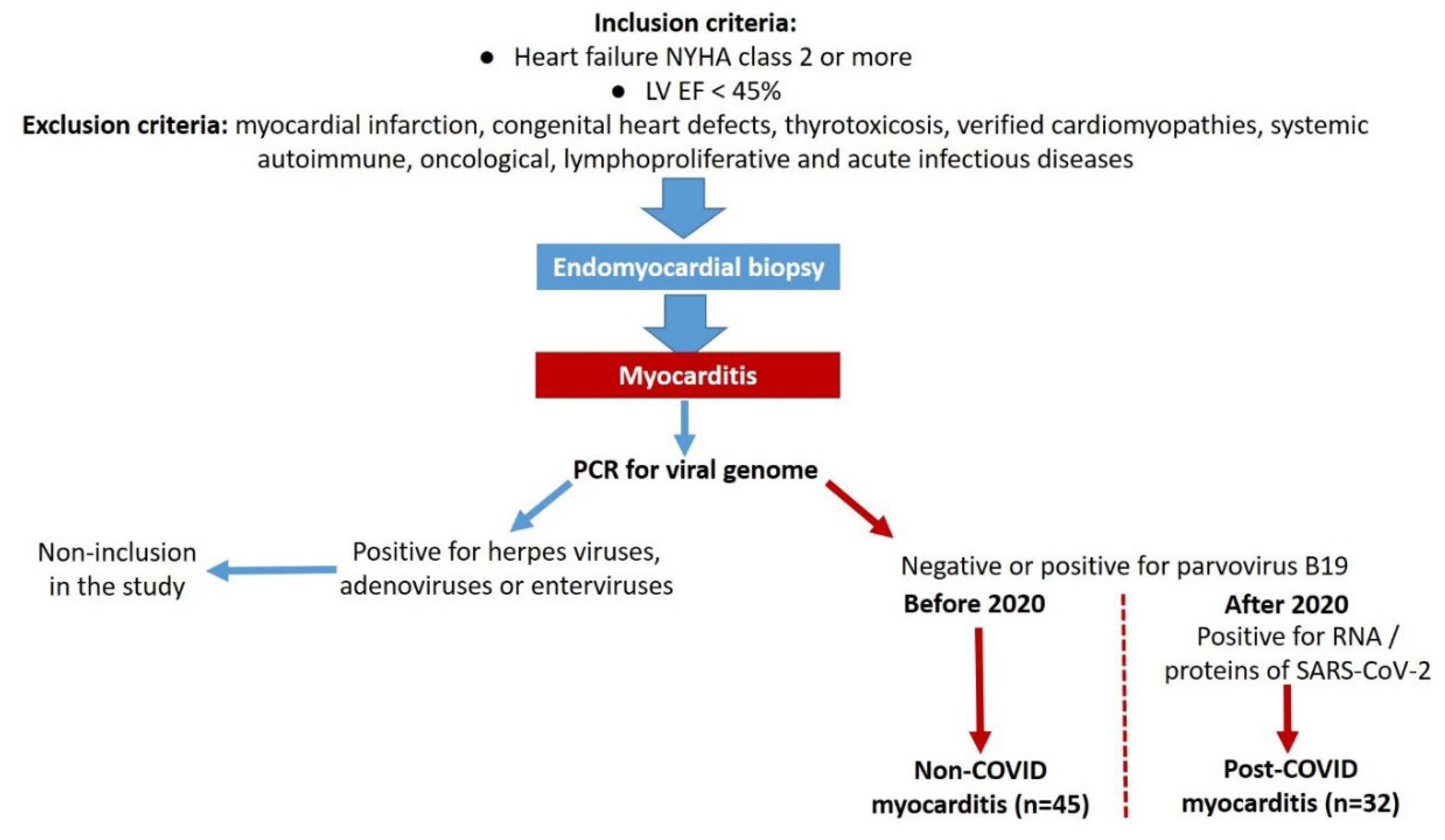
Seventy-seven patients (52 male and 25 female, 48.7±11.7 y.o.) with biopsy proven myocarditis were included. In 45 patients, myocarditis was diagnosed before the SARS-CoV-2 pandemic (non-COVID myocarditis). Other 32 patients had post-COVID myocarditis positive for RNA or/and proteins of SARS-CoV-2.
Additional inclusion criteria were the symptoms of heart failure NYHA class two or more, left ventricular ejection fraction (LV EF) < 45%, despite the maximally possible medical therapy for at least two months.
A history of myocardial infarction, congenital heart defects, thyrotoxicosis, verified cardiomyopathies (hypertrophic, restrictive, arrhythmogenic, familial or genetically proven dilated cardiomyopathy), systemic autoimmune, oncological, lymphoproliferative and acute infectious diseases were exclusion criteria.
In addition, viral genome detection in the myocardium, except for parvovirus B19 and/or SARS-CoV-2, pregnancy, mental disorders and refusal of the patient from participation in the study were a criterion for non-inclusion in the studies.
Research Methods
All patients underwent physical and standard laboratory tests, investigation of the anti-heart antibodies in the blood by the method of indirect immunofluorescence, echocardiography (EchoCG), Holter monitoring of an electrocardiogram, and also coronary angiography (n = 42) or multispiral computed tomography (MSCT) of the heart (n = 25), magnetic resonance imaging (MRI) of the heart (n = 44). To exclude systemic viral infection, a blood test was performed using the polymerase chain reaction (PCR) method.
Myocarditis was diagnosed in all patients by right ventricular EMB using Cordis biopsy forceps, followed by histological (staining with hematoxylin-eosin and Van Gieson), immunohistochemical (IHC, with antibodies to CD3, CD45, CD20, CD68) and virological (PCR for DNA of parvovirus B19, adenoviruses, enteroviruses and all viruses of the herpes group) investigations. From 2020 performed also PCR for coronavirus RNA or / and IHC study for coronavirus proteins (nucleocapsid and spike proteins). Total RNA was extracted from myocardial fragments using the RNeasy Mini Kit (Qiagen, Germany). A QuantiTect single-step PCR kit (Qiagen) was used to identify SARS-CoV-2. Only patients with a positive result were included in the study after 2020 (the subgroup of post-COVID-myocarditis). The diagnosis of active / borderline myocarditis was based on Dallas criteria supplemented by IHC criteria.
