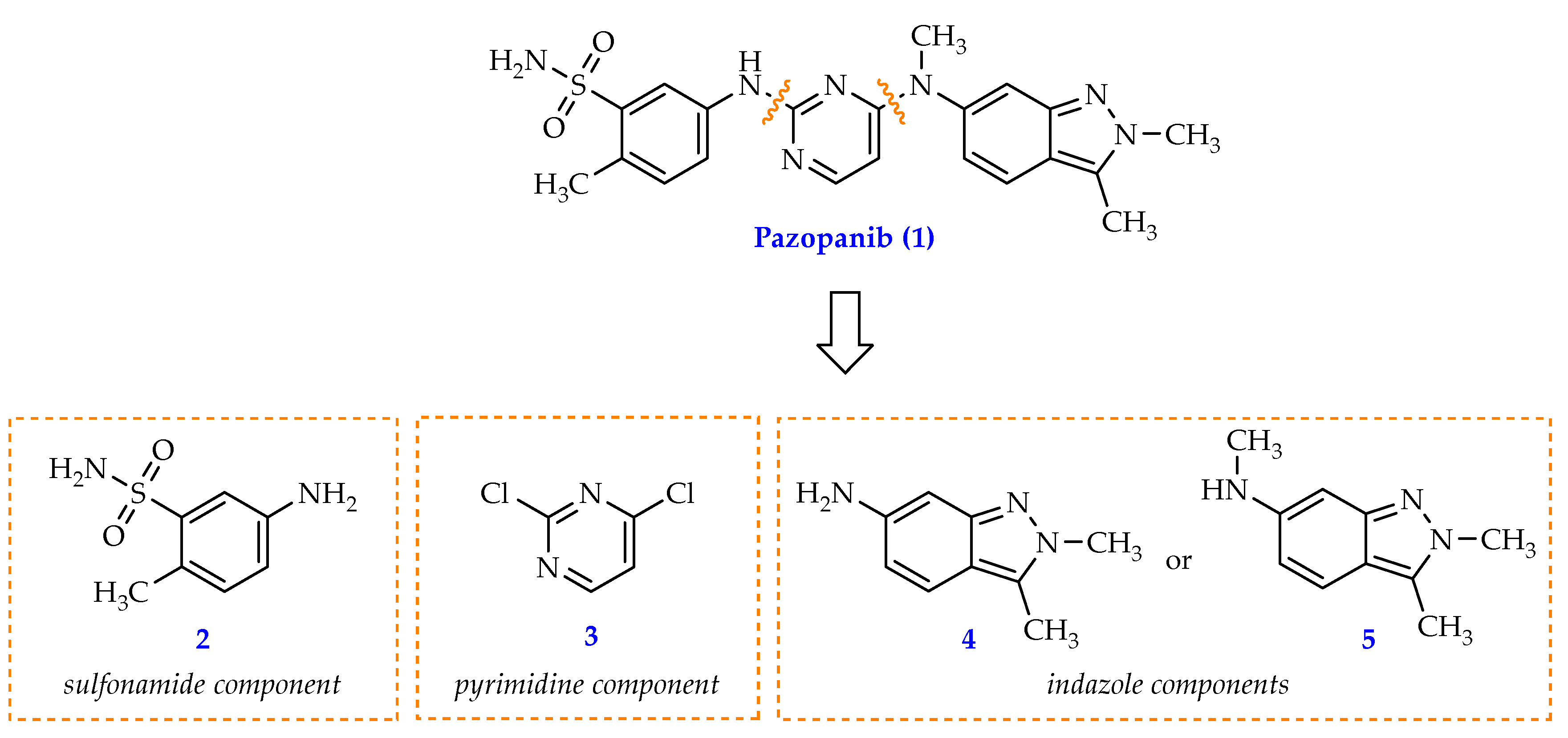
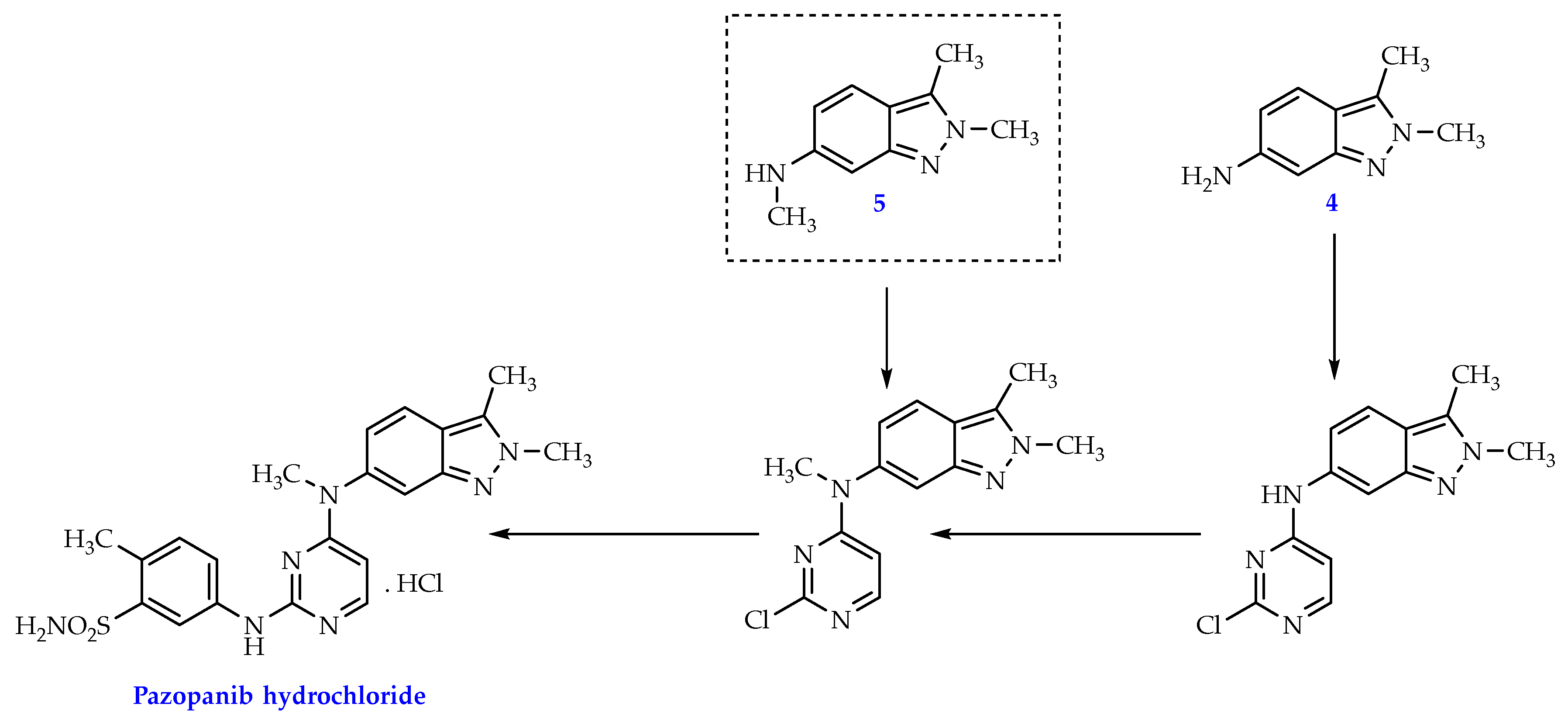
3.1. General Information
3-Methyl-6-nitro-1H-indazole (compound 6) was synthesized in the lab. Trimethyl orthoformate (98.0 %) was purchased from Aladdin Company (China). Potassium carbonate (K2CO3) (99.0 %) and paraformaldehyde (≥ 95.0 %) were purchased from Guangdong Guanghua Sci-Tech, China. Ethyl acetate (99.5%) was purchased from Samchun, Korea. Sodium borohydride (NaBH4) (98.0 %), n-hexane (≥ 98.0 %), methanol (MeOH) (99.5 %), isopropanol (IPA) (99.7 %), anhydrous sodium sulfate, toluene (99.5 %), tin(II) chloride (SnCl2.2H2O) 98.0 % and N,N-dimethylformamide (DMF) (99,5 %) meeting analytical reagent (AR) purity standards were obtained from Xilong (China). All chemicals were used without further purification.
