1. Introduction
Heart transplantation (HT) is the gold standard therapy for advanced heart failure (ADHF) [
1]. Unfortunately, most patients who reach this stage have formal contraindications to be transplanted, thus alternative approaches are needed. Durable left ventricular assist devices (LVAD) have emerged in the last decades as a reasonable alternative in those considered not suitable to receive a HT, especially after the development of the last generation of centrifugal continuous-flow and full magnetic levitation devices [
2]. Older age, poor renal and right ventricular function, and other comorbidities are frequent in ADHF and are some of the commonest reasons for not considering a patient for LVAD therapy. In fact, most patients with ADHF, especially the elderly, never receive a HT or a LVAD, so alternative strategies are required as patients are usually highly symptomatic, with poor quality of life, and have frequent hospital admissions. Intermittent ambulatory levosimendan administration has shown in some small, randomized clinical trials that it can reduce HF hospitalizations in ADHF patients in the short term [
3,
4]. Real-life data about the regular, intermittent use of this drug as a last resort in end-stage HF patients not candidates for advanced therapies also suggest that it might decrease HF hospitalizations [
5]. Levosimendan has the advantage of the ambulatory administration over other intravenous inotropes and patients referred to this strategy are usually less sick than those referred to milrinone and dobutamine [
6,
7]; thus, it is uncertain whether the results of the comparison of inotropes with older generation LVAD would have the same outcomes in the current era of less sick but older, ADHF treated with levosimendan patients.
In this paper, we compare the use of two therapeutic strategies for end-stage HF in patients not candidates for HT: Repetitive intermittent Levosimendan vs. LVAD as destination therapy, a scenario that has not been re-explored in the last years after changes in ADHF patient´s profile and LVAD technology. To do so, we compare two multicenter cohorts of real-life patients from Spain: the LEVO-D Registry and the REGALAD registry.
2. Materials and Methods
The LEVO-D [
5] is a multicenter, retrospective study of patients over 18-year-old diagnosed with ADHF, not candidates for HT or LVAD. 23 tertiary hospitals in Spain participated in the registry, including patients who received at least one dose of ambulatory levosimendan between 1st January 2015 and 1st September 2020. Patients needed to be on optimal medical therapy (OMT) according to their treating physician. Patients with de novo HF or who underwent any procedure which could improve prognosis or clinical outcome (coronary revascularization, valve repair or replacement, cardiac resynchronization therapy -CRT- device implantation or any other procedure that under investigator criteria could improve prognosis or quality of life) after levosimendan was started, were not included in the registry. OMT was defined according to current guidelines and did not include sodium glucose co-transporter type 2 (SGLT2) inhibitors as patients of this registry came from a pre-SGLT2 inhibitors era.
The REGALAD [
8] registry is an observational and multicenter study that includes all long-term LVAD procedures performed in adults in Spain from 2007 to the 31th of December 2021. All Spanish hospitals performing long-term LVAD implantation participated in this registry. In each centre, a local physician and a surgeon undertook to introduce all LVAD procedures into the REGALAD registry. For the current analysis we only included those devices used as destination therapy. This included the following LVAD intracorporeal continuous flow LVAD models for isolated left ventricular support: INCOR (Berlin Heart GmbH), HeartMate II (Abbott, United States), and Jarvik 2000 (Jarvik Heart Inc, United States), which provide axial flow, and HeartWare HVAD (Medtronic, United States) and HeartMate 3 (Abbott), the latest generation pumps that provide centrifugal continuous flow.
2.1. Centers Involved
All centers involved in both registries had a specialized or ADHF unit. 23 tertiary hospitals in Spain participated in the LEVO-D registry and 22 in the REGALAD registry. 12 hospitals participated in both registries. All hospitals involved in the REGALAD registry had the capacity for HT or LVAD implant, but only 12 of the hospitals that included patients in the LEVO-D were doing at least one of these advanced therapies at the time the patients were included in the registry.
2.2. LEVO-D Data Collection
Baseline data was collected on the day of the first dose of levosimendan, with blood pressure and heart rate measured before the drug was administered. Routine urgent laboratory data such as blood count, renal function or N-terminal pro-brain natriuretic peptide (NT-ProBNP) were from the day of the first scheduled infusion. Other laboratory parameters not usually performed in an urgent blood sample were allowed if the sample was taken up to 21 days before first levosimendan administration. Echocardiographic data was the closest before the first infusion. Data were collected in an anonymous database and analysed after the approval of the regional ethic committee. Patients were followed-up under their clinician’s judgement. Outcomes were updated up to June 2021.
