Submitted:
18 July 2024
Posted:
19 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Lentiviral Cell Transduction
2.3. qPCR Analysis
2.4. Western Blot Analysis
2.5. ROS Detection Assays
2.6. Statistical Analysis
3. Results
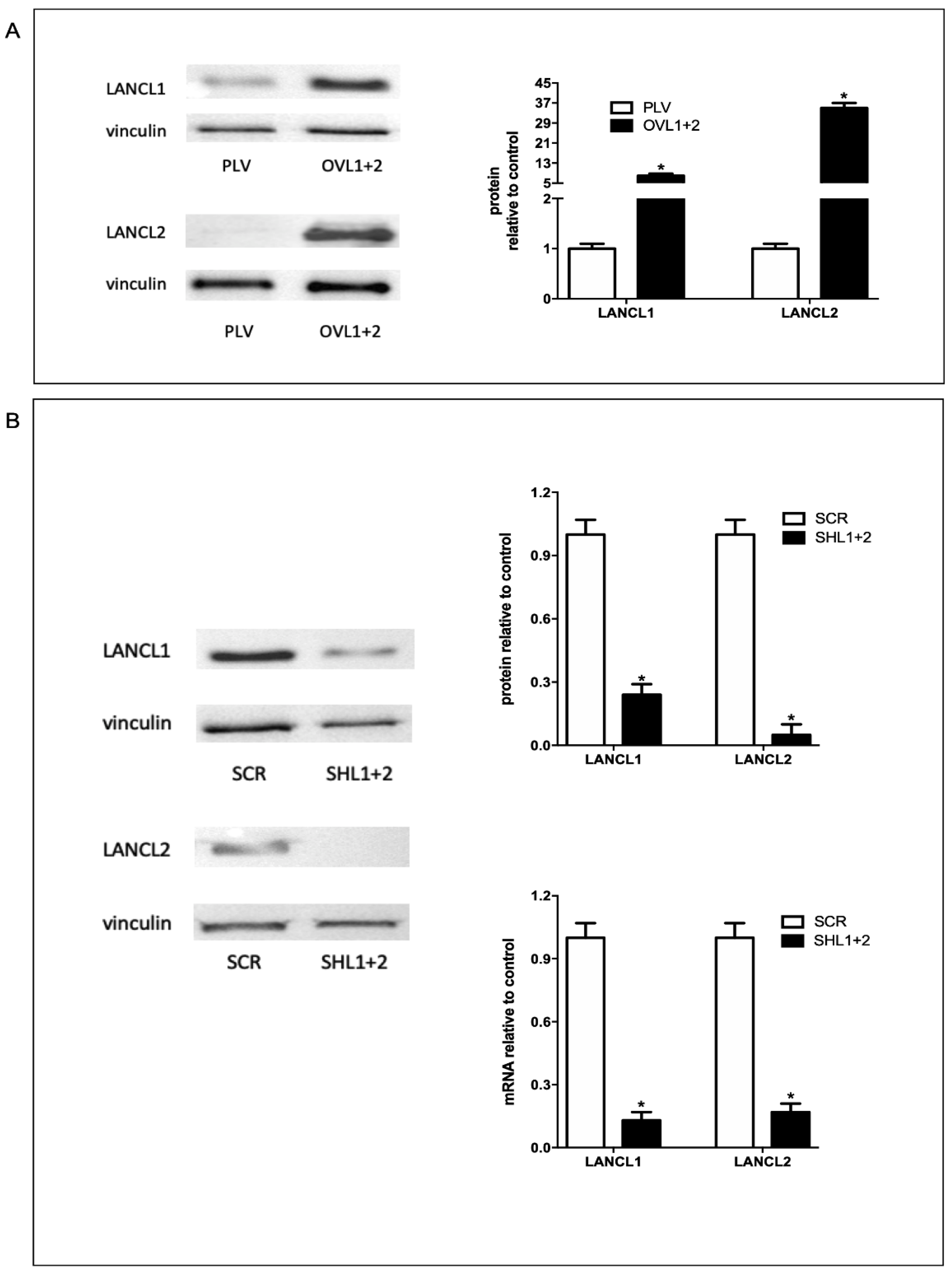
3.1. Overexpression and Silencing of LANCL1 or LANCL2 in H9c2 Cells
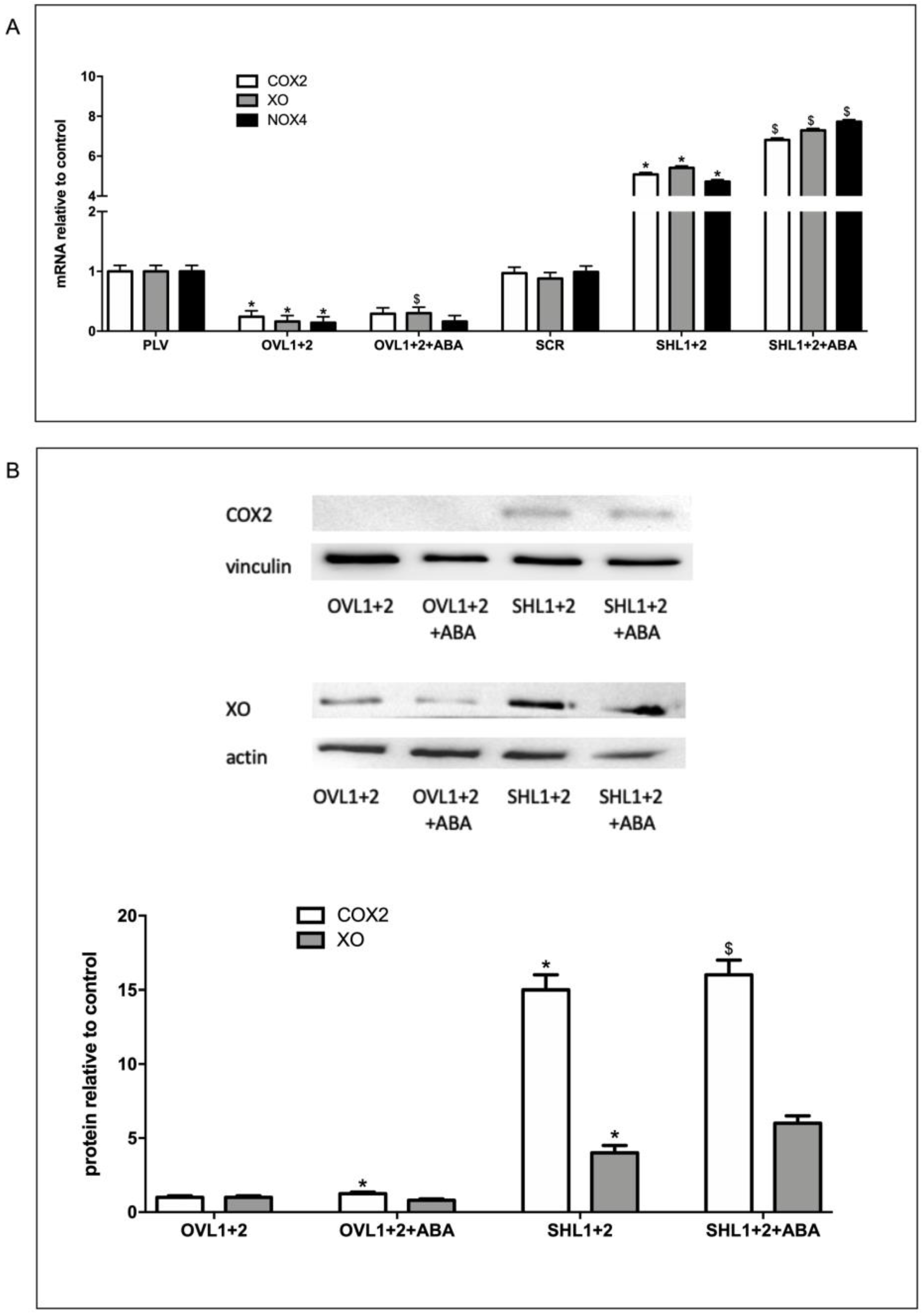
3.2. Overexpression of LANCL1 and LANCL2 Decreases, While Their Combined Silencing Increases, Transcription and Expression of Radicals-Generating COX2, XO and NOX4
3.3. LANCL1/2-Overexpression Increases, While Their Double-Silencing Decreases, Transcription, and Expression of the Radical Scavenging Enzymes SOD2 and GPX4
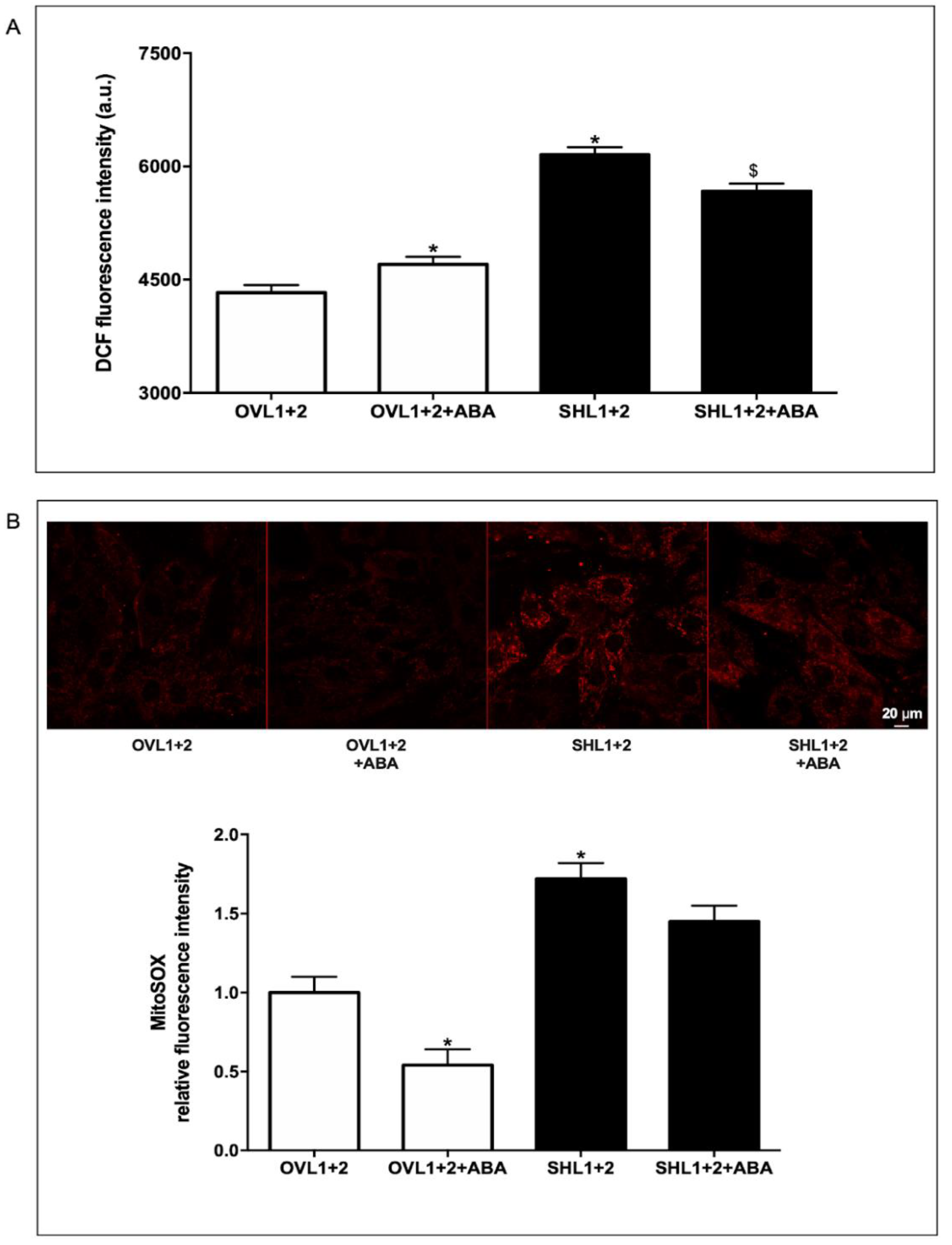
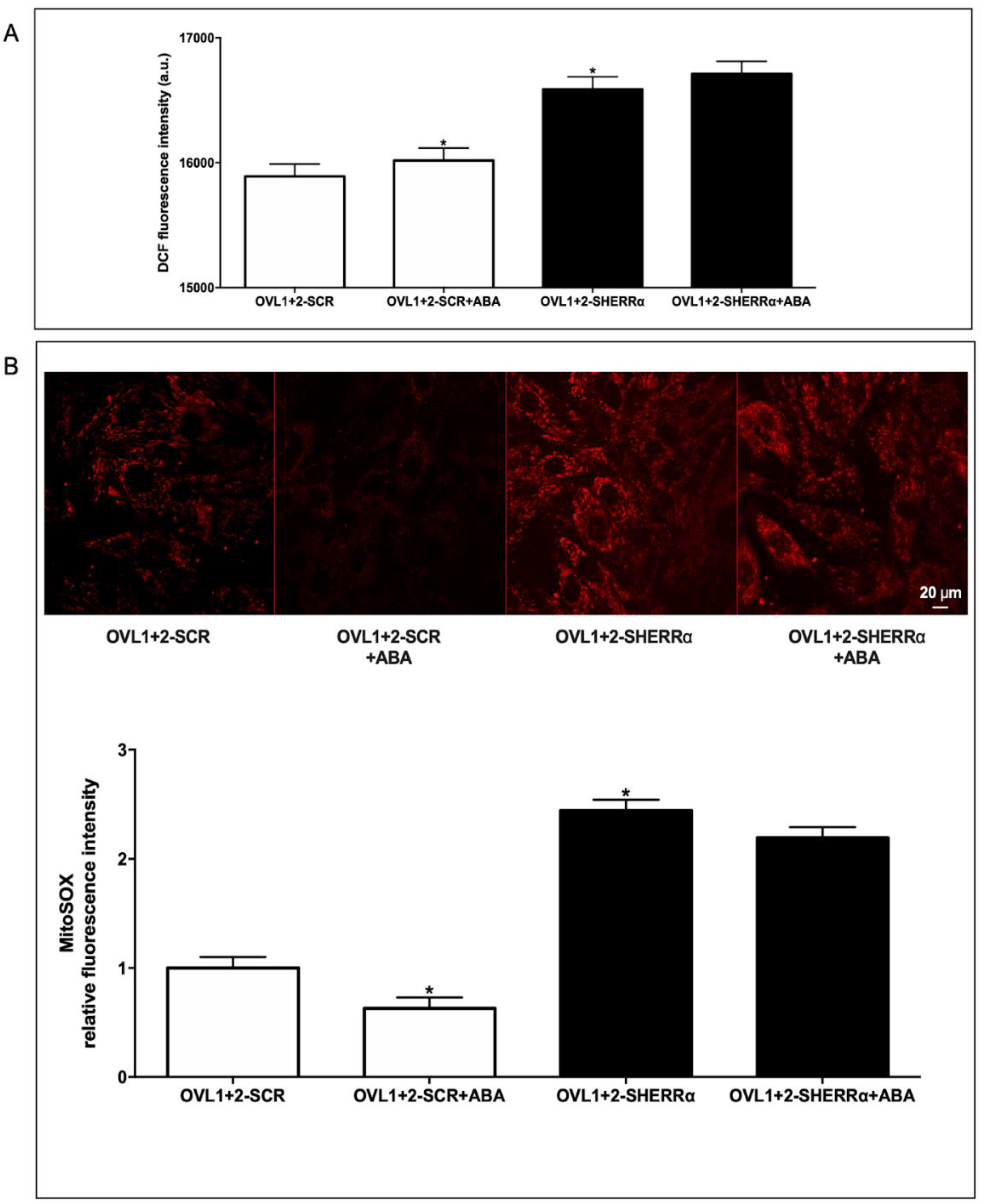
3.4. LANCL1/2-Overexpression Decreases and Their Combined Silencing Conversely Increases ROS Content in H9c2 Cells
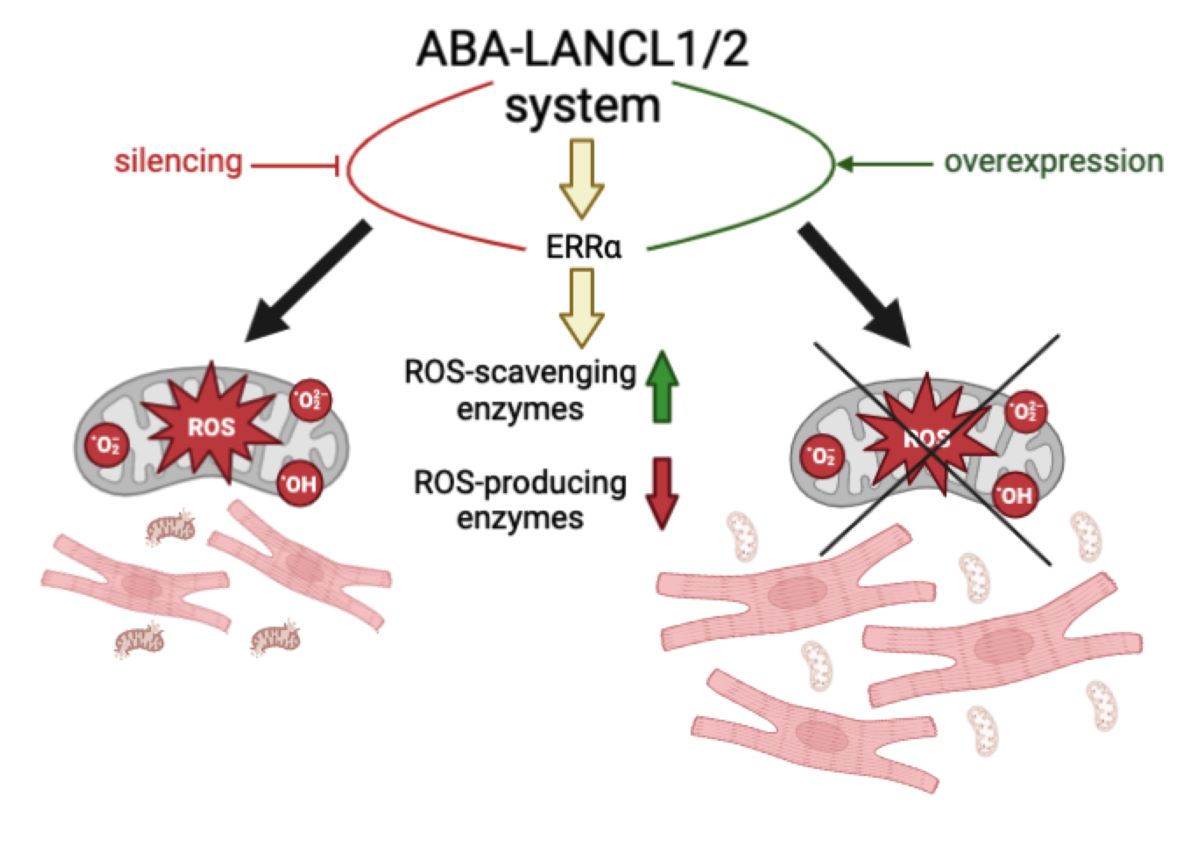
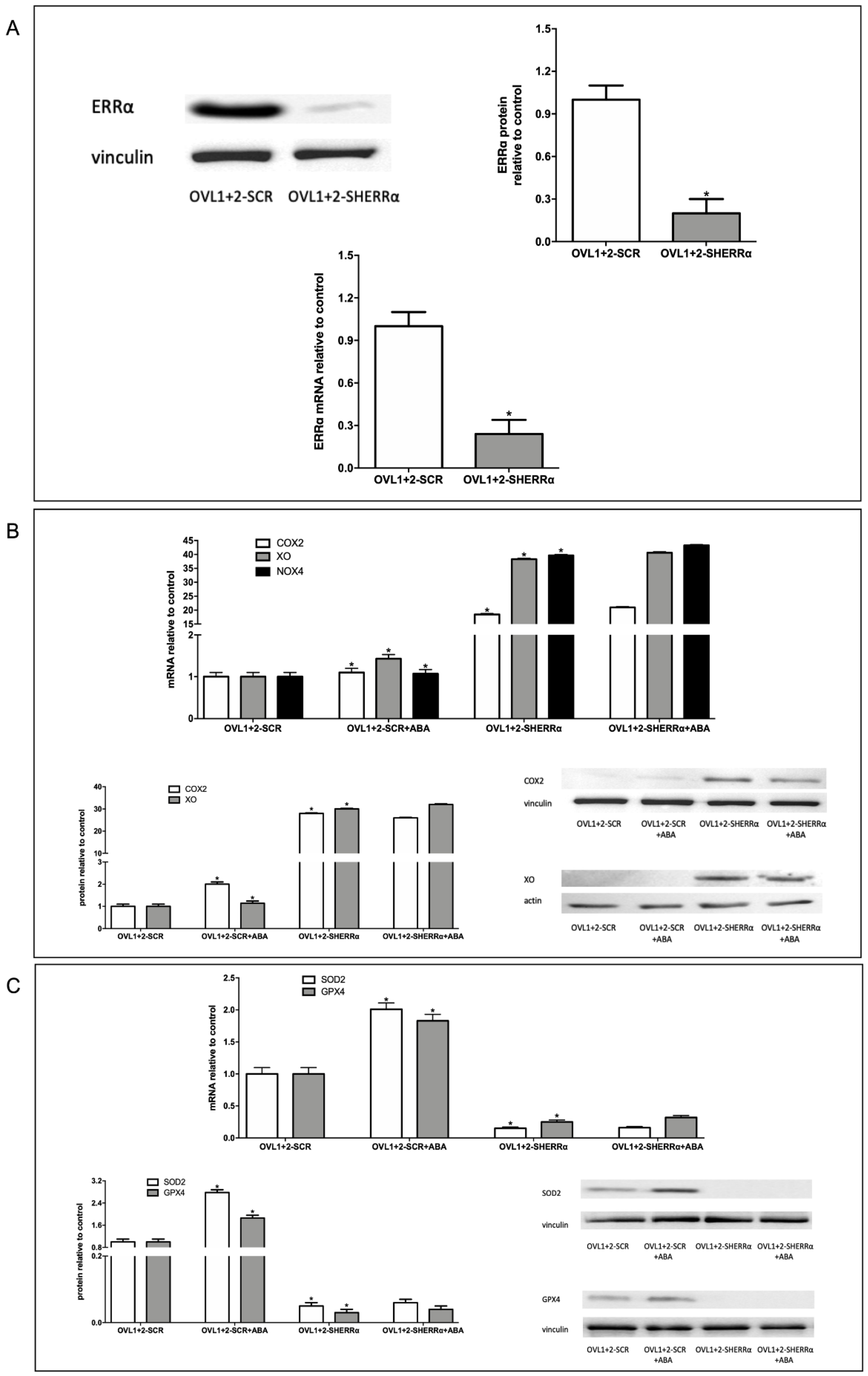
3.5. The ABA/LANCL1-2 System Controls ROS Metabolism via the Transcription Factor ERRα
3.6. ERRα Silencing Increases ROS Production in LANCL1/2-Overexpressing Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.Y.; Lin, P.Y.; Al-Babili, S. On the biosynthesis and evolution of apocarotenoid plant growth regulators. Semin. Cell Dev. Biol. 2021, 109, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, S.; Ameri, P.; Briatore, L.; Mannino, E.; Basile, G.; Andraghetti, G.; Grozio, A.; Magnone, M.; Guida, L.; Scarfì, S.; et