Submitted:
18 July 2024
Posted:
19 July 2024
You are already at the latest version
Abstract
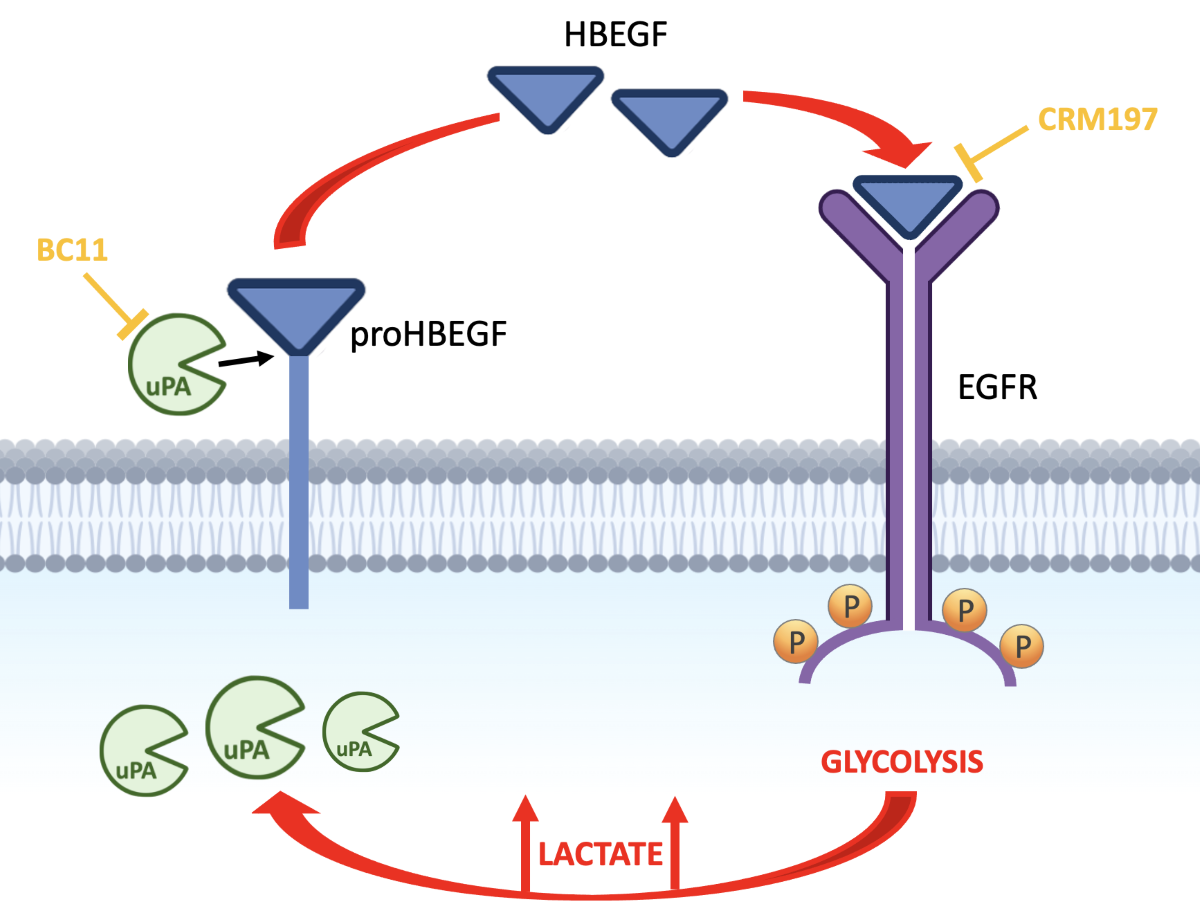
Keywords:
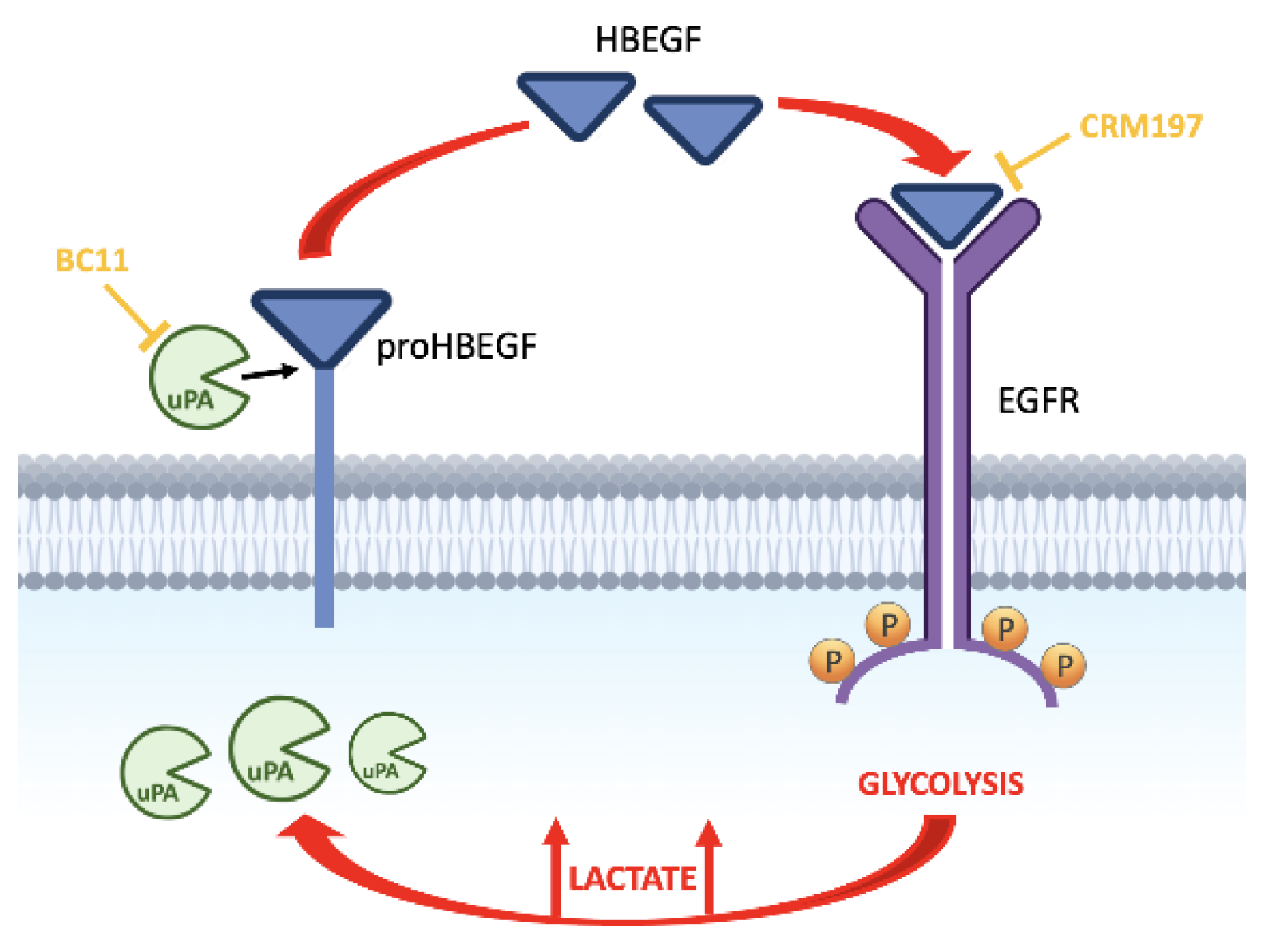
1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Treatments
2.2. Real-Time PCR
2.3. Immunoblotting Experiments
2.4. ELISA for the Detection of HBEGF Released in Culture Medium
2.5. Cell Proliferation Experiments
2.6. Assay of Lactate Levels
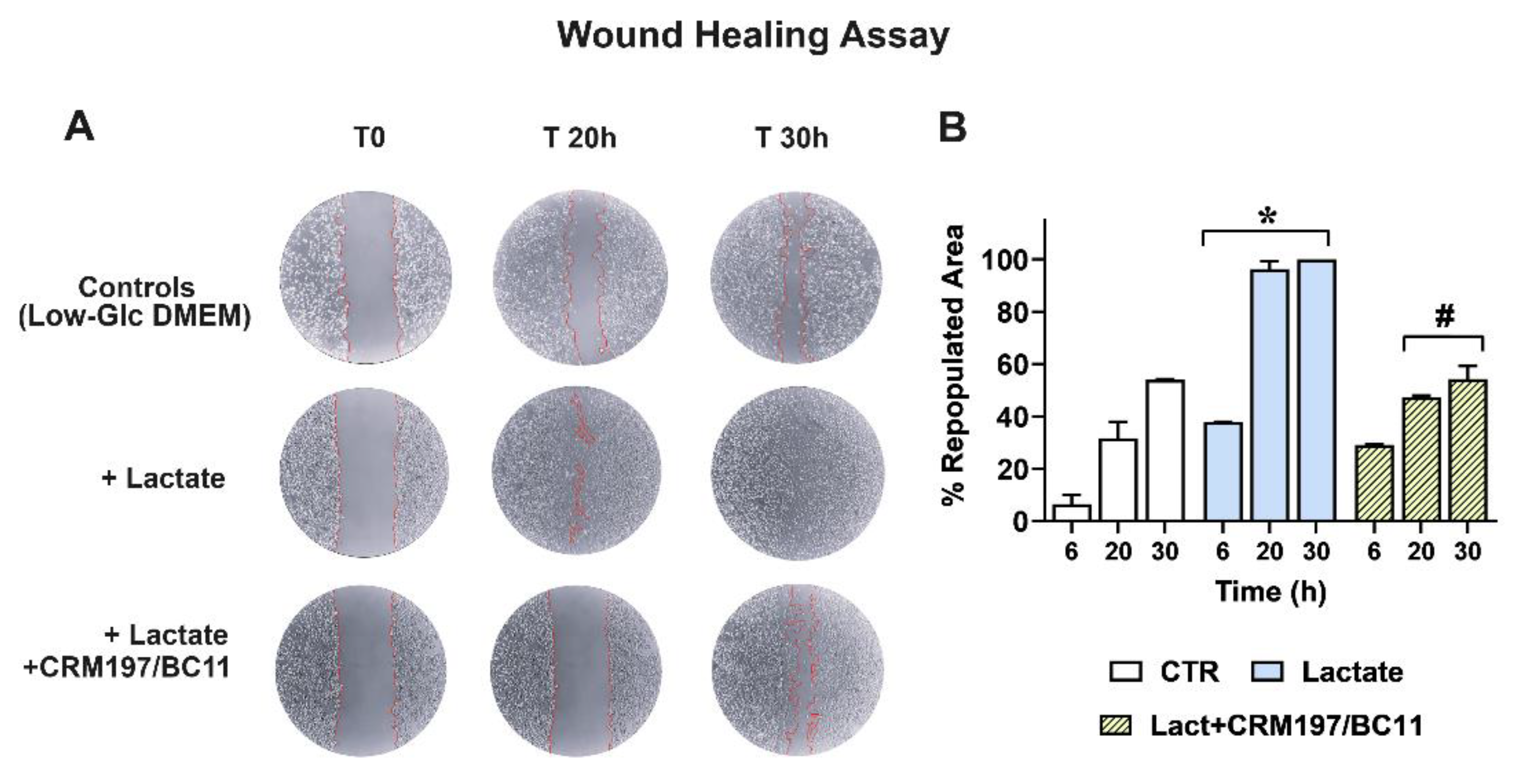
2.7. Wound Healing Assay
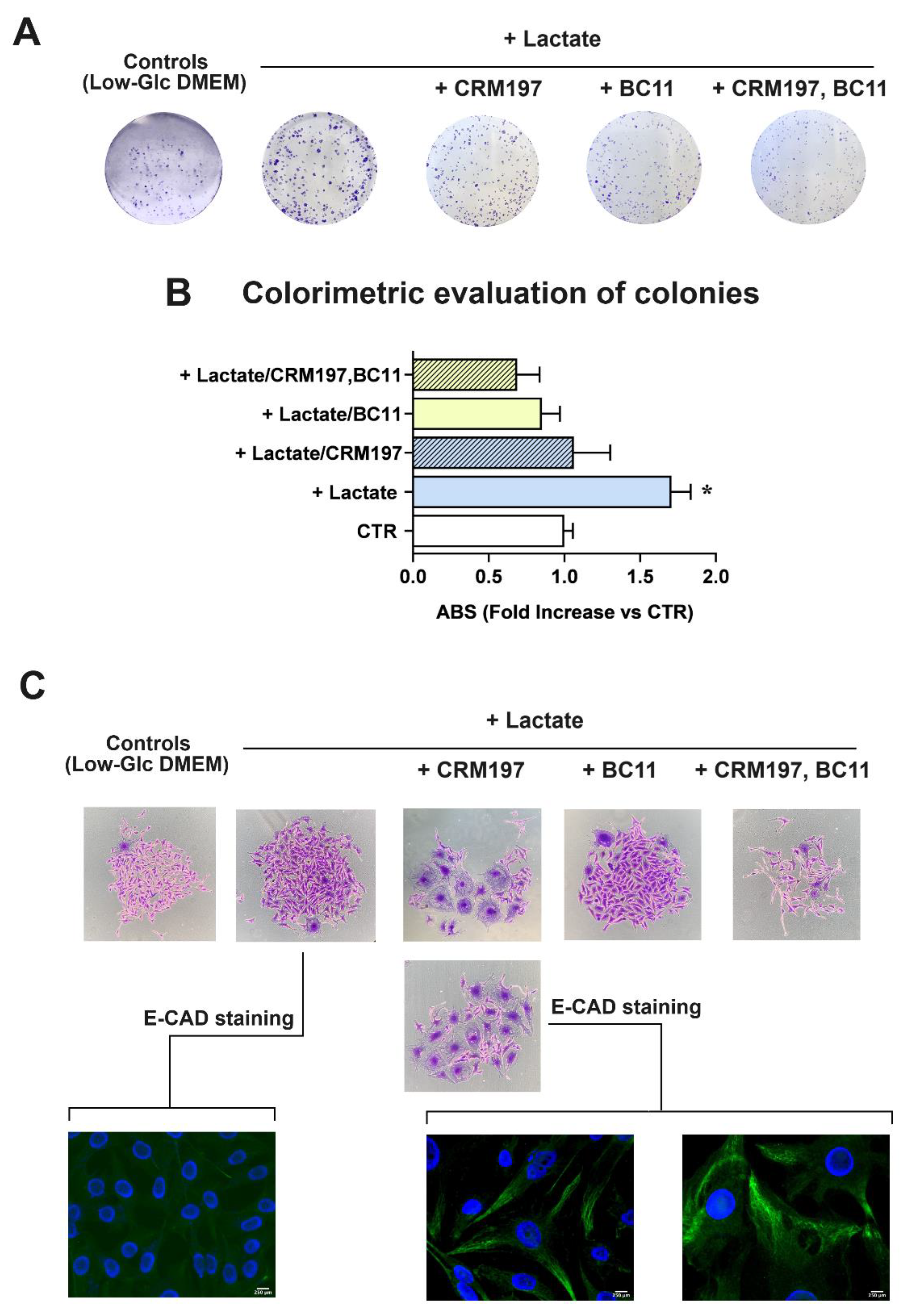
2.8. Clonogenicity Assay
2.9. E-Cadherin Immunostaining
2.10. Statistical Analysis
3. Results
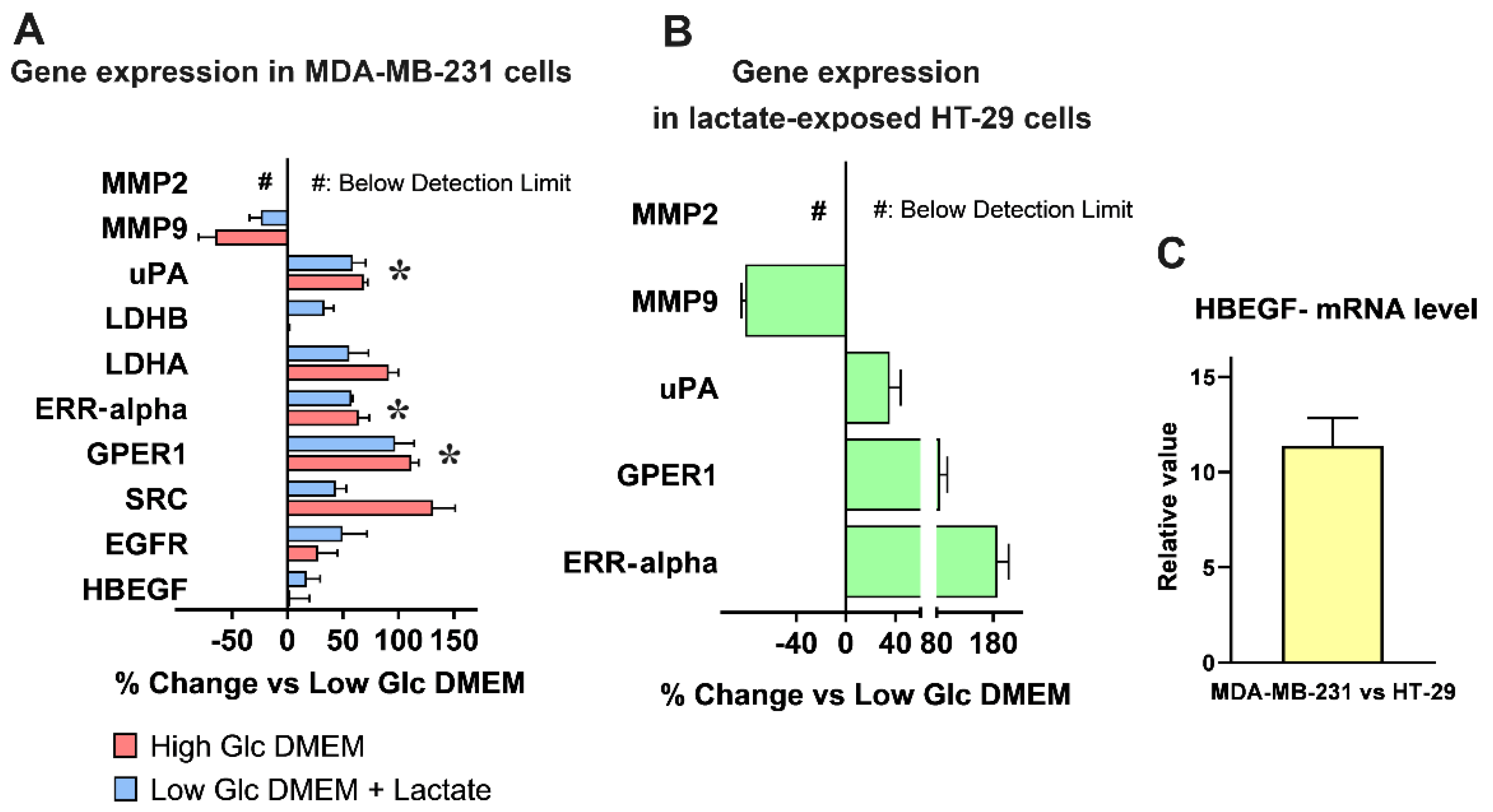
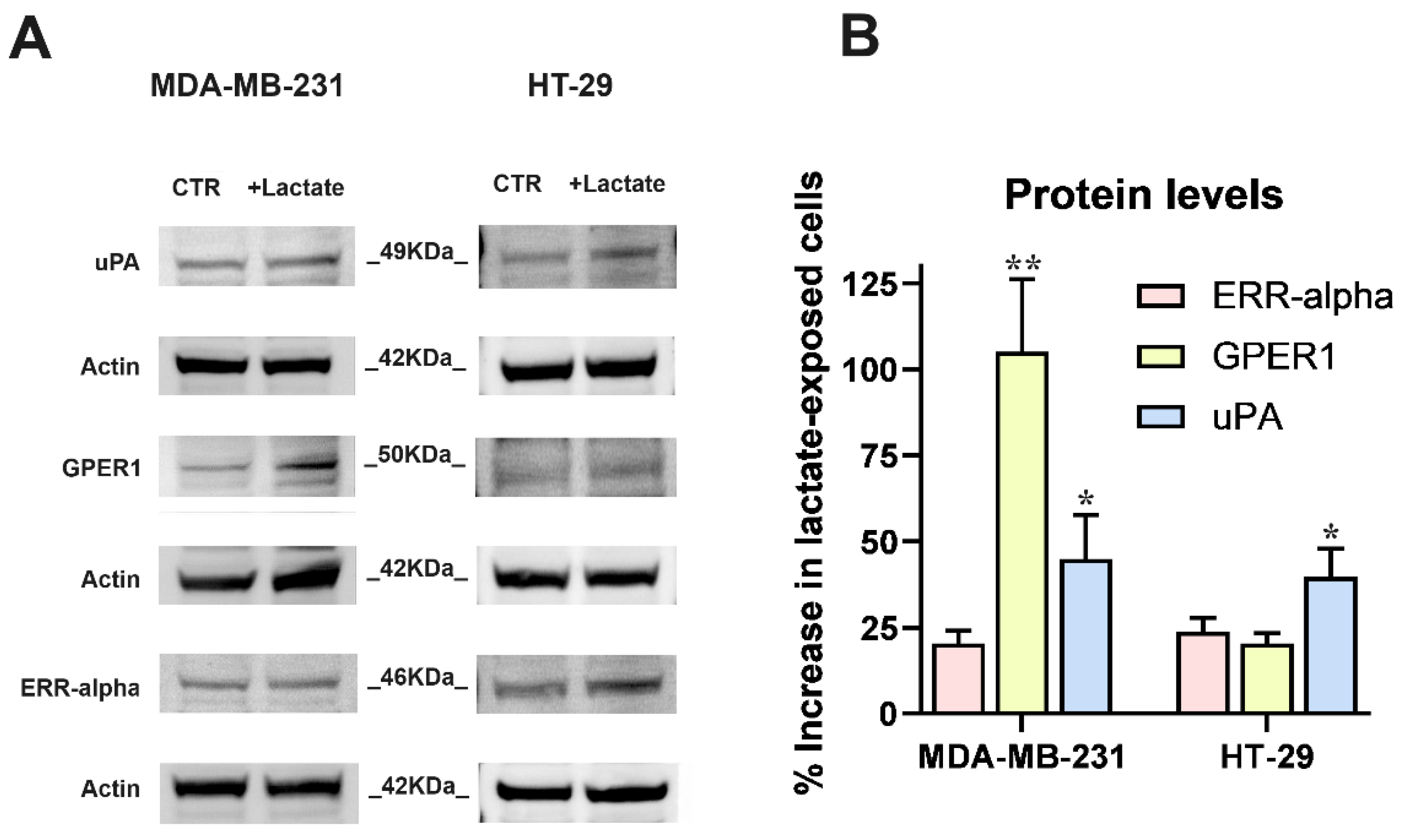
3.1. Lactate Upregulates Urokinase-Type Plasminogen Activator (uPA), Leading to HBEGF Shedding
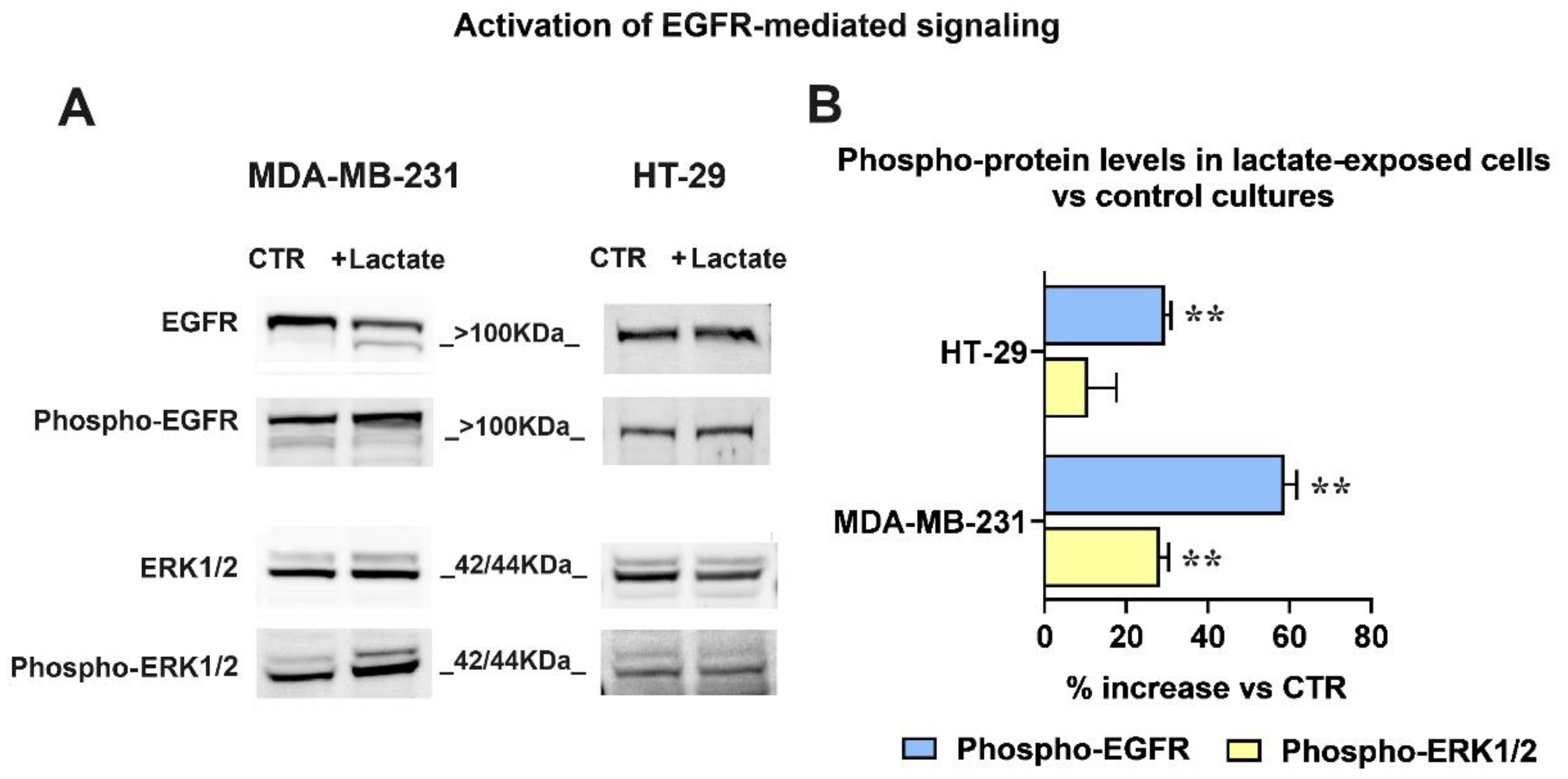
3.2. Lactate-Exposed Cells Show Signatures of Activated EGFR Pathway and Reduced Response to Cisplatin
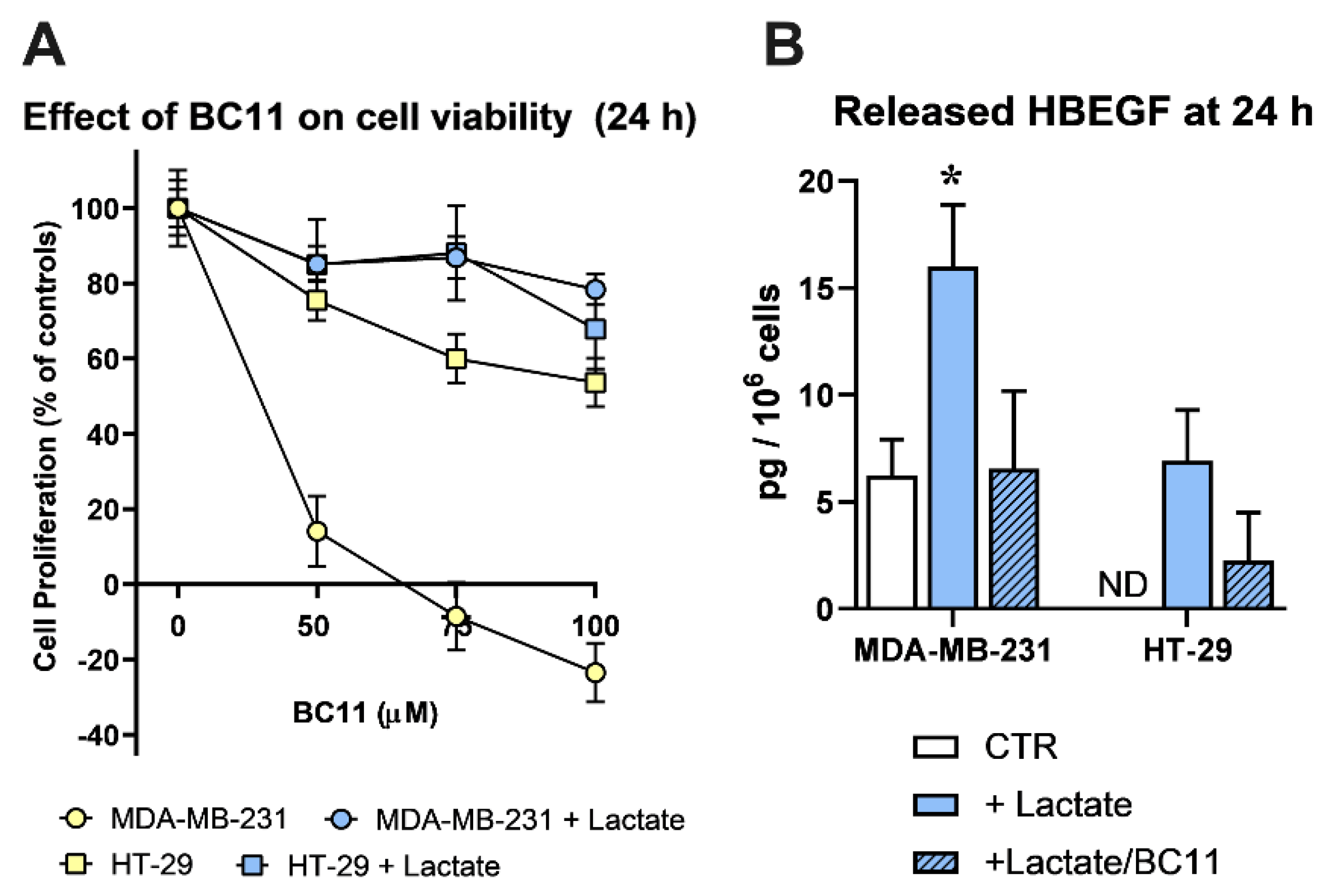
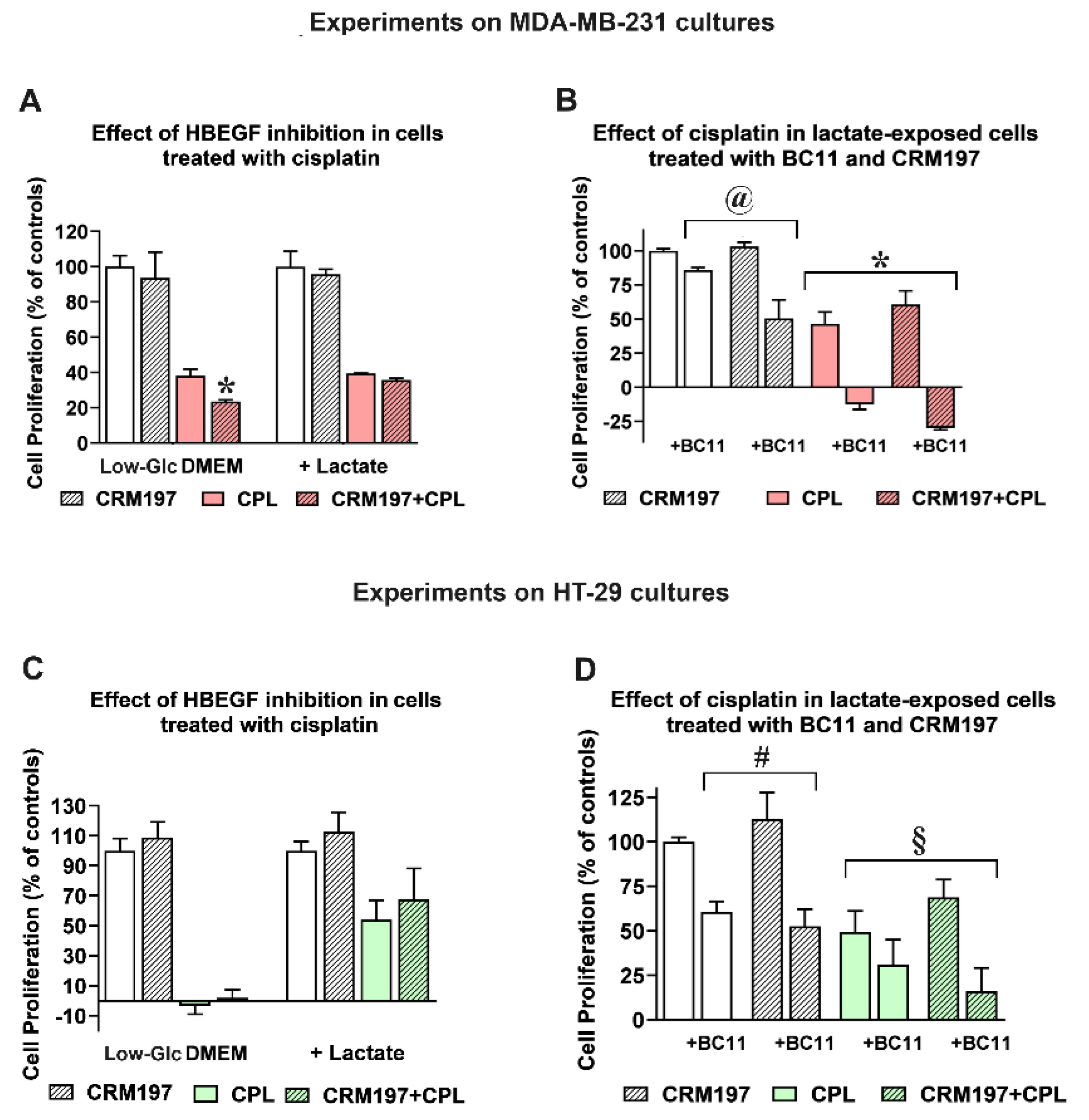
3.3. The Combined Inhibition of HBEGF Shedding and Function Shows Antineoplastic Potential in MDA-MB-231 Cultures
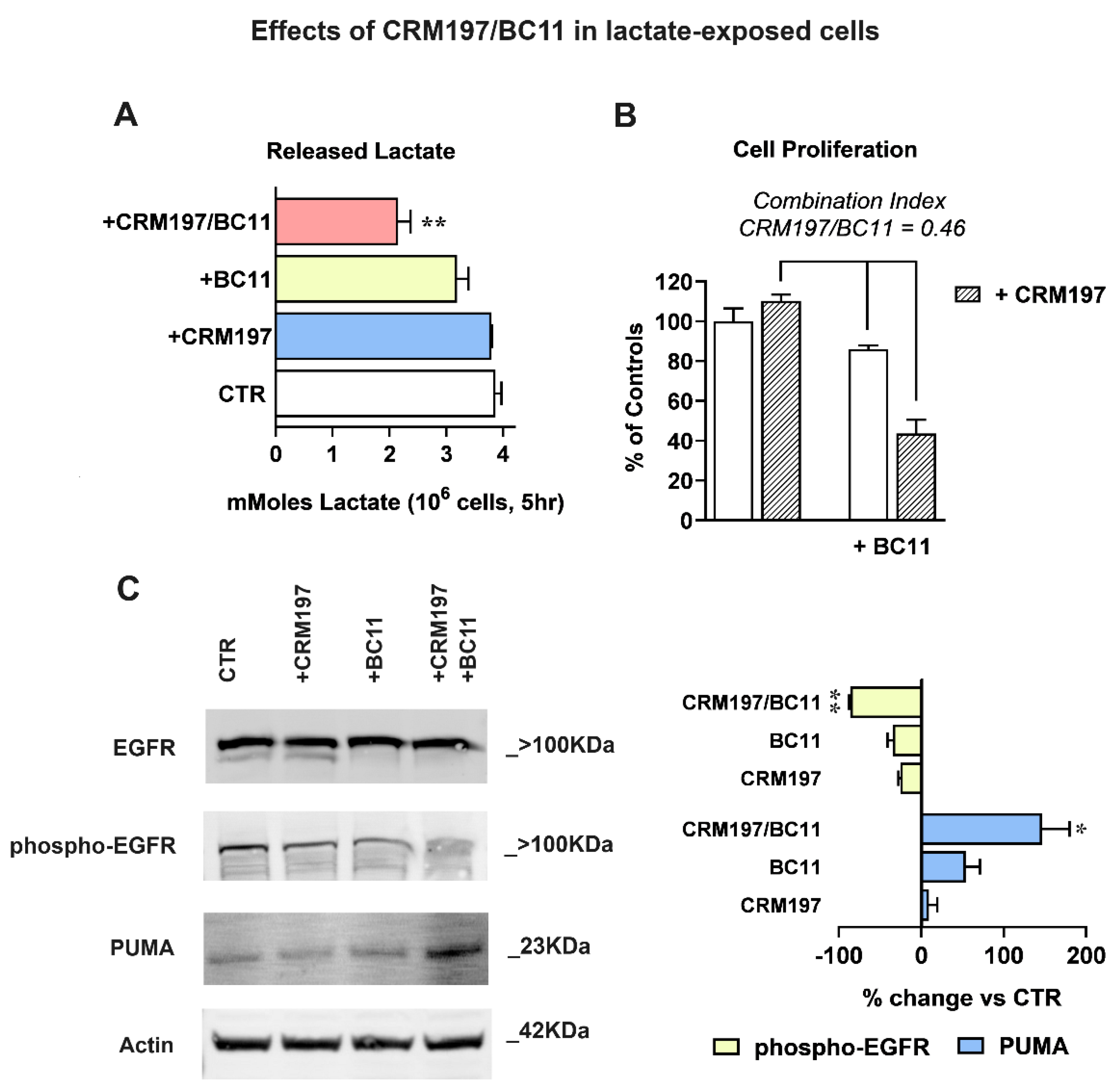
3.4. Effects of the CRM197/BC11 Association on Infiltrative Growth and Cell Clonogenicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z. ErbB Receptors and Cancer. Methods Mol. Biol. 2017, 1652, 3–35. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol. Res. 2014, 79, 34–74. [Google Scholar] [CrossRef] [PubMed]
- Appert-Collin, A.; Hubert, P.; Crémel, G.; Bennasroune, A. Role of ErbB receptors in cancer cell migration and invasion. Front. Pharmacol. 2015, 6, 283. [Google Scholar] [CrossRef]
- Al Moustafa, A.E.; Achkhar, A.; Yasmeen, A. EGF-receptor signaling and epithelial-mesenchymal transition in human carcinomas. Front. Biosci. 2012, 4, 71–84. [Google Scholar] [CrossRef]
- Lorch, J.H.; Klessner, J.; Park, J.K.; Getsios, S.; Wu, Y.L.; Stack, M.S.; Green, K.J. Epidermal growth factor receptor inhibition promotes desmosome assembly and strengthens intercellular adhesion in squamous cell carcinoma cells. J. Biol. Chem. 2004, 279, 37191–37200. [Google Scholar] [CrossRef]
- Nicholson, R.I.; Gee, J.M.W.; Harper, M.E. EGFR and cancer prognosis. Eur. J. Cancer 2001, 37 (Suppl. 4), 9–15. [Google Scholar] [CrossRef]
- Huang, S.M.; Harari, P.M. Epidermal growth factor receptor inhibition in cancer therapy: biology, rationale and preliminary clinical results. Invest. New Drugs 1999, 17, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.O.; Li, C.W.; Xia, W.; Lee, H.H.; Chang, S.S.; Shen, J.; Hsu, J.L.; Raftery, D.; Djukovic, D.; Gu, H.; Chang, W.C.; Wang, H.L.; Chen, M.L.; Huo, L.; Chen, C.H.; Wu, Y.; Sahin, A.; Hanash, S.M.; Hortobagyi, G.N.; Hung, M.C. EGFR signaling enhances aerobic glycolysis in triple-negative breast cancer cells to promote tumor growth and immune escape. Cancer Res. 2016, 76, 1284–1296. [Google Scholar] [CrossRef]