2.3. REGALAD Data Collection
REGALAD includes most variables from the IMACS (International Society for Heart and Lung Transplantation Registry for Mechanically Assisted Circulatory Support) and EUROMACS (European Registry for Patients with Mechanical Circulatory Support) registries, as well as additional variables considered pertinent by the registry founders. These variables include patients’ demographic, clinical, laboratory, echocardiographic, and hemodynamic characteristics, implantation data, and 3- month, 1-year, and annual follow-up data. Adverse events associated with the device were specifically recorded. HF severity at implantation was graded using the scale proposed by INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) [
9]. Patients were followed-up until death or study closure on December 31, 2021 (8).
2.4. Endpoints
We analysed all-cause death during follow-up and the combined endpoint of death/HF hospitalizations at 1 year after receiving at least one dose of levosimendan or the LVAD implant.
2.5. Statistical Analysis
Results are expressed as mean +/- standard deviation, medians (interquartile range -IQR-) or percentages, depending on each variable. Statistical differences were analysed with Student T-test (gaussian distribution), nonparametric Mann-Whitney U (non-gaussian distribution), nonparametric McNemar or Chi-square as appropriate. Simple survival analysis was performed with Kaplan-Meier curves and long-rank test was used to evaluate statistical significance. Missing data was managed by performing multiple imputations of all relevant parameters in the entire population. SPSS version 25 was used for multiple imputations using the automated function. A 20% limit for the missing data was set to exclude variables with excessive missing data. Results were expressed as hazard ratio (HR) with 95% confidence interval (95%CI). A p-value < 0.05 was considered as significant.
2.6. Propensity Matching
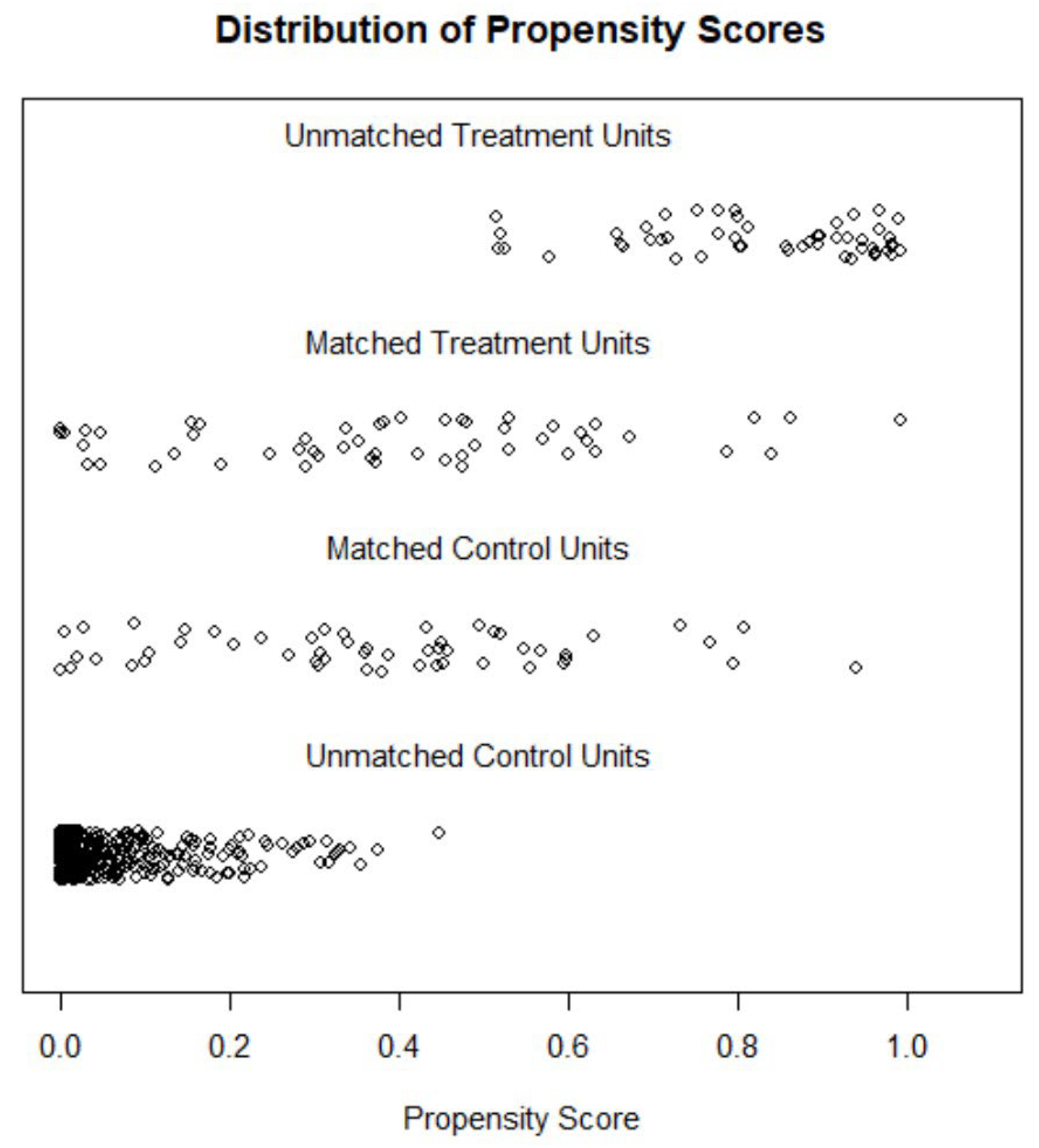
A propensity score matching (PSM) was performed to minimize the bias inherent to observational studies. Firstly, the propensity score was used to assess the probability of each patient to be treated with levosimendan or LVAD, according to baseline characteristics. PSM was then performed to match the characteristics of both groups (LEVO-D vs REGALAD). We used a 1:1 protocol without replacement, and calipers of width equal to 0.2 of the standard deviation of the logit of the PSM. All the measured covariates were well balanced across the comparator group (
Figure 1).
