1. Introduction
Winter 2022-2023 witnessed a massive increase in severe group A streptococcal (GAS) infections in children and adolescents in many parts of Europe [
1] and abroad [
2]. Its cause is incompletely understood, but the disrupted circulation of respiratory tract pathogens brought about by COVID-19 pandemic and the appearance of genetically distinct GAS clones were likely contributors. Most notable was the emergence of previously unknown clades of the M1
UK lineage [
3]. Accordingly, it was difficult to predict how case frequencies would evolve from mid-2023 onwards and the scientific community was left with closely monitoring GAS disease activity in order to gain a better understanding of this outbreak.
We recently reported epidemiologic and clinical data on pediatric GAS disease requiring hospital admission to our tertiary care center in Switzerland over a 10-year observation period ending on 30 June 2023 [
4]. Our data reflected developments similarly reported from numerous other sites across Europe. Apart from a threefold increase in overall GAS hospitalizations in 2022-2023 compared with the prepandemic years 2013-2020, we noted a six-fold increase in invasive GAS infections (iGAS). Among these, pleural empyema stood out with the most dramatic surge and analysis of concomitant viral circulation identified influenza A and B as the viral species circulating synchronously in our in-patient population. A concomitant increase in GAS pleural empyema cases had been reported from many other sites in Europe [
5].
In this report we describe the evolution of GAS hospitalizations in children and adolescents during the most recent 2023-2024 epidemiologic year ending on 30 June 2024.
2. Methods
Our institution is the only provider of pediatric in-patient services for a population of 1.2 million inhabitants (14% of the Swiss population). We monitored all hospitalizations to our institution with GAS infection as the main discharge diagnosis in patients below 16 years of age as previously described [
4]. An epidemiologic year was defined as a period of one year beginning on 1 July. Comparator cohorts of the 2023-2024 cohort consisted of the cases in the prepandemic period extending form 1 July 2013 to 30 June 2020 and the 2022-2023 cohort, respectively. iGAS was defined as previously described (
Table S1, supplementary data file [
4]). For GAS organ involvement the following definitions were used: The term “head” infection comprised suppurative GAS infections of the eye (e.g., orbital cellulitis), ear (e.g. mastoiditis), throat (e.g., peritonsillar- or retropharyngeal abscess) or neck (e.g. cervical lymphadenitis). “Skin and soft tissue” infection included abscesses, cellulitis or necrotizing fasciitis. “Lower respiratory tract” infection denoted pleural empyema or bacteremic pneumonia. “Bone, joint, muscle” infections consisted of osteomyelitis, septic arthritis or bacterial myositis.” Systemic-onset” infection denoted invasive GAS infections without an identified focal site of infection other than acute pharyngitis. Clinical parameters extracted for each case are listed in
Table 1. In-house viral surveillance of nasopharyngeal secretions of all patients admitted for acute respiratory tract illness was carried out using a direct immunofluorescence panel (Influenza A and B, rhino/enterovirus, Respiratory Syncytial Virus [RSV]), parainfluenza types 1-3, human metapneumovirus, adenovirus) [
6] and SARS-CoV-2 PCR (from 2020 onwards) until 30 November 2023. It was changed to a four-plex PCR panel (Influenza A, Influenza B, RSV, SARS-CoV-2) on 1 December 2023. For statistical analyses of ordinal data we used the Mann-Whitney U test or the Kruskal Wallis-test as appropriate. Contingency tables (2x2 and 2x3) were used for frequency data. VassarStats (
www.vassarstats.net) and GraphPad Prism version 10.0.0 for Windows (GraphPad Software, Boston, Massachusetts USA,
www.graphpad.com) were used for analyses and figure drawing.