- Menchikov, L.G.; Shestov, A.A.; Popov, A.V. Warburg effect revisited: embodiment of classical biochemistry and organic chemistry. current state and prospects. Biochemistry 2023, 88 (Suppl. 1), S1–S20. [Google Scholar] [CrossRef]
- Alquraishi, M.; Puckett, D.L.; Alani, D.S.; Humidat, A.S.; Frankel, V.D.; Donohoe, D.R.; Whelan, J.; Bettaieb, A. Pyruvate kinase M2: A simple molecule with complex functions. Free Radic. Biol. Med. 2019, 143, 176–192. [Google Scholar] [CrossRef]
- Holm, E.; Hagmüller, E.; Staedt, U.; Schlickeiser, G.; Günther, H.J.; Leweling, H.; Tokus, M.; Kollmar, H.B. Substrate balances across colonic carcinomas in humans. Cancer Res. 1995, 55, 1373–1378. [Google Scholar] [PubMed]
- Dhup, S.; Dadhich, R.K.; Porporato, P.E.; Sonveaux, P. Multiple biological activities of lactic acid in cancer: influences on tumor growth, angiogenesis and metastasis. Curr. Pharm. Des. 2012, 18, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- San-Millán, I.; Julian, C.G.; Matarazzo, C.; Martinez, J.; Brooks, G.A. Is Lactate an oncometabolite? Evidence supporting a role for lactate in the regulation of transcriptional activity of cancer-related genes in MCF7 breast cancer cells. Front. Oncol. 2020, 9, 1536. [Google Scholar] [CrossRef] [PubMed]
- Govoni, M.; Rossi, V.; Di Stefano, G.; Manerba, M. Lactate upregulates the expression of DNA repair genes, causing intrinsic resistance of cancer cells to cisplatin. Pathol. Oncol. Res. 2021, 27, 165. [Google Scholar] [CrossRef]
- Rossi, V.; Govoni, M.; Farabegoli, F.; Di Stefano, G. Lactate is a potential promoter of tamoxifen resistance in MCF7 cells. Biochim. Biophys. Acta Gen. Subj. 2022, 1866, 130185. [Google Scholar] [CrossRef] [PubMed]
- Rossi, V.; Govoni, M.; Di Stefano, G. Lactate can modulate the antineoplastic effects of doxorubicin and relieve the drug’s oxidative damage on cardiomyocytes. Cancers (Basel) 2023, 15, 3728. [Google Scholar] [CrossRef]
- Latham, T.; Mackay, L.; Sproul, D.; Karim, M.; Culley, J.; Harrison, D.J.; Hayward, L.; Langridge-Smith, P.; Gilbert, N.; Ramsahoye, B.H. Lactate, a product of glycolytic metabolism, inhibits histone deacetylase activity and promotes changes in gene expression. Nucleic Acids Res. 2012, 40, 4794–4803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; Ding, J.; Czyz, D.; Hu, R.; Ye, Z.; He, M.; Zheng, Y.G.; Shuman, H.A.; Dai, L.; Ren, B.; Roeder, R.G.; Becker, L.; Zhao, Y. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ye, Z.; Li, Z.; Jing, D.S.; Fan, G.X.; Liu, M.Q.; Zhuo, Q.F.; Ji, S.R.; Yu, X.J.; Xu, X.W.; Qin, Y. Lactate-induced protein lactylation: A bridge between epigenetics and metabolic reprogramming in cancer. Cell Prolif. 2023, 56, e13478. [Google Scholar] [CrossRef]
- Kuo, P.L.; Huang, M.S.; Cheng, D.E.; Hung, J.Y.; Yang, C.J.; Chou, S.H. Lung cancer-derived galectin-1 enhances tumorigenic potentiation of tumor-associated dendritic cells by expressing heparin-binding EGF-like growth factor. J Biol Chem. 2012, 287, 9753–9764. [Google Scholar] [CrossRef]
