1. Introduction
The preservation of genetic material through sperm cryopreservation will be very useful in future breeding programs in dogs to preserve the fertility of superior males and reduce the transport costs of live dogs [
1,
2,
3]. Freezing extenders are a basic and vital factor in improving sperm cryopreservation efficiency. Freezing extenders are used to improve the freezing ability and post-thaw characteristics of spermatozoa by protecting the sperm from unfavorable conditions during freezing [
4]. Certain components of the freezing extender, including nutrients, buffers, antibiotics, and cryoprotectants, are required for sperm cryopreservation [
5]. The most important element in the extender is the cryoprotectant, and glycerol is the most commonly used cryoprotectant. However, it is permeable and toxic to sperm during freezing [
6]. Therefore, a combination of non-permeable cryoprotectants, such as polysaccharides, poly-L-lysine, and hydroxyl ethylene starch, has been shown to improve post-thaw sperm recovery compared with glycerol alone [
5,
7,
8].
Carboxymethyl cellulose (CMC) is a cellulose derivative with a carboxymethyl group that contributes to its water solubility. CMC also has other characteristics such as being impermeable, osmotically inert, having low viscosity, and having no toxic effects [
6]. Recently, supplementation with CMC as a freezing extender for ram semen was found to reduce sperm membrane damage, including membrane integrity and mitochondrial membrane potential [
6]. Therefore, CMC may be helpful in reducing damage to dog sperm during freezing using a glycerol-supplemented extender. To the best of our knowledge, this is the first time that CMC has been used for dog sperm cryopreservation.
Gene and protein expression, mRNA stability, and epigenetic content of spermatozoa are modified throughout the freeze-thaw process [
4]. Hence, to have access to a safe approach for freezing, it may be essential to promote cryopreservation by studying a wide variety of semen quality biomarkers, including gene and protein expression [
9,
10]. Quantitative PCR-based quantification is an important technique for determining alterations in gene expression in sperm resulting from cryopreservation [
11,
12,
13].
Therefore, the purpose of this study was to investigate the effect of CMC supplementation in freezing extenders on dog sperm cryopreservation. We evaluated post-thaw sperm motility, viability, and acrosomal integrity. In addition, the cryoprotective effect of CMC was focused on evaluating gene expression levels as a marker of cryodamage occurring during freezing of dog spermatozoa. Therefore, we determined gene expression levels related to: apoptosis (B-cell lymphoma, BCL2; BCl2-associated X protein, BAX; Annexin2, ANAXA2); cold shock (Y-Box Binding Protein 3, YBX3); motility (β-actin, BACTIN); ROS (catalase, CAT; reactive oxygen species modulator 1, ROMO1; spermine oxidase, SMOX).
2. Materials and Methods
2.1. Chemicals
All reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated.
2.2. Animals
Four healthy male dogs aged 3–5 years (two beagles and two Pomeranians) were used in this study. The dogs had palpable normal genitalia and normal libido, and were housed in separate cages at the College of Veterinary Medicine, Jeonbuk National University. The dogs were fed commercial dog food (BioMill®, Jindo, South Korea) daily and given fresh drinking water ad libitum. All the dogs were trained for routine semen collection. The dogs used in this study were treated and received care under the Guiding Principles for the Care and Use of Research Animals established by Jeonbuk National University.
2.3. Preparation of Semen Extender
Semen extender was prepared using the method described by Linde-Forsberg [
14]. The composition of extender 1 was: 3.025 g Tris, 1.7 g citric acid, 1.25 g fructose, 0.06 g Na benzylpenicillin, 0.1 g streptomycin sulphate and 20 mL egg yolk per 100 mL ultra-pure water (ProGen life sciences, NY, United States). Extender 2 was composed of extender 1 and 8 % glycerol (final concentration of 4 %). Both extenders were supplemented with 0, 0.1, 0.25, 0.5 and 0.75 % (w/v) CMC as a final concentration.
2.4. Semen Collection
Semen from each of the four dogs was obtained by digital manipulation, and the second fraction was collected using a glass funnel and a 15-ml sterilized, previously warmed (at 37 °C) conical tube (SPL Life Sciences Co., Gyeonggi, Korea). Normal ejaculates with sperm concentrations ≥ 200 × 106 sperm/mL, motility ≥ 70 % and normal morphology ≥ 80 % were included in this study. For each replicate, ejaculates from the four dogs were centrifuged at 300 × g for 12 min and the supernatant was removed. The pellets were then pooled into a single final sample.