al. The plant hormone abscisic acid increases in human plasma after hyperglycemia and stimulates glucose consumption by adipocytes and myoblasts. FASEB J. 2012, 26, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Magnone, M.; Leoncini, G.; Vigliarolo, T.; Emionite, L.; Sturla, L.; Zocchi, E.; Murialdo, G. Chronic Intake of Micrograms of Abscisic Acid Improves Glycemia and Lipidemia in a Human Study and in High-Glucose Fed Mice. Nutrients 2018, 10, 1495. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, S.; Magnone, M.; Guida, L.; Sturla, L.; Zocchi, E. The ABA-LANCL hormone-receptor system in the control of glycemia, of cardiomyocyte energy metabolism and in neuro-protection: A new ally in the treatment of diabetes mellitus? Int J Mol Sci 2023, 24, 1199. [Google Scholar] [CrossRef] [PubMed]
- Magnone, M.; Spinelli, S.; Begani, G.; Guida, L.; Sturla, L.; Emionite, L.; Zocchi, E. Abscisic acid improves insulin action on glycemia in insulin-deficient mouse models of type 1 diabetes. Metabolites 2022, 12, 523. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, S.; Guida, L.; Vigliarolo, T.; Passalacqua, M.; Begani, G.; Magnone, M.; Sturla, L.; Benzi, A.; Ameri, P.; Lazzarini, E.; et al. The ABA-LANCL1/2 Hormone-Receptors System Protects H9c2 Cardiomyocytes from Hypoxia Induced Mitochondrial Injury via an AMPK- and NO-Mediated Mechanism. Cells 2022, 11, 2888. