1. Introduction
Atopic dermatitis (AD) is an inflammatory, pruritic, chronic, or chronically relapsing skin disease. The complex pathogenesis of AD is a result of genetic predisposition, skin barrier dysfunction, immunological factors, skin microbiome dysbiosis, environmental factors, and strong psychosomatic influence [
1]. The increasing incidence of AD in recent decades may be explained by changes in lifestyle and diet, in particular, a shift in the balance between dietary polyunsaturated fatty acids (PUFAs) with increased dietary intake of omega-6 fatty acids (omega-6 FA) intake, mainly linoleic acid (LA) from vegetable oils and a decreased intake of omega-3 fatty acids (omega-3 FA), mainly alpha-linolenic acid (ALA) from fish and algae [
2]. The ideal omega-6 FA to omega-3 FA ratio appears to be from 1:1 to 6:1, however the Western diet produces ratios of more than 25:1. This change in balance could influence inflammatory responses [
2,
3,
4,
5,
6,
7]. Omega-3 FA and omega-6 FA compete for the same enzymes during conversion into active metabolites. Omega-6 FA is a precursor of arachidonic acid (AA), a lipid with potential of inflammatory pathway activation following oxygenation during injury or irritation through release of pro-inflammatory eicosanoids which can exacerbate the inflammatory processes underlying AD [
2,
3,
8]. Omega-3 FA, ALA is converted to eicosapentaenoic acid (EPA) and then docosahexaenoic acid (DHA). These two acids have greater biological potential compared to ALA and are competitors of AA for cyclooxygenase-1 and lipoxygenase. Eicosanoids derived from EPA and DHA have high anti-inflammatory potential [
2,
8].
High intake of omega-6 FA is known to enhance pro-inflammatory response by inducing the production of pro-inflammatory eicosanoids. Omega-6 FA, LA, is converted to AA, the precursor of inflammatory prostaglandins and leukotrienes, while ALA, an omega-3 FA, is converted to EPA and subsequently to DHA. These FAs are known to be the most biologically potent precursors of anti-inflammatory mediators. However, LA, before it converts to AA, goes through a stage of gamma-linolenic acid (GLA) and dihomo-gamma-linolenic acid (DGLA), both involved in the induction of anti-inflammatory eicosanoids [
9].
In patients with AD a reduced enzyme activity of delta-6-desaturase was observed leading to higher levels of LA and lower levels of GLA resulting in lower concentrations of anti-inflammatory GLA and its metabolites [
10,
11]. This concept was supported by Swedish study that showed elevated linoleic acid concentrations, and on the other hand, significantly reduced concentrations of DGLA, AA and DHA [
12]. Moreover, LA and GLA have an essential role in maintaining the integrity of the skin barrier. GLA has been shown to regenerate the human skin barrier, increase ceramide synthesis, and prevent excessive water loss [
13,
14]. This explains the importance of the balance in consuming omega-3 FA and omega-6 FA and their role in the pathogenesis of AD through effects on inflammation, immune modulation, and skin barrier function. This nutritional disbalance could contribute to the higher production of pro-inflammatory cytokines and elevate the risk of development of atopic diseases [
15].
Most of the published studies on the role of omega-3 FA in AD demonstrated their pivotal preventive role in the context of atopic diseases and allergies, however, their role in the treatment of atopic diseases, e.g. AD, is still unclear [
16,
17,
18].
The treatment of AD includes proper education, avoiding triggers (clinically relevant allergens and irritants), regular use of emollients, and topical anti-inflammatory medication (reactive or proactive). After topical treatments are unsuccessful in patients with moderate-to-severe disease, the next approaches typically include either phototherapy or systemic therapies such as conventional immunosuppressants, biologics, or small molecules like JAK inhibitors. The use of PUFA in the treatment of AD is an area of ongoing research. While some studies suggest potential benefits, the evidence is not yet strong enough to establish standardized guidelines for PUFA supplementation in the treatment of AD [
19]. Studies suggest that dietary supplementation with omega-3 FA can help manage symptoms of AD by reducing lesion severity, skin inflammation, dryness and itching [
2]. The typical Western diet is characterized by a high intake of processed food and therefore high intake of omega-6 FA, primarily from vegetable oils like soybean and corn oil, and a relatively low intake of omega-3 FA, primarily from fish. Increasing omega-3 FA intake while reducing omega-6 FA intake could help to modulate the inflammatory processes associated with AD.
Therefore, the aim of this study was to evaluate the effect of supplementation with omega-3 FA form fish oil in combination with GLA form blackcurrant seed oil in children with AD.
