1. Introduction
The three-toed (
Bradypus spp.) and two-toed (
Choloepus spp.) sloths are obligate arboreal species in the Neotropics [
14]. Regarding feeding habits, both genera are folivorous, but the two-toed sloth is considered a generalist, while the three-toed sloth is more individually specialized [
20,
23]. In different habitats, studies have revealed dietary preferences of members of the Bradypodidae family for some plant species, such as those from the Apocynaceae and Sapotaceae families [
6], as well as Cecropiaceae, Clethraceae, and Clusiaceae [
33].
Although some plant species are more consumed and common in the diet of the sloths than others, the factors that influence the choice of plant species remain unclear [
16]. Differences in the physiological and nutritional needs of the diverse types of sloths can have a significant influence on these individual feeding choices [
2]. In this context, the maintenance of sloths
Bradypus spp. in ex-situ environments is considered challenging, as their preferred types of feed are not always available, compromising the ideal nutritional status of these animals [
27], leading to severe clinical disorders [
3].
Nevertheless, recent findings have demonstrated that free-living three-toed sloths are capable of consuming a great diversity and proportion of plant species [
16], and also their diet can change or be adapted according to the tree species available in their habitat, suggesting that a greater variety of potential plant species can be consumed by these animals under captivity environments [
24]. Digestibility tests In vivo with
B. variegatus corroborate this hypothesis, in which providing a greater diversity of plants in the diet of the sloths can favor their selective behavior, increasing nutrient intake and digestibility in mixed diets, up to 300 g kg
-1 of neutral detergent fiber (NDF) in dry matter of the feed [
1].
The nutritional assessment of plant species with potentialities for feeding sloths of the gender (
Bradypus spp.) in captive conditions is essential to increase food options for these animals [
13,
36]. In different regions of Central and South America, the most common diets in ex-situ conditions are based on leaves of
Cecropia sp. [
27], perhaps because they are known to have an ecological relationship or because they are one of the few plants with good acceptability by all
Bradypus, regardless of individual preferences [
23]. However, low levels of dry matter intake are observed when these plants are solely supplied [
7], which may be associated with its high fiber content, especially lignin [
13]. In contrast, leaves of
Inga sp. and
Pterodon sp. are among the species most consumed by three-toed sloths in the wild [
16,
24] and have a wide distribution in South America [
17,
26].
In nutritional terms, the leaves of Inga sp. were already reported to display values of 118g kg
-1 of crude protein (CP) and 335 g kg
-1 of NDF [
17], while
Pterodon sp. values of 110 and 322g kg
-1 for the same parameters respectively [
26]. From the perspective of potential foods for feeding captive sloths, it is necessary to know their rates and extents of degradation using in vivo models that can show the potential of these foods in promoting an increase in the consumption and digestibility of DM, and favor the transit of digesta in sloths. These parameters are also linked to the soluble carbohydrate content of these foods, which positively affected fermentation in the stomach of the sloths [
1].
The objective of this study was to evaluate the nutritional composition, digestibility, and in vitro degradation kinetics of the leaves of Cecropia sp., Inga sp., and Pterodon sp. to feed sloths of the species Bradypus variegatus in captivity.