2.5. Sperm Freezing Process and Post-Thaw Sperm Evaluation
Semen freezing was performed with some modifications, as previously described by Rahman et al. [
15]. The sperm pellets were resuspended in two steps. In the first step, the sperm pellet was diluted in extender 1 at 32 °C (digital dry bath incubator; Fisher Scientific, Iowa, USA) to a concentration of 200 × 10
6 sperm/mL and allowed to equilibrate for 1 h at 4 °C. The next step, after the equilibration period, was to add an equal volume of extender 2 that had been cooled to 4 °C to the sperm suspension (100 × 10
6 sperm/mL). After an additional equilibration period of 30 min at 4 °C, the samples were loaded into 0.5 mL French straws (IMV Technologies, Saint Quen-Surlton, France) and sealed. Straws were then horizontally frozen in liquid nitrogen vapor (7 cm above liquid nitrogen) for 20 min and stored in liquid nitrogen at -196 °C until evaluation [
15].
For sperm evaluation, frozen straws were thawed by placing them in 37 °C water for 25 s. Subsequently, thawed samples were transferred to a 1.5 mL Eppendorf tube and maintained at 37 °C for 5 min. Sperm quality (motility, viability, and acrosomal integrity) and gene expression levels were analyzed as described below.
2.6. Motility Assessment
Motility was measured by a computer-assisted sperm analysis system (CASA), using Sperm Class Analyzer (SCA
®) version 6.3.0.32 software. Microptic S.L., Barcelona, Spain). A phase-contrast microscope (Nikon Eclipse E-200; Nikon Corporation, Kanagawa, Japan) was used. The microscope was equipped with a Basler camera (acA1300-200uc, Ahrensburg, Germany) and an attached heating stage set at 37 °C (Tokai Hit Co., Ltd., Shizuoka-ken, Japan). A 2 µL of thawed spermatozoa was placed into a preheated (37 °C) Leja standard-count 8 chambered slide with a 20 μm (Leja Products B.V., NieuwVennep, The Netherlands). For each sperm sample, five videos lasting three seconds each were recorded in different fields. Sperm motility parameters were analyzed, including total motility (MOT, %), progressive motility (PMOT, %), average path velocity (VAP, μm/sec), curvilinear velocity (VCL, μm/s), straight line velocity (VSL, μm/s), linearity (LIN, %), straightness (STR, %), wobble (WOB, %), amplitude of the lateral head displacement (ALH, μm), and beat cross frequency (BCF, Hz). As described by Dorado et al.[
16], with minor modifications, the manufacturer's recommended software settings were adjusted to obtain a clear identification of the individual spermatozoa as follows: grid distance: 100 μm, frame rates: 25 frames/sec, number of images: 25, depth of sample chamber: 20 μm, volume per chamber: 3 μl, temperature of analysis: 38 °C, area (min): 10 μm
2, area (max): 80 μm
2, static: 10 μm/s, slow-medium: 25 μm/s, rapid: > 45 μm/s, progressive: STR > 45 %, connectivity: 14 pixels, VAP points: 5 pixels, sperm to analyze: 500, fields to analyze: 5 fields.
2.7. Assessment of Sperm Viability
Viability based on sperm plasma membrane integrity was assessed using propidium iodide (PI) and SYBR-14 kit (LIVE/DEAD™ Sperm Viability Kit, Thermo Fisher Scientific, Oregon, USA) using the procedure described by Yu [
17]. Briefly, SYBR-14 (5 μl, 100 nM) was added to 50 μl of frozen-thawed spermatozoa, and the mixture was incubated for 5 min at room temperature in the dark; 5 μl of PI (12 μM) was then added, and the mixture was incubated for an additional 5 min. For each sample, at least 500 spermatozoa were examined under a fluorescence microscope (Axio, Carl Zeiss, Göttingen, Germany). Sperm cells labeled green were considered live cells with an intact membrane, whereas those labeled red were considered dead cells with damaged membranes.
2.8. Assessment of Acrosome Integrity
Sperm acrosome integrity was evaluated using fluorescein isothiocyanate—Pisum sativum agglutinin (FITC-PSA), as previously reported by Yu [
17]. Briefly, frozen-thawed spermatozoa were stained with 30 μl FITC-PSA (100 mg/mL); the smear was covered with parafilm for 20 min, placed in a distilled water container for 15 min, and then dried. After staining, at least 200 spermatozoa were assessed under the above-mentioned microscope. Spermatozoa with intact acrosomal membranes (stained intense green fluorescence in the anterior acrosomal region of the spermatozoa), whereas spermatozoa with defective or damaged acrosomes stained pale green.
2.9. Gene Expression Level
2.9.1. RNA Extraction
RNA was extracted from the spermatozoa using RiboEx (GeneAll Biotechnology Co., Ltd., Seoul, Korea) according to the manufacturer’s instructions. RNA samples were quantified and assessed for quality using an Epoch Microplate Spectrophotometer (BioTec Instruments Inc., Winooski, USA). Quantitative real-time PCR (qPCR) was conducted to assess the transcript abundance using oligonucleotide primer sequences (
Table 1).