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, S.; Cossu, V.; Passalacqua, M.; Hansen, J.B.; Guida, L.; Magnone, M.; Sambuceti, G.; Marini, C.; Sturla, L.; Zocchi, E. The ABA/LANCL1/2 Hormone/Receptor System Controls Adipocyte Browning and Energy Expenditure. Int. J. Mol. Sci. 2023, 24, 3489. [Google Scholar] [CrossRef] [PubMed]
- Scarfì, S.; Fresia, C.; Ferraris, C.; Bruzzone, S.; Fruscione, F.; Usai, C.; Benvenuto, F.; Magnone, M.; Podestà, M.; Sturla, L.; et al. The plant hormone abscisic acid stimulates the proliferation of human hemopoietic progenitors through the second messenger cyclic ADP-ribose. Stem. Cells. 2009, 27, 2469–2477. [Google Scholar] [CrossRef] [PubMed]
- Sturla, L.; Fresia, C.; Guida, L.; Bruzzone, S.; Scarfì, S.; Usai, C.; Fruscione, F.; Magnone, M.; Millo, E.; Basile, G.; et al. LANCL2 Is Necessary for Abscisic Acid Binding and Signaling in Human Granulocytes and in Rat Insulinoma Cells. J Biol Chem 2009, 284, 28045–28057. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, S.; Begani, G.; Guida, L.; Magnone, M.; Galante, D.; D’Arrigo, C.; Scotti, C.; Iamele, L.; De Jonge, H.; Zocchi, E.; et al. LANCL1 binds abscisic acid and stimulates glucose transport and mitochondrial respiration in muscle cells via the AMPK/PGC-1α/Sirt1 pathway. Mol. Metab. 2021, 53, 101263. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signaling agents. Nat Rev Mol Cell Biol 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Cadenasa, S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic. Biol. Med. 2018, 117, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Shenshu Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.K.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Jia, L.; Liu, T.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Scholtes, C.; Giguère, V. Transcriptional Regulation of ROS Homeostasis by the ERR Subfamily of Nuclear Receptors. Antioxidants 2021, 10, 437. [Google Scholar] [CrossRef]
- Huss, J.M.; Garbacz, W.G.; Xie, W. Constitutive activities of estrogen-related receptors: Transcriptional regulation of metabolism by the ERR pathways in health and disease. Biochim Biophys Acta 2015, 9, 1912–1927. [Google Scholar] [CrossRef] [PubMed]
- Vernier, M.; Dufour, C.R.; McGuirk, S.; Scholtes, C.; Li, X.; Bourmeau, G.; Kuasne, H.; Park, M.; St-Pierre, J.; Audet-Walsh, E.; et al. Estrogen-related Receptors are Targetable ROS sensors. Genes Dev 2020, 34, 544–559. [Google Scholar] [CrossRef] [PubMed]
- Giguère, V. Transcriptional Control of Energy Homeostasis by the Estrogen-related Receptors. Endocr. Rev. 2008, 29, 677–696. [Google Scholar] [CrossRef] [PubMed]