PSM was performed using binary logistic regression in which the dependent variable was the ADHF therapy (LVAD vs levosimendan) and the explanatory variables were age, sex, body mass index, hypertension, diabetes mellitus, atrial fibrillation/flutter, ischemic heart disease, NYHA class, INTERMACS, systolic blood pressure, systolic pulmonary arterial pressure, heart rate, LVEF, right ventricular dysfunction, moderate-severe mitral regurgitation, moderate-severe tricuspid regurgitation, ≥ 3 HF hospitalizations, and medical therapy (Beta-blockers, Angiotensin-converting-enzyme inhibitors/Angiotensin receptor blockers -ACEI/ARB-, angiotensin receptor/neprilysin inhibitor -ARNI, mineralocorticoid receptor antagonist -MRA-, amiodarone, digoxin, hydralazine, thiazide, furosemide, oral anticoagulation).
3. Results
715 patients coming from the 2 registries where found, 403 from the LEVO-D and 312 from the REGALAD. Of the latter, 104 patients were considered to have a LVAD implanted as destination therapy. This group had an LVAD as destination therapy or as bridge to candidacy considered unlikely to be transplanted by their medical team and never underwent HT. The majority (91.3%) of patients of the REGALADreceived a third-generation continuous flow centrifugal device (56.7% HeartMate 3 and 34.6% HeartWare). Of the remaining patients, 1 received a Jarvik 2000, 5 a HeartMate II and 3 a BerlinHeart Incor. Although the REGALAD database included patients from 2007 and onwards, 92.3% of patients of the REGALADhad the implant performed between 2015 and 2020, and 95.1% between 2014 and 2020; thus, the majority of patients were contemporary to the LEVO-D registry. Full cohort characteristics of the LEVO-D and REGALAD registries have been published elsewhere (5,8).
3.1. Demographics
Table 1 compares the main characteristics of the two groups. Patients referred for destination inotropes were slightly older, LVEF and pulmonary pressures were higher for LEVO-D and left ventricular end-diastolic diameter (LVEDD) was smaller. Furthermore, LEVO-D showed worse renal function, bilirubin, and natriuretic peptides but more patients were in NYHA IV in the REGALADcohort. More LEVO-D patients had atrial fibrillation or flutter and therefore received more frequently anticoagulation. More REGALAD patients were on amiodarone and had an implantable cardioverter defibrillator -ICD- implanted. Regarding neurohormonal blockade, we found differences only in betablockers; 65% of patients of the REGALAD cohort were on betablockers compared to 78.9% on the LEVO-D.
