Submitted:
20 July 2024
Posted:
22 July 2024
You are already at the latest version
Abstract
Keywords:
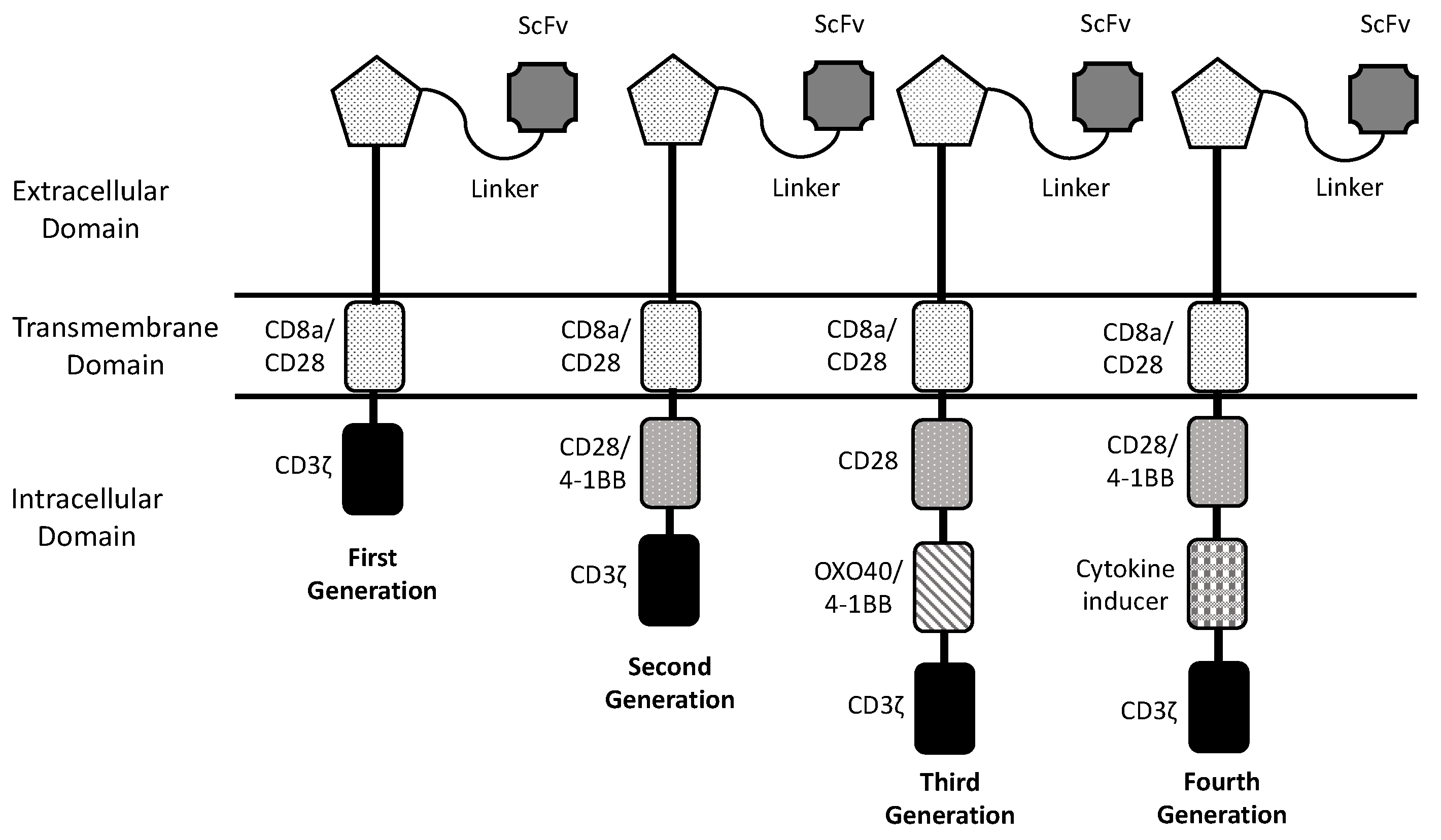
1. Introduction
2. Experimental and Clinical Studies of CAR-T Cell Therapy in Brain Tumors
2.1. HER-2
2.2. EGFRvIII
2.3. Interleukin-13 Receptor Alpha2 (IL-13Rα2)
2.4. Disialogangloside (GD2)
2.5. B7-H3
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sadelain, M.; Brentjens, R.; Rivière, I. The basic principles of chimeric antigen receptor design. Cancer Discov 2013, 3, 388-398. [CrossRef]
- Roselli, E.; Faramand, R.; Davila, M.L. insight into next-generation CAR therapeutics: designing CAR T cells to improve clinical outcomes. J Clin Invest 2021, 131, e142030. [CrossRef]
- Ceja, M.A.; Khericha, M.; Harris, C.M.; Puig-Saus, C.; Chen Y.Y. CAR-T cell manufacturing: major process parameters and next-generation strategies. J Exp Med 2024, 221, e20230903. [CrossRef]
- Labanieh, L.; Mackall, C.I. CAR immune cells: design principles, resistance and the next generation. Nature 2023, 614, 635-648. [CrossRef]
- Savoldo, B.; Grover, N.; Dotti G. CAR T cells for hematological malignancies. J Clin Invest 2024, 134, e177160. [CrossRef]
- Khan, A.N.; Asija, S.; Pendhari, J.; Purwar R. CAR-T cell therapy in hematological malignancies: where are we now and are we heading for? Esur J Haematol 2024, 112, 112, 6-18.