The melting point (M.p) was measured by using the capillary tube method with an SRS EZ-Melt apparatus (Stanford Research Systems, Sunnyvale, CA, USA). Mass spectra of compounds were recorded on an LC-MSD Trap SL machine, ionized by ESI (electrospray ionization) method. The FT-IR spectrum was recorded by a Shimadzu spectrometer (Kyoto, Japan). Nuclear magnetic resonance (1H, 13C, HSQC, HMBC, NOESY) experiments were measured on a Bruker Ascend spectrometer (Billerica, MA, USA) at 500 MHz for proton and 125 MHz for carbon-13 using CDCl3 as the solvent and tetramethylsilane (TMS) as an internal standard. The reaction mixtures were monitored, and the purity of the compounds was checked by thin-layer chromatography (TLC) on silica gel 60 F254 plates (Merck, Darmstadt, Germany).
3.2. Synthetic Procedure
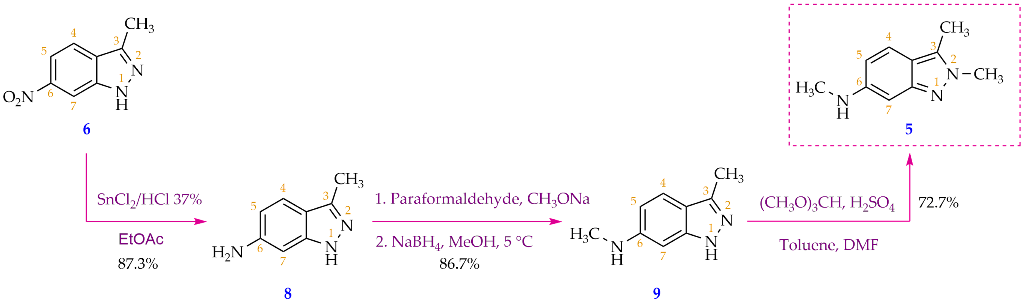
3-Methyl-1H-indazol-6-amine (8)
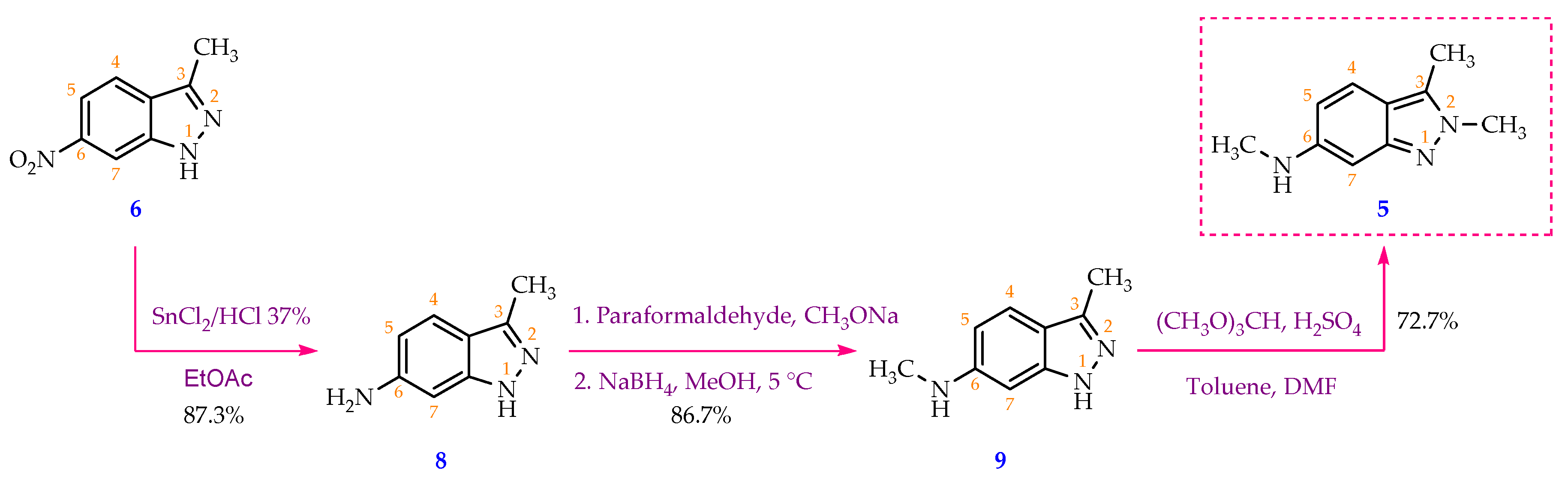
In a 100 mL round-bottom flask, 3-methyl-6-nitro-1H-indazole (6) (2.00 g, 11.3 mmol, 1 eq) was dispersed into ethyl acetate (10 mL) at 0 °C, then slowly added tin(II) chloride dihydrate (10.19 g, 45.2 mmol, 4 eq). This was followed by dropwise addition of concentrated HCl (1 mL) over 1 min, keeping the reaction temperature below 10 °C with an ice bath. The ice bath was taken out when HCl addition was finished, and the mixture was stirred for three more hours. After the reaction was completed (as shown by TLC), 50 milliliters of ethyl acetate was added and then