Primers were designed and tested for specificity using the Primer Designing Tool (
http://www.ncbi.nlm.nih.gov/tools/primerblast/primertool.cgi). The mRNA expression levels of apoptotic genes (BCL2, BAX, and ANAXA2), cold shock protein gene (YBX3), motility (BACTIN), and ROS-related genes (ROMO1, SMOX, and CAT) were analyzed using qPCR.
2.9.2. Quantitative RT-PCR
Relative quantification was performed using the One Step TB Green® PrimeScriptTM RT-PCR kit II (Takara Bio Inc., Shiga, Japan). A One-Step TB PrimeScript RT-PCR Kit II (Perfect Real Time) was used to synthesize cDNA from RNA using PrimeScript Reverse Transcriptase, followed by PCR with Takara Ex Taq HS DNA Polymerase in one tube. The PCR amplification products were monitored in real time using TB Green as an intercalator. The reactions were conducted using a QuantStudioTM Real-Time PCR instrument (Applied Biosystems, Life Technologies Holdings Pte Ltd., Marsiling, Singapore) according to the manufacturer's instructions. The expression of each target gene was quantified relative to that of the housekeeping gene GAPDH as an internal control. The RT-PCR was performed in 20 µl of solution containing 10 μl of 2X One Step TB Green RT-PCR Buffer, 0.8 μl of PrimeScript 1 Step Enzyme Mix, 0.8 μl of PCR Forward Primer (10 μM), 0.8 μl of PCR Reverse Primer (10 μM), 0.4 μl of ROX Reference Dye II (50X), 2 μl of total RNA and 5.2 μl of RNase Free dH
2O. The PCR experiments were performed using the following run method setup: stage 1 and 2 reverse transcription: 5 min at 42 °C and then 10 s at 95 °C; stage 3, PCR reaction: 40 cycles of 5 s at 95 °C and 34 s at 60 °C; stage 4, dissociation stage: 15 s at 95 °C, 1 min at 60 °C and 15 s at 95 °C. The formation of single products in the TB Green assay was confirmed by analyzing the melting curves of the amplicons. The data were analyzed using the Livak and Schmittgen equation, where fold change = 2
−ΔΔCt [
18].
2.10. Experimental Design
The present study was conducted to evaluate the effect of CMC supplementation in a sperm-freezing extender on the cryopreservation of dog sperm.
First, we determined the optimal concentration of CMC in the sperm-freezing extender by assessing general sperm parameters. CMC was added to the dog sperm freezing extenders at final concentrations of 0 (control), 0.1, 0.25, 0.5 or 0.75 % (w/v). Sperms were treated as described above. After freeze-thawing, the total motility, progressive motility, and kinematic parameters of motility were measured using the CASA system. In addition, viability and acrosome integrity were evaluated after freeze–thaw cycles.