- Albelda, S.M. CAR T cell therapy for patients with solid tumors: key lessons to learn and unlearn. Nat Rev Clin Oncol 2024, 21, 47-66.
- Amoròs-Perez, B.; Rivas-Pardo, B.; del Moral M.G.; Subiza, J.L.; Martinez-Naves, E. State of the art in CAR-T cell therapy for solid tumors: is there a sweeter future? Cells 2024, 13, 725. [CrossRef]
- Del Baldo, G.; Del Bufalo, F.; Pinacchio , C.; Carai, A.; Quintarelli, C.; De Angelis, B.; Merli, P.; Cacchione, A.; Locatelli, F.; Mastronuzzi A. The peculiar challenge of bringing CAR-T cells into the brain: perspective in the clinical application to the treatment of pediatric central nervous system tumors. Front Immunol 2023, 14, 1142597. [CrossRef]
- Brown, C.E.; Aguilar, B.; Starr, R.; Yang , X.; Chang, W.C.; Weng, L. Optimization of IL13Rα2-targeted chimeric antigen receptor T cells for improved anti-tumor efficacy against glioblastoma. Mol Ther 2018, 26, 31-44.
- Liu, G.; Ying, H.; Zeng , G.; Wheelr, C.J.; Black, K.L.; Yu, J.S. HER-2, gp100, and MAGE-1 are expressed in human glioblastoma and recognized by cytotoxic T cells. Cancer Res 2004, 64, 4980-4986. [CrossRef]
- Ramezani, M.; Siami, S.; Rezaei, M.; Khazaei, S.; Sadeghi, M. An immunohistochemical study of HER2 expression in primary brain tumors. Biomedicine 2020, 10, 21-27. [CrossRef]
- Mineo, J.F.; Bordron, A.; Baroncini, M.; Maurage, C.A.; Ramirez, C.; Siminsky, R.M.; Brethou, C.; Hieu, P.D. Low HER2-expressing glioblastomas are often secondary to anaplastic transformation of low-grade glioma. J Neuroncol 2007, 85, 281-287. [CrossRef]
- Ahmed, N.; Salsman, S.V.; Kew, Y.; Shaffer, D.; Powell, S.; Zhang, Y.; Grossman, R.G.; Heslop, H.E.; Gottschalk, S. HER2-sepcific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin Cancer Res 2010, 16: 474-485.
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Therapy 2010, 18: 843-851. [CrossRef]
- Ahmed, N.; Brawley, V.; Hedge, M.; Bielamowicz, K.; Kalra, M.; Landi, D.; Robertson, C.; Gray, T.L.; Diouf, O.; Wakefield, A.; et al. HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol 2017, 3, 1094-1101.
- Vitanza, N.A.; Johnson, A.J.; Wilson, A.L.; Brown, C.; Yokoyama, J.K.; Kunkele, A.; Chang, C.A.; Rawlings-Rhea, S.; Huang, W.; Seidel, K.; et al. Locoregional infusion of HER2-specific CAR T cells in children and young adults with recurrent or refractory CNS tumors: an interim analysis. Nat Med 2021, 27, 1544-1552. [CrossRef]
- Priceman, S.J.; Tilakawardane, D.; Jeang, B.; Aguilar, B.; Murad, J.P.; Park, A.K.; Chang, W.C.; Ostberg, J.R.; Neman, J.; Jandial, R.; et al. Regional delivery of chimeric antigen receptor-engineered T cells effectively targhets HER2+ breast cancer metastasis to the brain. Clin Cancer Res 2017, 24, 95-105.