the reaction mixture was cooled to 5 °C. Reaction mixture was then neutralized by saturated Na2CO3 solution to a pH of 9. The organic phase was separated, and two more fractions of ethyl acetate (2 x 50 mL) were sequentially added to carry out the extraction two more times. Following that, the organic phase was combined and rinsed three times with distilled water (3 x 20 mL). The organic phase was then transferred to a conical flask, in which 10 mL of a 20% hydrochloric acid solution was gradually added, and the mixture was shaken to facilitate the HCl salt formation which was soluble in the aqueous phase. The latter was separated and cooled to 5 °C and saturated Na2CO3 solution was added in small portions under stirring to pH= 9, which enabled the formation of a precipitate. The precipitate was collected by vacuum filtration, washed three times with distilled water (3 x 5 mL), and dried to obtain 3-methyl-1H-indazol-6-amine (8) as a yellow-brown solid (1.45 g, 87.3% yield). M.p: 203.8-204.5 °C. TLC: Rf = 0.45 (n-hexane/ethyl acetate, 3:7). MS (ESI, MeOH), m/z: Calculated for C8H9N3 [M+H]+: 148.08, found: 147.9. FT-IR (KBr), vmax (cm-1): 3382, 3180 (N-H); 1640 (C=N); 1520 (C=C); 1H-NMR (500MHz, CDCl3), δ (ppm): 9.44 (s, 1H, N-NH); 7.43 (d, J = 8.5 Hz, 1H, H-4); 6.57-6.55 (m, 2H, H-5, H- 7); 3.84 (s, 2H, NH2); 2.50 (s, 3H, H-1’).13C-NMR (125MHz, CDCl3), δ (ppm): 146.1 (C-6); 143.5; (C-7a); 142.9 (C- 3); 121.0 (C-4); 116.8 (C-3a); 111.9 (C-5); 92.4 (C-7); 11.9 (C-1’).