After determining the optimal concentrations, spermatozoa were frozen in extender containing 0 (control), 0.1, or 0.25 % CMC, as described above. The expression of apoptosis-related genes (BCL2, BAX, and ANAXA), cold shock protein (YBX3), motility (BACTIN), and ROS-related genes (CAT, ROMO1, and SMOX) were evaluated after freeze-thawing.
2.11. Statistical Analyses
Five replicates were performed for each experiment. All data are presented as mean ± SE. Data were analyzed using one-way ANOVA followed by Duncan's multiple range test, and all calculations were performed using Statistical Analysis System ver. 8x (SAS, Cary, NC, USA). Statistical significance was set at p<0.05. differences.
3. Results
3.1. Effect of CMC on Sperm Motility, Viability and Acrosome Integrity
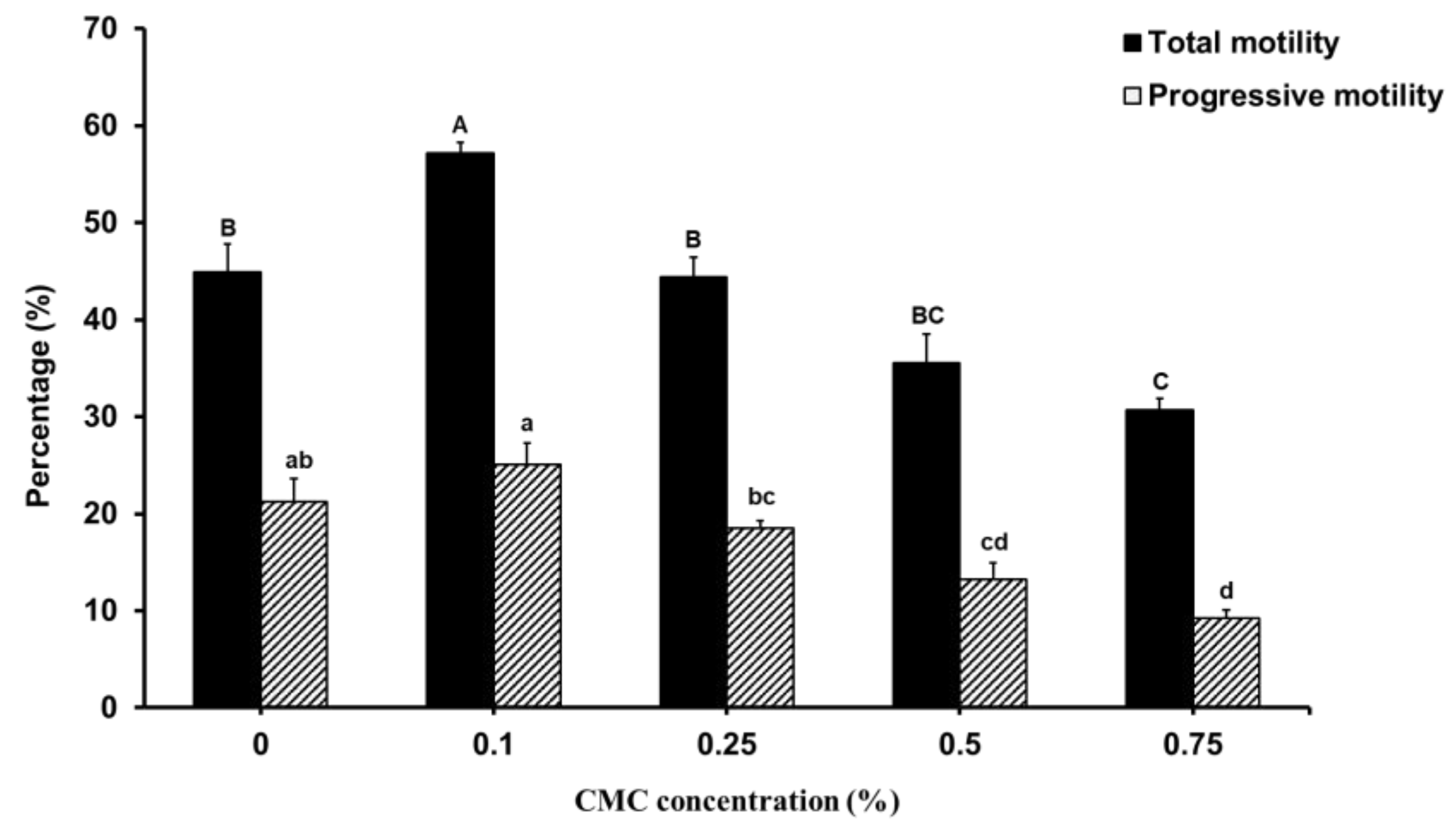
As shown in
Figure 1, the CASA results of cryopreserved sperm motion characteristics indicated that the extender supplemented with CMC concentration (0.1 %) significantly increased total motility compared to the control group and other groups.
CMC supplementation of 0.1 % and 0.25 % in the extender had no significant effect on progressive motility rates. On the contrary, there was a negative significant effect at a concentrations of 0. 5% and 0.75 % CMC when compared to control and 0.1 %. The data for the kinematic parameters (VCL, VAP, VSL, STR, LIN, WOB, ALH, and BCF) are listed in
Table 2.
No differences were detected in kinematic parameters between the groups.
Table 3 shows the results for the viability and acrosome integrity of the cryopreserved sperm.
No significant differences in viability or acrosomal integrity were observed between the CMC and control groups. However, the cell viability in the CMC group was slightly higher than that in the control.
3.2. Effect of CMC on Gene Expression
As shown in
Figure 2A, the relative expression of the antiapoptotic gene (BCL2) was significantly upregulated in the CMC group compared to that in the control group, whereas the expression of BAX was significantly lower in the CMC group than in the control group.
No significant differences were observed in the relative expression of ANAXA2. In the present study, the Bax/Bcl2 ratio was significantly lower in spermatozoa treated with 0.1 % and 0.25 % CMC compared to the control (
Figure 2B).
