1. Introduction
N-aryl piperazines are a class of molecules known to possess antihistamine, anti-inflammatory, and antihypertensive activities. They represent a fundamental scaffold of pharmaceutical chemistry and are the basis of several drugs involved, especially in neurodegenerative diseases. This is why, in recent years, interest in the synthesis of
N-arylpiperazine derivatives has increased and is currently growing [
1].
Most of these compounds have a flexible aliphatic chain that can vary in length, linking the arylpiperazine fragment to the second terminal pharmacophore group.
Piperazines are therefore considered important and biologically active elements; they are scaffolds consisting of a six-term ring and two nitrogen atoms placed at opposite ends of the ring. Structure-activity relationship studies have enhanced their pharmacokinetic properties and, since this moiety is involved in the structure of numerous drugs, their role in various pathways is known [
2]. Arylpiperazine derivatives are crucial for a variety of biological targets, particularly central nervous system receptors. Indeed, in the literature, their involvement in the regulation of the central nervous system is linked to their activity as possible agonists or antagonists of various serotonergic receptors [
3,
4].
This explains why N-1-substituted N-arylpiperazines (so-called “long-chain aryl piperazines”) have been thoroughly studied as a structural motif in the design of analogues for this type of receptor; in particular, this moiety is the most extensively studied class of 5-HT1A receptor ligands for serotonin (5-HT) receptors.
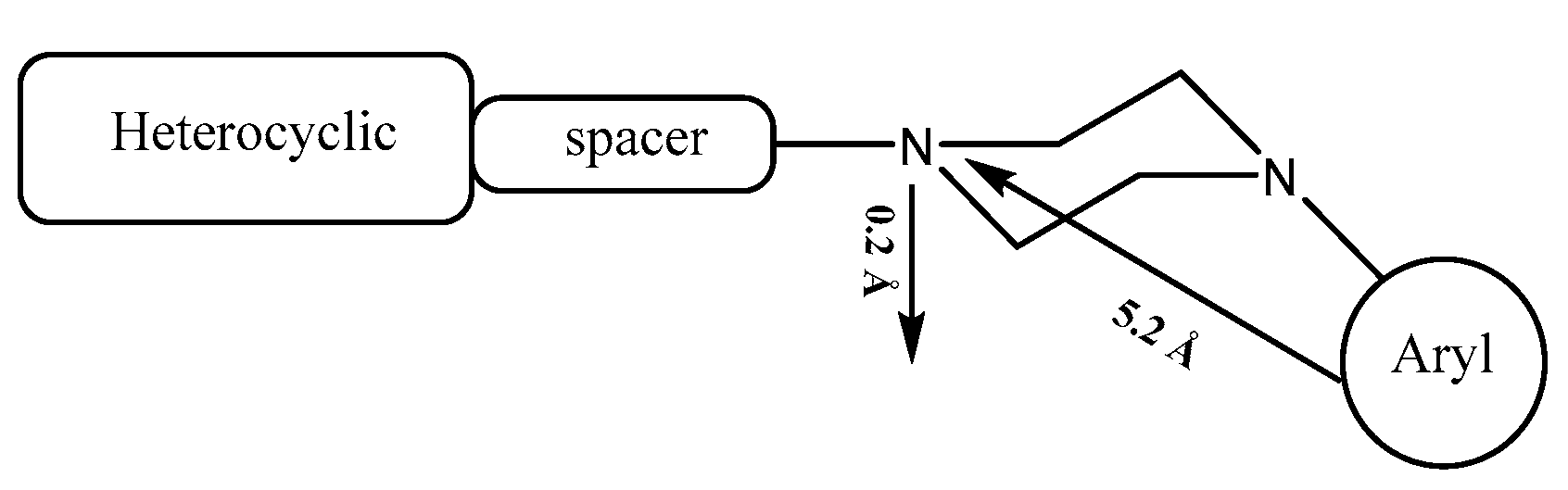
The pharmacophore of serotoninergic receptor agonists is characterized by an aromatic ring and a basic planar nitrogen, and it has been proven that there are two main interactions responsible for the affinity of
N-arylpiperazine with 5-HT
1A receptors (
Figure 1): on the one hand, the ionic bond between the protonated nitrogen atom of the piperazine ring and the carboxylic oxygen of the side chain of Asp3.32; on the other hand, an edge-to-face CH-π interaction between the aromatic ring and the Phe6.52 residue [
5].
Over the last few years, several novel compounds that target 5-HT1A receptors have advanced into phase II and phase III clinical trials or have already been released commercially as anxiolytics. Specifically, significant effort has been dedicated to understanding the role of the terminal component in the interaction between ligands and receptors.
Consequently, a wide variety of different fragments have been employed. An example is Buspirone, an anxiolytic from which other analogues have subsequently been derived [
6]. Another example of an
N-arylpiperazine derivative is Flibanserin, an ineffective antidepressant used for hypoactive sexual desire disorder (HSDD). Agonist 5-HT
1A and antagonist 5-HT
2A have activity on pyramidal neurons in the prefrontal cortex and can enhance dopamine (DA) and norepinephrine (NE) activity and reduce 5-HT activity in many brain regions. This results in improved symptoms of HSDD [
7].
To better emphasize the importance and potential of this scaffold, Ikwu et al. have performed a study based on a Quantitative Structure Activity Relationship (QSAR) model to design and predict the cytotoxic activity of arylpiperazine derivatives against LNCAP prostate cancer cell line. They explored their molecular docking interaction with the androgen receptor (LNCaP cells have been reported to be androgen-sensitive and depend on androgen for growth). This has indicated that some of these compounds are potent, and their properties are comparable to those of some drugs that are used for prostate cancer [
8].
Therefore, these derivatives have garnered significant interest and are being extensively studied and developed in the field of medicinal chemistry. In this context, the purpose of this review is to discuss the impact and the potential of N-arylpiperazine scaffold, and our attention is focused on representative examples reported in the literature with the aim to highlight the importance of these molecules and their use in cancer.
Nowadays, cancer is one of the most feared and life-threatening diseases; consequently, there is growing emphasis among medicinal chemists on developing innovative anticancer agents and refining treatment strategies to target cancer more precisely. Obviously, the main goal is to achieve highly selective targeting on cancer cells so as to radically decrease toxicity on non-transformed cells.
The limitations in this regard and the various aspects to be improved in cancer therapy are varied. First, the mortality associated with both the disease and the toxicity of the used drugs; then the bioavailability, half-life, and adverse effects that are not always on the patient’s side; and again, the poor quality of life to be led.
Presently, interest is progressively being directed toward small molecules, and the advances made so far have increasingly helped screening for molecules that have some affinity for tumor receptors [
9]. Obviously, a challenge to overcome always remains anti-cancer drug resistance linked to several mechanisms that often add up and for which multi-drug combination therapy is preferred; in this regard, once again, the efficacy of small molecules has been proven even in this case, although research is always moving forward [
10].
In this review, we shed more light on the recent literature and the most inspiring studies demonstrating the efficacy and especially the potential of arylpiperazine molecules in cancer. By focusing on the efficacy of these future drugs, their structure, and tumor localization, our goal is to place increasing attention on this promising and ever-present scaffold.
2. The Evidence of N-arylpiperazine Derivatives in Carcinogenic Pathways
The mechanisms related to carcinogenesis are numerous, but one of the purposes of this review is precisely to link arylpiperazines to cancer and try to investigate mechanisms related to proliferation. Among the most widely expressed receptors in different types of cancer is the serotoninergic receptor 5-HT
1A (
Table 1) [
11].
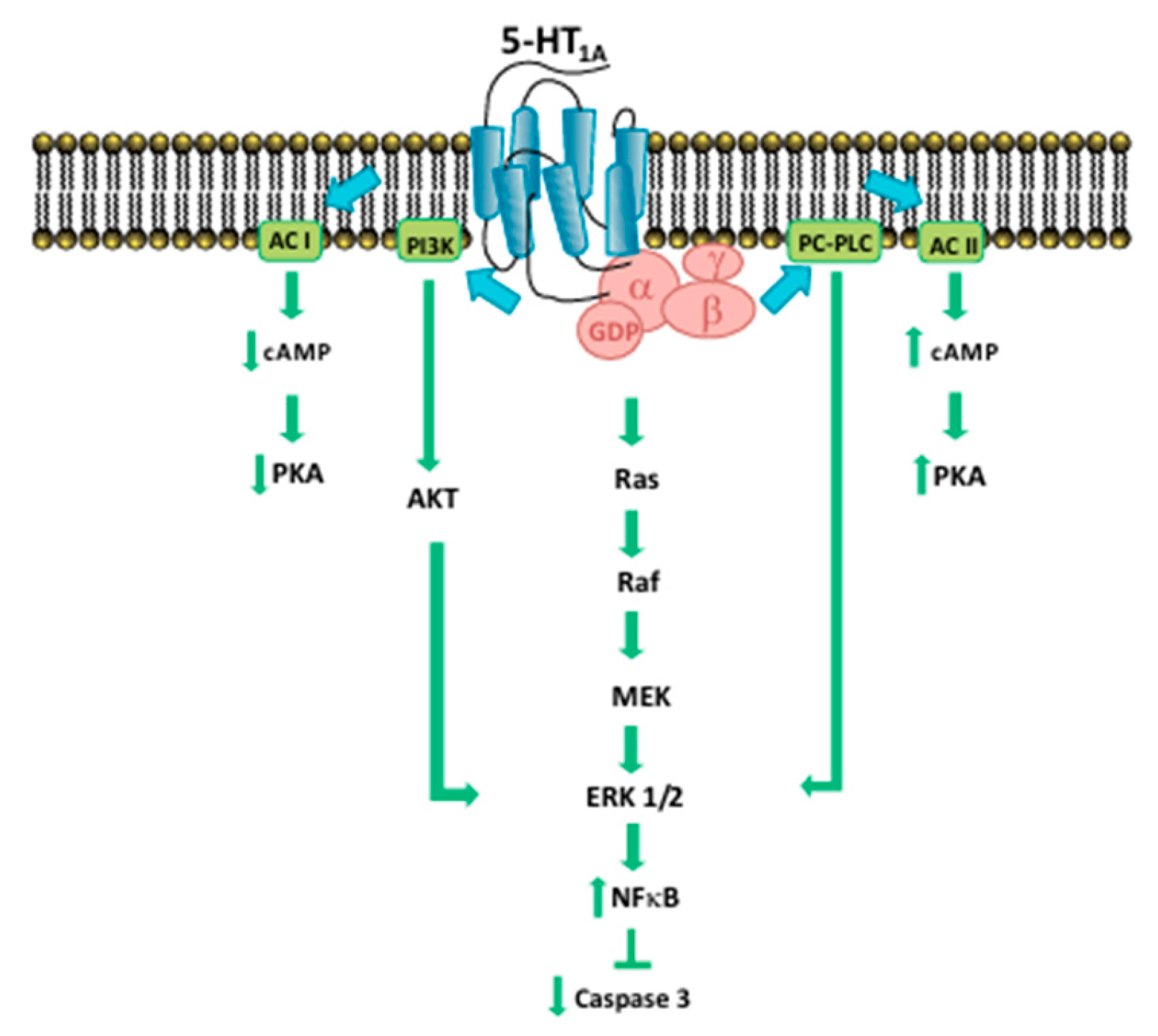
One of the mechanisms by which serotonin regulates proliferation is represented by MAPK/ERK and PI3K/Akt: the activation of serotonin receptors stimulates the molecules involved in this pathway (ERK1/2, Akt, NF-κB) (
Figure 2). Further studies have also highlighted apoptosis resulting from downstream activation of PLCβ, Ras and Raf-1 after stimulation of these receptors [
12,
13,
14].
First, it is widely known that prostate cancer is closely related to a number of neuroendocrine cells that release serotonin and a high concentration of 5-HT
1A receptors have been found in various prostate cancer cell lines (PC3, DU145, LNCaP) [
15]. To prove this, there are several 5-HT
1A antagonists, for example NAN-190 (
Figure 3) or Pindobind, some of which also have an arylpiperazinic scaffold, which have been shown to inhibit cell proliferation
in vitro.