N,3-Dimethyl-1H-indazol-6-amine (9)
In a 100 ml single neck round-bottom flask, 3-methyl-1H-indazol-6-amine (8) (2.00 g, 13.6 mmol, 1 equivalent) was dissolved in 20 ml of methanol. Then, CH3ONa (3.67 g, 67.9 mmol, 5 eq) and paraformaldehyde (2.04 g, 67.9 mmol, 5 eq) were added to the reaction vessel, respectively. The mixture was then refluxed for 5 minutes, and stirred at room temperature for 4 hours. After that, the reaction was cooled to 5 °C, and NaBH4 (2.06 g, 54.4 mmol, 4 eq) was slowly added under stirring for 5 minutes. The reaction mixture was refluxed for approximately 2 hours, and the solvent was removed by rotary evaporation. The residue was redistributed in water and washed three times with ethyl acetate (3x50 mL). The ethyl acetate extracts were combined and rinsed three times with purified water (3 x 30 mL). 20% HCl solution was added dropwise to the combined ethyl acetate layer under stirring until pH = 1. Subsequently, the aqueous phase was separated using a decanter. After the aqueous solution was cooled to room temperature, saturated Na2CO3 solution was gradually added under stirring until pH = 9, which enabled the precipitation of the base product (9). The precipitate was filtered using a Buchner filter funnel, collected, and dried under an infrared lamp. Compound 9 was obtained as a pink-white solid (1.90 g, 86.7 % yield). M.p: 215.6-217.3 °C. TLC: Rf = 0.60 (n-hexane/ethyl acetate, 3:7). MS (ESI, MeOH), m/z: Calculated for C9H11N3 [M+H]+: 162.10, found: 161.8. FT-IR (KBr), vmax (cm-1): 3338, 3221 (N-H); 2974, 2939, 2900 (C-H sp3), 1624 (C=N); 