2. Materials and Methods
Diets, Animals, and Food Analysis
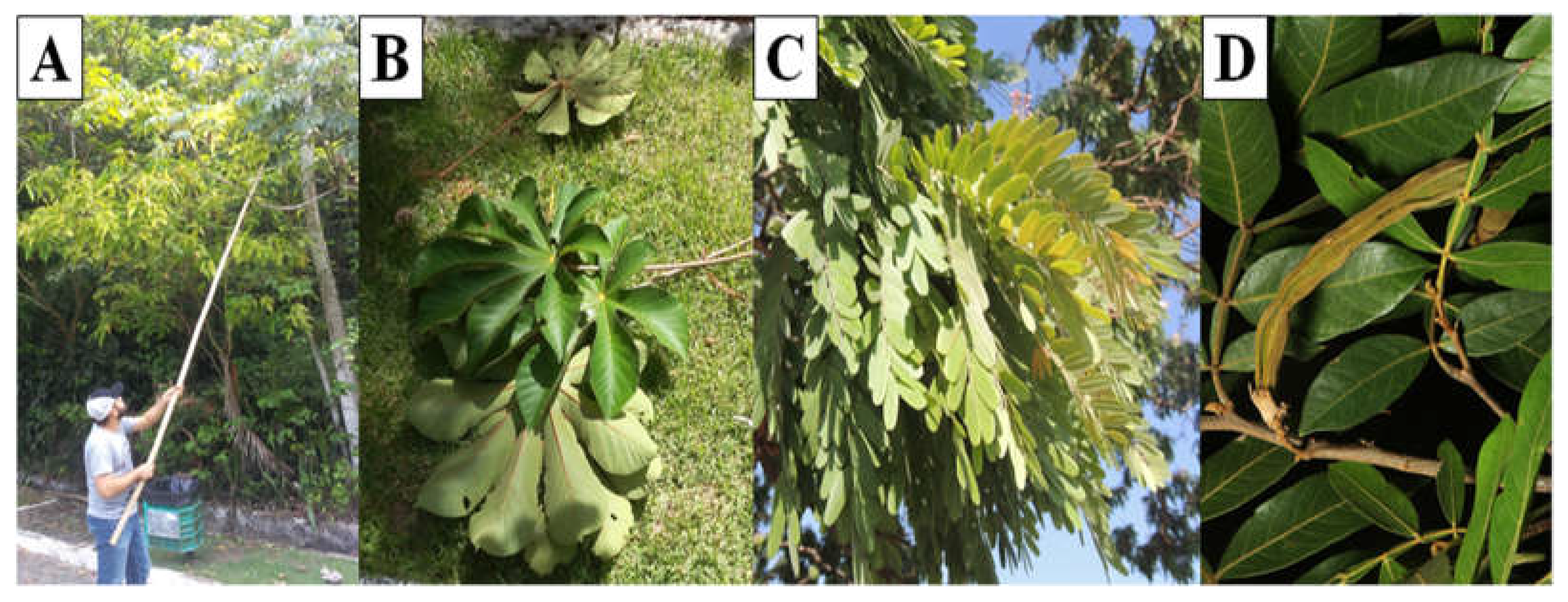
This research was divided into two stages. The first stage began with food management of three sloths (2.8±0.21kg), females, of the species
B. variegatus, kept in captivity in the Dois Irmãos State Park, Recife, Brazil (
Figure 1). These sloths were fed exclusively using the leaves of
Crecopia sp. (Cecropiacea).
Over 14 days the animals were adapted to a mixed diet composed of the leaves of
Crecopia sp.,
Inga sp. (Mimosoideae), and
Pterodon sp. (Fabaceae) (
Figure 2), in which 800g of each food was provided based on the fresh weight, considering up to 20% of leftovers. There was no restriction or limitation of the dry matter intake by the animals. Samples of these foods were collected for analysis purposes of bromatological composition and the analysis of in vitro kinetic parameters.
The samples were pre-dried in a forced ventilation oven, at 55 °C, for 72 hours and ground to 1- and 2-mm diameters, to estimate the contents of dry matter (DM), crude protein (CP), ashes, and ether extract (EE), according to [
10]. The fiber was quantified as neutral detergent fiber (NDFcp), corrected for ashes and protein, according to [
22].
Collection of the Digested Feed in the Stomach
After 14 days of feeding the diet, 500g of gastric contents were collected from each animal, using an esophageal probe in the cranial portion of the stomach. The samples were filtered through cotton fabric under CO
2 injection and placed in a thermos bottle with hermetic closure, at 39 °C. They were then taken to the laboratory, filtered again under CO
2 injection, and transferred to glass vials containing McDougall's buffer solution. To avoid fermentation before inoculation, the flasks were kept in a refrigerator at 4 ºC overnight. Five hours before inoculation, the flasks were removed from the refrigerator and taken to an oven at 39 ºC until inoculation [
19].
In vitro Digestibility Test
The technique for evaluating the in vitro dry matter digestibility (IVDMD) was adapted from the method proposed by [
30]. For the fermentative phase, 25 TNT (non-woven fabric) bags (5x12 cm) were incubated with 0.50g of samples of each food using glass bottles (2 L capacity). These were heated to 39 ºC in a McDougall buffer solution mixed with the filtered inoculum from the stomach of an adult sloth (400 mL). The medium was gasified using carbon dioxide (CO
2), and the bottles were sealed with rubber stoppers and aluminum washers. They were kept in the artificial rumen equipment DAISYII Incubator (ANKON
® Technology), remaining for 48 h. After this period, a solution containing 8g of pepsin and 40 mL of 6N HCl was added to each flask, remaining for another 24 h under digestion. The bags containing the residues were washed with distilled water and dried in an oven at 105 ºC, until constant weight and, then weighed to estimate the IVDMD coefficient.