2. Materials and Methods
This is longitudinal, prospective, randomized, triple blind, placebo-controlled parallel clinical trial approved by the Ethics Committee of Children’s Hospital Zagreb. Written informed consent was obtained from the parent or guardian of the child participating in the study.
The study was conducted during the 2-year period from autumn, winter, and spring 2021 to 2023 to avoid summer when AD usually improves.
2.1. Participants
The participants were pediatric patients diagnosed with AD and referred to a pediatric dermatology outpatient clinic. Children from 1 up to 8 years of age with AD were included. The diagnosis was based on the Rajka-Hanfin criteria [
20]. Children with moderate to severe AD according to SOCORAD (SCORing Atopic Dermatitis) index were eligible for the study [
21]. SCORAD is calculated using a 3-criteria questionnaire based on the involved area, intensity of eczema, and subjective symptoms (itching and sleeplessness). The total score ranges from zero to 103, where SCORAD 0 to 24 describes mild, 25 do 49 moderate and >50 severe form of AD. All children were enrolled by pediatric dermatologist.
The exclusion criteria were children younger than 1 year and older than 8 years of age, children with SCORAD ≤ 25, and children receiving any other supplements except vitamin D, those on or in need of phototherapy and systemic therapy and those allergic to fish.
Randomization of the study participants was done by using the Random Allocation Software to generate randomized blocks for assigning patients to 2 groups of equal size where every patient had a number assigned and received the labeled investigational product or placebo successively.
The investigational product was a citrus flavored syrup. Both preparations, active and placebo, were supplied by 4UPharma, Switzerland. 4UPharma had no involvement in the design, implementation, analysis and interpretation of the data. Products were packed in identical dark brown bottles different only in label (A or B). All participants, clinicians, data collectors, outcome adjudicators and data analysts did not have access to details of group assignment.
2.2. Study Product and Administration
The active study product (Mega Kid®) contained fish oil, EPA, DHA, GLA, vitamin D3 and, as inactive ingredients, medium chain triglycerides (MCT oil), lemon-lime flavoring and anhydrous citric acid. The placebo study product consisted of an identical formulation of inactive ingredients (MCT oil, lemon-lime flavor and anhydrous citric acid).
Both products, active and placebo, were of the same taste, color and smell. Products were stored on temperature below 25°C and the shelf life was 12 months. Once opened patients were instructed to store the product in the refrigerator while using it. Both the research staff and the patients were unaware of the nature of the product. Intervention period lasted 4 months. During the entire intervention period the subjects were not allowed to consume any other supplements except vitamin D. All participants were followed by a telephone call or an email every 4 weeks.
Children were assigned to one of the treatment groups (experimental or control) following a randomization procedure performed with computer-generated numbers. The daily dose of the investigational product was 5 ml with the active product containing 2 g of fish oil, 600 mg EPA, 400 mg DHA, 10 mg GLA, and 5 mcg of vitamin D3. Both groups received conventional AD treatment such as topical emollients and low potency topical corticosteroids (TCS, usually alclometasone dipropionate ointment or 40% and 60% alclometasone dipropionate in emollient).
2.3. Outcomes
Primary outcomes were change in severity of AD and the difference in TCS use. Secondary outcomes were change in itch intensity, sleep quality and FDLQI.
Demographic parameters, medical history, use of medication and supplements, AD severity assessed by SCORAD and PO-SCORAD (Patient Oriented – SCORAD) [
22], itch (assessed by numerical rating scale from 1 to 10, with 1 indicating low intensity and 10 highest intensity itch), sleep disturbance (using numerical rating scale 1 to 10, with 1 indicating best sleep quality and 10 the worst sleep quality), AD-related quality of life using the Croatian version of the Family Dermatology Life Quality Index (FDLQI) [
23] were recorded at baseline and at the end of treatment period (4 months).
The PO-SCORAD index is a self-assessment tool allowing parents to evaluate the severity of AD comprehensively, by using subjective and objective criteria derived from the SCORAD. The FDLQI is a dermatology-specific instrument that measures the disease effect on the health-related quality of life (QOL) of family members of patients with skin diseases. The 10 item questionnaire is designed to assess the different aspects of quality of life of parents, caregivers or partners of patients with various skin diseases, in particular emotional and physical well-being, relationships, social life, leisure activities, burden of care, effect on job or study, housework, and financial burden of the disease. Participants answer questions choosing form a 4-point scale: 0, not at all or not applicable; 1, a little; 2, a lot; 3, very much. The scores of individual items (0–3) are added to give a total score that ranges from 0 to 30; higher scores indicate poorer QOL. To obtain a valid Croatian version of the FDLQI, we followed the guidelines for the cross-cultural adaptation of health related QOL measures [
24].