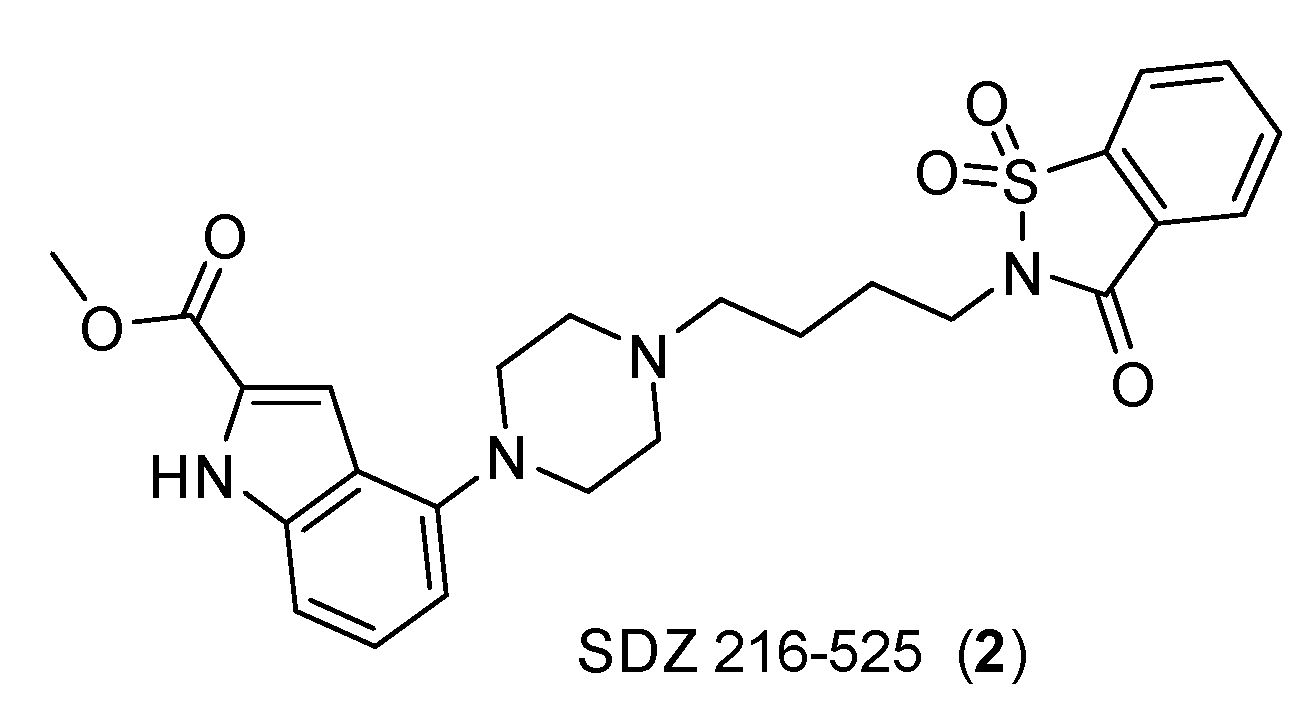
Similarly, there is evidence that serotonin is involved in bladder cancer and in small-cell lung carcinoma. The involvement of serotonergic receptors in the latter has been proven using antagonists such as SDZ 216-525 (
Figure 4).
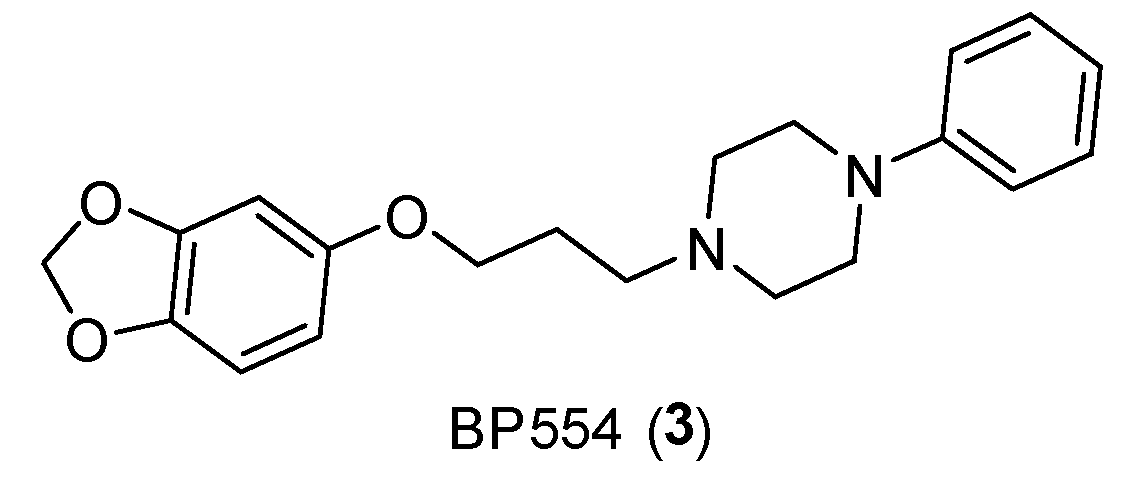
In addition, serotonin has demonstrated its mitogenic role in colorectal cancer, as the use of antagonists or SSRIs (such as BW501C or Citalopram and Fluoxetine) has reduced tumoral growth [
16]. In this case, having to consider the gastrointestinal tract, several receptor subtypes such as 5-HT
3, 5-HT
4 and 5-HT
1 receptors for colon cancer were studied. Additionally, the proliferative effects of selective receptor agonists (BP554) (
Figure 5) on HT29 cells were confirmed [
17].
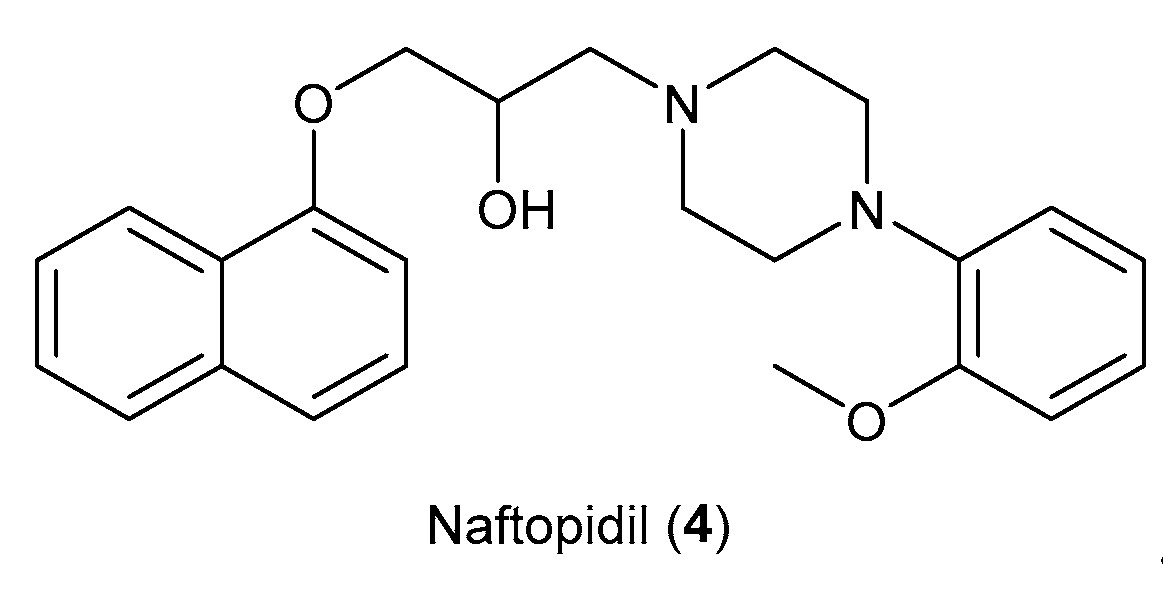
Another aspect to be considered as a starting point for future studies is the involvement of this scaffold in the design of selective derivatives of α1A and α1D receptor subtypes. An example could be represented by the drug repurposing of Naftopidil (
Figure 6), used for benign prostatic hyperplasia management. Naftopidil, an aryl-piperazine based α1-AR antagonist with a naphthalene group, in numerous studies has demonstrated its potential anticancer activities related to its pharmacological profile [
18]. This compound, used in Japan in the treatment of BPH, has emerged as a potential anticancer drug both because it is useful in arresting prostate cell growth but also in decreasing the cell viability of various cell lines such as bladder or renal cell lines. In fact, many research groups have been involved in the development of derivatives tested for their ability to block α1-ARs (specifically α1A, α1B, and α1D) [
19,
20].
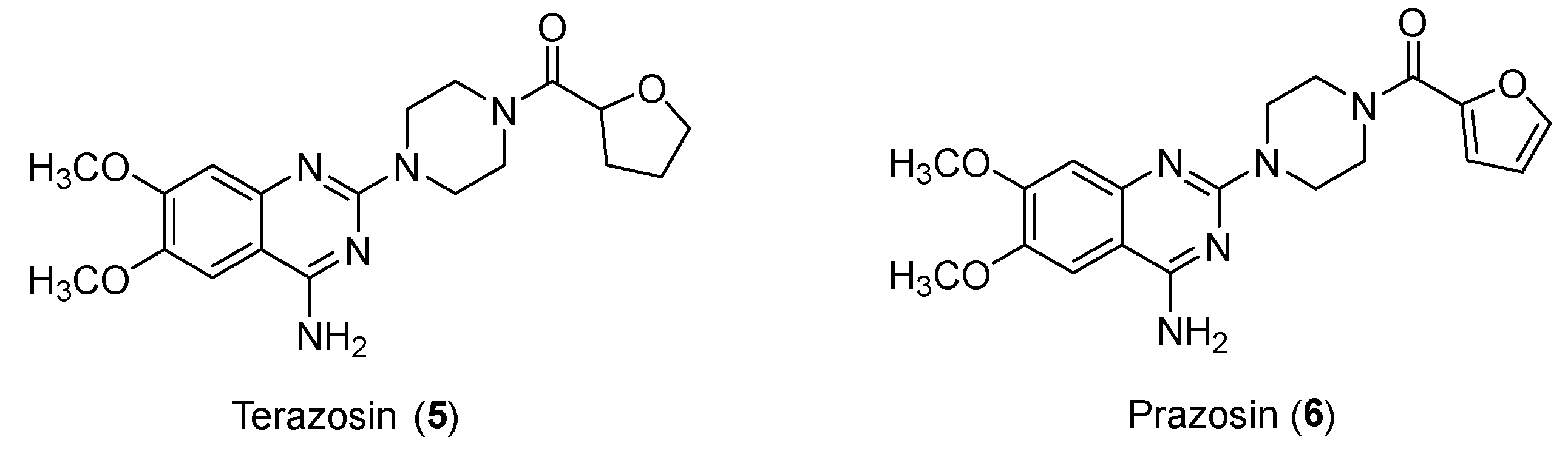
Several evidences concern the antitumor potential of α1 blockers. There are many relevant examples in the literature of antagonists such as Prazosin (used for the treatment of hypertension) or Terazosin (
Figure 7) (used in cases of hypertension or urinary symptoms due to benign prostatic hypertrophy); among the mechanisms highlighted are DNA damage stress induction for the former and cell growth inhibition for the latter [
21].