- Li, X.; Zhao, L.; Li, W.; Gao, P.; Zhang, N. HER2-targeting CAR-T cells show highly efficient anti-tumor activity against glioblastoma both in vitro and in vivo. Genes & Immunity 2024, in press. [CrossRef]
- Shabaneh, T.B.; Stevens, A.R.; Stull, S.M.; Shimp, K.R.; Seaton, B.W.; Gad, E.A.; Jaeger-Ruckstuhl, C.A.; Simon, S.; Koehne, A.L.; Price, J.P.; et al. Systemically administered low-affinity HER2 CAR T cells mediate entitumor efficacy without toxicity. J Immunother Cancer 2024, 12, e008566.
- Yeo, A.T.; Shah, R.; Aliazis K.; Pal, R.; Xu, T.; Zhang, P.; Rawal, S.; Rose, C.M.; Varn, F.S.; Appleman, V.A.; et al. Driver mutations dictate the immunologic landscape and response to checkpoint immunotherapy of glioblastoma. Cancer Immunol Res 2023, 11, 629-645. [CrossRef]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.; Martinez-Lage M.; Brem, S.; Maloney, E.; Shen, A.; et al. A single dose of peripherally infused EGFRvIII-directe CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Trans Med 2017, 9, eaaa0984.
- Goff, S.L.; Morgan, R.A.; Yang J.C.; Sherry R.M.; Robbins, P.F.; Restifo, N.P.; Feldman, S.A.; Lu, Y.C.; Zheng, Z.; Xi, L.; et al. Pilot trial of adoptive transfer of chimeric antigen receptor-transduced T cells targeting EGFRvIII in patients with glioblastoma. J Immunother 2019, 42, 126-135. [CrossRef]
- Choi, B.D.; Yu, X.; Castano, A.P.; Bouffard, A.A.; Schmidts, A.; Larson R.C.; Bailey, S.R.; Boroughs, A.C.; Frigault, M.J.; Leick, M.B.; et al. CAR-T cells secreting BiTEWs circumvent antigen escape without detectable toxicity. Nat Biotechnol 2019, 37, 1049-1058.
- Choi, B.D.; Gerstner, E.R.; Frigault, M.J.; Leick, M.B.; Mount, C.W.; Balaj, L.; Nikiforow, S.; Carter, B.S.; Curry, W.T.; Gallagher, K.; et al. Intraventricular CARv3-TEAM-E T cells in recurrent glioblastoma. N Engl J Med 2024, 390, 1290-1298. [CrossRef]
- Tang, O.Y.; Tian, L.; Yoder, T.; Xu, R.; Kulikovskaya, I.; Gupta, M.; Malenhorst, J.J.; Lacey, S.F.; O’Rourke, D.M.; Binder, Z.A. PD1 expression in EGFRvIII-directed CAR T cell infusion product for glioblastoma is associated with clinical response. Front Immunol 2022, 13, 872756.
- Bagley, S.J.; Binder, Z.A.; Lamrani, L.; Marinari, E.; Desai, A.S.; Nasrallah, M.L.; Maloney, E.; Brem, S.; Lustig, R.A.; Kurtz, G.; et al. Repeated peripheral infusions of anti-EGFRvIII CAR T cells in combination with pembrolizumab show no efficacy in glioblastoma: a phase 1 trial. Nat Cancer 2024, 5, 517-531.
- Bagley, S.J.; Logun, M.; Fraietta, J.A.; Wang, X.; Desai, A.S.; Bagley, L.J.; Nabavizadeh, A.; Jarocha, D.; Martins, R.; Maloney, F.; et al. Intratechal bivalent CART cell targeting EGFR and IL13Rα2 in recurrent glioblastoma: phase 1 trial interim results. Nat Med 2024, 30, 1320-1329.
- Abbott, R.C.; Iliopoulos, M.; Watson, K.A.; Arcucci, V.; Go, M.; Hughes-Parry, H.E.; Smith, P.; Call, M.J.; Cross, R.S.; Jenkins, M.R. Human EGFRvIII chimeric antigen receptor T cells demonstrate favorable safety profile and curative resonses in orthoptic glioblastoma. Clin Transl Immunol 2023, e1440.
- Joshi, B.H.; Plautz, G.E.; Puri, R.K. Interleukin-13 receptor α chain: a novel tumor-associated transmembrane protein in primary explants of human malignant gliomas. Cancer Res 2020, 60: 1168-1172.