During the intervention period data on tolerability and adverse events were collected.
2.4. Sample Size
Sample size was calculated based on previous data that showed that the itching score reported by patients at the end of 3 months in the intervention group was 2.6 ± 2.7, and in the placebo group the score was 5.2 ± 2.7 [
25]. The effect size based on these results was equal to 1.03. Based on these results calculated sample size was 42 participants (alpha 0.05, power 0.80). In order to account for possible drop-outs 4 additional patients were added.
2.5. Statistics
Descriptive statistics were used to describe the basic features including age, gender, duration of the intervention, and symptoms. Normality of the data distribution was analyzed with the Smirnov–Kolmogorov test. The Chi2 test was used to estimate differences in the distribution of qualitative variables. Differences in quantitative variables, according to their distribution, were analyzed with the parametric t test or the nonparametric Mann–Whitney test.
All statistical tests were two-tailed tests and performed at the 5% level of significance. Statistical software SPSS (version 23; SPSS, Chicago) was used for all statistical analyses. The difference between the study groups was considered significant when the p value was <0.05. All analyses were performed on the intention-to-treat basis, in which all of the participants in a trial are analyzed according to the intervention to which they were assigned.
3. Results
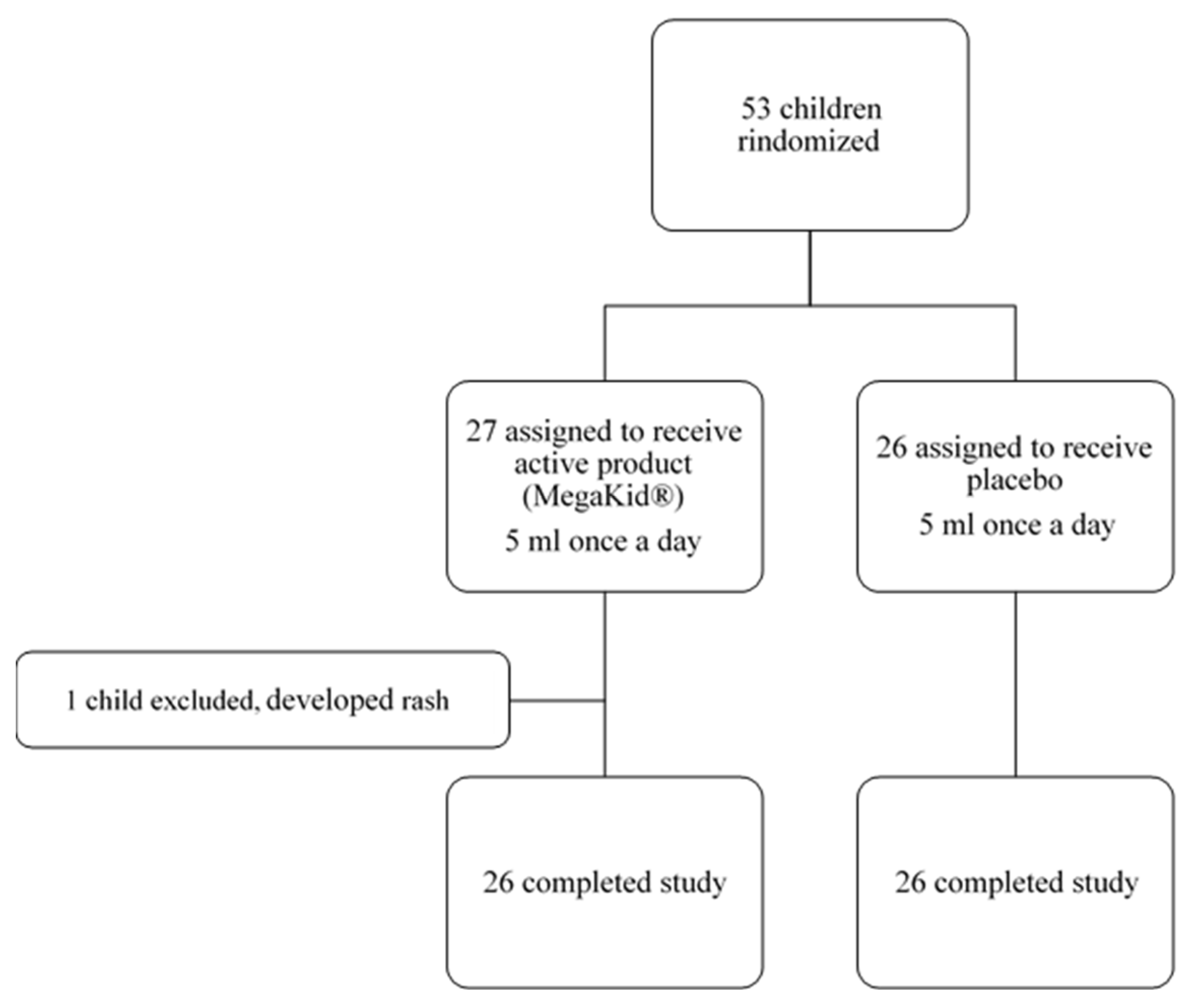
In this study, 53 patients were enrolled, with 52 patients completing the end-of-study follow-up visit as per protocol (26 in the intervention group and 26 in the placebo group) (
Figure 1).