3.2. Survival and HF events
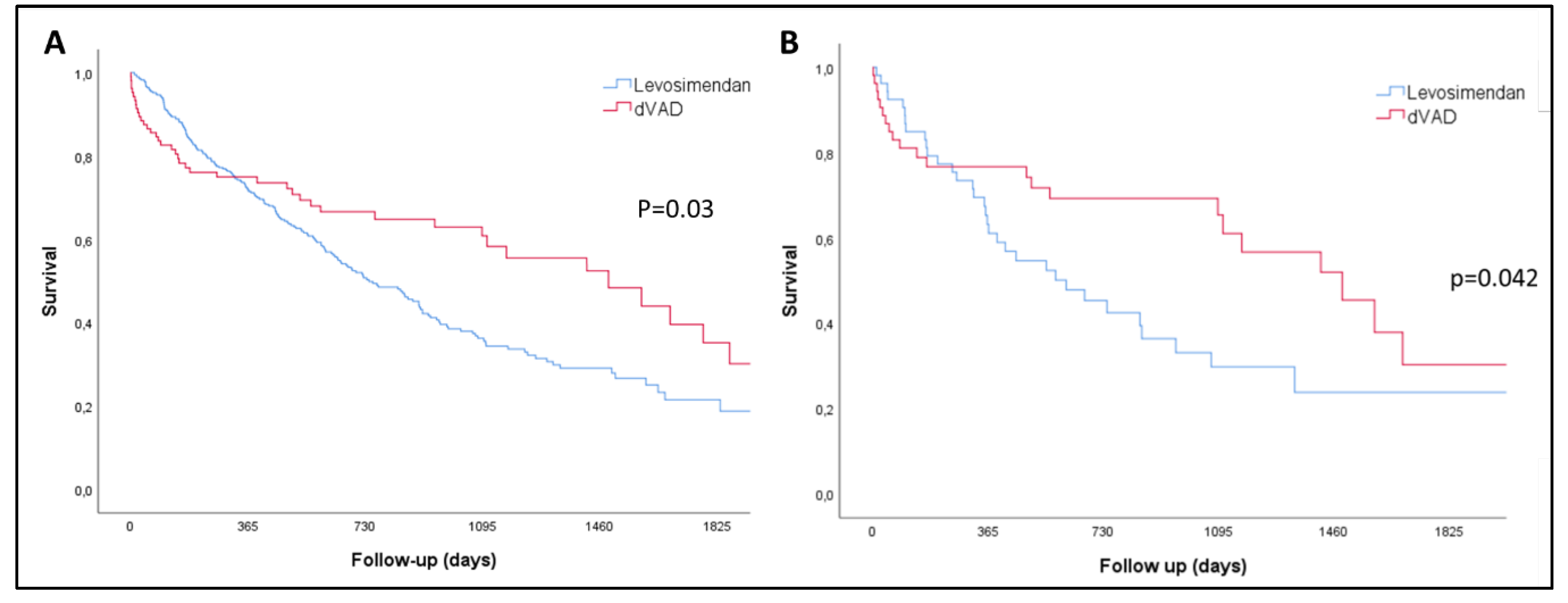
There were no patients lost to the follow-up.52.3% of the LEVO-D and 42.3% of patients of the REGALAD died during follow-up; thus, more patients in the inotrope group died despite a shorter follow-up (median, 458 days [CI95% 412 - 519] vs 494 days [CI95%357 - 730]; p=0.08). Non-adjusted median survival was shorter for LEVO-D patients (741 days [CI95% 611-870] vs 1486 days [CI95% 969-2002]; p=0.03); with the benefit for the LVAD seen after the first year of therapy when analyzing the Kaplan-Meier curves (
Figure 2A). On the other hand, within the first year of follow-up, the number of HF admissions was not different between groups (LEVO-D 1.12±1.78 vs REGALAD 0.94±1.64; p=0.35) and the percentage of patients alive without admissions was higher for LEVO-D patients within this first year (LEVO-D 49.6% vs REGALAD 33.7%; p=0.004).