- Knudson, K.M.; Hwang, S.; McCann, M.S.; Joshi, B.H.; Husain, S.R. Recent advances in IL-13Rα2-directed cancer immunotherapy. Front Immunol 2022, 13, 878365.
- Brown, C.E.; Badie, B.; Barish, M.E.; Weng, L.; Ostberg, J.R.; Chang, W.C.; Naranjo, A.; Starr, R.; Wagner, J.; Wright, C.; et al. Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin Cancer Res 2015, 21, 4062-4070.
- Brown, C.E.; Alizadeh, D.; Starr, R.; Weng, L.; Wagner, J.R.; Naranjo A.; Ostberg, J.R.; Blanchard, M.S.; Kilpatrick, J.; Simpson, J. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med 2016, 375, 2561-2569. [CrossRef]
- Alizadeh, D.; Wong, R.A.; Gholamin, S.; Maka, M.; Aftabizadeh, M.; Xang, W.; Pecoraro, J.R.; Jeppson, J.D.; Wang, D.; Aguilar, B.; et al. IFNγ is critical for CAR T cell-mediated myeloid activation and induction of endogenous immunity. Cancer Discov 2021; 11, 2248-2265.
- Brown, C.E.; Hibbard, J.C.; Alidazeh, D.; Blanchard, M.S.; Natri, H.M.; Wang, D.; Ostberg, J.R.; Aguilar, B.; Wagner, J.R.; Paul, J.A.; et al. Locoregional delivery of IL-13Rα2-targeting CAR-T cells in recurrent high-grade glioma: a phase 1 trial. Nat Med 2024, 30, 1001-1012.
- Brown, C.E.; Rodriguez, A.; Palmer, J.; Ostberg, J.R.; Naranjo, A.; Wagner, J.R.; Aguilar, B.; Starr, R.; Weng, L.; Synold, T.W.; et al. Off-the-shelf, steroid-resistant, IL13Rα2-specific CAR T cells for treatment of glioblastoma. Neuro-Oncol 2022, 24, 1318-1330.
- Stern, L.A.; Gholamin, S.; Moraga, I.; Yang, X.; Saravanakumar, S.; Cohen, J.R.; Cohen, J.R.; Starr, R.; Aguilar, B.; Salvary, V.; et al. Engineered IL13 variants direct specificity of IL13Rα2-targeted CAR T cell therapy. Proc Natl Acad Sci USA 2022, 119, e2112006119.
- Kim, K.; Gwak, H.S.; Han, N.; Hong, E.K.; Choi, B.K.; Lee, S.; Choi, S.; Park, Y.H.; Seok, J.H.; Jeon, Y.; et al. Chimeric antigen receptor T cells with modified interleukin-13 preferentially recognize IL13Rα2 and suppress malignant glioma: a preclinical study. Fron Immunol 2021, 12, 715000.
- Leland, P.; Degheidy, H.; Lea, A.; Bauer, S.R.; Puri, R.K.; Joshi, B.H. Identification and characterization of novel CAR-T cells to target IL13Rα2 positive human glioma in vitro and in vivo. Clin Transl Med 2024, 14, e1664.
- Weller, M.; Wen, P.Y.; Chang, S.M.; Dirven, L.; Lim, M.; Monje, M. Glioma Nat Rev Dis Prim 2024, 10, 33.
- Mount, C.W.; Majzner, R.G.; Sundaresh, S.; Arnold, E.P.; Kadapakkam, M.; Haile, S.; Labanieh, L.; Hulleman, E.; Woo, P.J.; Rierberg, S.P.; et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-H27M+ diffuse midline gliomas. Nat Med 2018, 24, 572-579. [CrossRef]
- Majzner, R.G.; Ramakrishna, S.; Yeom, K.W.; Patel, S.; Chinnasamy, H.; Schultz, L.M.; Richards, R.M.; Jiang, L.; Brasan, V.; Mancusi, R.; et al. GD2-CAR T cell therapy for K3K227M-mutated diffuse midline gliomas. Nature 2022, 603, 934-941. [CrossRef]
- Majzner, R.G.; Mahdi, J.; Ramakrishna, S.; Patel, S.; Chinna Samy, H.; Yeom, K.; Schultz, L.; Barsan, V.; Richards, R.; Conjar, C.; et al. Major tumor regressions in H3K27M-mutated diffuse midline glioma (DNG) following sequential intravenous (IV) and intracerebroventricular (ICV) delivery of GD2-CAR T cells. Cancer Res 2022, 81(suppl. 7), CT001.