1589, 1510 (C=C); 1H-NMR (500 MHz, CDCl3), δ (ppm): 7.42 (d, J = 8.50 Hz, 1H, H-4); 6.52 (dd, J1 = 8.75 Hz, J2 = 1.75 Hz, 1H, H- 5); 6.43 (d, J = 1.50 Hz, 1H, H-7); 2.90 (s, 3H, H-2’); 2.52 (s, 3H, H-1’).13C-NMR (125 MHz, CDCl3), δ (ppm): 149.1 (C-7a); 143.3 (C-3); 120.7 (C- 4); 115.7 (C-3a); 111.5 (C-5); 87.9 (C-7); 30.8 (C-2’); 11.9 (C-1’).
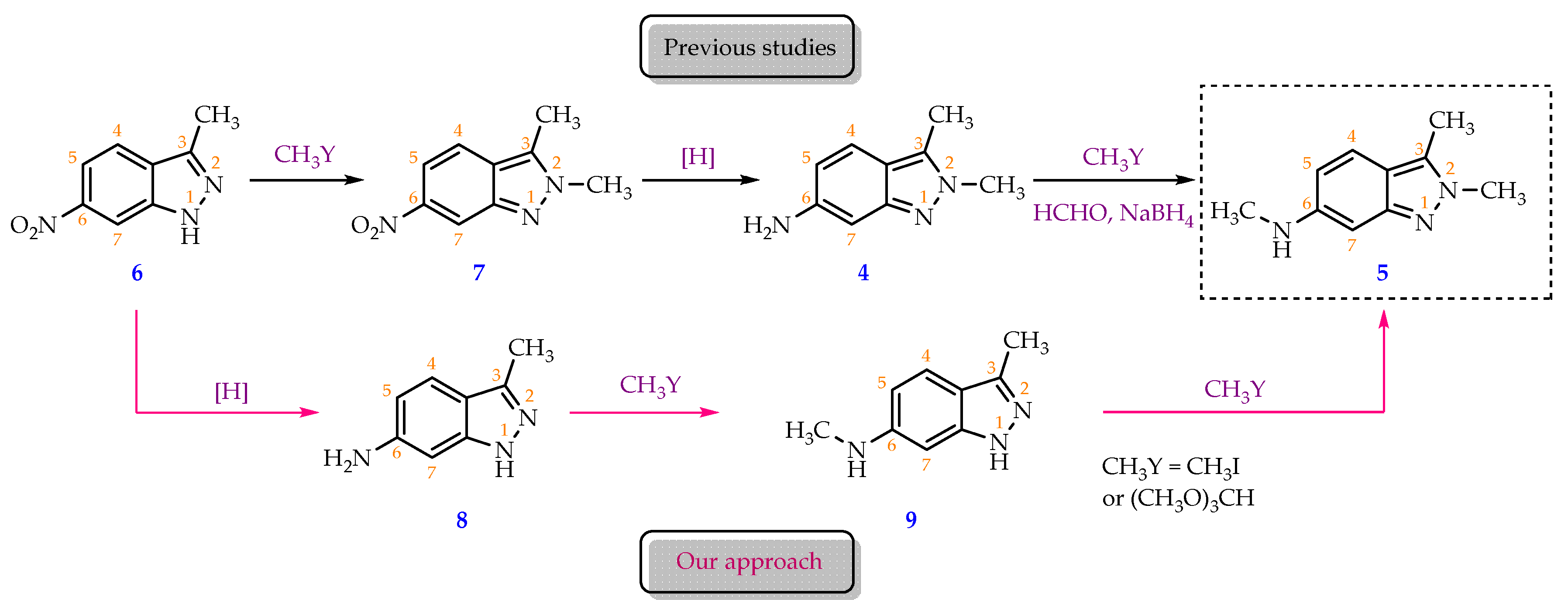
N,2,3-Trimethyl-2H-indazol-6-amine (5)
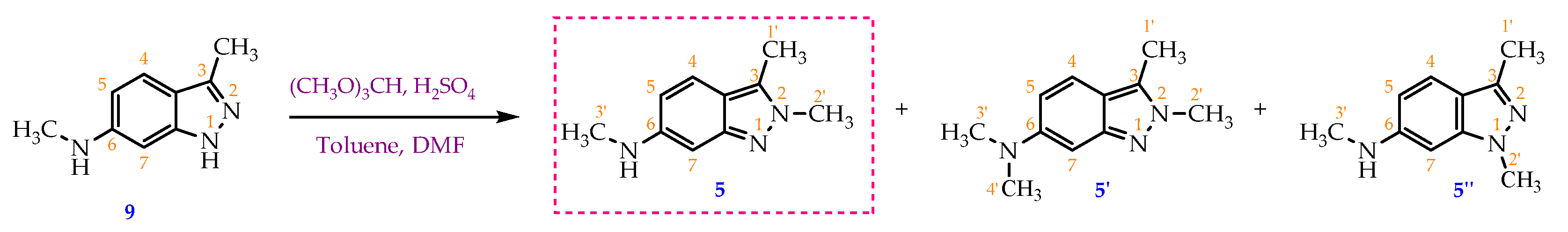
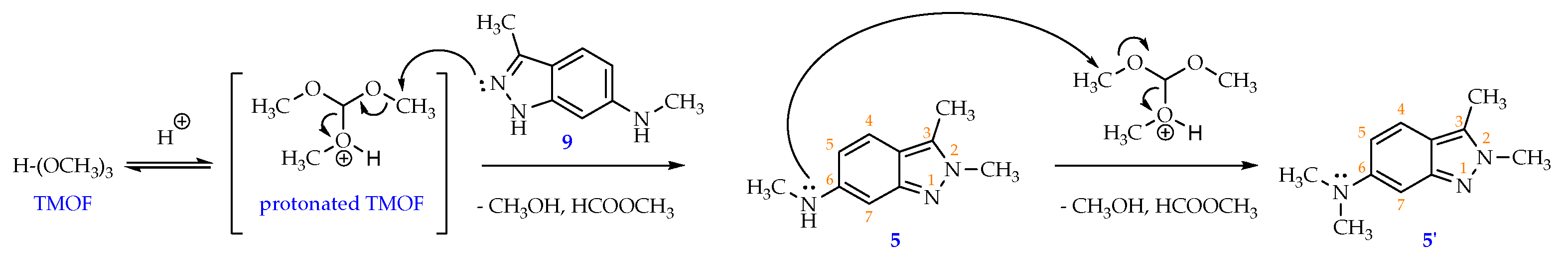
30 mL of toluene, 3 mL of N,N-dimethylformamide (DMF) and 5.4 mL of trimethyl orthoformate (49.6 mmol; 4 eq) were charged to a 50 mL round-bottom flask under stirring, 0.5 mL of 98 % H2SO4 solution (8.7 mmol; 0.7 eq) was then dropwise added to the mixture which was stirred at 5 °C for 5 minutes before 2.00 g of N,3-dimethyl-1H-indazol-6-amine (9) (12.4 mmol; 1 eq) was added into the reaction vessel. The reaction mixture was then heated and stirred at 60 °C for 5 hours. Solvent was removed under reduced pressure and a thick, dark pink residue was obtained. 100 mL of distilled water was then added to this residue to form a clear, wine-red solution. The solution was washed with ethyl acetate (3 times x 120 mL/time) and the aqueous phase was separated. Saturated Na2CO3 solution was slowly added to the aqueous phase until the pH = 8. The resulting aqueous solution was washed with ethyl acetate (3x120mL). The ethyl acetate fractions were combined and then washed with distilled water (5x70mL) to remove DMF and water-soluble impurities. Finally, the organic phase was dried with