Additionally, the expression of TLR types 4 and 9 in the myocardium was measured (Monoclonal Mouse Antibody anti-TLR4, clone 76B357.1, GeneTex, dilution 1:200; Monoclonal Mouse Antibody anti-TLR9, clone 5G5, GeneTex, dilution 1:100). The results of the reactions were appraised by a semi-quantitative method: grade 0 was attributed to absence of TLR, grade 1 to 1–15% positive cells in myocardial biopsy (vascular endothelium, endocardial cells, lymphocytes, macrophages); grade 2 to 16–30%, grade 3 to 31–45%, grade 4 to 46-60%, grade 5 to 60-80% and grade 6 >60% positivity.
Study Design
The study was a single-center prospective cohort study. After confirming the diagnosis of myocarditis, excluding viral infection (with the exception of parvovirus B19 and SARS-CoV-2) and evaluating other inclusion and exclusion criteria, the patients were classified into two groups - non-COVID myocarditis (n = 45) and post-COVID myocarditis (n = 32),
Figure 1.
The fast all patients with non-COVID lymphocytic myocarditis (n=44) received methylprednisolone at an initial dose of 24-40 mg / day (in average 24 [24; 30] mg / gay) orally for 1 month, followed by a decrease to a maintenance dose (4-8 mg / day). Thirty-four patients with non-COVID lymphocytic myocarditis were additionally treated with a cytostatic: azathioprine (average dose 150 [93.75; 150] mg / day) or mycophenolate mofetil (2 g/ day). One patient each with giant cell and lymphocytic non-COVID myocarditis were not treated with IST due to the terminal heart failure and absolute indications for emergency heart transplantation.
The all patients with post-COVID myocarditis (one with giant cell myocarditis, three with eosinophilic and the rest with lymphocytic myocarditis) were treated with methylprednisolone at a starting dose 24-40 mg / day (in average 24 [24; 24] mg / day) followed by its tapering to a maintenance dose 4-8 mg / day. Eight patients were later additionally prescribed azathioprine at a dose 100 mg / day, three patient received mycophenolate mofetil 2 g / day.
All patients also received standard therapy of heart failure, which included beta-blockers, angiotensin-converting enzyme inhibitors (ACE inhibitors) or sacubitril-valsartan, mineralocorticoid receptor antagonists (AMR), dapagliflozin and loop diuretics. Сonsidering the time after COVID and the absence of systemic manifestations of the disease, the infectious diseases specialist did not recommend antiviral therapy.
The average follow-up period was 15.0 [6.0; 35.5] month, at least 6 months for patient. Every six months and at the end of the observation, a follow-up examination was carried out (assessment of the functional status, standard blood tests, anti-heart antibodies, EchoCG, Holter monitoring).
Study endpoints were death / heart transplantation and response to therapy: a 10% or more increase in EF was considered as an excellent response (1 point), a 5-9% increase as a good response (2 points), and a less than 5% increase or decrease in EF as no response to therapy (3 points). The dynamics of NYHA class, other structural and functional parameters of the heart were also assessed.
Statistical analysis was carried out using the SPSS software package version 23. Quantitative characteristics are presented as M ±δ (mean ± one standard deviation) or as a median indicating the 1st and 3rd quartiles. The normality was assessed using the Kolmogorov-Smirnov test, the significance of the differences - using the Student’s test, χ2 or Fisher’s test, Mann-Whitney, Wilcoxon tests. Spearman’s coefficient was used to assess correlations. Linear regression was performed to identify predictors of treatment response. Differences were considered statistically significant at p <0.05.
Ethical approval. The investigation is complies with the principles outlined in the Declaration of Helsinki. The study was approved by the local ethics committee of the Sechenov University. All patients signed informed consent for EMB and subsequent IST.
Results
Comparative clinical characteristics of patients with non-COVID and post-COVID myocarditis presented in
Table 1.
Patients with non-COVID and post-COVID myocarditis did not differ in the manifestation of heart failure and the degree of structural and functional cardiac lesions. Patients with post-COVID myocarditis presented by delayed onset of symptoms: median time after acute COVID-19 was 5.5 [2.75; 13.5] months. The diagnosis of new coronavirus infection was confirmed by positive PCR during the infection (43% of patients) and the appearance of diagnostic titres of antiviral antibodies (in all patients). Bilateral viral pneumonia was diagnosed at CT in 7 patients, respiratory failure developed in only four patients. The severity of acute COVID-19 did not correlate with subsequent post-COVID myocarditis.
The overall duration of the disease was not significantly different in patients with non-COVID and post-COVD myocarditis and was on average 10-11 months. There was a tendency for a more significant increase in titres of anti-heart antibodies in the post-COVID myocarditis, while inflammatory changes were generally absent in both groups. High pulmonary hypertension due to pulmonary embolism were not found as a cause of myocardial dysfunction.
In MRI, only one patient in each group showed signs of oedema. The majority of the patients who underwent this examination had a one or two criteria of myocarditis: subepicardial or intramyocardial late gadolinium enhancement mainly in LV myocardium, increased native myocardial relaxation time in T1 mode, increased extracellular volume, excessive amount of fluid in pericardium. There were also no differences between the groups.
Significant differences in cardiotropic therapy were associated with the time of EMB and myocarditis diagnosis - before or after 2020. Accordingly, patients with post-COVID myocarditis significantly more often were treated with sacubitril-valsartan and dapagliflozin. Their follow-up period was significantly shorter than in patients with non-COVID myocarditis (see
Table 1).
Morphological examination in patients with non-COVID and post-COVID myocarditis.