- Miyamoto, S.; Yagi, H.; Yotsumoto, F.; Kawarabayashi, T.; Mekada, E. Heparin-binding epidermal growth factor-like growth factor as a novel targeting molecule for cancer therapy. Cancer Sci. 2006, 97, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Raab, G.; Klagsbrun, M. Heparin-binding EGF-like growth factor. Biochim. Biophys. Acta 1997, 1333, F179–F199. [Google Scholar] [CrossRef]
- Van Hiep, N.; Sun, W.L.; Feng, P.H.; Lin, C.W.; Chen, K.Y.; Luo, C.S.; Dung, L.N.; Van Quyet, H.; Wu, S.M.; Lee, K.Y. Heparin binding epidermal growth factor-like growth factor is a prognostic marker correlated with levels of macrophages infiltrated in lung adenocarcinoma. Front. Oncol. Erratum in: Front. Oncol. 2022, 12, 1106553. 2022, 12, 963896. [Google Scholar] [CrossRef]
- Wang, F.; Liu, R.; Lee, S.W.; Sloss, C.M.; Couget, J.; Cusack, J.C. Heparin-binding EGF-like growth factor is an early response gene to chemotherapy and contributes to chemotherapy resistance. Oncogene. 2007, 26, 2006–2016. [Google Scholar] [CrossRef]
- Yotsumoto, F.; Yagi, H.; Suzuki, S.O.; Oki, E.; Tsujioka, H.; Hachisuga, T.; Sonoda, K.; Kawarabayashi, T.; Mekada, E.; Miyamoto, S. Validation of HB-EGF and amphiregulin as targets for human cancer therapy. Biochem. Biophys. Res. Commun. 2008, 365, 555–5661. [Google Scholar] [CrossRef] [PubMed]
- Rivetti, S.; Lauriola, M.; Voltattorni, M.; Bianchini, M.; Martini, D.; Ceccarelli, C.; Palmieri, A.; Mattei, G.; Franchi, M.; Ugolini, G.; Rosati, G.; Montroni, I.; Taffurelli, M.; Solmi, R. Gene expression profile of human colon cancer cells treated with cross-reacting material 197, a diphtheria toxin non-toxic mutant. Int. J. Immunopathol. Pharmacol. 2011, 24, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS One 2020, 15, e0232565. [Google Scholar] [CrossRef]
- de la Cruz-López, K.G.; Castro-Muñoz, L.J.; Reyes-Hernández, D.O.; García-Carrancá, A.; Manzo-Merino, J. Lactate in the regulation of tumor microenvironment and therapeutic approaches. Front. Oncol. 2019, 9, 1143. [Google Scholar] [CrossRef]
- Chetta, P.; Sriram, R.; Zadra, G. Lactate as key metabolite in prostate cancer progression: What are the clinical implications? Cancers (Basel) 2023, 15, 3473. [Google Scholar] [CrossRef]
- Wagner, W.; Ciszewski, W.M.; Kania, K.D. L- and D-lactate enhance DNA repair and modulate the resistance of cervical carcinoma cells to anticancer drugs via histone deacetylase inhibition and hydroxycarboxylic acid receptor 1 activation. Cell. Commun. Signal. 2015, 13, 36. [Google Scholar] [CrossRef]
- Zhou, Z.N.; Sharma, V.P.; Beaty, B.T.; Roh-Johnson, M.; Peterson, E.A.; Van Rooijen, N.; Kenny, P.A.; Wiley, H.S.; Condeelis, J.S.; Segall, J.E. Autocrine HBEGF expression promotes breast cancer intravasation, metastasis and macrophage-independent invasion in vivo. Oncogene 2014, 33, 3784–3793. [Google Scholar] [CrossRef]
- Carroll, M.J.; Kapur, A.; Felder, M.; Patankar, M.S.; Kreeger, P.K. M2 macrophages induce ovarian cancer cell proliferation via a heparin binding epidermal growth factor/matrix metalloproteinase 9 intercellular feedback loop. Oncotarget 2016, 7, 86608–86620. [Google Scholar] [CrossRef]