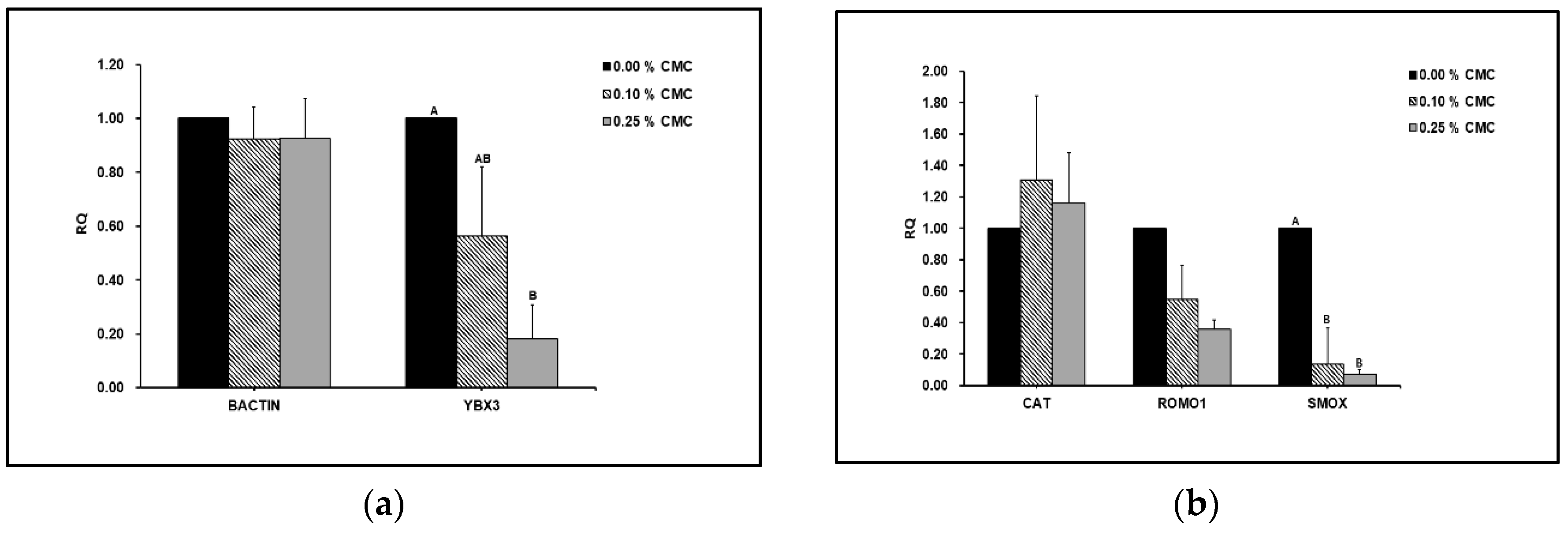
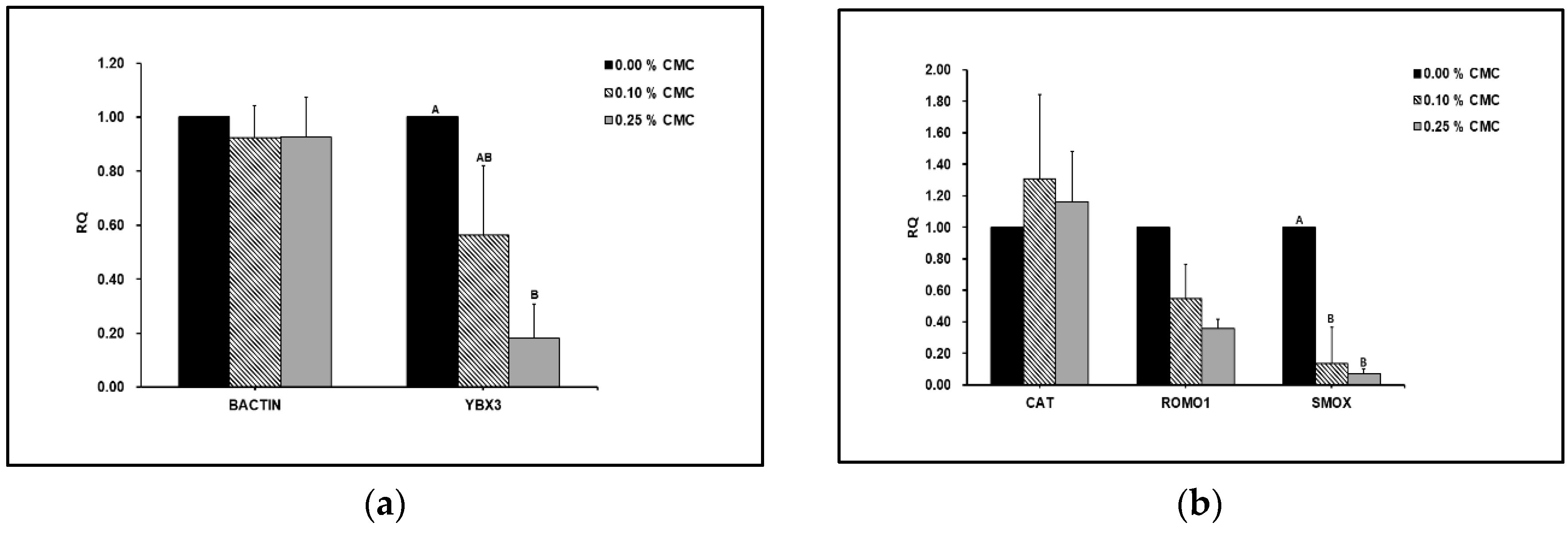
Figure 3A shows that the expression of YBX3 genes was significantly decreased with CMC supplementation.
However, there was no significant difference in the BACTIN levels between the groups. SMOX expression was significantly lower in the CMC-treated group than that in the untreated control group (
Figure 3B). However, there were no significant differences in the relative expression of CAT and ROMO1 between the groups.