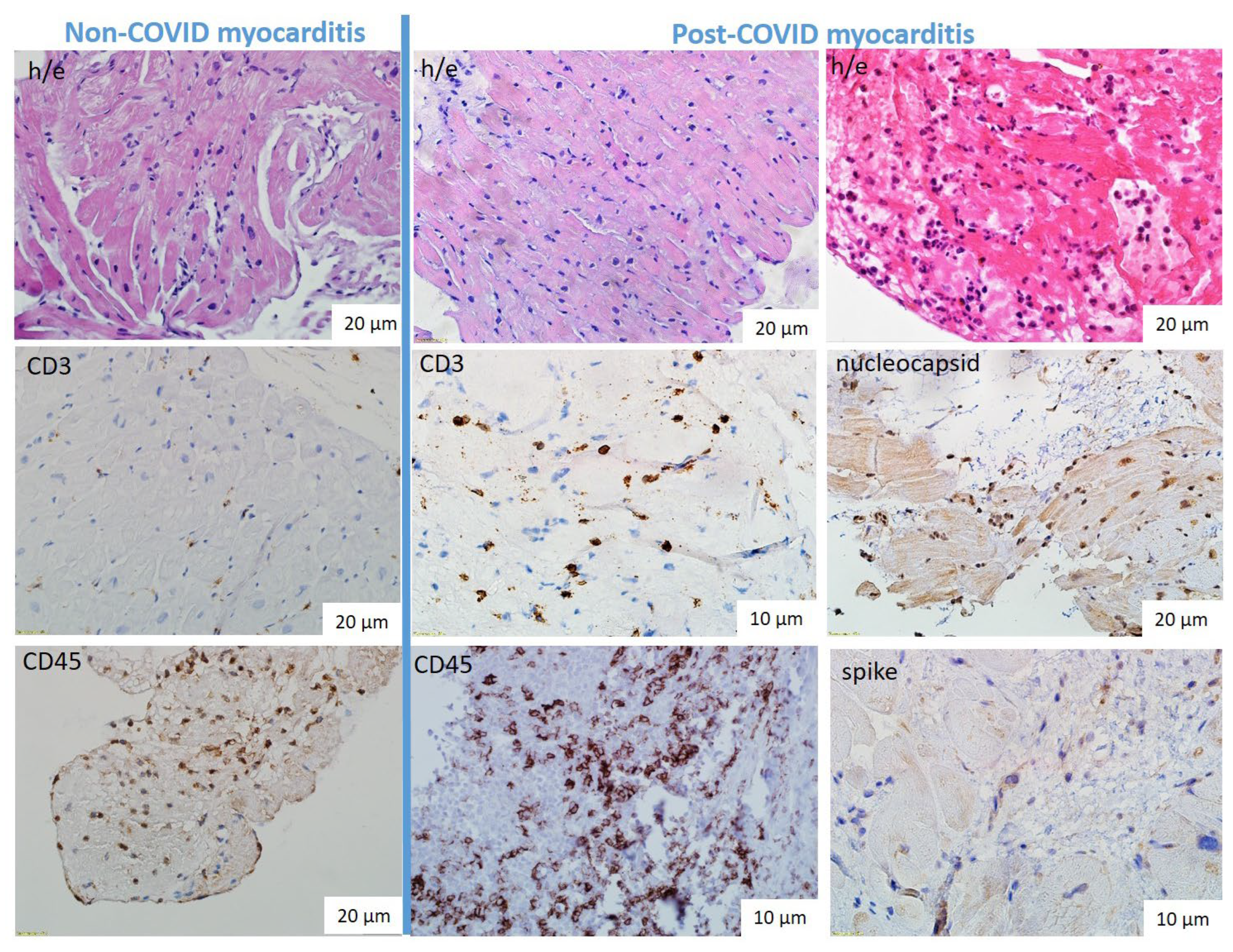
The lymphocytic variant was predominant in both non-COVID and post-COVID myocarditis (
Figure 2a,b): active in 82% and 97%, borderline in 18% and 3% respectively. One patient from each group had giant cell myocarditis. In addition, three eosinophilic myocarditis were diagnosed only among patients with post-COVID myocarditis (
Figure 2c). The specific characteristics of post-COVID myocarditis were also a high incidence of endocarditis (19% vs 7% in non-COVID myocarditis) and vasculitis (80% мс 54%) with microvascular thrombosis in 23% of cases vs only 8% in non-COVID myocarditis.