- Tang, L.; Han, X. The urokinase plasminogen activator system in breast cancer invasion and metastasis. Biomed. Pharmacother. 2013, 67, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, M.; Tobar, N.; Guerrero, J.; Smith, P.C.; Martínez, J. c-jun-NH2JNK mediates invasive potential and EGFR activation by regulating the expression of HB-EGF in a urokinase-stimulated pathway. J. Cell. Biochem. 2008, 103, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Sarno, F.; Goubert, D.; Logie, E.; Rutten, M.G.S.; Koncz, M.; Deben, C.; Niemarkt, A.E.; Altucci, L.; Verschure, P.J.; Kiss, A.; Berghe, W.V.; Rots, M.G. Functional validation of the putative oncogenic activity of PLAU. Biomedicines 2022, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Lappano, R.; De Marco, P.; De Francesco, E.M.; Chimento, A.; Pezzi, V.; Maggiolini, M. Cross-talk between GPER and growth factor signaling. J. Steroid Biochem. Mol. Biol. 2013, 137, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Scaling, A.L.; Prossnitz, E.R.; Hathaway, H.J. GPER mediates estrogen-induced signaling and proliferation in human breast epithelial cells and normal and malignant breast. Horm. Cancer 2014, 5, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Xu, Y.; Wang, L.; Zhou, K.; He, J.; Lu, J.; Huang, Q.; Sun, P.; Wang, T. Estrogen-Related Receptor α (ERRα) and G Protein-Coupled Estrogen Receptor (GPER) Synergistically Indicate Poor Prognosis in Patients with Triple-Negative Breast Cancer. Onco Targets Ther. 2020, 13, 8887–8899. [Google Scholar] [CrossRef]
- Cai, Q.; Lin, T.; Kamarajugadda, S.; Lu, J. Regulation of glycolysis and the Warburg effect by estrogen-related receptors. Oncogene 2013, 32, 2079–2086. [Google Scholar] [CrossRef]
- Han, L.; Liu, B.; Jiang, L.; Liu, J.; Han, S. MicroRNA-497 downregulation contributes to cell proliferation, migration, and invasion of estrogen receptor alpha negative breast cancer by targeting estrogen-related receptor alpha. Tumour Biol. 2016, 37, 13205–13214. [Google Scholar] [CrossRef]
- Luo, J.; Zou, H.; Guo, Y.; Tong, T.; Ye, L.; Zhu, C.; Deng, L.; Wang, B.; Pan, Y.; Li, P. SRC kinase-mediated signaling pathways and targeted therapies in breast cancer. Breast Cancer Res. 2022, 24, 99. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct. Target Ther. 2024, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Webb, D.J.; Jo, M.; Gonias, S.L. Endogenously produced urokinase-type plasminogen activator is a major determinant of the basal level of activated ERK/MAP kinase and prevents apoptosis in MDA-MB-231 breast cancer cells. J Cell Sci. 2001, 114, 3387–3396. [Google Scholar] [CrossRef]
- Longo, A.; Librizzi, M.; Chuckowree, I.S.; Baltus, C.B.; Spencer, J.; Luparello, C. Cytotoxicity of the urokinase-plasminogen activator inhibitor carbamimidothioic acid (4-boronophenyl) methyl ester hydrobromide (BC-11) on triple-negative MDA-MB231 breast cancer cells. Molecules 2015, 20, 9879–9889. [Google Scholar] [CrossRef] [PubMed]