4. Discussion
Recently, CMC was used as a cryoprotectant to manufacture bio-safe hydrofilm [
19]. Moreover, the synergistic effects of CMC and glycerol has been investigated in ram sperm freezing [
6]. However, CMC has not been used as a cryoprotectant for the cryopreservation of dog sperm, and the molecular effects of CMC on frozen dog spermatozoa have not been determined.
Prior to assay of gene expression in dog frozen sperm, general assay was also evaluated in this study. Supplementation with 0.1 % CMC led to a significant increase in the post-thawed dog sperm total motility, compared to control and other higher CMC concentrations in this study. However, viability and acrosome membranes were not significantly increased in the CMC-supplemented groups. On the contrary, the CMC (0.5 %) significantly affected the membranes of plasma, acrosomes, and mitochondrial potential in ram frozen sperm [
6]. However, the sperm motility did not increase with CMC supplementation. CMC has viscosity properties, and a slight increase in the viscosity of the extender improves semen quality [
6,
20,
21,
22]. However, different results have been reported for sperm motility between the present and previous studies [
6]. In finding the possible reason, the one obvious aspect might start from the composition of basic extender, TES-Tris-fructose extender with 5 or 6 % glycerol in previous study [
6], and Tris-fructose extender with 4 % glycerol in this study were used respectively. TES has a high molecular weight. Therefore, the basic extender used in sheep sperm freezing is denser than the extender used in dog sperm freezing. Additionally, supplementation with CMC intensified the viscosity of the extender and acted as a non-permeating cryoprotectant. As a result, sperm viability increased, but sperm movement was slower because the higher viscosity of CMC limited sperm mobility. In the present study, dog sperm motility increased at lower concentration of CMC. However, higher than 0.1 % CMC showed significant reduction in motility. However, the viability of dog sperm frozen with CMC supplementation was slightly higher in the CMC groups than in the control group, without significance. We infer that dog spermatozoa are still alive but do not move freely under conditions of higher viscosity. Therefore, we suggest that supplementation of the freezing extender with CMC could protect dog sperm viability during freezing. However, motility of dog sperm decreases as concentration of CMC increases higher than 0.25 %.
In addition, the other purpose of this research was to investigate the effect of CMC on gene expression of dog frozen sperm. We measured the expression of genes related to apoptosis (BCL2, BAX, and ANAXA), cold shock (YBX3), motility (BACTIN), and ROS-related genes (CAT, ROMO1, and SMOX). As a result, the relative expression level of the antiapoptotic gene (BCL2) was significantly upregulated, whereas the expression of BAX was significantly lower levels in the CMC groups (0.1 and 0.25 %) than those observed in the control group. BCL2 overexpression prevents apoptosis and improves cryo-resistance [
23]. As a second regulator of cellular response, the pro-apoptotic protein BAX accelerates apoptosis in response to apoptotic stimuli [
24]. By binding to their BH3-domains, anti-apoptotic BCL2-family proteins inhibit the activation of pro-apoptotic BAX proteins [
25]. In the present study, the increased levels of the antiapoptotic gene BCL2 and reduced levels of the proapoptotic gene BAX indicated that CMC reduced apoptosis during sperm freezing. Moreover, the Bax/Bcl2 ratio was significantly lower in spermatozoa treated with 0.1% and 0.25 % CMC compared to the control. The Bax/Bcl2 ratio exhibits an anti-apoptotic effect based on the interactions between the proapoptotic protein Bax and the antiapoptotic protein Bcl-2. A decreased Bax/Bcl-2 ratio increases the cellular resistance to apoptotic stimuli, resulting in decreased cell death [
26].
To the best of our knowledge, this is the first study to measure YBX3 expression in freeze-thawed canine spermatozoa. YBX3 is a Y-box gene expressed in response to environmental stress such as cold stress [
27]. In addition, YBX3, like other members of the YBX family, is involved in a variety of biological processes, including embryonic development and spermatogenesis [
28]. In this study, the expression of YBX3 was significantly lower in the CMC-treated group than in the untreated control group. Downregulation of the YBX3 gene in the CMC groups led to CMC reducing the damage caused by cold shock during cryopreservation of dog sperm.
We found that the expression of the ROS-related gene (SMOX), we found that it was significantly lower in the CMC-treated group than in the untreated control group. SMOX is an oxidative status marker [
29]. According to Amendola et al. [
30] and Pegg [
31], decreased SMOX expression indicates a reduced response to oxidative stress. ROMO1 expression was significantly downregulated in the CMC group. ROMO1 is implicated in the production of ROS, and its increase can excessively release ROS into the mitochondria. Consequently, ROMO1 expression enhanced oxidative stress, inflammation, and cell death [
32]. In addition, the gene expression of CAT can be related to oxidative stress, which affects sperm fertility. The gene expression of CAT was significantly associated with sperm motility [33]. In the present study, the gene expression was found to be significantly upregulated. Overall, we suggest that CMC acts as a non-permeating cryoprotectant during dog sperm freezing by improving sperm motility. Additionally, CMC supplementation reduced cryostress by regulating the expression of genes related to ROS, apoptosis, and cold shock during sperm freezing.