The
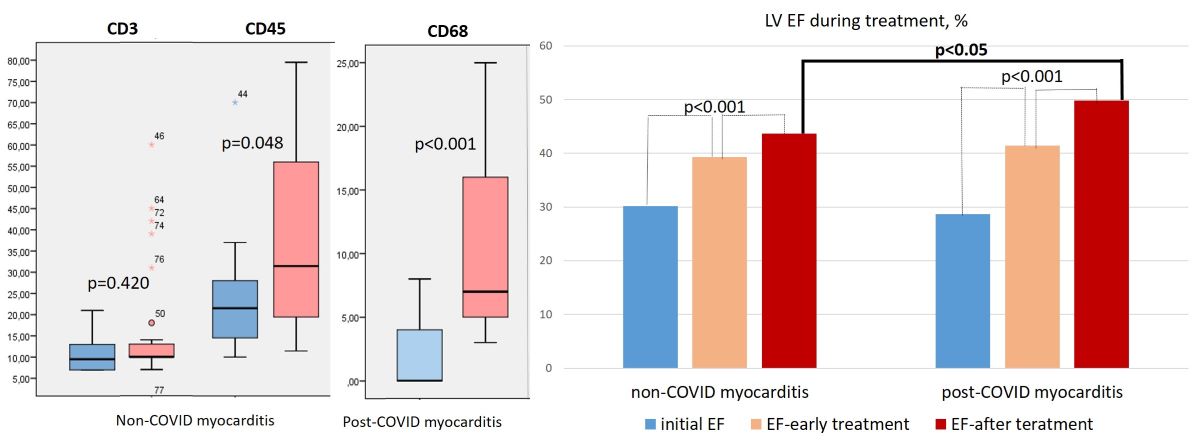
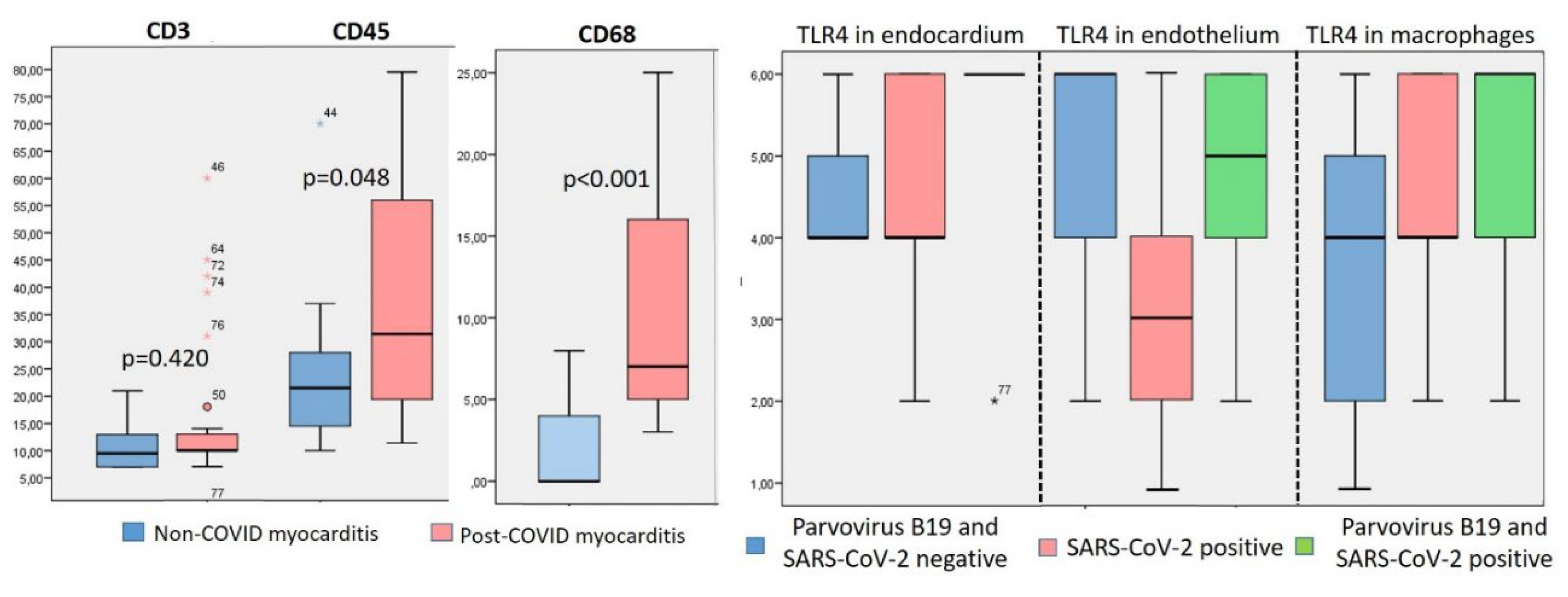
IHC study showed a non-significantly higher number of CD3-positive lymphocytes and a significantly higher number of CD45- and CD-68-positive cells in the infiltrates in patients with post-COVID myocarditis compared with non-CPVID myocarditis (
Table 2,
Figure 2d,e,g,h and
Figure 3, left panel). CD-20 positive lymphocytes were in fact absent in the infiltrates of both groups of patients.
The number of cells was counted per 1 mm2, and TLR expression was scored on a scale of 0 to 6. Explanation in the text.
Virological examination was performed at the stage of enrolment in the study. When viral genome was detected in myocardium, patients were not included in the study. The exclusion was made for patients with DNA of parvovirus B19 (in both groups). Coronavirus nucleocapsid and spike proteins with predominant deposition in endothelium, infiltrate cells (lymphocytes, macrophages) and, in lower degree, in cardiomyocytes were detected in all patients with post-COVID myocarditis (
Figure 3f,i). PCR for SARS-CoV-2 RNA was performed in 14 patients and was positive in 12 (86% of cases). The mean time from COVID-19 onset to myocardial biopsy was 7 [2.75; 18] months. The maximum time after acute COVID-19 in a patient with positive PCR for SARS-CoV-2 was 18 months.