3. Results
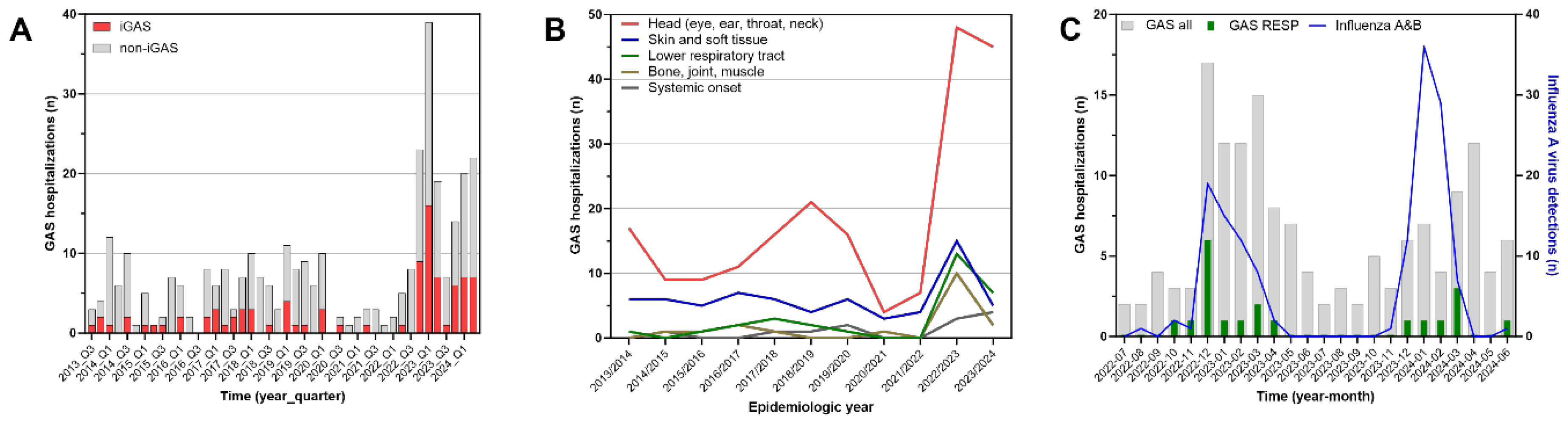
The evolution of case counts over time since 1 July 2013 is depicted in
Figure 1A. In the 2023-2024 epidemiologic year, these were lower (63 cases) than in 2022-2023 (89 cases), but continued to exceed the prepandemic annual median (25 cases, range 17-28) by 2.5-fold (
Table 1). iGAS cases evolved similarly (2013-2020, median 4 cases, range 3-8; 2022-2023, 32 cases; 2023-2024, 21 cases). A total of 19 cases including 3 iGAS cases occurred intrapandemically between 1 July 2020 and 30 June 2022. The lowest quarterly activity during the entire outbreak period from 2022-2024 was observed in the third quarter of 2023 (7 cases). In 2013-2020, the third quarter median was 3 cases (range 2-10). In terms of clinical manifestations, resolving the time series since 2013 according to the primary organ system involved resulted in the findings shown in
Table 1 and
Figure 1B. GAS infections of the head accounted for 61% of cases overall and fluctuated considerably. After the major surge in 2022-2023 with 48 cases, the case count was 45 in 2023-2024. In contrast, skin and soft tissue infections, infections of bone, joints and muscles, and lower respiratory tract infections promptly returned declined to lower figures after the peak in 2022-2023. In both outbreak years combined, lower respiratory tract disease predominantly consisted of pleural empyema (17 cases), while bacteremic pneumonia without empyema was infrequent (3 cases). Skin and soft tissue infections observed during the entire study period mostly consisted of cellulitis or skin abscesses. Necrotizing fasciitis was rare (2 cases in 2022-2023, both associated with varicella).
While the case frequencies in the two outbreak years strongly exceeded the case counts in the preceding decade, there was only limited evidence for greater case severity among hospitalized patients. A significantly greater proportion of cases was bacteremic and fulfilled the criteria of iGAS (
Table 1). This, however, did not translate to a greater proportion of cases being diagnosed with septic and/or toxic shock compared with the prepandemic period. Accordingly, there were no significant differences in key outcome markers listed in
Table 1, which remained stable throughout the entire observation period. An exception was the non-significant increase in inotrope use among ICU patients in 2023-2024.