Digestion Kinetic Assay
The in vitro gas production test was carried out at the Embrapa Semi-arid Gas Production Laboratory, in Petrolina, Pernambuco. Approximately 1 g of each food sample was incubated anaerobically, at 39 °C, with 100 mL of stomach fluid of an adult sloth (buffered, buffer/fluid ratio 8:2), and sealed in glass vials (160 mL capacity), with rubber caps. Gas measurement was carried out following the semi-automatic gas production technique described by [
18]. Pressure and volume readings were taken at increasing times 0, 2, 4, 6, 8, 10, 12, 15, 24, 30, 36, and 48h after incubation, using a pressure transducer connected to an outlet valve in the bottles.
The cumulative gas production data were analyzed using the bicompartmental model of [
29]: V(t) = Vf1/[1+e
(2-4kd1(L-T)] + Vf2/[1+e
(2-4kd2(L-T)], where V(t) represents the maximum total volume of gases produced; Vf1 the maximum volume of the gas for the faster digestible fraction; Vf2 the maximum volume of gas for the slow digestible fraction; kd1 the digestion rate for the fast digestible fraction; kd2 digestion rate for the slowly digestible fraction; L the duration of the initial digestion events (latency phase), common to both phases; and T is the fermentation time.
The bromatological composition and in vitro digestibility parameters were evaluated by analysis of variance, followed by the Tukey test, when the F test was significant, using a significance level of 5%. The parameters of the gas production models were estimated using nonlinear regression procedures, seeking the prediction equation that best fits the data obtained. Statistical analyses were performed using the software Statistical Analysis System (SAS) (Version 9.1, 2003).
3. Results
There was a significant difference (P<0.05) between the average dry matter content of the foods, with the leaves of Inga sp. displayer greater average values, followed by
Cecropia sp. and
Pterodon sp. (
Table 1). Values for neutral detergent fiber were higher in
Cecropia sp. (P<0.05), followed by Inga sp., and
Pterodon sp. (
Table 1). Non-fibrous carbohydrate contents were greater in
Pterodon sp. (P<0.05), followed by
Inga sp. and
Cecropia sp. The cellulose concentration was higher and the hemicellulose was lower in the leaves of
C. pachystachya (P<0.05) compared to the other foods, which did not differ from each other.
The in vitro digestibility results demonstrated a significant difference (P<0.05) only for crude protein, with greater digestibility for
Inga sp., followed by
Pterodon sp. and
Cecropia sp. (
Table 2). However, there was no difference in the IVDMD and in vitro digestibility of the NDF between foods (P>0.05).
Regarding kinetic parameters, greater gas production was observed from the digestion of
Pterodon sp. (P<0.05), followed by
Inga sp. and
Cecropia sp., which did not differ from each other (
Table 3). Regarding the volume of gases produced and the rate of digestion of fibrous carbohydrates, a statistical difference was observed between the foods (P<0.05). The volume of gases (vFC) was greater for
Pterodon sp. (68.35 mL), followed by
Cecropia sp. (61.69 mL) and Inga sp. (59.34 mL), respectively (
Table 3).
4. Discussion
The values of dry matter recorded in the present study corroborate the percentages observed in the literature for these foods [
1,
7] and exceed the values indicated as minimum levels (250 to 350 g kg
-1) for some ideal anaerobic fermentations [
34]. No significant differences were observed between the average crude protein values of the foods, but all were higher than the minimum of 7% recommended by [
34], to promote effective microbial fermentation in other gastric fermenters.
The results of NDF for
Cecropia sp. are in agreement with analytical data from other studies. However, attention should be paid to the considerably high levels of NDFcp in this plant, as feeding sloths exclusively with
Cecropia sp, may limit food intake. Studies have observed that values above 400g kg
-1 of NDF can limit food intake [
7] and reduce digest transit [
13]. In fibrous diets, cellulose and hemicellulose are indispensable sources for obtaining liquid energy in the form of ATP, used for the maintenance and growth of the microbes in the digestive tract, and the animal system [
34].
Studies in animals with a similar gastric system indicate that the efficiency in the digestion and use of exogenous crude protein for the synthesis of microbial protein is related to the availability of energy in the fermentative environment, especially fast and easily digestible non-fibrous carbohydrates or soluble carbohydrates, for example, simple sugars [
28]. Therefore, it is reasonable to speculate that the greater digestibility of crude protein observed in these foods may have occurred due to a greater supply of energy to the microbes involved in the digestion, this energy may have originated from the greater content of NFC in the feed.