- Licitra, L.; Perrone, F.; Tamborini, E.; Bertola, L.; Ghirelli, C.; Negri, T.; Orsenigo, M.; Filipazzi, P.; Pastore, E.; Pompilio, M.; Bossi, P.; Locati, L.D.; Cantù, G.; Scaramellini, G.; Pilotti, S.; Tagliabue, E. Role of EGFR family receptors in proliferation of squamous carcinoma cells induced by wound healing fluids of head and neck cancer patients. Ann. Oncol. 2011, 22, 1886–1893. [Google Scholar] [CrossRef]
- Dutta, P.R.; Maity, A. Cellular responses to EGFR inhibitors and their relevance to cancer therapy. Cancer Lett. 2007, 254, 165–177. [Google Scholar] [CrossRef]
- Kopacz-Bednarska, A.; Król, T. Cisplatin — properties and clinical application. Oncol. Clin. Pract. 2022, 18, 166–176. [Google Scholar] [CrossRef]
- Mitamura, T.; Higashiyama, S.; Taniguchi, N.; Klagsbrun, M.; Mekada, E. Diphtheria toxin binds to the epidermal growth factor (EGF)-like domain of human heparin-binding EGF-like growth factor/diphtheria toxin receptor and inhibits specifically its mitogenic activity. J. Biol. Chem. 1995, 270, 1015–1019. [Google Scholar] [CrossRef]
- Peng, J.; Cui, Y.; Xu, S.; Wu, X.; Huang, Y.; Zhou, W.; Wang, S.; Fu, Z.; Xie, H. Altered glycolysis results in drug-resistant in clinical tumor therapy. Oncol. Lett. 2021, 21, 369. [Google Scholar] [CrossRef]
- Breitinger, H.G. Drug Synergy – Mechanisms and Methods of Analysis. In Toxicity and Drug Testing, Acree, B. Ed.; InTech 2012, ISBN: 978-953-51-0004-1. http://www.intechopen.com/books/toxicityand-drug-testing/drug-synergy-mechanisms-and-methods-of-analysis. [CrossRef]
- Ryu, J.; Yoon, N.A.; Lee, Y.K.; Jeong, J.Y.; Kang, S.; Seong, H.; Choi, J.; Park, N.; Kim, N.; Cho, W.J.; Paek, S.H.; Cho, G.J.; Choi, W.S.; Park, J.Y.; Park, J.W.; Kang, S.S. Tristetraprolin inhibits the growth of human glioma cells through downregulation of urokinase plasminogen activator/urokinase plasminogen activator receptor mRNAs. Mol. Cells 2015, 38, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Hapach, L.A.; Carey, S.P.; Schwager, S.C.; Taufalele, P.V.; Wang, W.; Mosier, J.A.; Ortiz-Otero, N.; McArdle, T.J.; Goldblatt, Z.E.; Lampi, M.C.; Bordeleau, F.; Marshall, J.R.; Richardson, I.M.; Li, J.; King, M.R.; Reinhart-King, C.A. Phenotypic heterogeneity and metastasis of breast cancer cells. Cancer Res. 2021, 81, 3649–3663. [Google Scholar] [CrossRef] [PubMed]
- Schwager, S.C.; Mosier, J.A.; Padmanabhan, R.S.; White, A.; Xing, Q.; Hapach, L.A.; Taufalele, P.V.; Ortiz, I.; Reinhart-King, C.A. Link between glucose metabolism and epithelial-to-mesenchymal transition drives triple-negative breast cancer migratory heterogeneity. iScience 2022, 25, 105190. [Google Scholar] [CrossRef] [PubMed]
- Levantini, E.; Maroni, G.; Del Re, M.; Tenen, D.G. EGFR signaling pathway as therapeutic target in human cancers. Semin. Cancer Biol. 2022, 85, 253–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Targeting epidermal growth factor receptor for cancer treatment: abolishing both kinase-dependent and kinase-independent functions of the receptor. Pharmacol. Rev. 2023, 75, 1218–1232. [Google Scholar] [CrossRef] [PubMed]