- Villena, J.A.; Hock, M.B.; Chang, V.Y.; Barcas, J.E.; Gigue, V.; Kralli, A. Orphan nuclear receptor estrogen-related receptor is essential for adaptive thermogenesis. Proc Natl Acad Sci U S A 2007, 104, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Deblois, G.; Smith, H.W.; Tam, I.S.; Gravel, S.P.; Caron, M.; Savage, P.; Labbé, D.P.; Bégin, L.R.; Tremblay, M.L.; Park, M.; et al. ERRα Mediates Metabolic Adaptations Driving Lapatinib Resistance in Breast Cancer. Nat. Commun. 2016, 7, 12156. [Google Scholar] [CrossRef] [PubMed]
- Scarpulla, R.C. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta 2011, 1813, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Wang, R.; Xue, Y.; Liu, X.; Zhang, H.; Chen, Y.; Fang, F.; Chang, Y. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS ONE 2010, 22, e11707. [Google Scholar]
- Magnone, M.; Emionite, L.; Guida, L.; Vigliarolo, T.; Sturla, L.; Spinelli, S.; Buschiazzo, A.; Marini, C.; Sambuceti, G.; De Flora, A.; et al. Insulin-independent stimulation of skeletal muscle glucose uptake by low-dose abscisic acid via AMPK activation. Sci. Rep. 2020, 10, 1454. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, S.; Guida, L.; Passalacqua, M.; Magnone, M.; Cossu, V.; Sambuceti, G.; Marini, C.; Sturla, L.; Zocchi, E. Abscisic Acid and Its Receptors LANCL1 and LANCL2 Control Cardiomyocyte Mitochondrial Function, Expression of Contractile, Cytoskeletal and Ion Channel Proteins and Cell Proliferation via ERRα. Antioxidants 2023, 12, 1692. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, S.; Bruschi, M.; Passalacqua, M.; Guida, L.; Magnone, M.; Sturla, L.; Zocchi, E. Estrogen-Related Receptor α: A Key Transcription Factor in the Regulation of Energy Metabolism at an Organismic Level and a Target of the ABA/LANCL Hormone Receptor System. Int J Mol Sci 2024, 25, 4796. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Fernandes, E.; Lima, J.L. Fluorescence probes used for detection of reactive oxygen species. J Biochem Biophys Methods 2005, 65, 45–80. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.M.; Janes, M.S.; Pehar, M.; Monette, J.S.; Ross, M.F.; Hagen, T.M.; Murphy, M.P.; Beckmanet, J.S. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci U S A 2006, 103, 15038–15043. [Google Scholar] [CrossRef] [PubMed]
- Vigliarolo, T.; Guida, L.; Millo, E.; Fresia, C.; Turco, E.; de Flora, A.; Zocchi, E. Abscisic acid transport in human erythrocytes. J. Biol. Chem. 2015, 290, 13042–13052. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Borisenko, G.G.; Osipov, A.; Martin, I.; Chen, R.; Shvedova, A.A.; Sorokin, A.; Tyurina, Y.Y.; Potapovich, A.; Tyurin, V.A.; et al. Arachidonic acid-induced carbon-centered radicals and phospholipid peroxidation in cyclo-oxygenase-2-transfected PC12 cells. J Neurochem 2004, 90, 1036–1049. [Google Scholar] [CrossRef] [PubMed]