3.3. Propensity Score Matching
Two groups of 53 matched patients were created. The predictive power of the model used to generate the propensity score was 0.91, with adequate calibration (Hosmer-Lemeshow test, P = 0.292). The survival advantage for the LVAD cohort was also true after analysis of the matched cohort (
Table 2) in the propensity matching analysis. Of note, all the patients included in the PSM from the REGALAD were implanted from 2015 and onwards, thus contemporary to LEVO-D patients. LVAD patients showed a significantly better survival compared to the LEVO-D cohort (median, days, 1574 [CI95% 1048 - 1923] vs 612 [296 - 927]; p=0.042) but, as in the non-matched analysis, the survival benefit was mainly shown after one year of follow up (
Figure 2B). However, the suggested 1-year advantage of the combined event of death and HF hospitalization for the LEVO-D cohort in the first year, was not consistent in the PSM as the percentage of patients alive without admissions within the first year was similar (LEVO-D 37.7% vs REGALAD 39.6%; p=0.84).
4. Discussion
HT is the gold standard for AHF but in the western world most of patients with ADHF have formal contraindications for HT mostly due to age, thus alternatives are needed to decrease symptoms, reduce admissions and improve quality of life of patients with ADHF. Ambulatory inotropes and LVAD as destination therapy are two commonly used options for these ADHF patients without HT options but this scenario has not been re-explored in the last years after changes in the ADHF patient´s profile and LVAD technology. The data we present tries to bring some light to this real-life clinical dilemma.
Our analysis of two contemporary multicentre real-life registries shows how non-heart transplant candidates with outpatient ADHF are being referred to have a LVAD or ambulatory inotropes in a country with a relatively high heart transplantation availability and aging population. The vast majority of patients from the LEVO-D and REGALAD were contemporary (from 2015 and onwards) which was true for all the patients included in the PSM. With our findings we can conclude that patients with end-stage HF treated today with either LVAD or intermittent inotropes as destination therapy have a different clinical profile, similar 1-year outcomes but long-term survival is better for LVAD patients both in unmatched and PS matched comparison.
4.1. Patients Characteristics
Patients referred to have levosimendan as destination therapy were more comorbid showing worse renal function, higher pulmonary pressures, more atrial arrhythmias and more heterogenous HF aetiology, whether LVEF was better, had less mitral regurgitation and the left ventricle was less dilated. Although LEVO-D patients were older, differences were not as marked as expected but, in any case, both groups were in their seventies. In view of the baseline characteristics, it may be interpreted that those referred for an LVAD have more low output syndrome and less comorbidities (something that is reinforced by the fact that less REGALAD patients were on betablockers, which might reflect that they were withdrawn due to low cardiac output), while LEVO-D patients were more heterogeneous and with more cardiorenal syndrome. It is also likely that ventricular arrhythmia burden was higher for REGALAD as more patients were on amiodarone and had an ICD- implanted.
4.2. Long and Short-Term Outcomes
When these differences were adjusted by PS matching analysis, the elderly REGALAD patients got the benefit of a significantly better long-term survival. This means, that every effort should be done to carefully identify patients within the group of ambulatory ADHF non-HT candidates who can benefit from an LVAD, even among the elderly and comorbid ones. LVAD recipients older than 70 years show worse survival compared to younger patients, but their quality of life improvement has been shown to be at least as good and increased functional capacity superior [
10] to younger patients. Nowadays, the decision to implant an LVAD in this elderly population should be balanced with the increased risk of early mortality, thus a careful evaluation of the candidate should be undertaken. In fact, age is one of the components of the recent HeartMate 3 survival risk score [
11] and should be considered as well as the usual multiparameter case evaluation and right heart failure prediction.
Although our work shows, non-surprisingly, that an LVAD is superior to inotropes in the long term, it also shows that intermittent levosimendan could be as good in terms of survival and HF events as the LVAD within the first year, even in the adjusted population, as there were no differences in the rate of death/HF admission within the first year. This should be considered, when evaluating LVAD candidature for these elderly HF patients, specifically in patients with multiple non-cardiac comorbidities that may limit 1 year survival and when the risk of right heart failure after LVAD is high. Levosimendan infusions have been linked to a significant reduction of heart failure hospitalizations and increased QoL in small, randomized trials and in real life registries [
3,
4,
5,
12], showing significant improvements in survival if we compare it with historical data in ADHF [
13]. For these reasons, intermittent inotropic support with levosimendan has become a popular tool in some regions for ADHF patients, as administration can be easily performed in the ambulatory setting following protocols such as that from the LION HEART study [
3]. It can be interpreted that this is the reason why patients referred to levosimendan as destination therapy are significantly less sick than those referred to continuous intravenous inotropes; thus, the threshold for levosimendan in ADHF patients in some European countries seems to be lower than in other countries that use continuous ambulatory infusions of other inotropes like milrinone, with much higher 1-year mortality rates [
7].