- Monje, M.; Mahdi, J.; Majzner, R.; Yeom, K.; Schultz, L.M.; Richards, R.M.; Barsan, V.; Song, K.W.; Kamens, J.; Baggott, K.; et al. Sequential intravenous and intracerebroventricular GD2-CAR T-cell tharapy for H3K27M-mutated diffuse midline gliomas. medRxIV 2024, in press.
- Ramakrishna, S.; Good, Z.; Desai, M.; Zamler, D.; Mancusi, R.; Mahdi, J.; Majzner, R.; Schulz, L.; Richards, R.; Kamen, J.; et al. Immune signatures of GD2 CAR T cell activity in H3K27M+ diffuse midline glioma patients. Cancer Res 2023, 23 (suppl.7), 959. [CrossRef]
- Lin, F.Y.; Stuckert, A.; Tat, C.; White, M.; Ruggieri, L.; Zhang, H.; Mehta, B.; Lapteva, N.; Mei, Z.; Major, A.; et al. Phase I trial of GD2.CART cells augmented with constitutive interleukin-7 receptor for treatment of high-grade pediatric CNS tumors. J Clin Oncol 2024, in press. [CrossRef]
- Shum, T.; Omer, B.; Tashiro, H.; Kruse, R.L.; Wagner, D.L.; Parikh, K.; Yi, Z.; Sauer, T.; Liu, D.; Oarihar, R.; et al. Constitutive signaling from an engineered IL7 receptor promotes durable tumor elimination by tumor-redirected T cells. Cancer Discov 2017, 7, 1238-1247. [CrossRef]
- Liy, Z.; Zhou, J.; Yang, X.; Liu, Y.; Zou, C.; Lv, W.; Chen, C.; Cheng, K.K.; Chen, T.; Chang, L.J.; et aL. Safety and antitumor activity of GD2-specific 4SCAR-T cells in patients with glioblastoma. Mol Cancer 2023, 22, 3. [CrossRef]
- Chiavelli, C.; Prapa, M.; Rovesti, C.; Slingardi, M.; Neri, G.; Pugliese, G.; Trudu, L.; Dall’Ora, M.; Golinelli, G.; Griseni, G.; et al. Autologous anti-GD2 CART cells efficiently target primay human glioblastoma. NPJ Precis Oncol 2024, 8, 26.
- Gargett, T.; Ebert, L.M.; Truong, N.; Kollis, P.M.; Sedivakova, K.; Yu, W.; Yeo, E.; Wittver, N.L.; Gliddon, B.L.; Tea, M.N.; et al. GD2-targheting CAR-T cells enhanced by transgenic IL-15 expression are an effective and clinically feasible therapy for glioblastoma. Immunother Cancer 2022, 10, e005187.
- Zhang, G.; Zhao, Y., Liu, Z.; Liu, W.; Wu, H.; Wang, X.; Chen, Z. GD2 CAR-T cells in combination with Nivolumab exhibit enhanced antitumor efficacy. Transl Oncol 2023, 32, 101663. [CrossRef]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 antibody with GM-CSF, interleukemi-2, and isotretinoin for neuroblastoma. N Eng J Med 2010, 363, 1324-1334. [CrossRef]
- Straathof, K.; Flutter, B.; Wallace, R.; Jain, N.; Loka, T.; Depani, S.; Wright, G.; Thomas, S.; Cheung, G.W.K.; Gileadi, T.; et al. Antitumor activity without on-target off-tumor toxicity of GD2-chimeric antigen receptor T cells in pateints with neuroblastoma, Sci Transl Med 2020, 12, eabd6169.
- Del Bufalo, F.; De Angelis, B.; Cornana, I.; Del Baldo, G.; De Ioris, M.A.; Serra, A.; Mastronuzzi, A.; Cefalo, M.G.; Pagliara, D.; Amicucci, M.; et al. GD2-CART001 for relapsed or refractory high-risk neuroblastoma. N Engl J Med 2023, 388, 1284-1295. [CrossRef]
- Yu, L.; Huang, L.; Lin, D.; Lai, X.; Wu, L.; Liao, X.; Liu, J.; Zeng, Y.; Liang, L.; Zhang, G.; et al. GD2-specific chimeric antigen receptor-modified T cells for the treatment of refractory and/or recurrent neuroblastoma in pediatric patients. J Cancer Res Clin Oncol 2022, 148, 2643-2652. [CrossRef]
- Heczey, A.; Xu, X.; Courtney, A.N.; Tian, G.; Barragan, G.A.; Guo, L.; Amador, C.M.; Ghatwai, N.; Rathi, P.; Wood, M.S.; et al. Anti-GD2 CAR-NKT celsl in erlapsed or refractory neuroblastoma: updated phase 1 trial interin results. Nat Med 2023, 29, 1379-1388.