Temporal association with the circulation of influenza viruses was examined in
Figure 1C. While evolving synchronously with GAS counts in 2022-2023, the epidemic curves in in 2023-2024 were discordant. In this year, the respective monthly peak frequencies occurred three months apart. Green bars identifying lower respiratory tract infections occurred almost exclusively during the influenza epidemics. The seasonal activities of RSV and SARS-CoV-2 did not correlate with the GAS cases (
Figure S1, supplementary data file).
4. Discussion and Conclusion
The data presented here provide evidence of an ongoing excessive occurrence of severe pediatric GAS infections including iGAS in the second year following the abrupt onset of the outbreak in fall of 2022. A similar persistence has recently been reported from Norway [
7] and we expect that additional reports from other locations in Europe will likely draw the same picture. However, while the data from Norway indicate an important further increase of pediatric iGAS cases in 2023-2024, we find the opposite with a moderate decrease of pediatric GAS hospitalizations in general and, specifically, of iGAS cases by approximately one third compared with 2022-2023. This evolution mainly reflects a decrease in skin and soft tissue infections, bone, joint and muscle infections and pleural empyema, while suppurative GAS infections in the head and neck region (peritonsillar abscess, mastoiditis, orbital cellulitis etc.) with or without bacteremia and cases with septic/toxic shock remained essentially stable. Our data are unsuitable to identify potential reasons for this divergent development, but the overall decrease of both iGAS and bacteremia cases may herald that the outbreak is on the decline. Hypothetical explanation may include a recovering population immunity against GAS in general or against specific outbreak clones. This would be supported by our observation that systemic GAS infections appear to decline more quickly than complications adjacent to the pharyngeal carriage site of GAS. Moreover, the observed decline of severe respiratory disease, i.e., mostly pleural empyema, could be explained by the epidemiology of influenza. Under the assumption that a preceding influenza virus infection is a major risk factor for complicated GAS lower respiratory tract disease [
4,
8], the poor overlap of the 2023-2024 GAS and influenza epidemic curves, respectively, may have restricted the risk phase for pleural empyema to a shorter period than in 2022-2023 (
Figure 1C). For instance, Lassoued and co-workers similarly observed a coincidence of GAS pleural empyema and influenza activity in 2022-2023 in France and were able to demonstrate that the majority of pleural empyema patients were indeed co-infected with influenza virus [
9]. A rather specific role of influenza restricted to the pathogenesis of GAS pleural empyema - but not other GAS disease - could also explain observations indicating that non-invasive GAS infections in the 2022-2023 outbreak year did not appear to be related to the circulation of influenza, e.g. in Portugal [
10].
Our study has several limitations. Apart from the single site design, which reflects the epidemiology of a demographically stable, but geographically small area, the case figures in the subgroup analyses were small, allowing for wide margins of error. The data on viral co-circulation refer to the shapes of the respective epidemic curves, but not the co-occurrence of pathogens in the individual patients at the time of GAS infection.
In conclusion, however, our observations spanning 11 years of GAS hospitalizations in patients below 16 years of age demonstrate that the outbreak of severe GAS disease beginning in late 2022 continued through June 2024, however with a decline of cases in the second winter season. The decline mainly affected distant body sites and systemic infections, while the classic suppurative complications in the head area remained largely unchanged.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org. Figure S1: Monthly hospitalization figures for all GAS infections (grey bars), GAS lower respiratory tract disease (green bars), and detection rates of Respiratory Syncytial Virus (blue line) and SARS-CoV-2 (red line) at the Departments of Pediatrics and Pediatric Surgery, Bern University Hospital, Switzerland, between 1 July 2022 and 30 Jun3 2024.; Table S1: Definition of invasive group A streptococcal disease (iGAS).
Author Contributions
CA, NS and PKAA conceived the study. AD, KK, MH, AB and MVK provided input for case identification, clinical characterization and additional methodological issues. CA wrote the first draft of the manuscript. All authors contributed to subsequent modifications and approved the final version.
Funding
No funding was received for conducting this study.