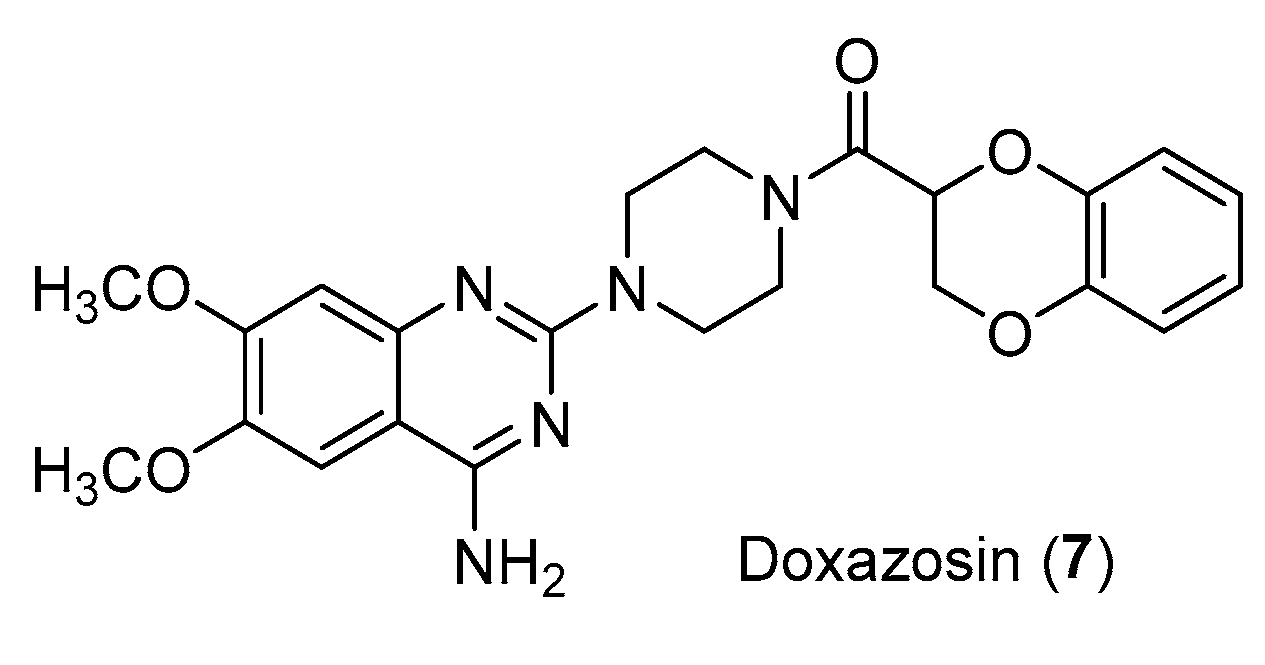
Similarly, the antitumor effect of Doxazosin (
Figure 8), an antihypertensive drug and used to treat benign prostatic hyperplasia, has been further investigated. This drug has the aryl piperazine scaffold in its structure and its antiproliferative effects have been demonstrated in several cell lines. It could act by different mechanisms such as activation of TGF and IkB, inhibition of PKB/AKT activation and angiogenesis or by autophagy and Suzuki et al., proved that Doxazosin sensitizes different tumor cells to Osimertinib, tyrosine kinase inhibitor [
22].
Additionally, androgens involved in normal prostate development and also in prostate cancer act through the androgen receptor (AR). Reason why the development of similar molecules are of clinical utility as chemotherapeutic agents for prostate cancer. These receptors are highly expressed in prostate cancer cells; in fact, they have long been studied as tumor targets for drug development. Evidence of this are AR- antagonist drugs such as flutamide, hydroxyflutamide, bicalutamide, and also arylpiperazine derivatives (
Figure 9) that have demonstrated efficacy on this pathway [
23].
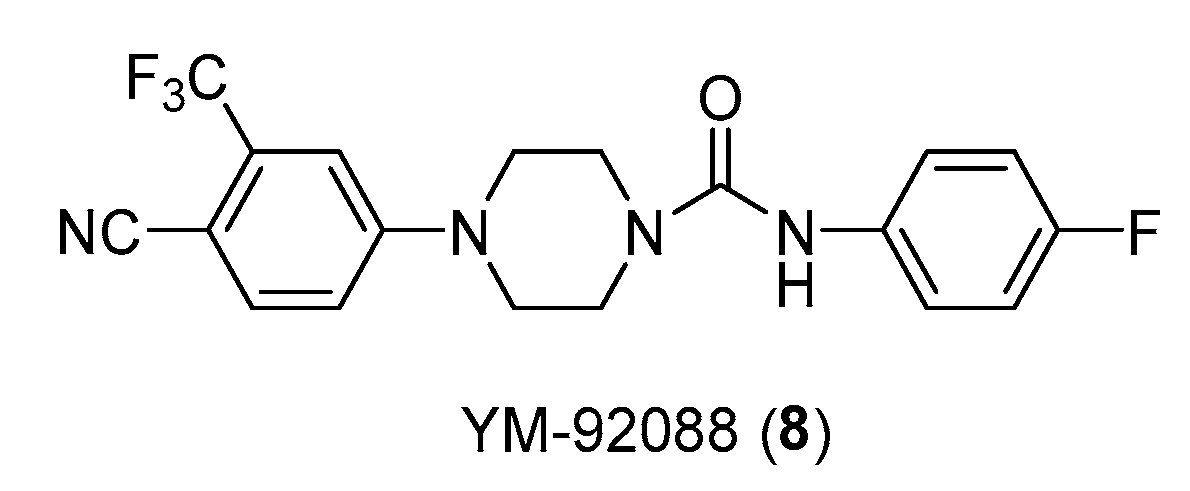
Therefore, it is well known the androgenic rule in physiological male development and in associated disorders. Another example is compound YM-92088 (
Figure 10), with high AR antagonist activity, with an IC
50 value of 0.47 μM, thus more potent than bicalutamide (IC
50 value of 0.89 μM) [
24].
2.1. N-aryl Piperazine Derivatives in Prostate Cancer
Prostate cancer is one of the most common cancers in the world, counting about 1-3 million new cases annually [
25]. Considering cases with or without PSA screening, it is the cause of death in 1-2% of the male population [
26].
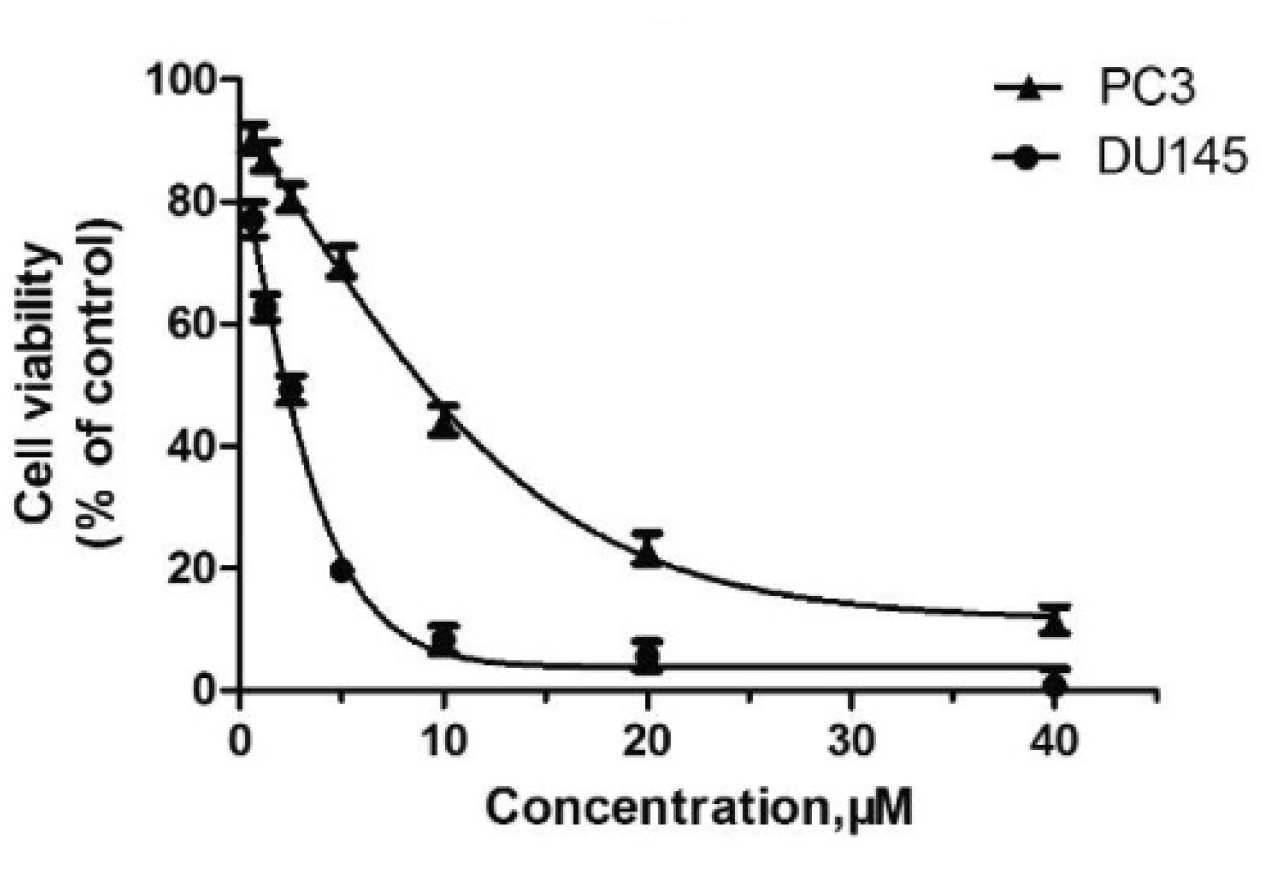
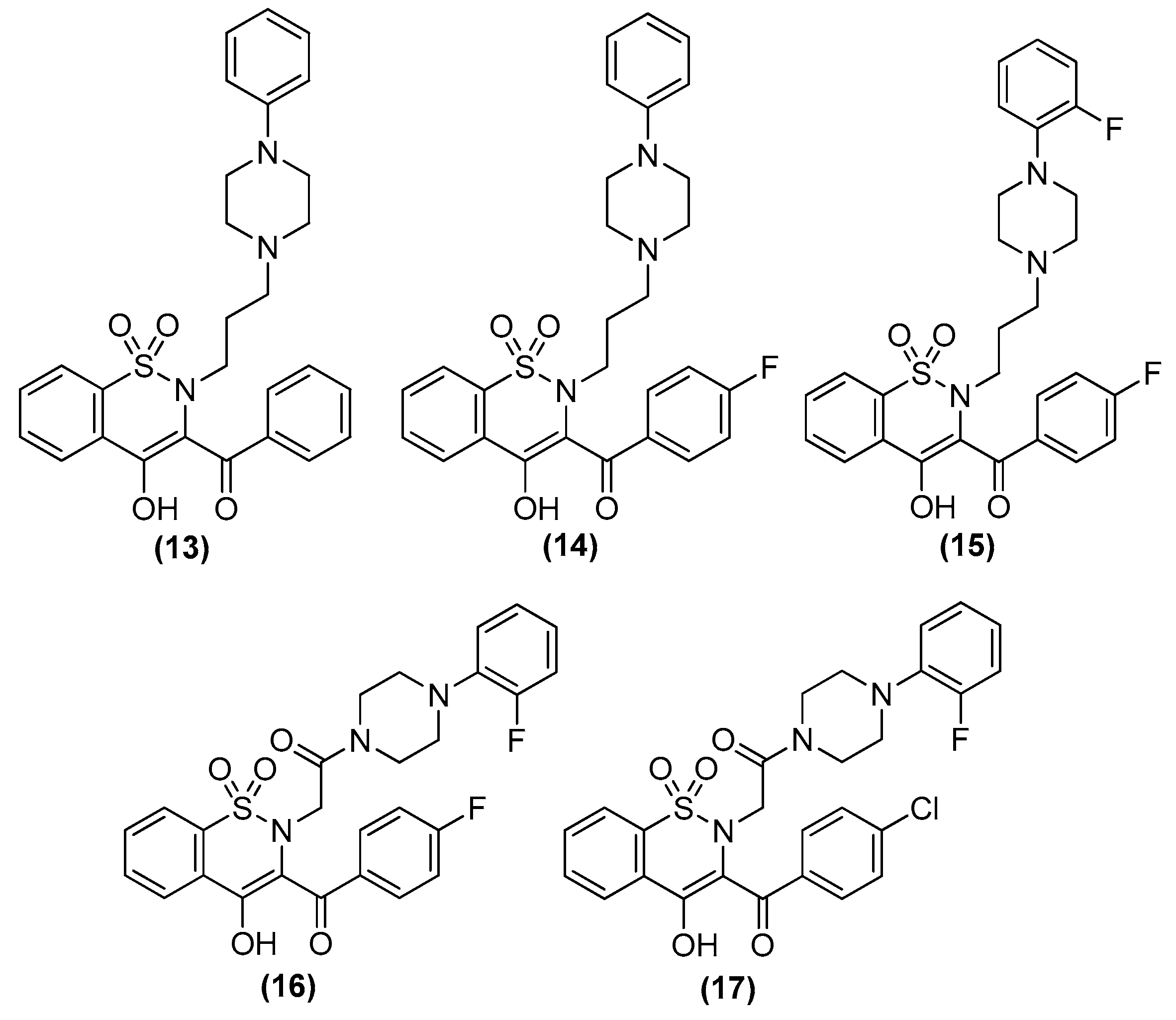
In recent studies Hong Chen et al. have presented a library of naftodipil-based arylpiperazine derivatives. These novel hybrids have been synthesized, characterized, and evaluated against prostate cancer cell lines (PC-3, LNCaP, and DU145) and their cytotoxicity has been compared to the effects of these compounds in non-cancer human prostate cells WPMY-1. Many of these compounds, have exhibited significant cytotoxic activities against LNCaP cells and DU145 cells (more active even than naftodipil and finasteride against DU145 cells), low cytotoxic profile toward WPMY-1, and have showed α1-ARs selectivity. Compounds
13 and
17 (
Table 2) have been evaluated for their effects on cell cycle progression and the result is that compound
17 has greatly increased the number of DU145 cells in the G0/G1 phase (
Figure 11), unlike compound
13 (9).