- Heczey, A.; Courtney, A.N.; Montalbano, A.; Robinson, S.; Liu, K.; Li, M.; Ghatway, N.; Dakhova, O.; Liu, B.; Raveh-Sadka, T.; et al. Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: an interin analysis. Nat Med 2020, 26, 1686-1690.
- Pule, M.A.; Savoldo, B.; Meyrs, G.D.; Rossig, C.; Russell, H.V.; Dotti, G.; Huls, M.H.; Liu, E.; Gee, A.P.; Mei, Z.; et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med 2008, 14, 1264-1270. [CrossRef]
- Louis, C.U.; Savoldo, B.; Dotti, G.; Pule, M.; Yvon, E.; Myers, G.D.; Rossig, C.; Russell, H.V.; Diouf, O.; Liu, E.; et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 2011, 118, 6050-6056. [CrossRef]
- Che-Hsing, L.; Sharma, S.; Heczey, A.A.; Steffin, D.; Louis. C.U.; Grilley, B.J.; Thakkar, S.G.; Wu, M.; Wang, T.; Rooney, C.M.; et al. Eighteen-year survival after GD2-direcetd chimeric antigen receptor-modified immune effector cell treatment for neuroblastoma. Res Sq 2024, rs.3.ers-4232549.
- Paret, C.; Ustjanzew, A.; Ersali, S.; Seidmann, L.; Jennemann, R.; Ziegler, N.; El Malki, K.; Russo, A.; Wingerter, A.; Ortmuller, F.; et al. GHD2 expression in medulloblastoma and neuroblastoma for personalized immunotherapy: a matter of subtype. Cancers 2022, 14, 6059.
- Ciccone, R.; Quintarelli, C.; Camera, A.; Pezzella, M.; Caruso, S.; Manni, S.; Ottaviani, A.; Guercio, M.; Del Bufalo, F.; Quadraccia, M.C.; et al. GD2-targeting CAR T-cell therapy for patients with GD2+ medulloblastoma. Clin Cancer Res 2024, 30, 2545-2557. [CrossRef]
- Haydar, D.; Houke, H.; Chiang, J.; Yi, Z.; Odé, Z.; Caldwell, K.; Zhu, X.; Mercer, K.S.; Stripay, J.L.; Shaw, T.I.; et al. Cell-surface antigen profiling of pediatric brain tumots: B7-H3 is consistently expressed and can be targeted via local or systemic CAR T-cell delivery. Neuro Oncology 2021, 23, 991-1101.
- Nehama, D.; Di Ianni, N.; Musio, S.; Du, H.; Patané, M.; Pollo, B.; Finocchiaro, G.; Park, J.; Dunn, D.E.; Edwards, D.S.; et al. B7-H3-redirected chimeric antigen receptor T cells target glioblastoma and neuropspheres. E BioMedicine 2019, 47, 33-43.
- Tang, X.; Zhao, S.; Zhang, Y.; Wang, Y.; Zhang, Z.; Yang, M.; Zhu, Y.; Zhang, G.; Guo, G.; Tong, A.; et al. B7-H3 as a novel CAR-T therapeutic target for glioblastoma. Mol Ther Oncolytics 2019, 14, 279-285. [CrossRef]
- Vitanza, N.A.; Wilson, A.L.; Huang, W.; Seidel, K.; Brown, C.; Gustafson, J.A.; Yokoyama, J.K.; Johnson, A.J.; Baxter, B.A.; Koning, R.W.; et al. Intraventricular b7-H3 CART cells for diffuse intrinsic pontine glioma: preliminary first-in-human bioactivity and safety. Cancer Discov 2023, 13, 114-131.
- Vitanza, N.A.; Ronsley, R.; Huang, W.; Seidel, K.; Thomsen, A.; Gust, J.; Hauptman, J.; Choe, M.; Crotty, E.; Leary, S.; et al. Intravenous B7-H3 CAR T cells for diffuse intrinsic pontine glioma: safety and efficacy report from the completed phase 1 trial BrainChild-03. Neuro Oncol 2024, 26 (suppl.4), TRLS-01.