Institutional Review Board Statement
The study has been approved by the Cantonal Ethics Committee (project no. 2023-00520). General or project-specific written informed consent was given by the legal guardians of all patients.
Informed Consent Statement
The study has been approved by the Cantonal Ethics Committee (project no. 2023-00520). General or project-specific written informed consent was given by the legal guardians of all patients.
Acknowledgments
We thank the physician staff of the Division of Pediatric Radiology and the Department of Ear, Nose and Throat diseases at our institution for their diagnostic and therapeutic contributions in each patient.
Conflicts of Interest
The authors have no competing interests to declare that are relevant to the content of this article.
References
- Bamford, A.; Whittaker, E. Resurgence of group A streptococcal disease in children. BMJ 2023, 380, 43. [Google Scholar] [CrossRef] [PubMed]
- Abo, Y.N.; Oliver, J.; McMinn, A.; Osowicki, J.; Baker, C.; Clark, J.E.; Blyth, C.C.; Francis, J.R.; Carr, J.; Smeesters, P.R.; et al. Increase in invasive group A streptococcal disease among Australian children coinciding with northern hemisphere surges. Lancet Reg Health West Pac 2023, 41, 100873. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.; Wan, Y.; Ryan, Y.; Li, H.K.; Guy, R.L.; Papangeli, M.; Huse, K.K.; Reeves, L.C.; Soo, V.W.C.; Daniel, R.; et al. Rapid expansion and international spread of M1(UK) in the post-pandemic UK upsurge of Streptococcus pyogenes. Nat Commun 2024, 15, 3916. [Google Scholar] [CrossRef] [PubMed]
- Schobi, N.; Duppenthaler, A.; Horn, M.; Bartenstein, A.; Keitel, K.; Kopp, M.V.; Agyeman, P.; Aebi, C. Preadmission course and management of severe pediatric group A streptococcal infections during the 2022-2023 outbreak: a single-center experience. Infection 2024. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, U.; Bloch, J.; Dungu, K.H.S.; Vollmond, C.; Buchvald, F.F.; Nielsen, K.G.; Kristensen, K.; Poulsen, A.; Vissing, N.H. Incidence and aetiology of Danish children with community-acquired pneumonia treated with chest tube drainage in 2022-2023 versus the previous three decades. Arch Dis Child 2023. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, C.D.; Aebi, C.; Gorgievski-Hrisoho, M.; Muhlemann, K.; Barbani, M.T. Twelve years' detection of respiratory viruses by immunofluorescence in hospitalised children: impact of the introduction of a new respiratory picornavirus assay. BMC Infect Dis 2011, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Valcarcel Salamanca, B.; Cyr, P.R.; Bentdal, Y.E.; Watle, S.V.; Wester, A.L.; Strand, A.M.W.; Boas, H. Increase in invasive group A streptococcal infections (iGAS) in children and older adults, Norway, 2022 to 2024. Euro Surveill 2024, 29. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, K.; Herbener, A.; Blaschke, A.J.; Heyrend, C.; Poritz, M.; Korgenski, K.; Rolfs, R.; Jain, S.; Carvalho Mda, G.; Pimenta, F.C.; et al. Association of 2009 pandemic influenza A (H1N1) infection and increased hospitalization with parapneumonic empyema in children in Utah. Pediatr Infect Dis J 2010, 29, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Lassoued, Y.; Assad, Z.; Ouldali, N.; Caseris, M.; Mariani, P.; Birgy, A.; Bonacorsi, S.; Bidet, P.; Faye, A. Unexpected Increase in Invasive Group A Streptococcal Infections in Children After Respiratory Viruses Outbreak in France: A 15-Year Time-Series Analysis. Open Forum Infect Dis 2023, 10, ofad188. [Google Scholar] [CrossRef] [PubMed]
- De Beir, J.; Lucas, M.; Jesus, A.R.; Gata, L.; Finn, A.; Rodrigues, F. Postpandemic Rebound in Noninvasive Group a Streptococcal Disease is not Synchronous with Winter RSV and Influenza Epidemics. Pediatr Infect Dis J 2024, 43, e106–e108. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).