There were no significant differences in respect to gender, age, food allergy, vitamin D supplementation, and severity of AD between the intervention and placebo group (
Table 1).
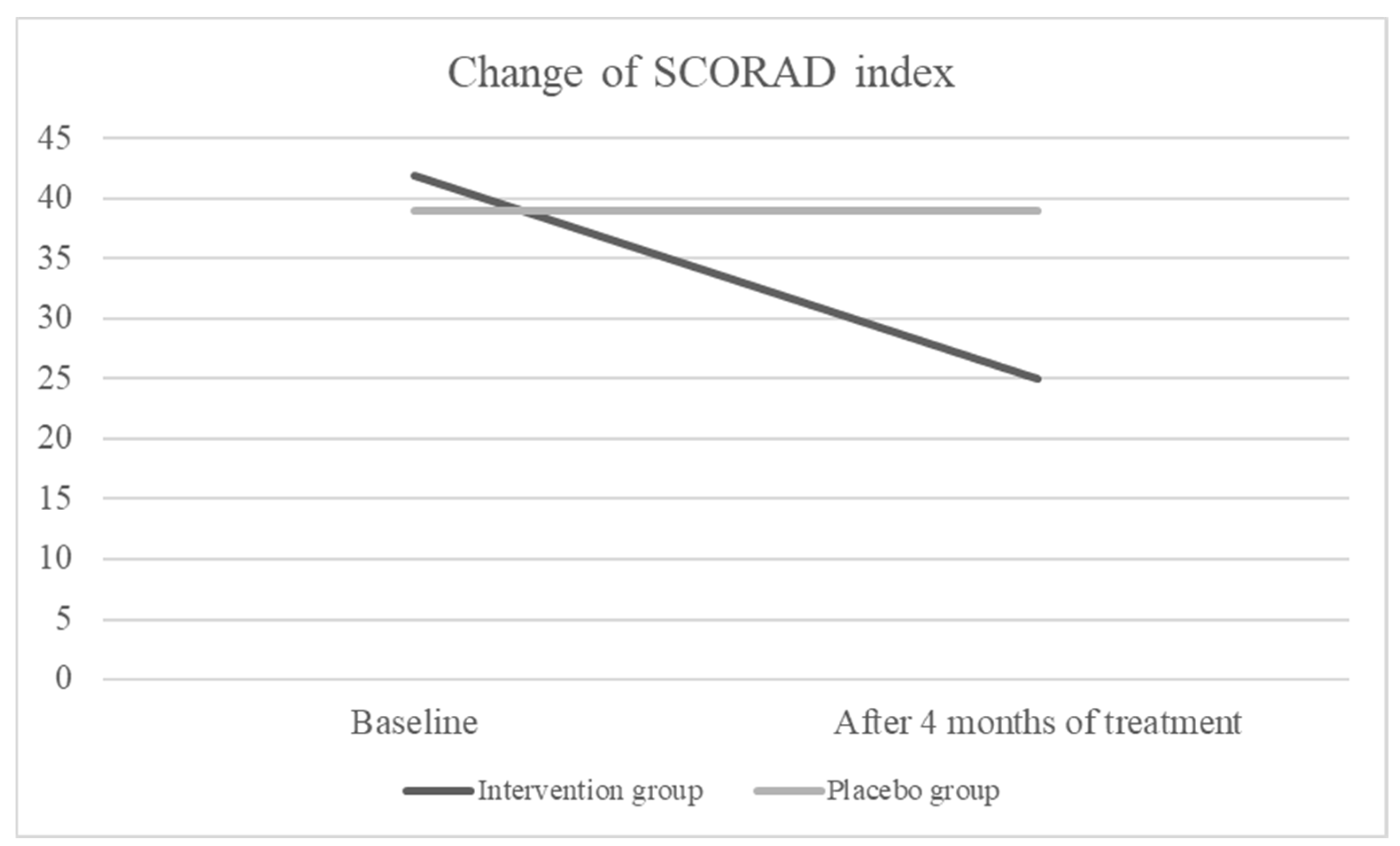
Intention-to-treat analysis showed that after 4 months of treatment, the SCORAD index was decreased significantly in the intervention group (form