Always considering the treatment of benign prostatic hyperplasia, prior research indicates that arylpiperazine derivatives could potentially act as α1a and/or α1a- + α1d- selective ligands. On this, they have undertaken additional assessments to explore antagonistic effects utilizing dual-luciferase reporter assays. Their goal was to identify potential subselective antagonist candidates aimed at treating benign prostatic hyperplasia (BPH), among arylpiperazine derivatives recognized for their potent anticancer properties. Finally, compounds 13 (
9) and 17 (
10) demonstrated heightened selectivity towards specific subtypes of α1-ARs and 17 [
27].
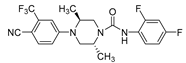
Kinoyama et al. have described the synthesis of a series of N-arylpiperazine derivatives and the correlated results as these compounds were then subjected to an assessment concerning their androgen antagonist profile. This choice was obviously made because many antiandrogens are currently in use for the treatment of prostate cancer. They focused on modifying compound 5 (YM-92088,
11) (
Table 3) considering the piperazine scaffold. The resulting data show that both nitrogen atoms in the piperazine ring are essential for potency. The modifications on one of the compounds led to the synthesis of the 18g derivative (
12) that demonstrated the strongest antiandrogenic activity [
28].
2.2. N-aryl Piperazine Derivatives in Colorectal Cancer
Colorectal cancer is one of the most aggressive forms of cancer; targeted therapy offers a novel approach that has shown promise in significantly prolonging the survival of patients. The number of deaths associated with this type of cancer is considerable even though it has decreased through early screening [
29].
Szczuka et al., have focused their attention on the role of HSPA1 and HSP90AA1, whose levels turns out to be increased in cancerous colorectal lesions. In fact, the expression of HSPA1 and HSP90AA1, key heat shock proteins involved in facilitating neoplastic transformation and cancer development, is altered already in precancerous colorectal lesions and surrounding tissue, to degree dependent on polyp potential for malignancy. Consequently, the effect of piroxicam, meloxicam and new arylpiperazine analogues, previously synthesized as analgesic without any ulcerogenic activity [
30,
31] was tested on the expression of the already mentioned heat shock proteins in colorectal adenocarcinoma lines (HCT 116, Caco-2 and HT-29 cells). These classic drugs have repeatedly shown anti-cancer properties, acting through both COX-dependent and independent pathways. Nevertheless, the precise molecular mechanisms behind these effects remain to be fully elucidated. The following compounds (
Figure 12) first showed reduced cytotoxicity compared to the corresponding oxicam, and then showed the ability to differently decrease protein expression of HSPA1 in all cells under examination. In addition, the oxicam analogues exhibited effectiveness in downregulating the expression of HSP90AA1, a trait not observed in classic drugs.
In terms of chemical structure, all examined analogues deviate from conventional drugs due to substitutions of the arylpiperazine pharmacophore and benzoyl moiety at the thiazine ring [
31].
2.3. N-aryl Piperazine Derivatives in Pancreatic Cancer
Pancreatic cancer, most of the time, is diagnosed at an advanced stage or already metastatic given the hard-to-diagnose early stage [
32]. Research directed toward screening and new therapeutic strategies can only help in reducing the mortality of this fatal malignancy [
33].
Through different approaches, Hong Su et al. [
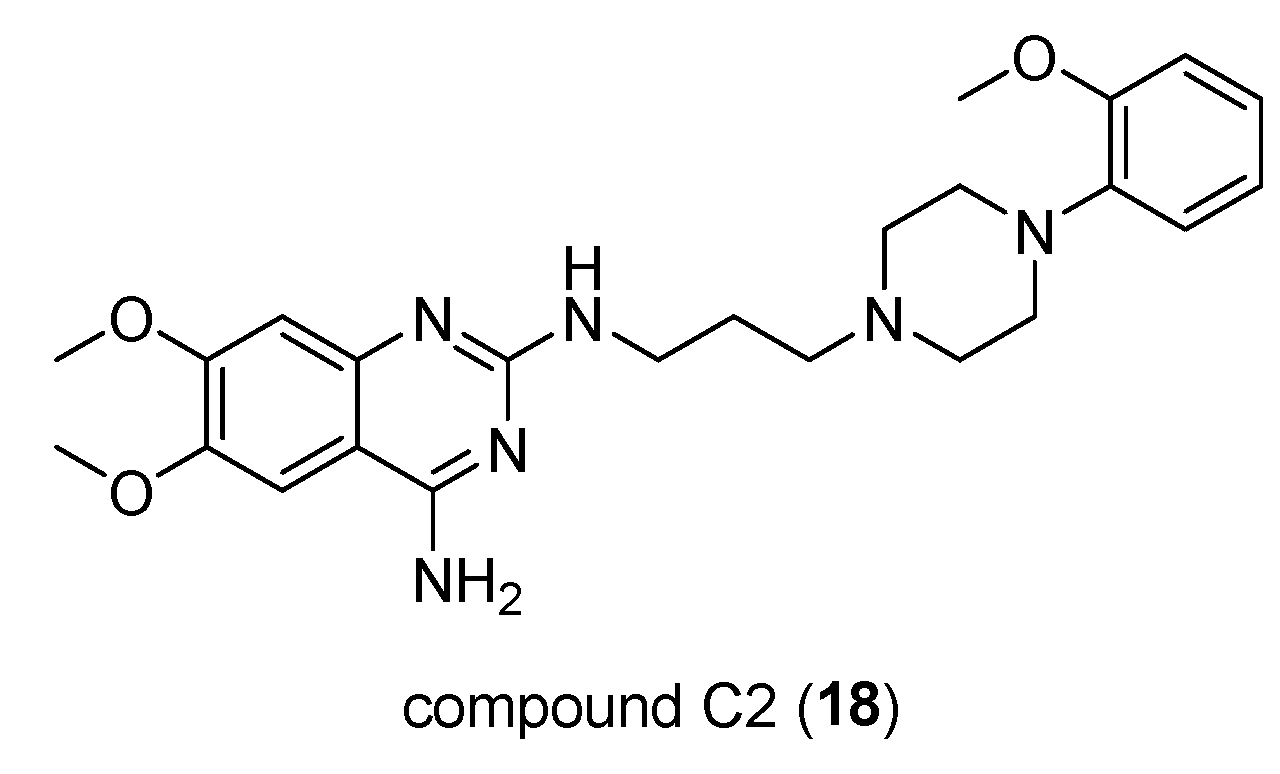
34] have explored new strategies for treating pancreatic cancer, as existing ones lead to short-term survival. First, they have considered Sunitinib (SUN), which is generally used to treat different types of cancer and their focus was to examinate the combination of SUN and an arylpiperazine derivative, compound C2 (
18,
Figure 13) that represents a D1DR agonist that was demonstreted able to reduce the cancer stem-like cells (CSC) frequency in both pancreatic cells and accordingly enhanced the response to SUN in the treatment of pancreatic cancer.
The discovery of new D1DR agonists that are chemically stable and available orally is essential. This is because several studies have shown that dopamine reduces the presence of cancer stem-like cells (CSC), closely associated with the progression, metastasis, and recurrence of pancreatic cancer. Indeed, gradual attention has been paid to the use of chemotherapy and targeting CSCs in the treatment of cancer. Also considered that, was deepened the role of dopamine [
35,
36,
37] in the reduction of CSC intratumor and D1DR as a target in anti-cancer therapy, CSC frequency inhibition by compound C2 in pancreatic cancer cells PANC-1 and SW1990 was confirmed. Additionally was found that this compound increases the cell level of cAMP in SW1990 xenograft (level that generally is increased by use of D1DR agonists or by D2DR antagonists), indicating, by molecular docking studies, the higher propensity for D1DR binding than D2DR. Definitely, C2 compound supporting an N-arylpiperazine moiety could be considered potential usefull in the treatment of pancreatic cancer also improving the response to Sunitinib [
34].
2.4. N-aryl Piperazine Derivatives in Breast Cancer
Different type of breast cancer (BC) are heterogeneous and classified according to subtype and corresponding therapy, and despite the reaserch efforts and increasing knowledge, BC represents the most common one along with lung cancer [
38].
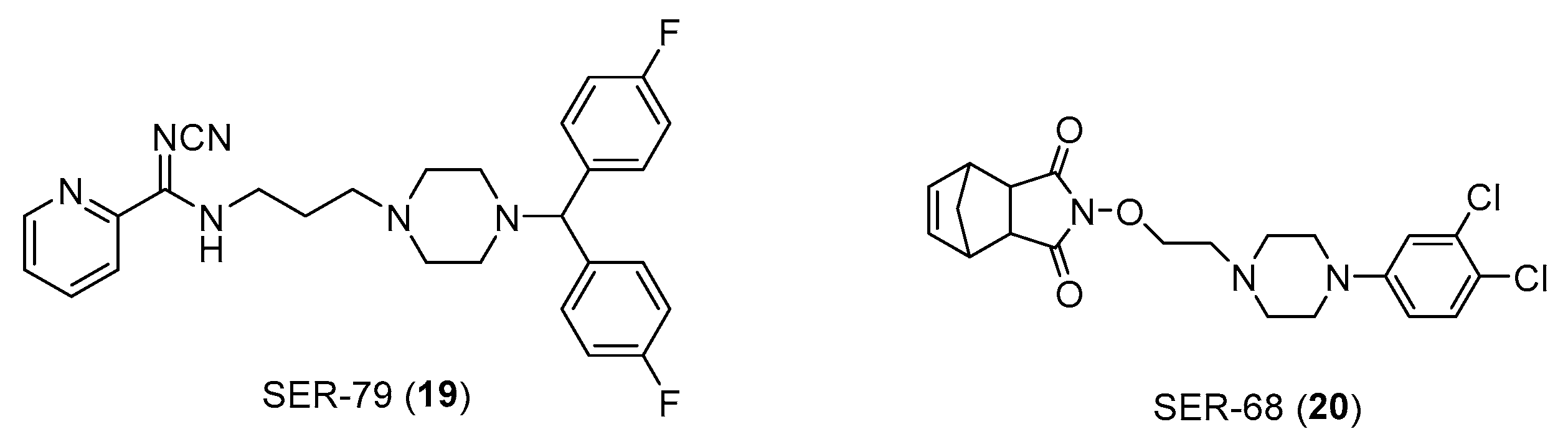
Also in this context the role of arylpierazine derivatives as serotonergic ligands emerges able to target serotonin and connective tissue growth factor (CTGF) signaling, also ameliorating the sensitivity to Tamoxifen in ER+ breast cancer cells. CTGF has been identified as a glucose-induced modulator of cell sensitivity to tamoxifen and the CTGF silencing induced a significant increase in tamoxifen sensitivity of BC cells grown in hyperglicemia, at levels like those obtained for cells cultured in normal levels of glycemia. The arylpiperazine derivatives (
Figure 14) improve the efficacy of tamoxifen on MCF7 breast cancer cells (ER+) by modulating the expression of CTGF [
39,
40,
41].