It was reasonably expected that, due to their higher levels of NDFcp,
Cecropia sp. demonstrated lower total DM digestibility values, however, this premise was not confirmed. One of the factors that could justify such findings would be a greater solubility of cellulose and hemicellulose from
Cecropia sp. leaves, which may have positively affected the total digestion of NDF in this food, as observed in other herbivores that ferment vegetal organic matter in their pre-stomachs [
4,
25].
The difference in total gas volume can be attributed to the fact that
Pterondon sp. displayed a greater proportion of soluble carbohydrates compared to other food analyzed (
Table 1). However, despite this difference, the average digestion rates estimated for non-fibrous carbohydrates (kdNFC) did not show a statistical difference among the foods, as did the volume of gas from these carbohydrates (vNFC) (
Table 3). The highest digestion rate of fibrous carbohydrates was also recorded for
Pterodon sp. This was possibly related to the lower NDFcp content and higher non-fibrous carbohydrate values. As previously discussed, these components contribute significantly to the initial energy input in the fermentative medium [
28].
Estimated digestion rates for fibrous carbohydrates in this study were slightly lower compared to assessments of fibrous foods in other gastric fermenters, which ranged from 0.039 to 0.045% h
-1 [
5]. It is important to highlight that, unlike other herbivores, sloths from the genus
Bradypus sp. have a stomach microbiota considered to be of low diversity, predominantly composed of the bacterial phyla Proteobacteria and Firmicutes [
11]. This characteristic may, in part, have contributed to the relatively lower digestion rates observed.
On the other hand, the greatest contribution to the total volume of gases originated from the fermentation of fibrous carbohydrates in all foods examined, according to the adjustment to the two-compartmental model (
Table 3). In general, foods with great NDFcp content and low NFC values are associated with lower digestion rates and lower gas production [
8,
9], which was not observed in this study. In all foods analyzed, the cumulative production of gases from non-fibrous carbohydrates was lower than the volume of gases from the fibrous fraction, indicating greater use of fibrous carbohydrates by microorganisms.
These events can be partially explained by the high content of cellulose and hemicellulose (NDF) in the foods evaluated (
Table 1). Such components, which ferment slower, probably contributed to the total gas production in longer periods. Normally, the fermentation of soluble carbohydrates occurs predominantly in the initial stages of fermentation to meet the energy needs of the microbiota, especially bacteria [
12,
28]. Furthermore, the notable levels of potentially digestible fiber in the foods evaluated may have favored the development of fibrolytic microbiota, intensifying the fermentation of fibrous carbohydrates.
5. Conclusions
Regarding the nutritive potentialities of the plant species tested to feed B. variegatus, the leaves of Pterodon sp. stood out with greater values of crude protein digestibility, total volume of gases, and digestion rates of fibrous carbohydrates, which was influenced by its high values of non-fibrous carbohydrates and lower levels of NDF. The leaves of Inga sp. also presented potential for feeding B. variegatus, due to their adequate DM, CP, and non-fibrous carbohydrate values. However, caution is advised when feeding Bradypus sp. with Cecropia sp., due to the high NDF content of this plant, which might affect consumption, despite the absence of impacts on dry matter digestibility.
6. Patents
This section is not mandatory but may be added if there are patents resulting from the work reported in this manuscript.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
I.L.C.M and MdCMML; investigation, JCdSN and GPA; supervision, JLPSI and PVdA; writing – original draft preparation, DBdN and JJC; data curation, MJAALA, ACLdM, RMPBC, CBVR, AGR and RASP; analysis, JCdSN; funding acquisition, JLPSI, GPA, JJC and ACLdM; writing-review and editing.
Funding
The Foundation for the Support of Science and Technology of the State of Pernambuco (FACEPE) for financial support in granting BIC scholarship number BIC-0552-5.04/22.
Acknowledgments
The authors would like to thank the Instituto Preguiça Garganta Marrom (IPGM), and Embrapa Semiárido – Petrolina for the subsidy in the in vitro gas production analyses. The Federal Rural University of Pernambuco (UFRPE) for the support of the technical team and the use of laboratories to carry out the other analyses in this research. The Foundation for the Support of Science and Technology of the State of Pernambuco (FACEPE) for financial support in granting BIC scholarship number BIC-0552-5.04/22.