median 42 to 25, p < 0.001) but there was no change in the placebo group (form median 39 to 39, p= 0.795) (
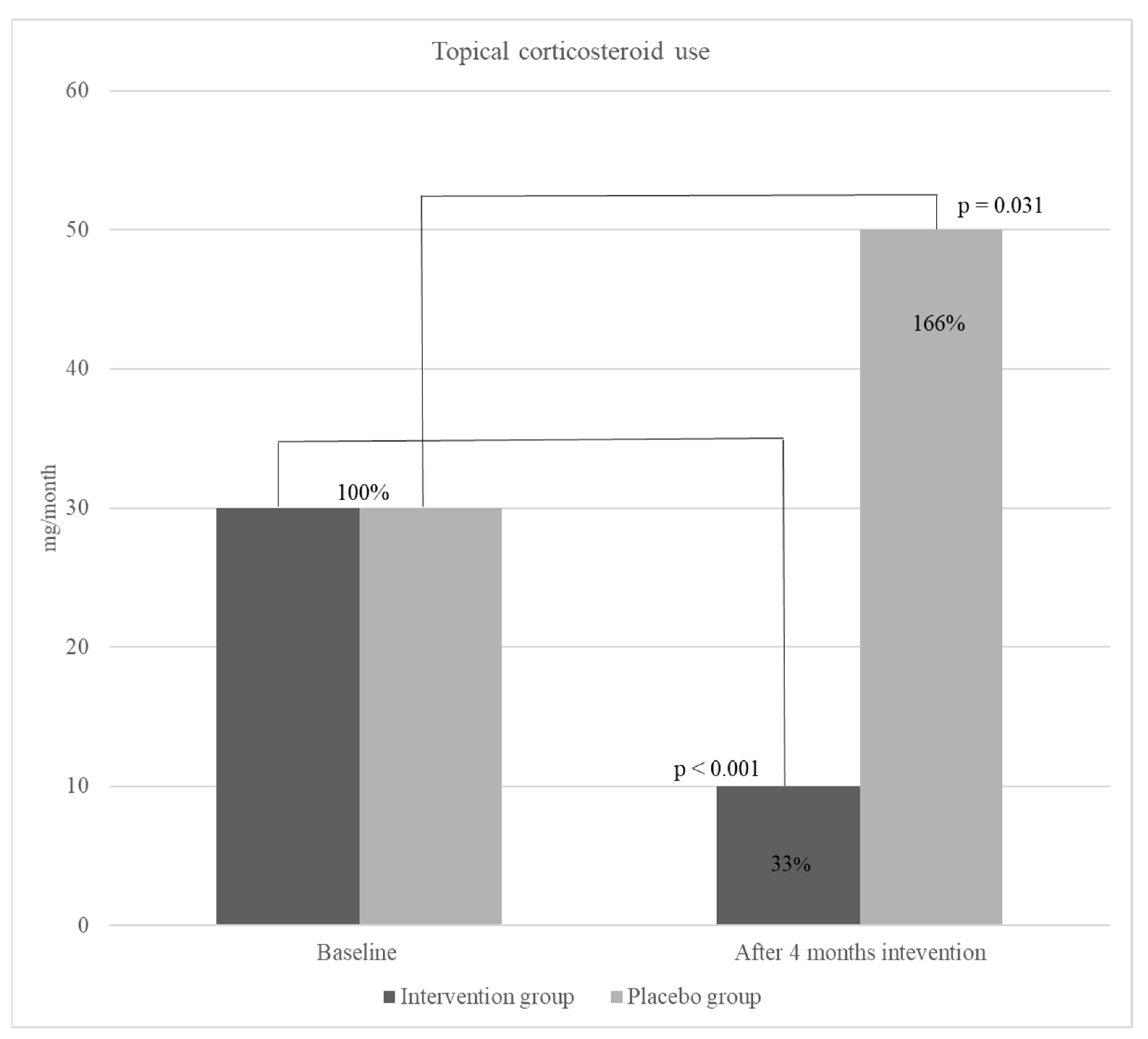
Figure 2.). Moreover, a significant decrease was detected in topical corticosteroid use in the intervention group, however, overall use of corticosteroids in the placebo group increased but not significantly (
Figure 3.)