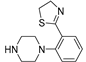
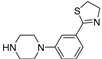
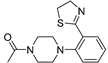
Successively a new arylpiperazine scaffold supporting a dihydrothiazole moiety in the different positions on the phenyl ring and with the meta position being the most favorable was designed and synthesized by Andreozzi et al. The synthesized compounds were subjected to binding assays on 5-HT1A receptors and pharmacological evaluation on breast and prostate cancer cells. Compared with prostate data, all thiazolinylphenyl-piperazine compounds showed a 50% reduction in breast cells viability with a concentration of at least 25 μM. The most interesting finding of this work, concerns the result of the
2a-c compounds on the MCF-7 cell line (
Table 4) and in addition a highly cytotoxic effect was observed on MDA-MB231 for both 2a-c and 3a-c acetylated derivatives simultaneously showing a significant selectivity towards non-transformed cells [
42].
Therefore, given the absence of therapeutic approaches devoid of cytotoxic effects, these results obtained on androgen-independent prostate cancer and triple negative breast cancer cells, highlight the potential innovation that these compounds can represent in combating extremely aggressive forms of tumors.
2.5. N-arylpiperazine Derivatives in Cervical Carcinoma
Cervical cancer is one of the most common causes of death in women [
43]. Cervical neoplasia begins as an intraepithelial alteration, and generally requires many years to progress into an invasive disease [
44].
Mao et al. [
45], in the context of arylpiperazine derivatives, focused on hybridization for the synthesis of new derivatives then tested in vitro for their anticancer activities. These new hybrid compounds have been tested on several cell lines: lung carcinoma (A549), cervical carcinoma (Hela), breast carcinoma (MCF-7) and gastric carcinoma (SGC7901). As shown in the
Table 5, preliminary anticancer activity of the reported compounds has been proven. From these data, however, it emerged that the compounds with the chlorine or trifluoromethyl substituents on the benzene ring are those that show most cytotoxic activity. So, they focused on just one compound, and they proved that this compound 13 (
27) exerts cytotoxic activity selectively against Hela.
2.6. N-arylpiperazine Derivatives in Leukemia
Considerable efforts have been made over the years to classify the different forms of hematopoietic neoplastic diseases and their respective treatment, but in some cases, it continues to represent a fatal malignancy. Some of these, called chronic, develop slowly, leading to high levels of circulating white blood cells. Acute leukemias are characterized by an early growth of white blood cells, and the disease is usually lethal in a short time [
46]. Choi et al. [
47] have synthesized many aryloxazole derivatives containing an arylpiperazine moiety and acting as vascular-targeting anticancer agents. The most interesting aspect is the dual effect of these compounds, the tumor vasculature disruption and mitotic arrest. Cytotoxic effects were studied considering human leukemia cells (HL-60), and a careful analysis was made on the importance of substituents and functional groups in this scaffold. In fact, replacing the arylpiperazine group with other heterocycles, they noticed a loss of cytotoxicity. This indicates that the piperazine nitrogen atoms and the substituted aryl group are critical for binding. The vascular-disrupting effect was then tested on selected derivatives that displayed low IC
50 values. Finally, to confirm the dual effect, they also demonstrated the ability of these compounds to inhibit tubulin polymerization during mitosis.
Therefore, considering all these data, there are conducted in vivo studies selecting compounds that showed the best profile in inhibition tests of growth cell. On these bases it was verified that compound 6-48 (
28,
Table 6) is a potential anticancer agent [
47] with an outstanding microsomal stability. This compound inhibited tubulin polymerization at low concentrations, suggesting that its biological activity comes from tubulin binding. Moreover compound 6-48 showed an excellent antimitotic effect and vascular-disrupting activity in vitro and demonstrated promising antitumor activity in vivo, possibly because of its metabolic stability.
2.7. N-arylpiperazine Derivatives in Melanoma
The incidence of melanoma cases has been increasing lately, especially in fair-skinned countries [
48] and the mortality rate is dramatically high [
49].
Romagnoli et al.[
50] have synthesized Cinnamic acid derivatives linked to arylpiperazine moieties with the aim of investigating the role of the enzyme tyrosinase in the melanogenic process, which is one of the most studied therapeutic targets for melanoma because it regulates melanin synthesis. Through structure-activity report they studied the influence of the substituent on the arylpiperazine scaffold, and the synthesized derivatives were tested by evaluating the inhibitory effect using mushroom tyrosinase, also evaluating cell viability and tyrosinase activity in A375 human melanoma cells. Finally, toxicity was evaluated by zebrafish assays considering depigmenting effects on zebrafish embryos. It was found that derivative 19r (
29,
Table 7) reduce melanogenesis without any toxicity effects up to 100 μM and at 5-fold reduced concentration (20μM) significantly decreased (60%) the activity of human tyrosinase in A375 cells that were stimulated with α-melanocortin (MSH)
2.8. Other Examples of Arylpiperazines in Cancer
Finally, there are many promising arylpiperazine that have been tested for their activity as anticancer agents on different cell lines.
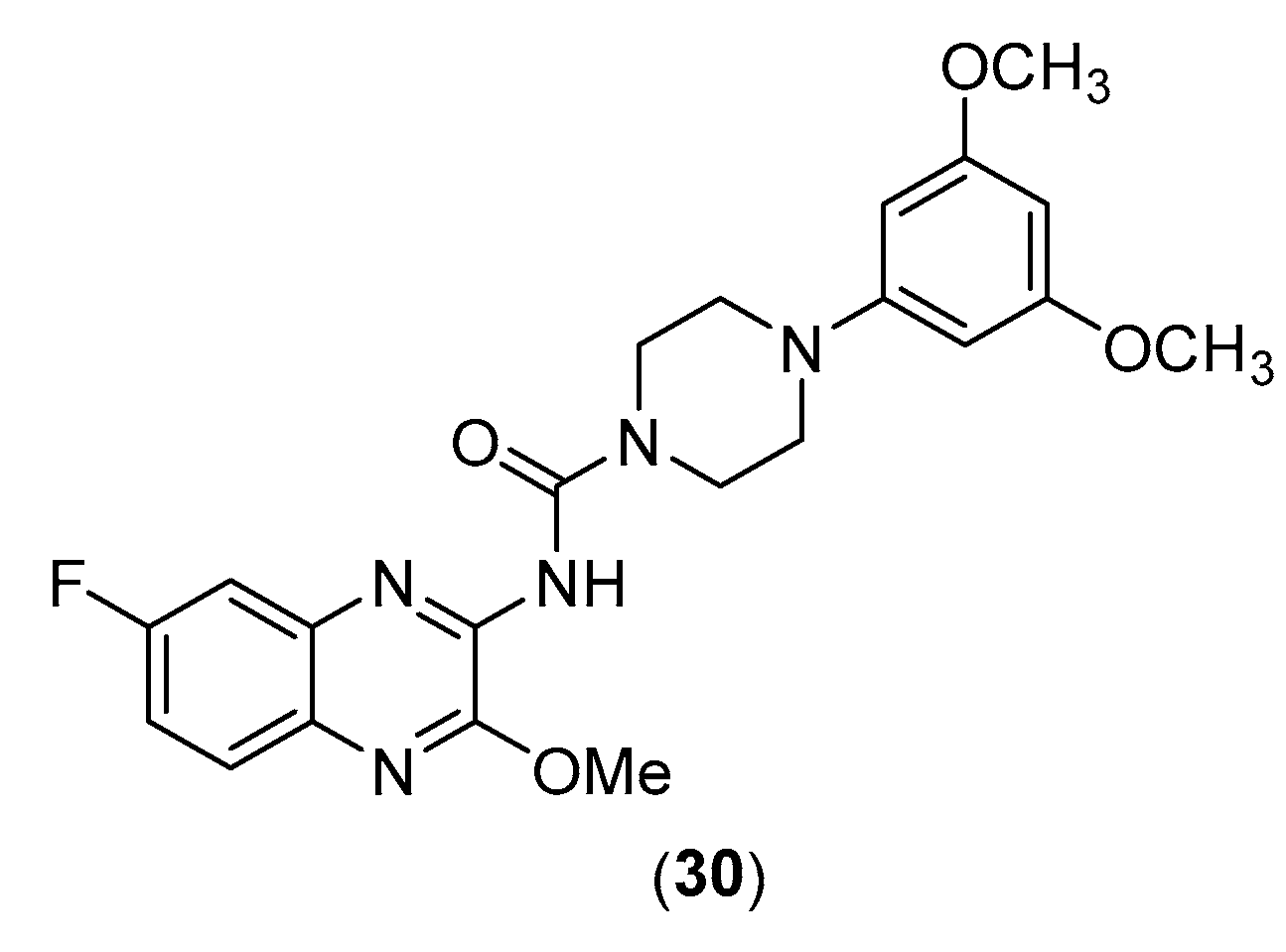
Lee et al. evaluated quinoxalinyl-piperazine derivatives as possible anticancer agents [
51]. Among them, they identified one as a growth inhibitor of cancer cells and specifically demonstrated that this compound is a G2/M-specific cell cycle inhibitor and inhibits anti-apoptotic Bcl-2 protein with p21 induction. Compound 25 (
30,
Figure 15) inhibited the proliferation of several lines of cancer cells, including breast cells, skin, pancreas, and cervix, as shown in the
Table 8. This inhibition is dose dependent.