- Abourehab, M.A.S.; Alqahtani, A.M.; Youssif, B.G.M.; Gouda, A.M. Globally approved EGFR inhibitors: insights into their syntheses, target kinases, biological activities, receptor interactions, and metabolism. Molecules 2021, 26, 6677. [Google Scholar] [CrossRef]
- Uribe, M.L.; Marrocco, I.; Yarden, Y. EGFR in cancer: signaling mechanisms, drugs, and acquired resistance. Cancers (Basel) 2021, 13, 2748. [Google Scholar] [CrossRef]
- Cooper, A.J.; Sequist, L.V.; Lin, J.J. Third-generation EGFR and ALK inhibitors: mechanisms of resistance and management. Nat. Rev. Clin. Oncol. 2022, 19, 499–514. [Google Scholar] [CrossRef]
- Shi, K.; Wang, G.; Pei, J.; Zhang, J.; Wang, J.; Ouyang, L.; Wang, Y.; Li, W. Emerging strategies to overcome resistance to third-generation EGFR inhibitors. J. Hematol. Oncol. 2022, 15, 94. [Google Scholar] [CrossRef]
- Mansour, M.A.; AboulMagd, A.M.; Abbas, S.H.; Abdel-Rahman, H.M.; Abdel-Aziz, M. Insights into fourth generation selective inhibitors of (C797S) EGFR mutation combating non-small cell lung cancer resistance: a critical review. RSC Adv. 2023, 13, 18825–18853. [Google Scholar] [CrossRef]
- Jung, K.H.; Lee, E.J.; Park, J.W.; Lee, J.H.; Moon, S.H.; Cho, Y.S.; Lee, K.H. EGF receptor stimulation shifts breast cancer cell glucose metabolism toward glycolytic flux through PI3 kinase signaling. PLoS One 2019, 14, e0221294. [Google Scholar] [CrossRef]
- Han, J.H.; Lee, E.J.; Park, W.; Ha, K.T.; Chung, H.S. Natural compounds as lactate dehydrogenase inhibitors: potential therapeutics for lactate dehydrogenase inhibitors-related diseases. Front. Pharmacol. 2023, 14, 1275000. [Google Scholar] [CrossRef]
- Apicella, M.; Giannoni, E.; Fiore, S.; Ferrari, K.J.; Fernández-Pérez, D.; Isella, C.; Granchi, C.; Minutolo, F.; Sottile, A.; Comoglio, P.M.; Medico, E.; Pietrantonio, F.; Volante, M.; Pasini, D.; Chiarugi, P.; Giordano, S.; Corso, S. Increased lactate secretion by cancer cells sustains non-cell-autonomous adaptive resistance to MET and EGFR targeted therapies. Cell Metab. 2018, 28, 848–865.e6. [Google Scholar] [CrossRef]
- Claps, G.; Faouzi, S.; Quidville, V.; Chehade, F.; Shen, S.; Vagner, S.; Robert, C. The multiple roles of LDH in cancer. Nat. Rev. Clin. Oncol. 2022, 19, 749–762. [Google Scholar] [CrossRef]
- Rani, R.; Kumar, V. When will small molecule lactate dehydrogenase inhibitors realize their potential in the cancer clinic? Future Med. Chem. 2017, 9, 1113–1115. [Google Scholar] [CrossRef]
- Tanida, S.; Joh, T.; Itoh, K.; Kataoka, H.; Sasaki, M.; Ohara, H.; Nakazawa, T.; Nomura, T.; Kinugasa, Y.; Ohmoto, H.; Ishiguro, H.; Yoshino, K.; Higashiyama, S.; Itoh, M. The mechanism of cleavage of EGFR ligands induced by inflammatory cytokines in gastric cancer cells. Gastroenterology 2004, 127, 559–569. [Google Scholar] [CrossRef]
- Takenobu, H.; Yamazaki, A.; Hirata, M.; Umata, T.; Mekada, E. The stress- and inflammatory cytokine-induced ectodomain shedding of heparin-binding epidermal growth factor-like growth factor is mediated by p38 MAPK, distinct from the 12-O-tetradecanoylphorbol-13-acetate- and lysophosphatidic acid-induced signaling cascades. J. Biol. Chem. 2003, 278, 17255–17262. [Google Scholar] [CrossRef]