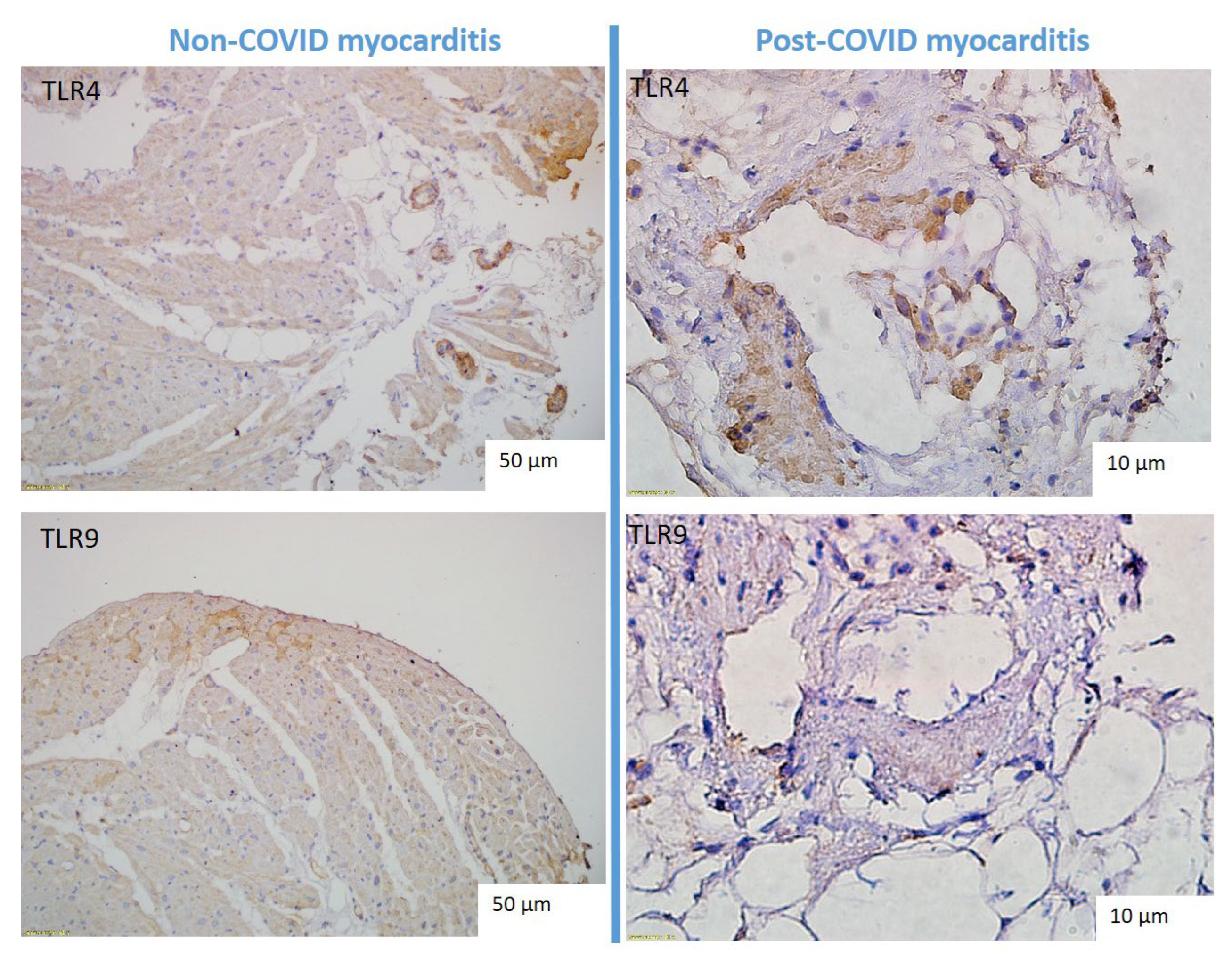
Comparison of TLR4 and TLT9 Expression in Myocardium in Patients with Non-COVID and Post-COVID Myocarditis
In patients with both non-COVID and post-COVID myocarditis, a marked expression of TLR type 4 and, to a lesser extent, TLR9 type 9 was detected (
Table 2). TLRs were expressed predominantly in the parietal endocardium, vascular endothelium, and macrophages (
Figure 4). We observed significant correlations of TLR9 expression and the number of CD3, CD68-positive cells in the infiltrates (r = 0.4, p<0.05). The expression of TLR4 and TLR 9 was not significantly higher in post-COVID myocarditis than in non-COVID myocarditis (
Table 2,
Figure 3, right panel). At the same time, TLR4 expression in endocardium and vascular endothelium was significantly correlated with detection of parvovirus B19 DNA in myocardium (r = 0.04, p < 0.05).
Comparison of response to the therapy in patients with non-COVID and post-COVID myocarditis.
Patients with non-COVID and post-COVID myocarditis were treated with comparable IST and complex therapy for heart failure (see
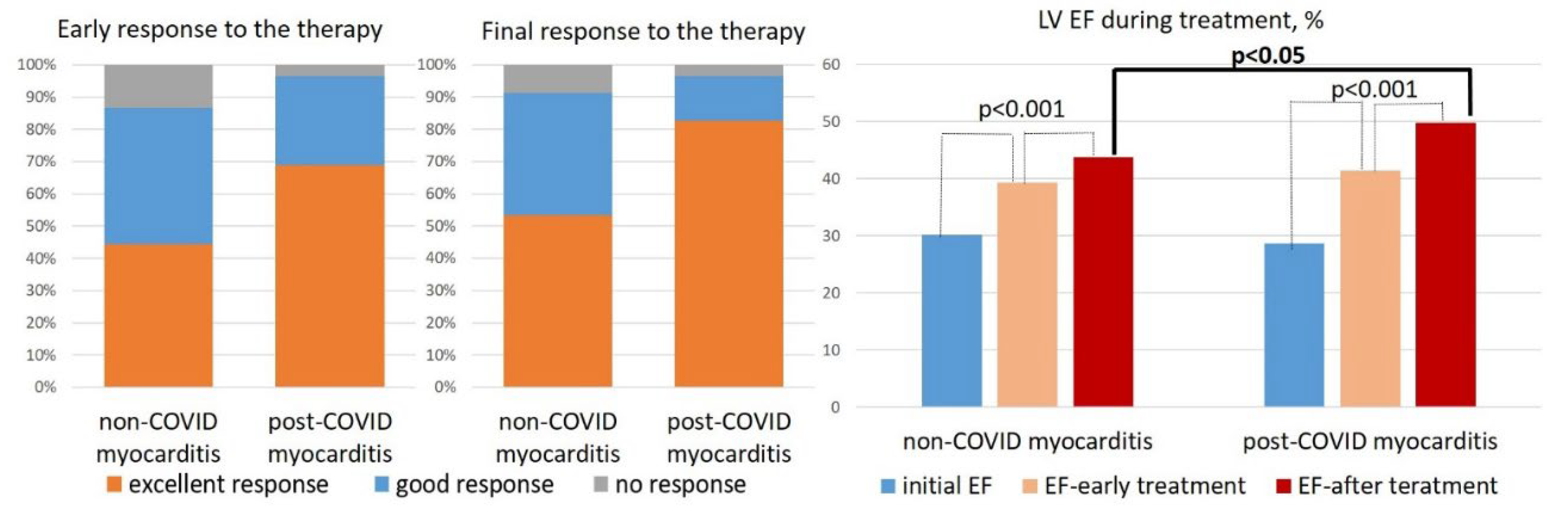
Table 1). At the same doses of methylprednisolone, patients with post-COVID myocarditis were treated more often with corticosteroid monotherapy (in 66%), whereas in non-COVID myocarditis a steroid and cytostatic combination was more often prescribed (in 76%). In the early treatment period (4-6 months after IST administration) and by the end of the follow-up, a significantly higher percentage of patients with excellent response to therapy (10% or more increase in LV EF) was observed in post-COVID myocarditis than in non-COVID myocarditis (
Figure 5, left panel). LV EF increased significantly in both groups, but by the end of the follow-up it was significantly higher in patients with post-COVID myocarditis (
Figure 5, right panel) in the absence of baseline differences (see
Table 1).