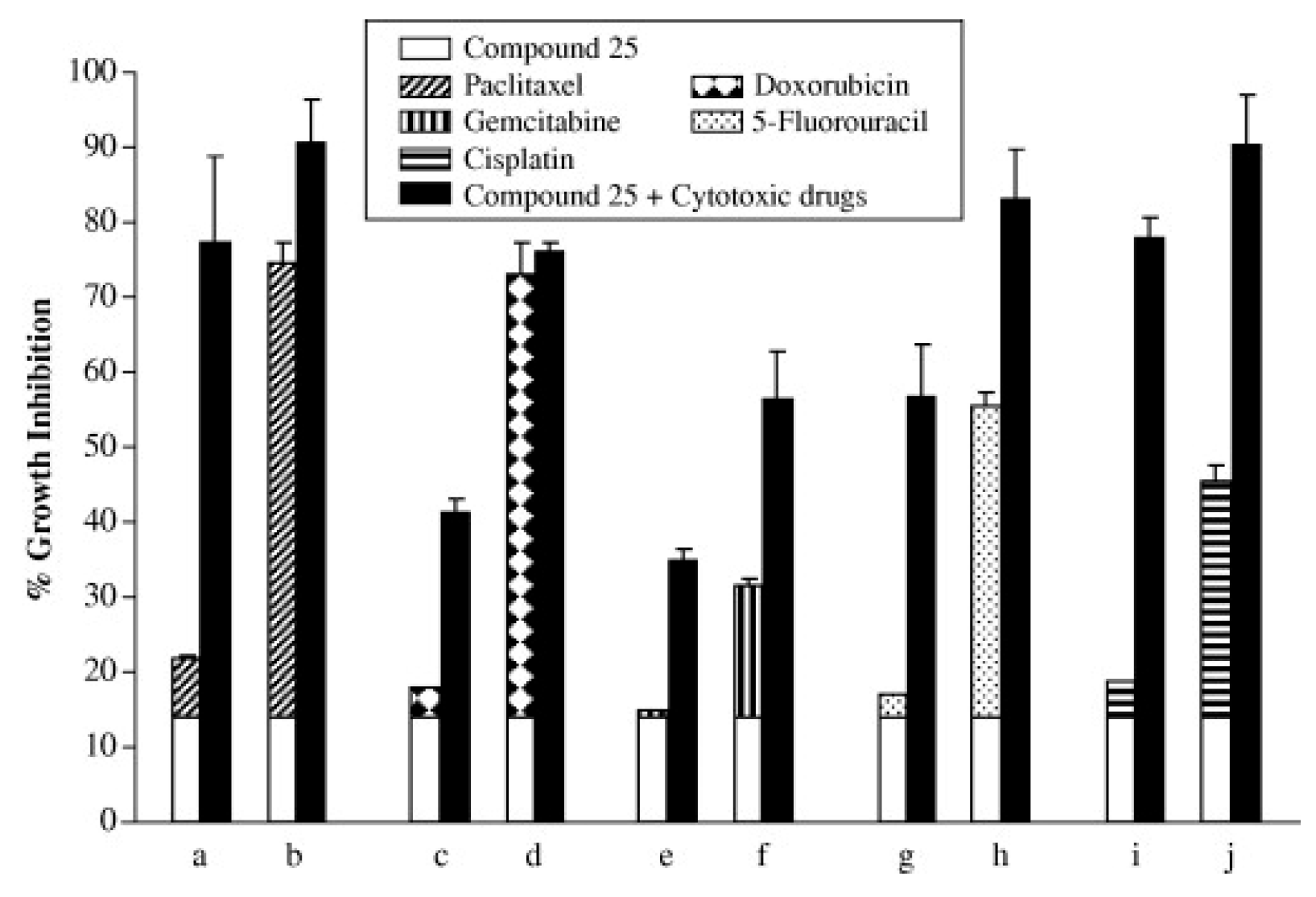
In addition, the association between this compound and other anticancer drugs (
Figure 16) such as taxanes, paclitaxel, a platinum derivative cisplatin, a topoisomerase II selective agent doxorubicin, gemcitabine, and fluorouracil, has led to a synergistic inhibitory effect.
3. Conclusions
Over the years, interest of
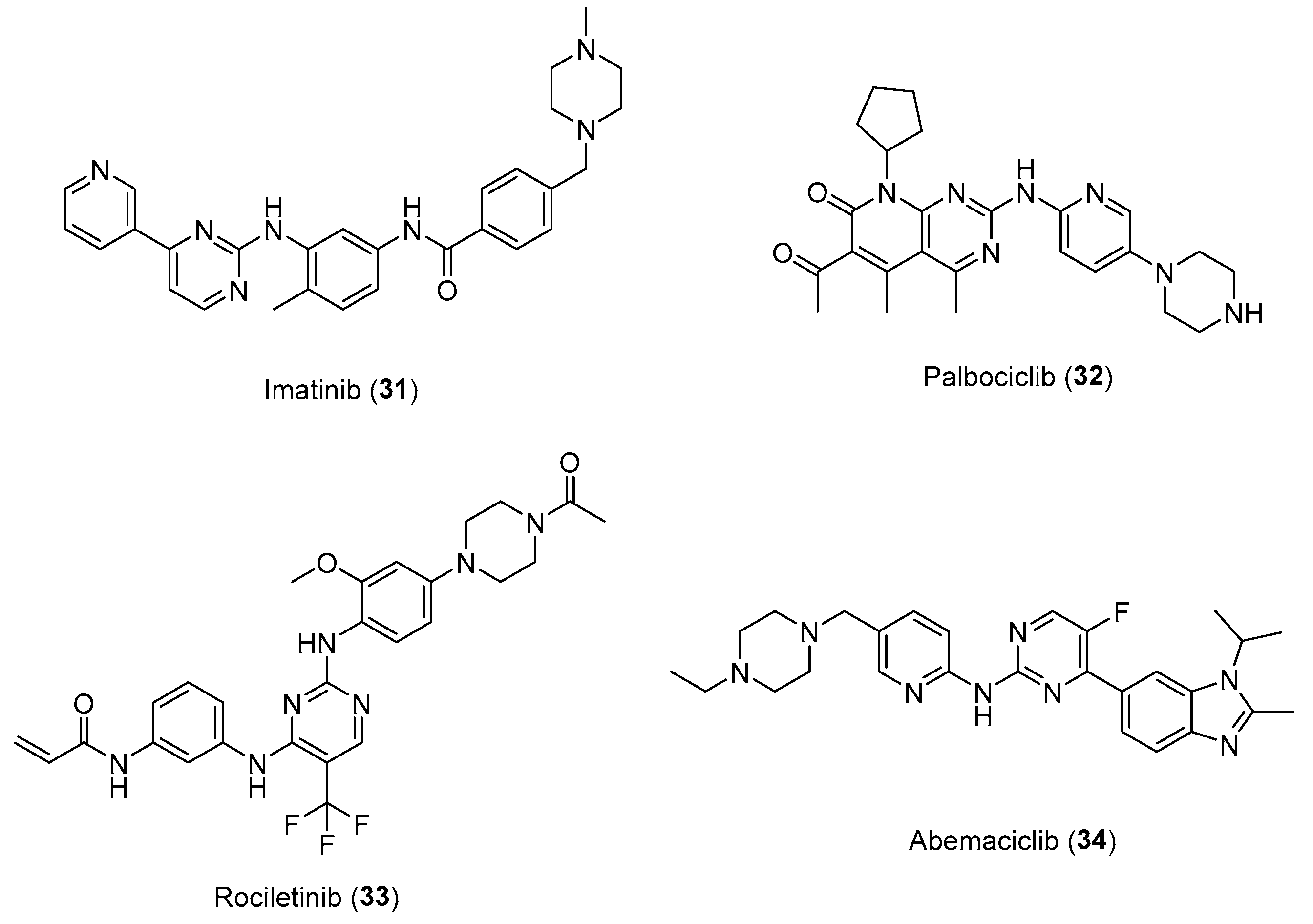
N-arylpiperazine derivatives in the field of pharmaceutical chemistry has been growing. At the beginning derivatives were mainly studied as serotonergic ligands, finding application in the development of drugs active on the central nervous system (CNS). Subsequently, also due to the chemical-physical characteristics of this basic scaffold, studies were undertaken on different pharmacological models including some molecular targets involved in carcinogenesis and tumor progression. In this context, many examples are reported in the literature which have shed more light on the potential of the arylpiperazine moiety useful in the development of new antitumor agents. Notably, these findings have also translated into the market entry of some arylpiperazine-based drugs, such as Imatinib (31), Palbociclib (32), Rociletinib (33) and Abemaciclib (34) (
Figure 17), as targeted therapies approved by FDA [
52] for the treatment of cancer. These results, together with the current studies described in this review, clearly emphasize the role of arylpiperazine derivatives not only on the CNS but also in the cancer.
Consequently, research in this area requires further efforts to clarify the molecular target, the transduction mechanism of inhibition of the pathways involved in the various tumor forms, and highlight the activity of the described molecules. Moreover it’s also desirable that in the field of medicinal chemistry more interest could be directed towards the design and synthesis of new chemical entity including arylpiperazine-based molecules. To this purpose in order to investigate all the different portions of this scaffold and the different substituents on the aromatic ring could be interesting to perform SAR studies that lead to develop novel compound characterized by increased selectivity against cancer cell lines and reduced toxicity. This last issue is of particular interest since also a synergistic use in combination with classic chemotherapeutics could be hypothesized for these molecules. In this way, the conventional anticancer therapy would be used with lower doses and consequent fewer toxic effects.
In conclusion the promising results presented in this review, and the presence of N-arylpiperazine moiety in several FDA-approved anticancer medications makes it a desirable scaffold with great potential for the develop of novel anticancer drugs.
Author Contributions
Conceptualization, G.A. F.Fi and A.C.; Resources, E.M., F.Fr., B.S., E.P.; writing—original draft preparation, G.C. and F.Fi; writing—review and editing, A.C. and B.S.; supervision, G.C. and V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
The authors declare no conflicts of interest
References
- Ravilla, L.; Venkata Subba Naidu, N.; Nagarajan, K. An Efficient Scale up Process for Synthesis of N-Arylpiperazines. Tetrahedron Lett 2015, 56, 4541–4544. [Google Scholar] [CrossRef]
- Jaziri, E.; Louis, H.; Gharbi, C.; Lefebvre, F.; Kaminsky, W.; Agwamba, E.C.; Egemonye, T.C.; Unimuke, T.O.; Ikenyirimba, O.J.; Mathias, G.E.; Ben Nasr, C.; Khedhiri, L. Investigation of Crystal Structures, Spectral (FT-IR and NMR) Analysis, DFT, and Molecular Docking Studies of Novel Piperazine Derivatives as Antineurotic Drugs. J Mol Struct 2023, 1278, 134937. [Google Scholar] [CrossRef]
- Karolak-Wojciechowska, J.; Fruzinski, A.; Mokrosz, M.J. Structure and Conformational Analysis of New Arylpiperazines Containing N-Butyl Chain. Elsevier 2001, 542, 47–56. [Google Scholar] [CrossRef]
- Weber, K.C.; Salum, L.B.; Honorio, K.M.; Andricopulo, A.D.; da Silva, A.B.F. Pharmacophore-Based 3D QSAR Studies on a Series of High Affinity 5-HT1A Receptor Ligands. Eur J Med Chem 2010, 45, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Quaglia, W.; Cifani, C.; Del Bello, F.; Giannella, M.; Giorgioni, G.; Micioni di Bonaventura,m.v.; Piergentili, A. 4WD to Travel Inside the 5-HT1A Receptor World. In Serotonin - A Chemical Messenger Between All Types of Living Cells; InTech, 2017, pp. 67-108.
- Caliendo, G.; Santagada, V.; Perissutti, E.; Fiorino, F. Derivatives as 5HT 1A Receptor Ligands-Past and Present; Current Medicinal Chemistry, 2005, 12, 763-771.
- Fiorino, F.; Severino, B.; Magli, E.; Ciano, A.; Caliendo, G.; Santagada, V.; Frecentese, F.; Perissutti, E. 5-HT1A Receptor: An Old Target as a New Attractive Tool in Drug Discovery from Central Nervous System to Cancer. J Med Chem 2014, 57, 4407–4426. [Google Scholar] [CrossRef] [PubMed]
- Ikwu, F.A.; Shallangwa, G.A.; Mamza, P.A. Ligand Based Design, ADMET and Molecular Docking Studies of Arylpiperazine Derivatives as Potent Anti-Proliferate Agents Against LNCAP Prostate Cancer Cell Lines. Chemistry Africa 2021, 4, 71–84. [Google Scholar] [CrossRef]
- Kaur, N.; Popli, P.; Tiwary, N.; Swami, R. Small Molecules as Cancer Targeting Ligands: Shifting the Paradigm. Journal of Controlled Release 2023, 355, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wu, J. : Liu, B. Therapeutic Strategies of Dual-Target Small Molecules to Overcome Drug Resistance in Cancer Therapy. Biochim Biophys Acta Rev Cancer 2023, 1878. [Google Scholar] [CrossRef] [PubMed]
- Corvino, A.; Fiorino, F.; Severino, B.; Saccone, I.; Frecentese, F.; Perissutti, E.; Di Vaio, P.; Santagada, V.; Caliendo, G.; Magli, E. The Role of 5-HT1A Receptor in Cancer as a New Opportunity in Medicinal Chemistry. Curr Med Chem 2018, 25, 3214–3227. [Google Scholar] [CrossRef] [PubMed]
- Cowen, D.S.; Sowers, R.S.; Manning, D.R. Activation of a Mitogen-Activated Protein Kinase (ERK2) by the 5-Hydroxytryptamine 1A Receptor Is Sensitive Not Only to Inhibitors of Phosphatidylinositol 3-Kinase, but to an Inhibitor of Phosphatidylcholine Hydrolysis; JBC, 1996.