- Theruvath, J.; Sotillo, E.; Mount, C.W.; Graef, C.M.; Delaidelli, A.; Heitzeneder, S.; Labanieh, L.; Dhingra, S.; Leruste, A.; Majzner, R.G.; et al. Locoregional administered B7-H3-targeted CAR T cells for treatment of atypical teratoid/rhabdoid tumors. Nat Med 2020, 26, 712-719. [CrossRef]
- Tang, X.; Wang, Y.; Huang, J.; Zhang, Z.; Liu, F.; Xu, J.; Guo, G.; Wang, W.; Tong, A.; Zhou, L. Administration of B7-H3-targeted chimeric antigen receptor-T cells induce regression of glioblastoma. Signal Transd Targetes Ther 2021, 6, 125. [CrossRef]
- Shang, X.; T-MAXIMUM Pharmaceutical. An exploratory clinical trial on intra-lumbar injection of B7-H3-specific allogeneic universal CAR-T cells in patients with recurrent high-grade gliomas. J Clin Oncol 2023, 141(suppl. 16), 2043.
- Zhang, Y.; Feng, R.; Chi, X.; Xian, N.; Chen, X.; Huang, N.; Zhang, Y.; Zhang, K.; Zhang, J.; Chen, L.; et al. Safety and efficacy of B7-H3 targeting CAR-T cell therapy for patients with recurrent GBM. J Clin Oncol 2024, 142 (suppl. 16), 2062. [CrossRef]
| Author | Trial and Phase | Target Antigen | Dose and Route of Administration |
Number of Patients (Age) |
Clinical Results | Adverse Events |
|---|---|---|---|---|---|---|
| Ahmed et al. 2017 | NCT 01109059 Phase I |
HER2 | 1x106 to 1x108 Intravenous without prior lymphodepletion |
Pediatric 7 (10-17 yr) Adult 10 (30-69 yr) Recurrent GBM |
1 partial response; 7 stable disease; 8 disease progression In adult pts: mOS 9.4 mo |
No severe adverse events related to treatment |
| O’Rourke et al. 2017 | NCT 02209376 Phase I |
EGFRvIII | 1.75x108 to 5x108 Intravenous after lymphodepletion |
11 Adult recurrent GBM |
1 SD; 10 no response Evidence of CAR-T cell trafficking to the tumor Reduction of target antigen |
No off-tumor toxicity No CRS |
| Goff et al. 2019 | NCT 01454596 Phase I |
EGFRvIII | 4pts 1x107 ; 3pts 1x108 5pts 1x109 ; 5pts 1x1010 Intravenous after lymphodepletion |
18 Adult recurrent GBM |
No objective response mPFS: 1.2 mo mOS: 6.9 mo |
Severe adverse events and dose-limiting toxicities in the group at 1x1010 CAR-T cells |
| Choi et al. 2024 | NCT 05660369 Phase I/Pilot |
EGFRvIII | Patient 1: 2 infusions of 10x106 CAR-T cells Patients 2 and 3: 1 infusion of 10x106 CAR-T cells |
3 Adult recurrent GBM |
All patients displayed rapid and dramatic radiographic tumor regression. In 2/3 pts, this response was transient | No dose-limiting toxicity No adverse events greater than 3 |
| Bagley et al. 2024 | NCT 03726515 Phase I |
EGFRvIII | 4.65x107 to 2x108 1-3 cycles of CAR-T cells 1 cycle Pembrolizumab |
7 (56-76 yr) Adult recurrent GBM |
No objective responses mPFS: 5.2 mo mOD: 11.8 mo |
No dose-limiting toxicity |
| Bagley et al. 2024 | NCT 05168423 Phase I |
EGFRvIII - IL-13Rα2 (bicistronic lentiviral vector) |
3 pts (1x107 cells/m2) 3 pts (2.5x107 cells/m2) Intrathecal |
6 (33-71 yr) Adult recurrent GBM |
Significant reduction of tumor size at MRI, but none with radiographic objective response | Low-grade CRS Early moderate-severe neurotoxicity |
| Brown et al. 2024 | NCT 02208362 Phase I |
IL-13Rα2 | 57pts From 2 to 200x106 IL13-CAR-T cells ICT or ICV or ICT and ICV infusions |
57pts (16-71 yr) 41pts GBM 2pts DMG 7pts gr4 Astrocytoma 7pts gr3 Glioma |
SD: 50% PR: 2pts CR: 2pts mOS: 7.7 mo GBM(all) mOS: 10.2 mo GBM(ICT and ICV) |