5. Conclusions
In conclusion, the addition of CMC (0.1 %) to the sperm freezing extender significantly increased total sperm motility and antiapoptotic gene expression (BCL2), whereas 0.1 and 0.25 % CMC significantly downregulated BAX and ROS gene expression. The cold shock-related gene (YBX3) was significantly downregulated by 0.25 % CMC. Therefore, supplementation of the freezing extender with CMC could improve sperm motility and alleviate apoptosis, reactive oxygen stress, and cold shock that occur during sperm freezing by regulating the expression of related genes.
Author Contributions
Conceptualization S.I.; methodology S.I.; visualization S.I. and Y.J.; formal analysis S.I. and Y.J; writing original draft S.I.; data curation S.I. and Y.J.; validation S.I. and Y.J; writing review I.Y.; editing S.I., I.Y.; resources I.Y.; supervision I.Y.; project administration Y.J. and I.Y.; funding acquisition I.Y.; Authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1F1A1052776).
Institutional Review Board Statement
The animal protocol was approved by the Institutional Animal Care and Use Committee of the Jeonbuk National University, Jeonju 54896, Korea (approval number JBNU 2021-069).
Data Availability Statement
The data supporting the findings of this study are available upon reasonable request from the corresponding author (I.Y.).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- England, G. C.; Millar, K. M. The ethics and role of AI with fresh and frozen semen in dogs. Reprod Domest Anim 2008, 43 Suppl 2, 165-71. [CrossRef]
- Thomassen, R.; Farstad, W. Artificial insemination in canids: a useful tool in breeding and conservation. Theriogenology 2009, 71, 190-9. [CrossRef]
- Puja, I. K.; Sawitri, N. M.; Maharani, N.; Heryani, L.; Dharmayudha, A.; Gunawan, I. Preservation of semen from Kintamani Bali dogs by freezing method. J Adv Vet Anim Res 2019, 6, 158-162. [CrossRef]
- Hezavehei, M.; Sharafi, M.; Kouchesfahani, H. M.; Henkel, R.; Agarwal, A.; Esmaeili, V.; Shahverdi, A. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reproductive BioMedicine Online 2018, 37, 327-339. [CrossRef]
- Oldenhof, H.; Gojowsky, M.; Wang, S.; Henke, S.; Yu, C.; Rohn, K.; Wolkers, W. F.; Sieme, H. Osmotic stress and membrane phase changes during freezing of stallion sperm: mode of action of cryoprotective agents. Biol Reprod 2013, 88, 68. [CrossRef]
- Paul, R. K.; Kumar, D.; Singh, R. Carboxymethyl cellulose and glycerol act synergistically as cryoprotectant during cryopreservation of ram semen. Cryobiology 2021, 101, 61-66. [CrossRef]
- Aisen, E. G.; Medina, V. H.; Venturino, A. Cryopreservation and post-thawed fertility of ram semen frozen in different trehalose concentrations. Theriogenology 2002, 57, 1801-8. [CrossRef]
- Tariq, A.; Ahmad, M.; Iqbal, S.; Riaz, M. I.; Tahir, M. Z.; Ghafoor, A.; Riaz, A. Effect of carboxylated poly l-Lysine as a cryoprotectant on post-thaw quality and in vivo fertility of Nili Ravi buffalo (Bubalus bubalis) bull semen. Theriogenology 2020, 144, 8-15. [CrossRef]
- Ezzati, M.; Shanehbandi, D.; Hamdi, K.; Rahbar, S.; Pashaiasl, M. Influence of cryopreservation on structure and function of mammalian spermatozoa: an overview. Cell Tissue Bank 2020, 21, 1-15. [CrossRef]
- Ďuračka, M.; Benko, F.; Tvrdá, E. Molecular Markers: A New Paradigm in the Prediction of Sperm Freezability. In International Journal of Molecular Sciences2023; Vol. 24. [CrossRef]
- Guerra, S. M.; Valcarce, D. G.; Cabrita, E.; Robles, V. Analysis of transcripts in gilthead seabream sperm and zebrafish testicular cells: mRNA profile as a predictor of gamete quality. Aquaculture 2013, 406, 28-33.
- Valcarce, D. G.; Cartón-García, F.; Herráez, M. P.; Robles, V. Effect of cryopreservation on human sperm messenger RNAs crucial for fertilization and early embryo development. Cryobiology 2013, 67, 84-90.
- Linde-Forsberg, C. Regulations and recommendations for international shipment of chilled and frozen canine semen. In Recent advances in small animal reproduction, International Veterinary Information Service (www. ivis. org), Document 2001; Vol. 1209.