4.3. Clinical Implications
The interpretation of old clinical trials comparing medical treatment including or not inotropes and LVAD is difficult for several reasons: contemporary patients have very different 1-year mortality rates with the current optimal medical therapy [
1], but LVAD technology, complications, and outcomes have also improved considerably [
2]. Patients randomized to medical treatment in the REMATCH trial showed a 1-year mortality of 75% [
13] and it was 89% in the INTREPID trial [
14]; compared to 26.5% in the LEVO-D registry [
5] or the 22% rate of death/LVAD/transplant in the RELEVANT-HF [
11] and 34% in the MedaMACS registry [
15]. A recent meta-analysis of ambulatory inotropic treatment suggested a 1-month mortality rate of 4.2% [
16]; although studies included were very heterogeneous and some of them separated by more than 20 years. It should also be noted that LVADs are completely different to those compared to medical treatment 15 years ago, but also patients are older and more comorbid, even more than those of the recent pivotal trials: REGALADpatients were more than 5 years older than DT patients from the MOMENTUM 3 [
17] and INTERMACS registry [
18]. All these reasons might be behind the low rate of LVAD implantation in some countries [
8], especially compared to the United States, because age is seen as an obstacle when referring patients to have a LVAD, as it is related to poorer outcomes, especially in those over 75 years old [
19]. However, when comparing adequately selected elderly patients, LVAD offers better long-term outcomes, and this must be taken into consideration when discussing therapeutic options in these elderly patients with ADHF.
We consider that our work brings some light to this situation comparing contemporary ADHF patients, treatments and technology, so that we can have a real picture of today´s management of ambulatory ADHF non-HT candidates and what to expect from the different therapeutic options. The ongoing SweVAD trial aims to compare LVAD to optimal medical therapy in patients with ADHF and may provide more clinical answers to the current management of these patients [
20].
4.4. Limitations
This is a retrospective study thus it is subject to bias by its nature. Propensity score analysis is more robust than regression analysis, but it has certain weaknesses as unmeasured confounding factors cannot be corrected. The relatively small numbers of the REGALAD registry, as the result of the low number of LVAD implants in Spain, as well as the differences in the two populations, imply that the N of the propensity analysis was small, but it should be noted that this is the only contemporary study comparing destination inotropes and LVAD. Patients were not matched by psychosocial factors, which can be reasons for a non LVAD implant decision without other medical contraindications, as although they have not been related to worse survival on support [
21,
22] they have been linked to increased risk of complications and decreased quality of life.
5. Conclusions
In elderly ADHF non-HT-candidates, LVAD therapy offers significantly better long-term outcomes when compared to intermittent levosimendan; thus, it should be considered in carefully selected candidates. On the other hand, in poor LVAD candidates or highly comorbid patients, intermittent inotropic support with levosimendan could be a reasonable alternative to LVAD as 1-year outcomes are similar.
Author Contributions
Conceptualization, D.D., M.G.B. and J.G.C.; methodology, S.R.; formal analysis, D.D. and S.R.; writing—original draft preparation, D.D; investigation, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
LEVO-D registry is investigator-initiated. Orion Pharma provided funding for the web-based data base, but the design, data collection and analysis were performed independently by the investigators.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Galicia (HUV-LEV-2020-01, 7th October 2020).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of this work that made obtaining informed consent not possible.
Data Availability Statement
The original data are not publicly available. However, research projects are evaluated centrally by an ad hoc committee
Conflicts of Interest
D.D, J.G.C., J.J.B., J.F. received speaker fees from Orion. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- McDonagh TA, Metra M, Adamo M; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599-3726
. [CrossRef]
- Mehra MR, Cleveland JC Jr, Uriel N; et al. Primary results of long-term outcomes in the MOMENTUM 3 pivotal trial and continued access protocol study phase: A study of 2200 HeartMate 3 left ventricular assist device implants. Eur J Heart Fail. 2021;23:1392-1400
. [CrossRef]
- Comín-Colet J, Manito N, Segovia-Cubero J; et al. LION-HEART Study Investigators. Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: The LION-HEART multicentre randomised trial. Eur J Heart Fail. 2018;20:1128-1136. [CrossRef]
- García-González MJ, Aldea Perona A, Lara Padron A; et al. Efficacy and safety of intermittent repeated levosimendan infusions in advanced heart failure patients: The LAICA study. ESC Heart Fail. 2021;8:4820-4831. [CrossRef]
- Dobarro D, Donoso-Trenado V, Solé-González E; et al. Intermittent inotropic support with levosimendan in advanced heart failure as destination therapy: The XXXX1 registry. ESC Heart Fail. 2023. [CrossRef]
- Laufer-Perl M, Sadon S, Zahler D, et al Repetitive milrinone therapy in ambulatory advanced heart failure patients. Clin Cardiol. 2022;45:488-494
.