No-dose limiting toxicity 2pts grade3 neurologic events |
| Majzner et al. 2022 | NCT 04196413 Phase I |
GD2 | IV (1x106 cells/Kg) Optional ICV infusions in responding patients |
4 (5-25 yr) DMG H3K2M7-mutated |
75% patients clinical and radiographic response | Manageable toxicity On target, off-tumor toxicity not observed |
| Majzner et al. 2022 | NCT 04196413 Phase I |
GD2 | 4 pts IV (1x106 cells/Kg) 9 pts IV (3x106 cells/Kg) |
13 (2-30 yr) DMG H3K2M7-mutated |
90% pts clinical and radiographic response. 2 pts with complete response | Grade 4 CRS in 3 pts at 3x106 cells/Kg. Transient tumor inflammation neurotoxicity |
| Lin et al. 2024 | NCT 04099797 | GD2 – IL-7R | 3pts: IV GD2-CAR-T cells 1x107 cells/m2 Ppts: IV CR7-GD2-CAR-T cells 1x107 cells/m2 |
11 (4-18 yr) 8 DMG H3K27M-mut 2 recurrent MB 1 ATRT (atypical teratoid rhabdoid tumor) |
3 pts: GD2-CAR-T group: PD 8pts CR7-GD2-CAR-T group: 2pts PR; 6pts SD |
CRS grade 1 75% Tumor inflammation-associated toxicity grade 1 88% |
| Liu et al. 2023 | NCT 03170141 Phase I |
GD2 | 8pts IV: 3x107 - 2.1x108 3pts IC: 2.6x106 - 6.4x106 4SCAR-T cells |
8 (3-63 yr) Recurrent GBM |
3pts: PD; 4pts: PR; 1pt: SD mOS from diagnosis: 19.7 mo; mOS from infusions: 11.5 mo |
Grade 2 or 3 neurologic events: 2pts |
| Del Bufalo et al. 2023 | NCT 03373097 Phase I/II |
GD2 | 17pts single infusion; 11pts multiple infusions 3x106 , 6x106,10x106 GD2-CART01 cells/Kg |
27 (2.7-18.6 yr) Refractory/relapsed Neuroblastoma |
CR: 33%; PR: 30%; SD: 19%; NR: 19% At 3yr: OS 67% LDB vs 0% HDB; EFS 58% LDB vs 0% HDB |
No-dose limiting toxicities Grade 1-2 CRS: 70% Grade 3 CRS: 4% Neurologic toxicity grade 1-2: 22% |
| Heczey et al. 2023 | NCT 03294954 Phase I |
GD2 | 3pts 3x106, 3pts 1x107, 3pta 3x107, 3pts 1x108 GD2-CAR-NKT cells/m2 8pts single infusion, 4pts double infusion |
12 (2-12 yr) Refractory/relapsed Neuroblastoma |
After first infusion: PR: 3pta; SD: 4pts; PD: 5pts After second infusion: CR: 1pt; PR: 1pt; PD: 2pts. |
|
| Pule et al. 2008 Louise et al. 2011 Che-Hsing et al. 2024 |
NCT 00085930 Phase I |
GD2 | 19pts IV infusion 1.2x107 cells/m2 5x107 cells/m2 3.1x108 cells/m2 |
19pts with R/R Neuroblastoma 11 with active disease 8 in remission |
After a follow-up of 8-14 years survived: 5/8 in remission 2/11 with active disease |
No-dose limiting toxicities |
| Brown et al. 2024 | NCT 02208362 Phase I |
IL-13Rα2 | From 2x106 to 200x106 IL13-CAR-T cells ICT or ICV or ICT and ICV infusions |
57pts 41pts GBM 2pta DMG 7pts Astrocytoma 7pts Glioma |
SD: 50% PR: 2pts CR: 2pts mOS: 7.7mo (GBM); 10.2mo (GBM ICT and ICV) |
No-dose limiting toxicity 2pts with grade 3 neurologic events |
| Vitanza et al. 2024 | NCT 04185038 Phase I Brain Child 03- Arm C |
B7-H3 | ICV infusions of 10x107 B7-H3 CAR-T cells Multiple infusions (median 7) |
21 pediatric DIPG | mOS for pts after progression: 9.7mo, before progression: 16.9mo 3pts alive 3yrs from diagnosis |
No-dose limiting toxicity Common neurologic events: headache, nausea, vomiting, fever |
| Zhang et al. 2024 | NCT 05241392 Phase I |
B7-H3 | ICT or ICV infusions of B7-H3 CAR-T cells 3x107 cells (3pts) 6x107 cells (4pts) 15x107 cells (6pts) |
13 adult R/R GBM patients | At 12mo: 83% OS mOS: 20.3mo 1pt: PR 1pt: CR |
No-dose limiting toxicity 2pts neurologic events gr.3 some pts CRS gr2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