Conflicts of Interest
The authors declare no conflicts of interest
Ethical Standards
The study was certified by the Ethics Committee on the Use of Animals (CEUA/UFRPE), number 23082.007646/2019-79 with licence 033/2019.
References
- Andrade G.P.; Albuquerque PV, Tschá MC, Alcântara SF, Miranda MELC, Nascimento JCS, Barros NFJ, Amorim MJAAL. Dietary neutral detergent fibre and lignin contents affect intake, digestibility and digesta retention in captive sloths (Bradypus variegatus). Journal of Animal Physiology and Animal Nutrition. 2022, 106, 910-921.
- Araújo M.S, Bolnick DI, Layman CA. The ecological causes of individual specialisation. Ecology Letters. 2011, 14, 948-958. [CrossRef]
- Arenales A, Silva FL, Miranda F, Guedes PEB, Werther K, Costa MELT, Tinoco HP, Coelho CM, Santos RL. (2020). Pathologic findings in 36 sloths from Brazil. Journal of Zoo and Wildlife Medicine. 2020, 51, 672-677. [CrossRef]
- Bergman, EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiological Reviews. 1990, 70, 567-590. [CrossRef]
- Cabral L.S, Valadares Filho S.D.C, Malafaia P.A.M, Lana R.P, Silva J.F.C, Vieira R.A.M., Pereira E.S. Frações de carboidratos de alimentos volumosos e suas taxas de degradação estimadas pela técnica de produção de gases. Revista Brasileira de Zootecnia, 2000, 29, 2087-2098.
- Chiarello A.G. Diet of the Atlantic forest maned sloth Bradypus torquatus (Xenarthra: Bradypodidae). Journal of Zoology. 1998, 246, 11-19.
- Cliffe R.N, Haupt RJ, Avey-Arroyo JA, Wilson RP. Sloths like it hot: ambient temperature modulates food intake in the brown-throated sloth (Bradypus variegatus). Peer J. 2015, 3, e875. [CrossRef]
- De Boever J.L, Aerts JM, Vanacker JM, De Brabander DL. (2005). Evaluation of the nutritive value of maize silages using a gas production technique. Animal Feed Science and Technology. 2005, 123, 255-265. [CrossRef]
- De Aville E.R, Givens DI. Use of the automated gas production technique to determine the fermentation kinetics of carbohydrate fractions in maize silage. Animal Feed Science and Technology. 2001, 93, 205-215. [CrossRef]
- Detmann E, et al. Métodos para Análise de Alimentos – 2ª Edição, Instituto Nacional de Ciência e Tecnologia de Ciência Animal (INCT-CA). 2021, 350p.
- Dill-McFarland K.A.; Weimer PJ, Pauli JN, Peery MZ, Suen G. (2016) Diet specialization selects for an unusual and simplified gut microbiota in two-and three-toed sloths. Environmental Microbiology. 2016, 18, 1391-1402.
- Fernandes A.M, Queiroz AC, Pereira JC, Lana RP, Barbosa MHP, Fonseca DM, Detmann E, Cabral LS, Pereira ES, Vittori A. Composição químico-bromatológica de variedades de cana-de-açúcar (Saccharum spp L.) com diferentes ciclos de produção (precoce e intermediário) em três idades de corte. Revista Brasileira de Zootecnia. 2003, 32, 977-985.
- Foley WJ, Engelhardt WV, Charles-Dominique P. The passage of digesta, particle size, and in vitro fermentation rate in the three-toed sloth Bradypus tridactylus (Edentata: Bradypodidae). Journal of Zoology. 1995, 236, 681-696.
- Gardner A.L. Mammals of South America. Chicago: University of Chicago Press, 2008.
- Goering H.K, Van Soest PJ. Forage fiber analyses (apparatus, reagents, procedures, and some applications). Washington, DC: USDA. 1970, 379p.
- Giné G.A.F.; Mureb LS, Cassano CR. Feeding ecology of the maned sloth (Bradypus torquatus): Understanding diet composition and preferences, and prospects for future studies. Austral Ecology. 2022, 47, 1124-1135.
- Leme M.C.J.; Durigan ME, Ramos A. Avaliação do potencial forrageiro de espécies florestais, Colombo. Anais. Colombo: EMBRAPA-CNPF. 1994, p. 147-155.