- Sofiullah, S.S.M.; Murugan, D.D.; Muid, S.A.; Seng, W.Y.; Abdul Kadir, S.Z.S.A.; Abas, R.; Ridzuan, N.R.A.; Zamakshshari, N.H.; Woon, C.K. Natural bioactive compounds targeting NADPH Oxidase pathway in cardiovascular diseases. Molecules 2023, 28, 1047. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim Biophys Acta 2013, 5, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Forcina, G.C.; Dixon, S.J. GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis. Proteomics 2019, 18, e1800311. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yan, Y.; Qi, C.; Liu, J.; Li, L.; Wang, J. The Role of Ferroptosis in Cardiovascular Disease and Its Therapeutic Significance. Front Cardiovasc Med 2021, 8, 733229. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Tsui, Y.M.; Ho, D.W.; Chung, C.Y.; Sze, K.M.; Lee, E.; Cheung, G.C.; Zhang, V.X.; Wang, X.; Lyu, X.; et al. LANCL1, a cell surface protein, promotes liver tumor initiation through FAM49B-Rac1 axis to suppress oxidative stress. Hepatology 2024, 79, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, J.; Shi, S.; Lan, X.; Cheng, X.; Li, L.; Zou, Y.; Jia, L.; Liu, W.; Luo, Q.; et al. LanCL2 Implicates in Testicular Redox Homeostasis and Acrosomal Maturation. Antioxidants 2024, 13, 534. [Google Scholar] [CrossRef] [PubMed]

| Rat genes | Accession N. | Forward Primer 5’-3’ | Reverse Primer 5’-3’ |
|---|---|---|---|
| Lancl1 | NM_053723 | TCTTGCTCCTCATCCTGCTCATC | CACTGTACTCGCCGAAGGTCTC |
| Lancl2 | NM_001014187 | GGTGCCACGGTGCTCCAG | CCTCGCTGCCAAATCACATCAC |