- Blesen, T.; Hawes, B.E.; Luttrell, D.K.; Krueger, K.M.; Touhara, K.; Porflrlt, E.; Sakauet, M.; Luttrell, L.M.; Lefkowitz, R.J. Receptor-tyrosine-kinase- and Gpy-mediated MAP kinase activation by a common signalling pathway LETTERS TO NATURE 1995, 376.
- Della Rocca, G.J.; Mukhin, Y. V.; Garnovskaya, M.N.; Daaka, Y.; Clark, G.J.; Luttrell, L.M.; Lefkowitz, R.J.; Raymond, J.R. Serotonin 5-HT1A Receptor-mediated Erk Activation Requires Calcium/Calmodulin-dependent Receptor Endocytosis. THE JOURNAL OF BIOLOGICAL CHEMISTRY, 1999, 4749-4753. [CrossRef]
- Shinka, T.; Onodera, D.; Tanaka, T.; Shoji, N.; Miyazaki, T.; Moriuchi, T.; Fukumoto, T. Serotonin Synthesis and Metabolism-Related Molecules in a Human Prostate Cancer Cell Line. Oncol Lett 2011, 2, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Tutton, P.J.M.; Steel, G.G. Influence of biogenic amines on the growth of xenografted human colorectal carcinomas; Br. J. Cancer, 1979; 40, 743-749.
- Matsuda, T.; Seong, Y. H.; Aono, H.; Kanda, T.; Baba, A.; Saito, K.; Tobe, A.; Iwata, H. Agonist Activity of a Novel Compound, 1-[3-(3,4-Methylenedioxyphenoxy) Propyl]-4-Phenyl Piperazine (BP-554), at Central 5-HT1A Receptors. Eur J Pharmacol 1989, 170, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Florent, R.; Poulain, L.; N’Diaye, M. Drug Repositioning of the A1-Adrenergic Receptor Antagonist Naftopidil: A Potential New Anti-Cancer Drug? Int J Mol Sci 2020, 21, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liang, X.; Xu, F.; Xu, B.; He, X.; Huang, B.; Yuan, M. Synthesis and Cytotoxic Activity Evaluation of Novel Arylpiperazine Derivatives on Human Prostate Cancer Cell Lines. Molecules 2014, 19, 12048–12064. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Chen, H.; Xu, J.; Liang, X.; He, X.; Shao, B.; Sun, X.; Li, B.; Deng, X.; Yuan, M. Synthesis, Structure-Activity Relationship and Biological Evaluation of Novel Arylpiperzines as A1A/1D-AR Subselective Antagonists for BPH. Bioorg Med Chem 2015, 23, 7735–7742. [Google Scholar] [CrossRef] [PubMed]
- Hanušová, V.; Skálová, L.; Kralova, V.; Matouskova, P. Potential Anti-cancer Drugs Commonly Used for Other Indications. Current Cancer Drug Targets 2015, 15, 35-52 2015. [CrossRef] [PubMed]
- Suzuki, S.; Yamamoto, M.; Sanomachi,T.; Togashi, K.; Sugai, A.; Seino, S.; Okada, M.; Yoshioka, T.; Kitanaka, C. Doxazosin, a Classic Alpha 1-Adrenoceptor Antagonist, Overcomes Osimertinib Resistance in Cancer Cells via the Upregulation of Autophagy as Drug Repurposing. Biomedicines, 2020; 8, 273. [CrossRef]
- Qi, Y.; Chen, H.; Chen, S.; Shen, J.; Li, J. Synthesis, Bioactivity, and Molecular Docking of Novel Arylpiperazine Derivatives as Potential AR Antagonists. Front Chem 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Kinoyama, I.; Taniguchi, N.; Yoden, T.; Koutoku, H.; Furutani, T.; Kudoh, M.; Okada, M. Synthesis and Pharmacological Evaluation of Novel Arylpiperazine Derivatives as Nonsteroidal Androgen Receptor Antagonists. Chem Pharm Bull (Tokyo) 2004, 52, 1330–1333. [Google Scholar] [CrossRef]
- Sandhu, S.; Moore, C.M.; Chong, E.; Beltran, H.; Bristow, R.G.; Williams, S.G. Prostate Cancer. The Lancet 2021, 398, 1075–1090. [Google Scholar] [CrossRef] [PubMed]
- Attard, G.; Parker, C.; Eeles, R.A.; Schroder, F.; Tomlins, S.A.; Tannok,I.; Drake, C.G.; de Bono, J.S. Prostate Cancer. The Lancet 2016, 387, 70–82.
- Chen, H.; Wang, C.L.; Sun, T.; Zhou, Z.; Niu, J.; Tian, X.; Yuan, M. Synthesis, Biological Evaluation and SAR of Naftopidil-Based Arylpiperazine Derivatives. Bioorg Med Chem Lett 2018, 28, 1534–1539. [Google Scholar] [CrossRef] [PubMed]
- Kinoyama, I.; Taniguchi.; Kawaminami, E.; Nozawa, E.; Koutoku, H.; Furutani, T.; Kudoh, M.; Okada, M. N-Arylpiperazine-1-Carboxamide Derivatives: A Novel Series of Orally Active Nonsteroidal Androgen Receptor Antagonists. Chem. Pharm. Bull. 2005, 53, 402-409.
- Torre, L.A.; Bray, F.; Siegel, R. L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. CA Cancer J Clin 2015, 65, 87–108.
- Szczęśniak-Sięga, B.M.; Mogilski, S.; Wigluszc, R.J.; Janczak, J.; Maniewska, J.; Malinka, W.; Filipek, B. Synthesis and Pharmacological Evaluation of Novel Arylpiperazine Oxicams Derivatives as Potent Analgesics without Ulcerogenicity. Bioorg Med Chem 2019, 27, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Szczuka, I.; Wierzbicki, J.; Serek, P.; Szczesniak-Siega, B.M.; Krzystek-Korpacka, M. Heat Shock Proteins Hspa1 and Hsp90aa1 Are Upregulated in Colorectal Polyps and Can Be Targeted in Cancer Cells by Anti-Inflammatory Oxicams with Arylpiperazine Pharmacophore and Benzoyl Moiety Substitutions at Thiazine Ring. Biomolecules 2021, 11, 1588. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Chu, M.; Song, G.; Zuo, Z.; Han, Z.; Chen, C.; Li, Y.; Wang, Z. Emerging Roles of Long Noncoding RNAs in Chemoresistance of Pancreatic Cancer. Semin Cancer Biol 2022, 83, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic Cancer; 2008, 395, 2008-20.
- Su, H.; Xue, Z.; Feng, Y.; Xie, Y.; Deng, B.; Yao, Y.; Tian, X.; An, Q.; Yang, L.; Yao, Q.; Xue, J.; Chen, G.; Hao, C.; Zhou,T. N-Arylpiperazine-Containing Compound (C2): An Enhancer of Sunitinib in the Treatment of Pancreatic Cancer, Involving D1DR Activation. Toxicol Appl Pharmacol 2019, 384, 114789. [CrossRef]
- Wang, C.; Niu, M.; Zhou, Z.; Zheng, X.; Zhang, L.; Tian, Y.; Yu, X.; Bu, G.; Xu, H.; Ma, Q.; Zhang, Y. VPS35 Regulates Cell Surface Recycling and Signaling of Dopamine Receptor D1. Neurobiol Aging 2016, 46, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Wang, S.; Zhu1, X.; Xue, J.; Li, J.; Wang, L.; Li, J.; Lu, W.; Zhou, T. Pharmacokinetic-Pharmacodynamic Modeling of the Anti-Tumor Effect of Sunitinib Combined with Dopamine in the Human Non-Small Cell Lung Cancer Xenograft. Pharm Res 2017, 34, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, S.; Ren, Y.; Li, J.; Guo, T.; Lu, W.; Zhou, T. Antitumor Effect of Axitinib Combined with Dopamine and PK-PD Modeling in the Treatment of Human Breast Cancer Xenograft. Acta Pharmacol Sin 2019, 40, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Wang, S.; Ali, N. Advanced Approaches to Breast Cancer Classification and Diagnosis. Front Pharmacol 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, M.R.; Magli, E.; Caliendo, G.; Sparaco, R.; Massarelli, P.; D’Esposito, V.; Migliaccio, T.; Mosca, G.; Fiorino, F.; Formisano, P. Serotoninergic Receptor Ligands Improve Tamoxifen Effectiveness on Breast Cancer Cells. BMC Cancer 2022, 22, 171. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, F.; Perissutti, E.; Severino, B.; Santagada, V.; Cirillo, D.; Terracciano, S.; Massarelli, P.; Bruni, G.; Collavoli, E.; Renner, C.; Caliendo, G. New 5-Hydroxytryptamine1A Receptor Ligands Containing a Norbornene Nucleus: Synthesis and in Vitro Pharmacological Evaluation. J Med Chem 2005, 48, 5495–5503. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, F.; Magli, E.; Ke˛dzierska, E.; Ciano, A.; Corvino, A.: Severino, B.; Perissutti, E.; Frecentese, F.; Di Vaio, P.; Saccone, I.; Izzo, A. A.; Capasso, R.; Massarelli, P.; Rossi, I.; Orzelska-Gòrkac, J.; Kotlin’ska, J.HJ; Santagada, V.; Caliendo, G. New 5-HT1A, 5HT2A and 5HT2C Receptor Ligands Containing a Picolinic Nucleus: Synthesis, in Vitro and in Vivo Pharmacological Evaluation. Bioorg Med Chem 2017, 25, 5820–5837.
- Andreozzi, G.; Ambrosio, M.R.; Magli, E.; Maneli, G.; Severino, B.; Corvino, A.; Sparaco, R.; Perissutti,E.; Le´ sniak, A.; Frecentese, F.; Santagada,V.; Bujalska-Zadrozny,M.; Caliendo, G.; Formisano, P.; Fiorino, F. Design, Synthesis and Biological Evaluation of Novel N-Arylpiperazines Containing a 4,5-Dihydrothiazole Ring. Pharmaceuticals 2023, 16, 1483.
- Ferrall, L.; Lin, K.Y.; Roden, R.B.S.; Hung, C.-F.; Wu, T.-C. Cervical Cancer Immunotherapy: Facts and Hopes. Clinical Cancer Research 2021, 27, 4953–4973. [Google Scholar] [CrossRef] [PubMed]
- Small, W.; Bacon, M.A.; Bajaj,A.; Chuang, L.T.; Fisher, B.J.; Harkenrider, M.M.; Jhingran,A.; Kitchener, H.C.; Mileshkin, L.R.; Viswanathan, A.N.; Gaffney, D.K. Cervical Cancer: A Global Health Crisis. Cancer 2017, 123, 2404–2412. [CrossRef]
- Mao, Z.W.; Zheng, X.; Lin, Y.-P.; Hu, C.-Y.; Wang, X.-L.; Wan, C.-P.; Rao, G.-X. Design, Synthesis and Anticancer Activity of Novel Hybrid Compounds between Benzofuran and N-Aryl Piperazine. Bioorg Med Chem Lett 2016, 26, 3421–3424. [Google Scholar] [CrossRef] [PubMed]
- Snyder, R. Leukemia and Benzene. Int J Environ Res Public Health 2012, 9, 2875–2893. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; No, E.S.; Thorat, D.A.; Jang, J.W.; Yang,H.; Lee,J.; Choo,H.; Kim,S.J.; Lee,C.S.; Ko,S.Y.; Lee,J.; Nam,G.; Pae, A.N. Synthesis and Biological Evaluation of Aryloxazole Derivatives as Antimitotic and Vascular-Disrupting Agents for Cancer Therapy. J Med Chem 2013, 56, 9008–9018. [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla,P.; Barsouk,A. Epidemiology of Melanoma. Med Sci (Basel) 2021, 9, 63.