Subjective assessment of AD course done by parents in context of AD severity, intensity of itch and sleep disturbance also showed significant change in the intervention group. PO-SCORAD decreased in the intervention group significantly in comparison to the placebo group where the median difference in baseline and after 4-month intervention PO-SCORAD index was 13 for the intervention group and 1 for placebo group (p<0.001). There was also changes in the intensity of itching, sleep disturbance and consequently on overall quality of life (FDLQI) in the intervention group (
Table 2).
Vitamin D serum values did not significantly change in both groups (intervention group: 66 to 76 nmol/L, p=0.567, placebo group: 78 to 66 nmol/L, p=0.211)
4. Discussion
We conducted a study on the efficacy of a specific blend of fish oil, DHA, EPA, GLA, and vitamin D on severity of AD. According to the results, the severity of AD in the group receiving the active investigational product was significantly lower compared to the placebo group at the end of the 4-month treatment period (p < 0.001). The SCORAD index significantly decreased in children receiving active product (
Figure 1).
Moreover, results showed a significant, 67 % (from a median of 30 to 10 mg per day) (p <0.001) decrease in the use of TCS in children receiving active product during the intervention study period. Although the studies on omega-3 effect on atopic diseases, especially AD are scarce, a recent study, conducted with a similar methodology to our study, has demonstrated the significant positive effect of EPA on the decrease of AD severity (SCORAD) and TCS use in children [
2]. Moreover, their results were in line with ours showing the same reduction in TCS use (67 %) already after 4 weeks of EPA supplementation. Also, the SCORAD index significantly decreased (49.65 ± 8.29 to 10.63 ± 9.58, p<0.001).
High dosages of fish oil or omega-3 FA (EPA and/or DHA) were used in subjects with psoriasis, however, the results of these studies vary. Some studies showed significant positive effect of treatment in term of improving itching and erythema [
25,
26,
27], while some did not confirm it [
28]. Regarding omega-3 effect on AD the data are scarce, few studies done in adults showed that dietary fish-oil supplementation may improve biomarkers of skin inflammation and oxidative-stress response [
29] and that 8 weeks DHA supplementation resulted in significant clinical improvement of atopic eczema in terms of a decrease in SCORAD (DHA: baseline 37.0 (17.9-48.0), week 8: 28.5 (17.6-51.0), control: baseline 35.4 (17.2-63.0), week 8: 33.4 (10.7-56.2)). Moreover, this study demonstrated that the DHA group showed an increase in plasma omega-3 FA and a decrease in the omega-6 to omega-3 FA ratio [
30]. However, although few, some studies did not show a significant therapeutic effect of omega-3 FA on AD which is why the question of the use of omega-3 FA as a complementary therapy is still open [
18].
Our study showed a significant positive change in clinical features of AD among patients receiving active product, consequently resulting in significant improvement in QOL. As AD symptoms (itching and sleep disturbance) improved, a subjective assessment tool family DLQI decreased responding to parents better QOL.
In children receiving placebo, there was an increase in TCS use, however, the result was not significant, and the SCORAD did not change. This was interesting, as one would expect the change in SCORAD due to TCS use. In comparison to study done with EPA [
2] where the corticosteroid use showed significant change in SCORAD, although smaller than the change in group receiving EPA.
The result of our study could be related to the longer intervention period (4 months) where the dynamics of the AD were changing, even worsening depending on the season. As we enrolled most of the children during September/October/November, their AD could have flared in December/January/February due to the winter period.
From interviews with parents of AD patients, we could conclude that most of the parents did not use adequate or recommended amounts of TCS as they were expecting the positive effect of the investigational product. There was no significant change in SCORAD and PO-SCORAD index, therefore there was no significant change in other assessed parameters (itch, sleep, FLDQI).
The taste of the product was acceptable. The citrus flavor very good camouflaged the fish oil taste, so most of the children accepted the product with no objections. There were no differences in adverse events between the placebo and intervention group.
We are however, aware of several possible limitations of the study mainly the small number of patients enrolled, although calculated sample size was reached and the lack of in-clinic (on site) follow-ups during the study period.
However, main strengths are that this is, by our best knowledge, the first study of its kind. Furthermore, patients were thoroughly followed and the adherence and compliance regularly checked by phone visits.
5. Conclusions
Omega-3 fatty acids in combination with GLA and vitamin D has a great beneficial effect in treatment of atopic dermatitis in children. Therefore, we can conclude that supplementation with this specific combination could be considered a safe and effective intervention that can significantly reduce the severity of AD in pediatric patients.