- Miyamoto, S.; Hirata, M.; Yamazaki, A.; Kageyama, T.; Hasuwa, H.; Mizushima, H.; Tanaka, Y.; Yagi, H.; Sonoda, K.; Kai, M.; Kanoh, H.; Nakano, H.; Mekada, E. Heparin-binding EGF-like growth factor is a promising target for ovarian cancer therapy. Cancer Res. 2004, 64, 5720–5727. [Google Scholar] [CrossRef]
- Mishima, K.; Higashiyama, S.; Asai, A.; Yamaoka, K.; Nagashima, Y.; Taniguchi, N.; Kitanaka, C.; Kirino, T.; Kuchino, Y. Heparin-binding epidermal growth factor-like growth factor stimulates mitogenic signaling and is highly expressed in human malignant gliomas. Acta Neuropathol. 1998, 96, 322–328. [Google Scholar] [CrossRef]
- Thøgersen, V.B.; Sørensen, B.S.; Poulsen, S.S.; Orntoft, T.F.; Wolf, H.; Nexo, E. A subclass of HER1 ligands are prognostic markers for survival in bladder cancer patients. Cancer Res. 2001, 61, 6227–6233. [Google Scholar]
- Kobrin, M.S.; Funatomi, H.; Friess, H.; Buchler, M.W.; Stathis, P.; Korc, M. Induction and expression of heparin-binding EGF-like growth factor in human pancreatic cancer. Biochem. Biophys. Res. Commun. 1994, 202, 1705–1709. [Google Scholar] [CrossRef] [PubMed]
- Prenzel, N.; Zwick, E.; Daub, H.; Leserer, M.; Abraham, R.; Wallasch, C.; Ullrich, A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 1999, 402, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Shinefield, H.R. Overview of the development and current use of CRM(197) conjugate vaccines for pediatric use. Vaccine 2010, 28, 4335–4339. [Google Scholar] [CrossRef] [PubMed]
- Aw, R.; Ashik, M.R.; Islam, A.A.Z.M.; Khan, I.; Mainuddin, M.; Islam, M.A.; Ahasan, M.M.; Polizzi, K.M. Production and purification of an active CRM197 in Pichia pastoris and its immunological characterization using a Vi-typhoid antigen vaccine. Vaccine 2021, 39, 7379–7386. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, P.; Liu, Y.; Xue, Y. Regulation of cellular growth, apoptosis, and Akt activity in human U251 glioma cells by a combination of cisplatin with CRM197. Anticancer Drugs 2012, 23, 81–89. [Google Scholar] [CrossRef]
- Kunami, N.; Yotsumoto, F.; Ishitsuka, K.; Fukami, T.; Odawara, T.; Manabe, S.; Ishikawa, T.; Tamura, K.; Kuroki, M.; Miyamoto, S. Antitumor effects of CRM197, a specific inhibitor of HB-EGF, in T-cell acute lymphoblastic leukemia. Anticancer Res. 2011, 31, 2483–2488. [Google Scholar]
- Tang, X.H.; Li, M.; Deng, S.; Lu, M.S. Cross-reacting material 197, a heparin-binding EGF-like growth factor inhibitor, reverses the chemoresistance in human cisplatin-resistant ovarian cancer. Anticancer Drugs 2014, 25, 1201–1210. [Google Scholar] [CrossRef]
- Tang, X.H.; Deng, S.; Li, M.; Lu, M.S. The anti-tumor effect of cross-reacting material 197, an inhibitor of heparin-binding EGF-like growth factor, in human resistant ovarian cancer. Biochem. Biophys. Res. Commun. 2012, 422, 676–680. [Google Scholar] [CrossRef]