- Hayward, N.K. Genetics of Melanoma Predisposition. Oncogene 2003, 22, 3053–3062. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, R.; Oliva, P.; Prencipe, F.; Manfredini, S.; Germano, M.P.; De Luca, L.; Ricci,F.; Corallo, D.; Aveic,S.; Mariotto, E.; Viola, G.;Bortolozzi, R. Cinnamic Acid Derivatives Linked to Arylpiperazines as Novel Potent Inhibitors of Tyrosinase Activity and Melanin Synthesis. Eur J Med Chem 2022, 231. [CrossRef]
- Lee, Y.B.; Gong, Y.D.; Yoon, H.; Ahn,C.H.; Jeon, M.K.; Kong,J.Y. Synthesis and Anticancer Activity of New 1-[(5 or 6-Substituted 2-Alkoxyquinoxalin-3-Yl)Aminocarbonyl]-4-(Hetero)Arylpiperazine Derivatives. Bioorg Med Chem 2010, 18, 7966–7974. [CrossRef]
- Bai, J.W.; Qiu, S.Q. & Zhang, G.J. Molecular and functional imaging in cancer-targeted therapy: current applications and future directions. Sig Transduct Target Ther 2023, 8, 89. [CrossRef]
Figure 1.
General structure of N-arylpiperazine and pharmacophoric model of 5-HT1A agonist.
Figure 1.
General structure of N-arylpiperazine and pharmacophoric model of 5-HT1A agonist.
Figure 2.
Signaling pathways of 5-HT1A receptor.
Figure 2.
Signaling pathways of 5-HT1A receptor.
Figure 3.
Structure of NAN-190 (1).
Figure 3.
Structure of NAN-190 (1).
Figure 4.
Structure of SDZ 216-525 (2).
Figure 4.
Structure of SDZ 216-525 (2).
Figure 5.
Structure of BP554 (3).
Figure 5.
Structure of BP554 (3).
Figure 6.
Structure of Naftopidil (4).
Figure 6.
Structure of Naftopidil (4).
Figure 7.
Structures of Terazosin (5) and Prazosin (6).
Figure 7.
Structures of Terazosin (5) and Prazosin (6).
Figure 8.
Structure of Doxazosin (7).
Figure 8.
Structure of Doxazosin (7).
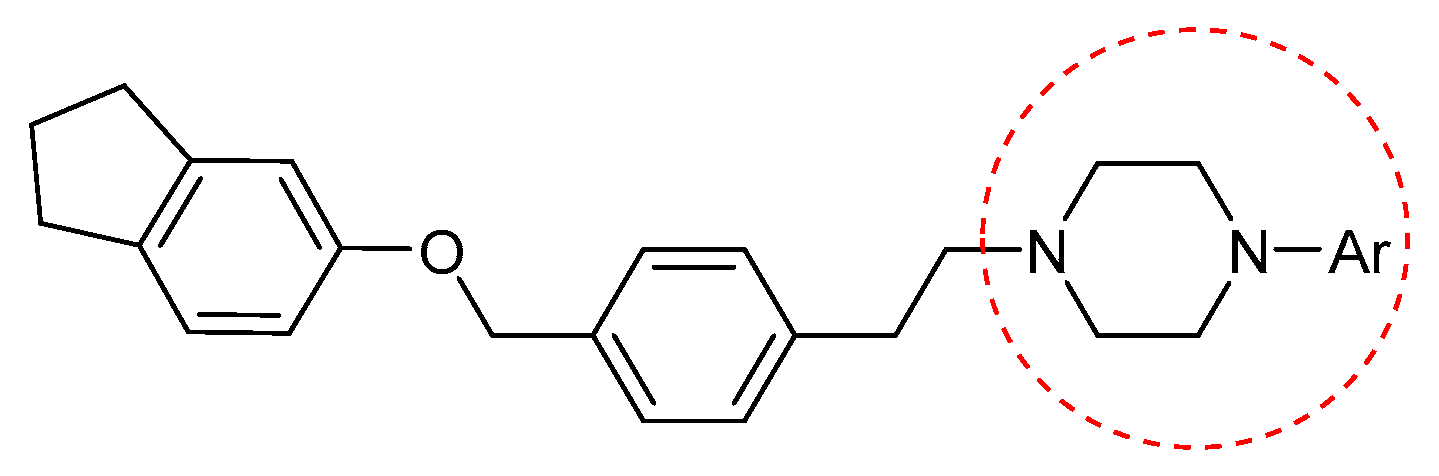
Figure 9.
General structure of arylpiperazine derivatives.
Figure 9.
General structure of arylpiperazine derivatives.
Figure 10.
Structure of androgen antagonist YM-92088 (8).
Figure 10.
Structure of androgen antagonist YM-92088 (8).
Figure 11.
Compound 17 (10) inhibited cell viability in prostate cell lines PC-3 and DU145. The all cells were exposed to escalating concentrations of arylpiperazine derivatives respectively for 24h, and the cell viability was detected by CCK-8 assay.
Figure 11.
Compound 17 (10) inhibited cell viability in prostate cell lines PC-3 and DU145. The all cells were exposed to escalating concentrations of arylpiperazine derivatives respectively for 24h, and the cell viability was detected by CCK-8 assay.
Figure 12.
Chemical structures of oxicams derivatives acting on colonrectal adenocarcinoma cell lines.
Figure 12.
Chemical structures of oxicams derivatives acting on colonrectal adenocarcinoma cell lines.
Figure 13.
Chemical structure of C2 (18).
Figure 13.
Chemical structure of C2 (18).
Figure 14.
Chemical structure of SER-79 (19) and SER-68 (20).
Figure 14.
Chemical structure of SER-79 (19) and SER-68 (20).
Figure 15.
Chemical structure of compound 25 (30).
Figure 15.
Chemical structure of compound 25 (30).
Figure 16.
Combination effect of compound 25 (30) with different cytotoxic drugs on the growth of MDA-MB-231 cancer cells.
Figure 16.
Combination effect of compound 25 (30) with different cytotoxic drugs on the growth of MDA-MB-231 cancer cells.
Figure 17.
Potent anticancer drugs bearing N-arylpiperazine moieties.
Figure 17.
Potent anticancer drugs bearing N-arylpiperazine moieties.
Table 1.
The expression of 5-HT1A receptor in cancer cells.
Table 1.
The expression of 5-HT1A receptor in cancer cells.
Type of
Cancer |
Cell Lines
Expressing
5-HT1AR |
Drugs |
Effects |
| Prostate Cancer |
PC3, DU145,
LNCap |
NAN-190
Pindobind
|
5HT1A antagonists that inhibit cell growth in vitro, inducing apoptosis. |
6-nitroquipazine
Zimelidine
Fluoxetine |
5HT uptake inhibitors that cause dose- dependent inhibition of cells proliferation. |
| Bladder Carcinoma |
SHT1376 |
NAN 190
SB224289 |
5HT1A (NAN-190) and 5HT1B (SB224289) antagonists that show an inhibitory effect on the serotonin induced growth cells. |
| Small Cell Lung Carcinoma |
GLC8 |
Spiperone
SDZ 216-525 |
5-HT1A (spiperone) and 5-HT7 (SDZ 216-525) antagonists that inhibit 8-OH-DPAT-induced mitogenic effect. |
| Colonrectal Carcinoma |
HT29 |
BW501C
Citalopram
Fluoxetine |
Serotoninergic antagonists (BW501C) and SSRIs (Citalopram and Fluoxetine) that retard the tumor growth. |
NAN 190
SB224289 |
5HT1A (NAN-190) and 5HT1B (SB224289)
antagonists that reduce cell growth acting as antiproliferative agents. |
| Cholangiocarcinoma |
Mz-chA1, HuH28,
HUVV-T1, CCLP-1,
SG231, TFK1. |
- |
- |
Table 2.
Compound 13 and 17: data of selectivity ratio and IC50 values.
Table 2.
Compound 13 and 17: data of selectivity ratio and IC50 values.
| Compd. |
Structure |
Selectivity ratio |
IC50 values (DU145) |
| 13 (9) |
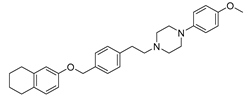 |
α1B/α1A
ratio = 16.7 |
0.93 ± 0.19 |
| 17 (10) |
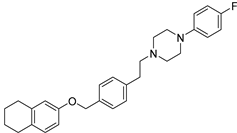 |
α1B/α1D
ratio = 10.9 |
0.90 ± 0.20 |
Table 3.
Compound 5 and 18g: AR antagonistic activities.
Table 3.
Compound 5 and 18g: AR antagonistic activities.
| Compd. |
Structure |
IC50 (μM)a
|
% inhibitionb
|
5
YM-92088
(11)
|
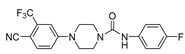 |
0.47 |
- |
18g
(12)
|
 |
0.20 |
85%**
ED50=1.1mg/kg |
Table 4.
IC50 values (μM) of novel thiazolinylphenyl-piperazines (2a–c; 3a-c) observed on breast cancer cell lines.
Table 4.
IC50 values (μM) of novel thiazolinylphenyl-piperazines (2a–c; 3a-c) observed on breast cancer cell lines.
| Compound |
Structure |
MCF-7 |
MDA-MB231 |
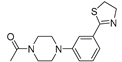
| 2a (21)
|
 |
14,7±1,9 |
31,37±5,1 |
| 2b (22)
|
 |
15,93±1,8 |
39,96±9,8 |
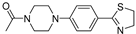
| 2c (23)
|
 |
19,47±2,3 |
36,32±7,7 |
| 3a (24)
|
 |
- |
23,27±3,4 |
| 3b (25)
|
 |
- |
34,6±5,4 |
| 3c (26)
|
 |
- |
47,15±6,7 |
Table 5.
In vitro cytotoxic activity of compound 13.
Table 5.
In vitro cytotoxic activity of compound 13.
| Compound 13 (27) |
 |
| Cell lines (IC50 μM) |
| A549 |
Hela |
MCF-7 |
SGC7901 |
| 5.73±1.22 |
0.03±0.04 |
12.38±3.62 |
6.17±1.62 |
Table 6.
Cytotoxic effect of 6-48 derivative (28) against Human Leukemia Cells (HL-60).
Table 6.
Cytotoxic effect of 6-48 derivative (28) against Human Leukemia Cells (HL-60).
| Compound |
Structure |
IC50 (nM) |
| 6-48 (28) |
 |
60.2 |
Table 7.
Effect of compound 19r (29) on the diphenolase activity of mushroom tyrosinase.
Table 7.
Effect of compound 19r (29) on the diphenolase activity of mushroom tyrosinase.
| Compound |
Structure |
IC50 (μM) |
| 19r (29) |
 |
0.51±0.10 |
Table 8.
Inhibition of cell growth (IC50, μM) by quinoxalinyl-piperazine compound 25 (30) against human cancer cell lines.
Table 8.
Inhibition of cell growth (IC50, μM) by quinoxalinyl-piperazine compound 25 (30) against human cancer cell lines.
| MDA-MB-231 |
0.012 |
| Caki-1 |
0.011 |
| UMRC2 |
0.013 |
| PANC-1 |
0.021 |
| A549 |
0.021 |
| MKN-45 |
0.020 |
| HepG2 |
0.019 |
| HCT116 |
0.020 |
| HT29 |
0.021 |
| PC-3 |
0.021 |
| U251 |
0.015 |
| HeLa |
0.021 |
| SK-MEL-28 |
0.020 |
| OVCAR-3 |
0.012 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).