Author Contributions
Conceptualization, T.N. and N.P.; methodology, T.N. and N.P.;.formal analysis, I.H..; investigation, T.N., N.P., S.O.B.; data curation, T.N. and N.P.; writing—original draft preparation, T.N. and N.P.; writing-review and editing, I.H. and S.O.B.; funding acquisition: T.N. and N.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by 4U pharma gmbh Switzerland, Limited liability company, Register number: CH-300.4.017.952-7.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and commenced after obtaining approval from the Children’s Hospital Zagreb Ethics Committee (No.02-23/24-1-20, approved 1st October 2020).
Informed Consent Statement
Informed consent was obtained from all parents/caregivers of subjects involved in the study. Informed written consent/assent was obtained from the parents/caregivers of children after informing them about the benefits and risks of the study. Autonomy was maintained by study participants, as participation in the trial was voluntary. Confidentiality was also maintained, as the participants’ names and personal details were recorded under the code.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic Dermatitis. The Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Mirrahimi, B.; Moazemi, M.; Eslami, N.; Jamshidi, E.; Mir, M.; Mohebbi, R.; Esmaily, H. Evaluating the Effect of Eicosapentaenoic Acid in Children With Atopic Dermatitis: A Randomized Triple-Blind Clinical Trial. Journal of Pediatric Pharmacology and Therapeutics 2023. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids. Biomedicine and Pharmacotherapy 2002. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016. [CrossRef]
- Simopoulos, A.P. The Omega-6/Omega-3 Fatty Acid Ratio: Health Implications. Oléagineux, Corps gras, Lipides 2010. [Google Scholar] [CrossRef]
- Liput, K.P.; Lepczyński, A.; Ogłuszka, M.; Nawrocka, A.; Poławska, E.; Grzesiak, A.; Ślaska, B.; Pareek, C.S.; Czarnik, U.; Pierzchała, M. Effects of Dietary n–3 and n–6 Polyunsaturated Fatty Acids in Inflammation and Cancerogenesis. International Journal of Molecular Sciences 2021. [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J. The Importance of Maintaining a Low Omega-6/Omega-3 Ratio for Reducing the Risk of Autoimmune Diseases, Asthma, and Allergies. Missouri medicine 2021, 118, 453–459. [Google Scholar] [PubMed]
- Balić, A.; Vlašić, D.; Žužul, K.; Marinović, B.; Mokos, Z.B. Omega-3 versus Omega-6 Polyunsaturated Fatty Acids in the Prevention and Treatment of Inflammatory Skin Diseases. International Journal of Molecular Sciences 2020. [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021. [CrossRef]
- Yen, C.H.; Dai, Y.S.; Yang, Y.H.; Wang, L.C.; Lee, J.H.; Chiang, B.L. Linoleic Acid Metabolite Levels and Transepidermal Water Loss in Children with Atopic Dermatitis. Annals of Allergy, Asthma and Immunology 2008. [Google Scholar] [CrossRef]
- Ziboh, V.A.; Miller, C.C.; Cho, Y. Metabolism of Polyunsaturated Fatty Acids by Skin Epidermal Enzymes: Generation of Antiinflammatory and Antiproliferative Metabolites. In Proceedings of the American Journal of Clinical Nutrition; 2000. [Google Scholar]
- Strannegård, I.L.; Svennerholm, L.; Strannegård, Ö. Essential Fatty Acids in Serum Lecithin of Children with Atopic Dermatitis and in Umbilical Cord Serum of Infants with High or Low IgE Levels. International Archives of Allergy and Immunology 1987. [Google Scholar] [CrossRef]
- Jung, J.Y.; Kwon, H.H.; Hong, J.S.; Yoon, J.Y.; Park, M.S.; Jang, M.Y.; Suh, D.H. Effect of Dietary Supplementation with Omega-3 Fatty Acid and Gamma-Linolenic Acid on Acne Vulgaris: A Randomised, Double-Blind, Controlled Trial. Acta Dermato-Venereologica 2014. [Google Scholar] [CrossRef]
- Muggli, R. Systemic Evening Primrose Oil Improves the Biophysical Skin Parameters of Healthy Adults. International Journal of Cosmetic Science 2005. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, S.; Newman, S.A. Review of Evidence for Dietary Influences on Atopic Dermatitis. Skin therapy letter 2014, 19, 5–7. [Google Scholar]