- Rahman, M. A.; Park, S. H.; Yu, I. J. Dog sperm cryopreservation using one step dilution with glycerol-free tris extender. Cryo Letters 2016, 37, 137-41. Retrieved from https://www.ingentaconnect.com/content/cryo/cryo/2016/00000037/00000002/art00009.
- Dorado, J.; Rijsselaere, T.; Munoz-Serrano, A.; Hidalgo, M. Influence of sampling factors on canine sperm motility parameters measured by the Sperm Class Analyzer. Syst Biol Reprod Med 2011, 57, 318-25.
- Yu, I. J. Canine sperm cryopreservation using glucose in glycerol-free Tris. Cryo Letters 2014, 35, 101-7.
- Livak, K. J.; Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402-8. [CrossRef]
- Bahr, M. M.; Amer, M. S.; Abo-El-Sooud, K.; Abdallah, A. N.; Shehab, G. G.; El-Tookhy, O. S. Proficiency of Carboxymethylcellulose as a Cryoprotectant. Clinical and Histological Evaluation of Cryopreserved Heterogenous Mesenchymal Stem Cell-Exosomal Hydrogel on Critical Size Skin Wounds in Dogs. Int J Hematol Oncol Stem Cell Res 2021, 15, 178-191. [CrossRef]
- Nagy, S.; Sinkovics, G.; Kovács, A. Viability and acrosome integrity of rabbit spermatozoa processed in a gelatin-supplemented extender. Animal Reproduction Science 2002, 70, 283-286. [CrossRef]
- Yániz, J.; Martí, J. I.; Silvestre, M. A.; Folch, J.; Santolaria, P.; Alabart, J. L.; López-Gatius, F. Effects of solid storage of sheep spermatozoa at 15 degrees C on their survival and penetrating capacity. Theriogenology 2005, 64, 1844-51. [CrossRef]
- Anaya, M. C. G.; Barón, F. J.; Guerrero, J. M.; García-Marín, L. J.; Gil, J. Increasing extender viscosity improves the quality of cooled boar semen. Journal of Agricultural Science 2014, 6, 12.
- Asa, E.; Ahmadi, R.; Mahmoodi, M.; Mohammadniya, A. Supplementation of freezing media with alpha lipoic acid preserves the structural and functional characteristics of sperm against cryodamage in infertile men with asthenoteratozoospermia. Cryobiology 2020, 96, 166-174. [CrossRef]
- Salamon, S.; Maxwell, W. M. C. Frozen storage of ram semen I. Processing, freezing, thawing and fertility after cervical insemination. Animal Reproduction Science 1995, 37, 185-249. [CrossRef]
- Oltvai, Z. N.; Milliman, C. L.; Korsmeyer, S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993, 74, 609-19. [CrossRef]
- Vaskivuo, T. E.; Stenbäck, F.; Tapanainen, J. S. Apoptosis and apoptosis-related factors Bcl-2, Bax, tumor necrosis factor-alpha, and NF-kappaB in human endometrial hyperplasia and carcinoma. Cancer 2002, 95, 1463-71. [CrossRef]
- Wang, X. L.; Zhang, Y. X.; Yang, C. G.; Zhang, B.; Chen, S. L. Cloning, characterization and expression analysis of a cold shock domain family member YB-1 in turbot Scophthalmus maximus. Fish Shellfish Immunol 2012, 33, 1215-21. [CrossRef]
- Snyder, E.; Soundararajan, R.; Sharma, M.; Dearth, A.; Smith, B.; Braun, R. E. Compound Heterozygosity for Y Box Proteins Causes Sterility Due to Loss of Translational Repression. PLoS Genet 2015, 11, e1005690. [CrossRef]
- Cervelli, M.; Amendola, R.; Polticelli, F.; Mariottini, P. Spermine oxidase: ten years after. Amino Acids 2012, 42, 441-50. [CrossRef]
- Amendola, R.; Cervelli, M.; Tempera, G.; Fratini, E.; Varesio, L.; Mariottini, P.; Agostinelli, E. Spermine metabolism and radiation-derived reactive oxygen species for future therapeutic implications in cancer: an additive or adaptive response. Amino Acids 2014, 46, 487-98. [CrossRef]
- Pegg, A. E. Functions of Polyamines in Mammals. J Biol Chem 2016, 291, 14904-12. [CrossRef]
- Amini, M. A.; Karimi, M.; Talebi, S. S.; Piri, H.; Karimi, J. The Association of Oxidative Stress and Reactive Oxygen Species Modulator 1 (ROMO1) with Infertility: A Mini Review. Chonnam Med J 2022, 58, 91-95. [CrossRef]
- Fallahi, S.; Rajaei, M.; Hesam, M. J.; Koolivand, M.; Malekzadeh, K. The effect of Phoenix dactylifera pollen on the expression of NRF2, SOD2, CAT, and GPX4 genes, and sperm parameters of fertile and infertile men: A controlled clinical trial. Int J Reprod Biomed 2021, 19, 545-558. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).