In view of some differences in the heart failure therapy and duration of treatment (see
Table 1), a multivariate analysis was performed to examine the impact of dapagliflozin, sacubitril-valsartan prescription, duration of follow-up, and post-COVID nature of myocarditis on the best response to therapy. Using linear regression method, we found that only the post-COVID myocarditis maintained independent significance with a significance level of p = 0.20.
Comparison of Disease Outcomes in Patients with Non-COVID and Post-COVID Myocarditis
There were no significant differences between the groups in lethality, transplantation rate and death + transplantation rate (
Table 3). The cause of death in four patients was terminal heart failure (heart transplantation could not be performed). One patient with non-COVID myocarditis had sudden cardiac death during the first month of treatment. In one patient with post-COVID myocarditis with a good response to treatment, the cause of death remained unclear.
There was an inverse correlation between unfavourable outcomes and treatment duration: all deaths, with one exception, occurred in the first months of treatment. Adverse outcomes correlated both with the degree of initial decrease in EF and with poor response to treatment. No significant correlations of TLR expression with response to treatment and unfavourable outcomes were found. The presence of parvovirus В19 DNA in the myocardium had no effect on treatment efficiency and outcomes.
Discussion
Our study is the first to associate the post-COVID nature of myocarditis with higher morphologic activity and better response to IST.
For the first time, we have shown that the number of not only macrophages but also CD45 positive cells in inflammatory infiltrates is significantly higher in post-COVID myocarditis compared with non-COVID myocarditis. The morphologic spectrum of myocarditis was wider in the post-COVID group: three cases of eosinophilic myocarditis and one giant cell myocarditis were diagnosed; the proportion of these highly immune forms was 12.5%. It is significantly higher than in normal myocarditis.
At the same time, the presence of coronavirus RNA and/or proteins in the myocardium was noted in all patients with post-COVID myocarditis, which was a criterion for the inclusion in this group. Coronavirus challenges the concept that eosinophilic and giant cell myocarditis are virus-negative and caused exclusively by immune mechanisms. Although most reports of COVID-associated eosinophilic myocarditis describe virus-negative cases due to vaccination [9], in one of the first case reports, fatal myocarditis developed at the peak of acute infection [10]. SARS-CoV-2 RNA was detected in both of our patients with giant cell and eosinophilic myocarditis who underwent PCR. A similar response of the organism to the spike protein of both native SARS-CoV-2 and mRNA vaccines can be hypothesized.
Small autopsy studies quantified cellular infiltrates in coronavirus myocarditis in comparison with pre-COVID myocarditis: a high number of CD68 positive cells and, in contrast, a decrease in the number of CD4 and CD8 positive lymphocytes were found [11]. CD68 within infiltrates were also described in post-СOVID virus-negative myocarditis [5]. Suggesting, resident alveolar macrophages are involved in the initial response to viral infection, and may disseminate antigenic material of SARS-CoV-2 to other organs. The mononuclear phagocyte system is considered as a major player in the hyperinflammation and procoagulation, which can lead to vascular endothelial damage and microthrombosis [12]. This is in agreement with our data about the high incidence of vasculitis and microvascular thrombosis.
However, high macrophage activity alone cannot fully explain the development of chronic post-COVID myocarditis. One of the comparative morphologic studies also showed a higher number of CD68 cells in the myocardium in patients who had COVID-19 compared with pre-COVID myocarditis. But criteria for post-CoVID myocarditis were found in less than half of these patients [13]. There were no significant differences in the expression of endothelial activation markers. PCR tests did not detect SARS-CoV-2 RNA in any cases, which may also explain the low incidence of myocarditis in this study; time after COVID-19 is not reported.
It can be hypothesized that virus persistence leads not so much to direct damage of cardiomyocytes as to activation of autoimmune processes. In our study, a tendency to higher titers of anti-heart antibodies in post-COVID myocarditis was noted. Their production was associated with persistence of RNA and/or virus proteins and can be considered as one of the leading mechanisms of myocarditis chronicization. There is some evidence of high autoimmunity in post-COVID myocarditis. One case of morphologically proved fulminant myocarditis one month after acute infection with human myxovirus resistance protein 1 (MxA) in the myocardium is described [14]. The authors named it «dermatomyositis-like myocarditis».
In view of the importance of both mechanisms (coronavirus and also parvovirus B19 persistence and autoimmune activation), the study of TLR expression in the myocardium was of especial interest. TLR – a family of innate immunity receptors that are on the immune competent cells’ surface and are activated under the influence of various infectious agents. In humans, 10 types of TLR, which trigger the synthesis of proinflammatory cytokines and the induction of genes responsible for inflammatory response of the cell. In a number of researches of TLR4 expression in patients with coxsackie myocarditis compared with control a correlation of TLR4 with the presence of viral RNA and viral replication was shown [15]. By silencing the TLR4 gene, the coxsackie virus B induced TLR4 production and severity of myocarditis were significantly decreased [16].