- Eaton RE, Kissling KT, Haas GJ; et al. Rehospitalization of Patients with Advanced Heart Failure Receiving Continuous, Palliative Dobutamine or Milrinone. Am J Cardiol. 2022;184:80-89
. [CrossRef]
- Gómez-Bueno M, Pérez de la Sota E, Forteza Gil A; et al. First report of the XXXX2 registry. Rev Esp Cardiol (Engl Ed). 2022
. Epub ahead of print. [CrossRef]
- Stevenson LW, Pagani FD, Young JB; et al. INTERMACS profiles of advanced heart failure: The current picture. J Heart Lung Transplant. 2009;28:535-41
. [CrossRef]
- Emerson D, Chikwe J, Catarino P; et al. Contemporary Left Ventricular Assist Device Outcomes in an Aging Population: An STS INTERMACS Analysis. J Am Coll Cardiol. 2021;78:883-894
.
- Mehra M.R., Nayak A., Morris A.A.; et al. Prediction of survival after implantation of a fully magnetically levitated left ventricular assist device. J Am Coll Cardiol HF 2022;10:948-959
. [CrossRef]
- Oliva F, Perna E, Marini M; et al. Scheduled intermittent inotropes for Ambulatory Advanced Heart Failure. The RELEVANT-HF multicentre collaboration. Int J Cardiol. 2018;272:255-259
.
- Rose EA, Gelijns AC, Moskowitz AJ; et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435-43
. [CrossRef]
- Rogers JG, Butler J, Lansman SL; et al. Chronic mechanical circulatory support for inotrope-dependent heart failure patients who are not transplant candidates: Results of the INTrEPID Trial. J Am Coll Cardiol. 2007;50:741-7
. [CrossRef]
- Ambardekar AV, Kittleson MM, Palardy M; et al. Outcomes with ambulatory advanced heart failure from the Medical Arm of Mechanically Assisted Circulatory Support (MedaMACS) Registry. J Heart Lung Transplant. 2019;38:408-417. [CrossRef]
- Nizamic T, Murad MH, Allen LA; et al. Ambulatory Inotrope Infusions in Advanced Heart Failure: A Systematic Review and Meta-Analysis. JACC Heart Fail. 2018;6:757-767
.
- Goldstein DJ, Naka Y, Horstmanshof D; et al. Association of Clinical Outcomes With Left Ventricular Assist Device Use by Bridge to Transplant or Destination Therapy Intent: The Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy With HeartMate 3 (MOMENTUM 3) Randomized Clinical Trial. JAMA Cardiol. 2020;5:411-419
.
- Shah P, Yuzefpolskaya M, Hickey GW; et al. Twelfth Interagency Registry for Mechanically Assisted Circulatory Support Report: Readmissions After Left Ventricular Assist Device. Ann Thorac Surg. 2022;113:722-737
. [CrossRef]
- Aleksova N, Alba AC, Fan CS; et al. The Effect of Age on Outcomes After Destination-Therapy Left Ventricular Assist Device Implantation: An Analysis of the IMACS Registry. Can J Cardiol. 2021;37:467-475
. [CrossRef]
- Karason K, Lund LH, Dalén M; et al. Randomized trial of a left ventricular assist device as destination therapy versus guideline-directed medical therapy in patients with advanced heart failure. Rationale and design of the SWEdish evaluation of left Ventricular Assist Device (SweVAD) trial. Eur J Heart Fail. 2020;22:739-750
.
- DeFilippis EM, Breathett K, Donald EM; et al. Psychosocial Risk and Its Association With Outcomes in Continuous-Flow Left Ventricular Assist Device Patients. Circ Heart Fail. 2020;13:e006910
. [CrossRef]
- Fakoya OA, McCorry NK, Donnelly M. Loneliness and social isolation interventions for older adults: A scoping review of reviews. BMC Public Health. 2020;20:129
. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).