- Huang, X.W.; Pang, S.W.; Yang, L.Z.; Han, T.; Chen, J.M.; Huang, C.W.; Liao, L.; Xie, P.J. TNFSF14 Mediates the Impact of Docosahexaenoic Acid on Atopic Dermatitis: A Mendelian Randomization Study. European Review for Medical and Pharmacological Sciences 2024. [Google Scholar] [CrossRef]
- Reese, I.; Werfel, T. Do Long-Chain Omega-3 Fatty Acids Protect from Atopic Dermatitis? JDDG - Journal of the German Society of Dermatology 2015. [Google Scholar] [CrossRef] [PubMed]
- Abdelnour, A.M.; Shareef, S.J.; Kreuser, K.; Ashack, K. 319 Effects of Omega-3 Fatty Acid Supplementation on Atopic Dermatitis. British Journal of Dermatology 2023. [Google Scholar] [CrossRef]
- Wollenberg, A.; Kinberger, M.; Arents, B.; Aszodi, N.; Barbarot, S.; Bieber, T.; Brough, H.A.; Pinton, P.C.; Christen-Zaech, S.; Deleuran, M.; et al. First Update of the Living European Guideline (EuroGuiDerm) on Atopic Eczema. Journal of the European Academy of Dermatology and Venereology 2023. [CrossRef]
- Hanifin, J.M.; Rajka, G. Diagnostic Features of Atopic Dermatitis. Acta Dermato-Venereologica 1980. [Google Scholar] [CrossRef]
- Kunz, B.; Oranje, A.P.; Labréze, L.; Stabler, J.F.; Ring, J.; Taïeb, A. Clinical Validation and Guidelines for the Scorad Index: Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 1997. [Google Scholar] [CrossRef]
- Stalder, J.F.; Barbarot, S.; Wollenberg, A.; Holm, E.A.; De Raeve, L.; Seidenari, S.; Oranje, A.; Deleuran, M.; Cambazard, F.; Svensson, A.; et al. Patient-Oriented SCORAD (PO-SCORAD): A New Self-Assessment Scale in Atopic Dermatitis Validated in Europe. Allergy: European Journal of Allergy and Clinical Immunology 2011. [Google Scholar] [CrossRef] [PubMed]
- Basra, M.K.A.; Edmunds, O.; Salek, M.S.; Finlay, A.Y. Measurement of Family Impact of Skin Disease: Further Validation of the Family Dermatology Life Quality Index (FDLQI). Journal of the European Academy of Dermatology and Venereology 2008. [Google Scholar] [CrossRef]
- Guillemin, F.; Bombardier, C.; Beaton, D. Cross-Cultural Adaptation of Health-Related Quality of Life Measures: Literature Review and Proposed Guidelines. Journal of Clinical Epidemiology 1993. [Google Scholar] [CrossRef] [PubMed]
- Bjørneboe, A.; Smith, A.K.; Bjørneboe, G.A.; Thune, P.O.; Drevon, C.A. Effect of Dietary Supplementation with N-3 Fatty Acids on Clinical Manifestations of Psoriasis. British Journal of Dermatology 1988. [Google Scholar] [CrossRef]
- Bittiner, S.B.; Cartwright, I.; Tucker, W.F.G.; Bleehen, S.S. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. The Lancet 1988. [Google Scholar] [CrossRef] [PubMed]
- Soyland, E.; Funk, J.; Rajka, G.; Sandberg, M.; Thune, P.; Rustad, L.; Helland, S.; Middelfart, K.; Odu, S.; Falk, E.S.; et al. Effect of Dietary Supplementation with Very-Long-Chain n-3 Fatty Acids in Patients with Psoriasis. New England Journal of Medicine 1993. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarski, M.; Cudowska, B.; Sawicka-Zukowska, M.; Bobrus-Chociej, A. Supplementation with Long Chain Polyunsaturated Fatty Acids in Treatment of Atopic Dermatitis in Children. Postepy Dermatologii i Alergologii 2013. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Niu, Y.; Jiang, Y.; Chen, B.; Peng, L.; Mi, T.; Huang, N.; Li, W.; Xu, D.; Chen, R.; et al. Protective Effects of Dietary Fish-Oil Supplementation on Skin Inflammatory and Oxidative Stress Biomarkers Induced by Fine Particulate Air Pollution: A Pilot Randomized, Double-Blind, Placebo-Controlled Trial. British Journal of Dermatology 2021. [Google Scholar] [CrossRef]
- Koch, C.; Dölle, S.; Metzger, M.; Rasche, C.; Jungclas, H.; Rühl, R.; Renz, H.; Worm, M. Docosahexaenoic Acid (DHA) Supplementation in Atopic Eczema: A Randomized, Double-Blind, Controlled Trial. British Journal of Dermatology 2008. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).