- Mauricio R.M.; Mould FL, Dhanoa MS, Owen E, Channa KS, Theodorou MK. A semi-automated in vitro gas production technique for ruminant feedstuff evaluation. Animal Feed Science and Technology. 1999, 79, 321-330. [CrossRef]
- Mauricio R.M.; Pereira LGR, Gonçalves LC, Rodriguez NM. Relação entre pressão e volume para implantação da técnica in vitro semi-automática de produção de gases na avaliação de forrageiras tropicais. Arquivo Brasileiro de Medicina Veterinária e Zootecnia. 2003, 55, 216-219.
- Mendoza J.E.; Peery MZ, Gutiérrez GA, Herrera G, Pauli JN. Resource use by the two-toed sloth (Choloepus hoffmanni) and the three-toed sloth (Bradypus variegatus) differs in a shade-grown agro-ecosystem. Journal of Tropical Ecology. 2015, 31, 49-55. [CrossRef]
- Mertens D.R. Regulation of forage intake. Forage quality, evaluation, and utilization. Madison: American Society of Agronomy, Crop Science Society of America, Soil Science Society of America. 1994, 450-493.
- Mertens D.R. Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: collaborative study. Journal of AOAC International. 2002, 85, 1217-1240.
- Montgomery G.G.; Sunquist ME. Habitat selection and use by two-toed and three-toed sloths. In G. G. Montgomery (Ed.), The ecology of arboreal folivores, 1978, (pp. 329–359). Smithsonian University Press.
- Mureb L.S.; Rocha-Santos L, Cassano CR, Lopes GS, Rosa B, Miranda FR, Giné GAF. Tree diversity mediates individual diet specialization of the maned sloth (Bradypus torquatus). Mammalian Biology. 2023, 103, 145-159.
- Nússio L.G.; Campos FP, Lima MLM. Metabolismo de carboidratos estruturais. In: BERCHIELLI, T. T. et al. (Ed.). Nutrição de Ruminantes. 2011, 193-238.
- Pott E.; Pott A. Níveis de nutrientes em plantas não-gramíneas pastejadas por bovinos na sub-região dos Paiaguás, do Pantanal Mato-Grossense. Pesquisa Agropecuária Brasileira, 1987, 22, 1293-1299.
- Raines J. Captive health and husbandry of the Bradypodidae. Zoo Biology, 2005, 24, 557-568. [CrossRef]
- Russell J.B.; O’connor JD, Fox DG, Van Soest PJ, Snifen CJ. A net carbohydrate and protein system for evaluating cattle diets: I. Ruminal fermentation. Journal of Animal Science, 1992, 70, 3551-3561.
- Schofield P.; Pitt RE, Pell An. Kinetics of fiber digestion from in vitro gas production. Journal of Animal Science, 1994, 72, 2980-2991.
- Silva F.F.; Gonçalves LC, Rodrigues JAS, Corrêa CES, Rodriguez NM, Brito AF, Mourão GB. Qualidade das silagens de híbridos de sorgo (Sorghum bicolor (L.) Moench). de portes baixo, médio e alto com diferentes proporções de colmo mais folhas/panícula. I. Avaliação do processo fermentativo. Revista Brasileira de Zootecnia, 1999, 28, 21-29.
- Tilley J.M.A.; Terry RA. A two-stage technique for the in vitro digestion of forage crops. Grass and Forage Science, 1963, 18, 104-111.
- Tomich T.R.; Pereira LGR, Guimarães Júnior R, Gonçalves LC. Adaptação de uma técnica "in vitro" para descrição da cinética de degradação ruminal da matéria seca de volumosos, 2006.
- Urbani B.; Bosque C. Feeding ecology and postural behaviour of the three-toed sloth (Bradypus variegatus flaccidus) in northern Venezuela. Mammalian Biology, 2007, 72, 321-329. [CrossRef]
- Van Soest P.J. Nutritional ecology of the ruminant, 1994, Cornell university press.
- Vaughan C.; Ramírez O, Herrera G, Guries R. Spatial ecology and conservation of two sloth species in a cacao landscape in Limón, Costa Rica. Biodiversity and Conservation, 2007, 16, 2293-2310.
- Vendl C.; Frei S, Dittman MT, Furrer S, Osmann C, Ortmann S, Munn A, Kreuzer M, Clauss M. Digestive physiology, metabolism and methane production of captive Linné's two-toed sloths (Choloepus didactylus). Journal of Animal Physiology and Animal Nutrition, 2016, 100, 552-564.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).