On the other hand, an increased expression of TLR type 4 has been proven in patients with virus-negative (autoimmune) myocarditis; they were a great predictor of a positive response to IST with a sensitivity of 100%, specificity of 90.9% and positive predictive value of 98% [8]. In a recent study of myocarditis in psoriasis, the same group of researchers found evidence of its autoimmune nature. In addition to increasing of anti-heart antibodies in the blood, TLR-4 and interleukin 17 overexpression vs. no-psoriatic myocarditis was found in the myocardium [17]. The viral genome was absent in the myocardium of all patients. Therapy with steroids, azathioprine and secukinumab resulted in rapid clinical improvement. When necrotizing coronary vasculitis was compared with myoraditis, TLR 4 was overexpressed in immune-mediated forms and poorly detected in viral forms [18].
We found no clear association between TLR expression and response to complex therapy. The lack of significant differences may be due to the small number of observations (TLR expression study was performed in 38 patients, only five of them showed a poor response to therapy). But it cannot be excluded that TLR expression reflected not only the degree of autoimmune activation but also the viral load. This can be proved by an identified significant correlation between the presence of parvovirus B19 in the myocardium and TLR4 expression in the endocardium and endothelium targeted by this virus.
In general, our patients with post-COVID myocarditis showed a significantly better response to treatment compared with non-COVID myocarditis, despite the absence of baseline differences and a lower frequency of cytostatic prescription (in combination with corticosteroids). We avoided prescribing cytostatics mainly in patients with positive PCR for SARS-CoV-2 RNA, although there are no specific studies in this regard. The use of cytostatics in a small proportion of post-COVID patients was not accompanied by marked differences in treatment results, but a larger group of patients should be analyzed.
All identified characteristics of post-COVID myocarditis formed the background for the high efficacy of IST. The presence of parvovirus B19 in the myocardium had no effect on the treatment results, in agreement with earlier studies of IST in parvovirus-positive patients [19]. There are no SARS-CoV-2 antiviral drugs with proven efficacy in the long term after COVID-19. There are also no specific studies on the efficacy of steroid therapy in post-COVID myocarditis, but their effectiveness was shown in single case reports [5]. In one such case, effective steroid and cytostatic therapy was discontinued after a retrospective positive myocardial PCR for coronavirus in 2020 [20]. That doesn’t seem reasonable today.
We previously reported the efficacy of steroid therapy in patients with post-COVID myocarditis [
7]. This study is the first to show a more significant positive response to IST in patients with post-COVID myocarditis compared to pre-COVID myocarditis, despite the rarer administration of cytostatics. We believe, that this result reflects the high immune activity of post-COVID myocarditis and its sensitivity to steroid therapy, regardless of the presence of virus in the myocardium. The benefit of simultaneous prescription of cytostatics requires further study.
Conclusions
SARS-CoV-2 infection can induce chronic myocarditis with persistent systolic dysfunction. The median time to symptom onset after acute COVID-19 was 5.5 [2.75; 13.5] months. Clinically, this myocarditis had no significant differences from non-COVID myocarditis, although a tendency to higher titers of anti-heart antibodies, more pronounced dilatation of the LV, and decreased LV EF was detected.
A wider spectrum of morphologic variants with a high frequency of hyperimmune forms (giant cell and eosinophilic myocarditis in 12.5%) was detected in EMB. Nucleocapsid and spike proteins of SARS-CoV-2 were found in all patients; PCR for RNA coronavirus was positive in 86% of cases. The duration of virus persistence in the myocardium reached 18 months and can be considered as one of the important mechanisms of post-CoV-2 myocarditis.
On the other hand, a more numerous of CD45 and CD68 positive cells were detected, and there was a tendency to more intense expression of TLR4 and TLR9, what may reflect high activity of immune inflammation. Steroid therapy (in combination with cytostatics in 34%) was associated with a higher rate of good response to therapy and a significantly higher increase in LV EF compared with non-COVID myocarditis. There were no differences in the rate of death/transplantation. No correlation between TLR expression and response to therapy was obtained, which may be due to the small number of patients with ineffective treatment.
Author Contributions
Conceptualization, O.B. and E.K.; Investigation, O.B., V.N., V.B., N.Zh and E.K.; Writing—original draft, O.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the local ethics committee of Sechenov University for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the staff of the virology and immunology laboratories, as well as the ultrasound and radiation diagnostics specialists who performed the range of necessary tests.
Conflicts of Interest
All authors declare no potential conflicts of interest warranting disclosure in this article.
The limitations of this study are the relatively small number of patients in each subgroup, especially for TLR expression evaluation.
Abbreviations
ACE - angiotensin-converting enzyme, AMR - antagonists of mineralocorticoid receptor, COVID-19 -coronavirus disease 2019, DNA - deoxyribonucleic acid, EF- ejection fraction, EMB - endomyocardial biopsy, IHC – immunohistochemical, IST -immunosuppressive therapy, LV - left ventricle, EchoCG - echocardiography, MSCT - multispiral computed tomography, MRI - magnetic resonance imaging, PCR - polymerase chain reaction, RNA - ribonucleic acid, SARS-CoV-2 - severe acute respiratory syndrome-related coronavirus 2, TLR 4/9 – toll-like receptors type 4/9.