- Dateoka, S.; Ohnishi, Y.; Kakudo, K. Effects of CRM197, a specific inhibitor of HB-EGF, in oral cancer. Med. Mol. Morphol. 2012, 45, 91–97. [Google Scholar] [CrossRef]
- Fiorentini, G.; Banfi, R.; Dentico, P.; Moriconi, S.; Turrisi, G.; Pelagotti, F.; Rossi, S.; Montagnani, F. Clinical experience of treatment of metastatic melanoma and solid tumours adopting a derivative of diphtheria toxin: cross-reacting material 197. In Vivo 2013, 27, 197–202. [Google Scholar]
- Sebastian, S.; Settleman, J.; Reshkin, S.J.; Azzariti, A.; Bellizzi, A.; Paradiso, A. The complexity of targeting EGFR signalling in cancer: from expression to turnover. Biochim. Biophys. Acta. 2006, 1766, 120–139. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H. EGFR ligands and their signaling scissors, ADAMs, as new molecular targets for anticancer treatments. J. Dermatol. Sci. 2009, 56, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Kenny, P.A. Tackling EGFR signaling with TACE antagonists: a rational target for metalloprotease inhibitors in cancer. Expert Opin. Ther. Targets 2007, 11, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Hedemann, N.; Rogmans, C.; Sebens, S.; Wesch, D.; Reichert, M.; Schmidt-Arras, D.; Oberg, H.H.; Pecks, U.; van Mackelenbergh, M.; Weimer, J.; Arnold, N.; Maass, N.; Bauerschlag, D.O. ADAM17 inhibition enhances platinum efficiency in ovarian cancer. Oncotarget 2018, 9, 16043–16058. [Google Scholar] [CrossRef]
| Gene | KiCqStart® ID | ID RefSeq | Exons |
|---|---|---|---|
| MMP2 | H_MMP2_2 | NM_004530 | 11-13 |
| MMP9 | H_MMP9_1 | NM_004994 | 9-11 |
| PLAU (uPA) | H_PLAU_1 | NM_001145031 | 7-8 |
| LDHB | H_LDHB_1 | NM_001174097 | 7-8 |
| LDHA | H_LDHA_1 | NM_001135239 | 6-7 |
| ESRRA (ERR-alpha) | H_ESRRA_2 | NM_004451 | 6-7 |
| GPER (GPER1) | H_GPER_1 | NM_001039966 | 2-3 |
| SRC | H_SRC_2 | NM_005417 | 5-6 |
| EGFR | H_EGFR_3 | NM_005228 | 2-3 |
| HBEGF | H_HBEGF_1 | NM_001945 | 4-5 |
| B2M | H_B2M_1 | NM_004048 | 2-3 |
| CYP33 (PPIE) | H_PPIE_1 | NM_006112 | 7-8 |
| RPS13 | H_RPS13_2 | NM_001017 | 3-4 |
| TUBA | H_TUBA1A_2 | NM_006009 | 2-3 |
| Experiment | Antibody | Host species | Catalog number |
Producer | Dilution |
|---|---|---|---|---|---|
| IB, primary | uPA | rabbit | A2181 | AB Clonal | 1:2000 |
| GPER1 | rabbit | A10217 | AB Clonal | 1:1000 | |
| ERR-alpha | rabbit | A14184 | AB Clonal | 1:2000 | |
| EGFR | rabbit | CPA4394 | Cohesion Biosciences | 1:1000 | |
| Phospho-EGFR (Tyr1068) | rabbit | AP0301 | AB Clonal | 1:1000 | |
| ERK1/2 | rabbit | 9102 | Cell Signaling | 1:1000 | |
| Phospho-ERK1/2 (Thr202/Tyr204) | rabbit | 4370 | Cell Signaling | 1:1000 | |
| PUMA α/β | mouse | sc-374223 | Santa Cruz | 1:500 | |
| Actin | rabbit | A2066 | Merck | 1:1000 | |
| IB, secondary | Rabbit IgG Cy5-labeled |
goat | 111-175-144 | Jackson Immuno Research | 1:2500 |
| Mouse IgG AlexaFluor 647-labeled |
donkey | 715-605-151 | Jackson Immuno Research | 1:1000 | |
| IF, primary | E-CAD | mouse | MAB8138 | R&D System | 1:100 |
| IF, secondary | Mouse poly-Ig FITC-labeled |
goat | F1010 | Merck | 1:400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