| Sod2 | NM_017051 | TAAGGGTGGTGGAGAACCCA | ACCTTGGACTCCCACAGACA |
| Nox4 | NM_053524 | CTGTACAACCAAGGGCCAGA | GCTCTGCTCAAACACAATCCT |
| Gpx4 | NM_017165 | CCGTCTGAGCCGCTTATTGA | AATCATCGCGGGATGCACA |
| Cox2 | S67722 | GTGAAAACTGTACTACGCCGAG | TACTGTGTTTGGGGTGGGCT |
| Xor | NM_017154 | TCCCTGCGTTTGGTAGCATC | CCAGGAAAAGAGGTGGCTCC |
| Hprt1 | NM_012583 | TTGGTCAAGCAGTACAGCCC | TGGCCTGTATCCAACACTTCG |
| Primary Antibody | Host | Concentrations | Manufacturer |
|---|---|---|---|
| Anti-LANCL1 | Rabbit | 1:250 | Novus Biologicals |
| Anti-LANCL2 | Mouse | 1:1000 | Reference [29] |
| Anti-XO | Mouse | 1:100 | Santa Cruz Biotechnology Inc., California |
| Anti-COX2 | Goat | 1:200 | Santa Cruz Biotechnology Inc., California |
| Anti-SOD2 | Rabbit | 1:5000 | Abcam |
| Anti-GPX4 | Mouse | 1:100 | Santa Cruz Biotechnology Inc., California |
| Anti-ERRα | Mouse | 1:200 | Santa Cruz Biotechnology Inc., California |
| Anti-vinculin | Rabbit | 1:1000 | Cell Signaling Technology, Danvers, MA |
| Anti-Actin | Mouse | 1:1000 | Santa Cruz Biotechnology Inc., California |
| Secondary Antibody | Concentrations | Manufacturer | |
| Anti-Mouse | 1:2000 | Santa Cruz Biotechnology Inc., California | |
| Anti-Rabbit | 1:1000 | Santa Cruz Biotechnology Inc., California | |
| Anti-Goat | 1:1000 | Santa Cruz Biotechnology Inc., California | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