References
- Wenzel P, Kopp S, Göbel S, Jansen T, Geyer M, Hahn F, Kreitner KF, Escher F, Schultheiss HP, Münzel T. Evidence of SARS-CoV-2 mRNA in endomyocardial biopsies of patients with clinically suspected myocarditis tested negative for COVID-19 in nasopharyngeal swab. Cardiovasc Res. 2020; 116(10):1661-1663. [CrossRef]
- Lledó GM, Sellares J, Brotons C, Sans M, Antón JD, Blanco J, Bassat Q, Sarukhan A, Miró JM, de Sanjosé S; Multidisciplinary Collaborative Group for the Scientific Monitoring of COVID-19 (GCMSC). Post-acute COVID-19 syndrome: a new tsunami requiring a universal case definition. Clin Microbiol Infect. 2022; 28(3):315-318. [CrossRef]
- Van Linthout S, Klingel K, Tschöpe C. SARS-CoV-2-related myocarditis-like syndromes Shakespeare’s question: what’s in a name? Eur J Heart Fail. 2020; 22(6):922-925. [CrossRef]
- Gyöngyösi M, Alcaide P, Asselbergs FW, Brundel BJJM, Camici GG, Martins PDC, Ferdinandy P, Fontana M, Girao H, Gnecchi M, Gollmann-Tepeköylü C, Kleinbongard P, Krieg T, Madonna R, Paillard M, Pantazis A, Perrino C, Pesce M, Schiattarella GG, Sluijter JPG, Steffens S, Tschöpe C, Van Linthout S, Davidson SM. Long COVID and the cardiovascular system-elucidating causes and cellular mechanisms in order to develop targeted diagnostic and therapeutic strategies: a joint Scientific Statement of the ESC Working Groups on Cellular Biology of the Heart and Myocardial and Pericardial Diseases. Cardiovasc Res. 2023;119(2):336-356. [CrossRef]
- Bohné M, Bohnen S, Willems S, Klingel K, Kivelitz D, Bahlmann E. Acute Lymphocytic Myocarditis in a Young Male Post-COVID-19. Case Rep Cardiol. 2023; 2023:7646962. [CrossRef]
- Pietsch H, Escher F, Aleshcheva G, Baumeier C, Morawietz L, Elsaesser A, Schultheiss HP. Proof of SARS-CoV-2 genomes in endomyocardial biopsy with latency after acute infection. Int J Infect Dis. 2021; 102:70-72. [CrossRef]
- Blagova O, Lutokhina Y, Kogan E, Kukleva A, Ainetdinova D, Novosadov V, Rud’ R, Savina P, Zaitsev A, Fomin V. Chronic biopsy proven post-COVID myoendocarditis with SARS-Cov-2 persistence and high level of antiheart antibodies. Clin Cardiol. 2022;45(9):952-959. [CrossRef]
- Chimenti C, Verardo R, Scopelliti F, Grande C, Petrosillo N, Piselli P, De Paulis R, Frustaci A. Myocardial expression of Toll-like receptor 4 predicts the response to immunosuppressive therapy in patients with virus-negative chronic inflammatory cardiomyopathy. Eur J Heart Fail. 2017; 19(7):915-925. [CrossRef]
- Frustaci A, Verardo R, Galea N, Lavalle C, Bagnato G, Scialla R, Chimenti C. Hypersensitivity Myocarditis after COVID-19 mRNA Vaccination. J Clin Med. 2022 Mar 16;11(6):1660. [CrossRef] [PubMed]
- Craver R, Huber S, Sandomirsky M, McKenna D, Schieffelin J, Finger L. Fatal Eosinophilic Myocarditis in a Healthy 17-Year-Old Male with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2c). Fetal Pediatr Pathol. 2020;39(3):263-268. [CrossRef]
- Fox SE, Falgout L, Vander Heide RS. COVID-19 myocarditis: quantitative analysis of the inflammatory infiltrate and a proposed mechanism. Cardiovasc Pathol. 2021; 54:107361. [CrossRef]
- Martinez FO, Combes TW, Orsenigo F, Gordon S. Monocyte activation in systemic Covid-19 infection: Assay and rationale. EBioMedicine. 2020; и59:102964. [CrossRef]
- Makarov I, Mayrina S, Makarova T, Karonova T, Starshinova A, Kudlay D, Mitrofanova L. Morphological Changes in the Myocardium of Patients with Post-Acute Coronavirus Syndrome: A Study of Endomyocardial Biopsies. Diagnostics (Basel). 2023; 13(13):2212. [CrossRef]
- Kinoshita H, Kurashige T, Fukuda T, Morita M, Maeda S, Kanegawa M, Sumimoto Y, Masada K, Shimonaga T, Sugino H. The impact that myocarditis for post-acute COVID-19 syndrome may be dermatomyositis-like myocarditis: A case report. Heliyon. 2023; 9(6):e16512. [CrossRef]
- Satoh M, Nakamura M, Akatsu T, Iwasaka J, Shimoda Y, Segawa I, Hiramori K. Expression of Toll-like receptor 4 is associated with enteroviral replication in human myocarditis. Clin Sci (Lond). 2003; 104(6):577-84. [CrossRef]
- Zhao Z, Cai TZ, Lu Y, Liu WJ, Cheng ML, Ji YQ. Coxsackievirus B3 induces viral myocarditis by upregulating toll-like receptor 4 expression. Biochemistry (Mosc). 2015 Apr;80(4):455-62. [CrossRef]
- Frustaci A, Galea N, Dominici L, Verardo R, Alfarano M, Scialla R, Richetta AG. Interleukin-17A-Correlated Myocarditis in Patients with Psoriasis: Cardiac Recovery following Secukinumab Administration. J Clin Med. 2023 Jun 12;12(12):4010. [CrossRef]
- Frustaci A, Alfarano M, Verardo R, Agrati C, Casetti R, Miraldi F, Galea N, Letizia C, Chimenti C. Myocarditis-associated necrotizing coronary vasculitis: incidence, cause, and outcome. Eur Heart J. 2021;42(16):1609-1617. [CrossRef]
- Tschöpe C, Elsanhoury A, Schlieker S, Van Linthout S, Kühl U. Immunosuppression in inflammatory cardiomyopathy and parvovirus B19 persistence. Eur J Heart Fail. 2019; 21(11):1468-1469. [CrossRef]
- Hudowenz O, Klemm P, Lange U, Rolf A, Schultheiss HP, Hamm C, Müller-Ladner U, Wegner F. Case report of severe PCR-confirmed COVID-19 myocarditis in a European patient manifesting in mid January 2020. Eur Heart J Case Rep. 2020;4(6):1-6. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).