sodium sulfate and solvent was removed via rotary evaporator to obtain a yellow powder (1.58 g, 72.7 % yield). M.p: 144.2-145.8 °C. TLC: Rf = 0.30 (n-hexane/ethyl acetate, 3:7); 0.65 (dichloromethane/methanol, 9:1). MS (ESI, MeOH), m/z: Calculated for C10H13N3 [M+H]+: 176.11, found: 175.9. FT-IR (KBr), vmax (cm-1): 3422 (N-H); 2982, 2904 (C-H sp3), 1645 (C=N); 1560, 1513 (C=C); 1H-NMR (500 MHz, CDCl3), δ (ppm): 7.28 (d, J = 9.00 Hz, 1H, H-4); 6.52 (s, 1H, H-7); 6.45 (dd, J1 = 8.75 Hz, J2 = 1.75 Hz, 1H, H-5); 3.97 (s, 3H, H-2’); 2.86 (s, 3H, H-3’); 2.49 (s, 3H, H-1’). 13C-NMR (125 MHz, CDCl3) δ (ppm): 149.6 (C-7a); 147.8 (C-6); 131.3 (C- 3); 119.9 (C-4); 115.5 (C-3a); 114.8 (C-5); 91.7 (C-7); 36.7 (C-3’); 30.8 (C-2’); 9.8 (C-1’).
N,N,2,3-Tetratramethyl-2H-indazol-6-amine (5’)
When using a larger ratio between TMOF: starting material 9 as (6.2:1), beside the target compound 5 (27.6%), a novel compound 5’ was isolated with 21.3% of yield.
Data for compound 5’: TLC Rf = 0.30 (n-hexane/ethyl acetate, 3:7); 0.70 (dichloromethane/methanol, 9:1). MS (ESI, MeOH), m/z: Calculated for C11H15N3 [M+H]+: 190.1, found: 189.9. FT-IR (KBr), vmax (cm-1): 2926 (C-H sp3); 1650 (C=N); 1559 (C=C). 1H-NMR (500 MHz, DMSO-d6), δ (ppm): 7.43 (d, J = 9.50 Hz, 1H, H-4); 6.77 (dd, J1 = 9.5 Hz, J2 = 1.50 Hz, 1H, H-5); 6.45 (d, J = 1.50 Hz,1H, H-7); 3.91 (s, 3H, H-2’); 2.88 (s, 6H, H-3’, H-4’), 2.50 (s, 3H, H-1’). 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 149.6 (C-7a); 148.9 (C-6); 131.2 (C- 3); 120.5 (C-4); 115.4 (C-3a); 113.2 (C-5); 94.9 (C-7); 41.4 (C-3’,4’); 37.1 (C-2’); 9.8 (C-1’).