1. Introduction
Exposure to toxins and drugs during pregnancy creates a suboptimal condition for fetal growth and development [
1,
2,
3]. It is well known that maternal cigarette smoking during pregnancy is one of the leading preventable causes of birth defects and impaired fetal development [
4]. Despite this information, in Australia in 2021 [
5], 8.3% reported smoking during the first 20 weeks of pregnancy, and of these, a substantial number of women (74%) reported smoking during the last 20 weeks of pregnancy. From 2011-2021, the number of women smoking during the first 20 weeks of pregnancy has slowly declined Nationally (from 12.9%); however the majority (70-74%) continued to smoke during the last 20 weeks of pregnancy [
5]. Encouragingly, the proportion of Australian First Nations mothers who smoked during pregnancy declined by 9.2% between 2011 and 2020 [
6]. However a concerning number of pregnant women continue to smoke in regional and remote regions (ranging from 13.3% - 35.5%) [
5].
Nicotine is the main addictive teratogen within cigarettes, which has been linked to adverse pregnancy outcomes and fetal development [
7]. Nicotine causes vasoconstriction of uteroplacental blood vessels, crosses the placental barrier to enter the fetal circulation, and accumulates in fetal tissues [
4,
8]. These mechanisms, directly and indirectly, impact pregnancy outcomes. Vasoconstriction of uteroplacental blood vessels reduces blood flow to the placenta, reducing blood flow to the fetus and reducing micronutrient and oxygen delivery [
4]. Aoyagi Y et al. [
9] found that in animal models, the groups exposed to nicotine prenatally demonstrated a reduction in blood supply to all fetal organs compared with the control group. This reduction in blood flow can potentially cause fetal tissue hypoxia, fetal growth restriction and adverse postnatal health effects [
9].
Additionally, nicotine is associated with other placental changes contributing to placental dysfunction. These alterations include thickening of the trophoblastic membrane, increased collagen content in the villous stroma, decreased angiogenesis, inhibition of trophoblast interstitial invasion, increased placental hypoxia and oxidative stress, and downregulation of labyrinth vascularisation [
10]. As the placenta functions as both a metabolic and endocrine organ, creating an effective maternal-fetal barrier, any disruption in placental function can potentially disrupt fetal growth and development [
7,
10]. Furthermore, nicotine concentration in fetal circulation is much greater than in maternal circulation. In adults, 70-80% of circulating nicotine is metabolised through the liver; however, in utero, the fetal liver is underdeveloped and does not express the appropriate enzymes to allow nicotine metabolism [
4,
7].
The impact of smoking on neonatal birth weight is well recognised. Kataoka et al. [
11]. showed that in full-term infants, birth weight decreased as the cigarette number per day increased, with a significant weight reduction of the category 6 to 10 cigarettes per day. Provision of smoking history to a health professional during antenatal visits and pregnancy is voluntary in most healthcare settings. It is often obtained as part of a broader consult with health professionals where other medical histories pertinent to the pregnancy are obtained. An important consideration is that the accuracy of the data on smoking and the number of cigarettes smoked per day during pregnancy may vary during the pregnancy period.
Cotinine, the major metabolite of nicotine, has become the biomarker of choice for assessing exposure to tobacco smoke (smoking) or environmental tobacco smoke (passive smoking) more accurately [
12,
13]. It has a long biological half-life of between 19 and 40 hours in the body compared with nicotine, which has a short half-life of about 30 minutes to 2 hours [
12]. Serum cotinine levels, measured using ELIZA assays, have been shown to reflect dosage, showing a good correlation between the levels found in all biological fluids, urine, saliva and cord blood. Considered less invasive, urinary cotinine levels are the most widely used marker of tobacco use and show high sensitivity [
12].
We carried out a study to determine if maternal urine cotinine measurements can be used to assess the impact of exposure to nicotine during pregnancy on infant birth weight. The primary outcome measure was infant birth weight, and a secondary outcome measured any differences between male and female neonates. We hypothesised that maternal urine cotinine levels negatively impact birth weight, affecting both male and female neonates equally.
2. Materials and Methods
2.1. Patient Population and Study Design
The data presented in this manuscript is from
The Relationship between Maternal Health and Infant Kidney Development and Function Study, which was a prospective, longitudinal cohort of mother-infant dyads from pregnancy up until the child is two years of age carried out at the Townsville University Hospital (TUH), Queensland, Australia. TUH serves a region with a population of over 240 000 and 10 000 births annually [
14]. The Townsville Hospital's Human Research Ethics Committee approved the study, and written consent was obtained from the women participating. The study was performed in accordance with the Declaration of Helsinki.
2.2. Study Participants and Recruitment
All pregnant women presenting to the hospital during recruitment (August 2019 to August 2021) were invited to participate. A detailed maternal medical history was obtained during the first presentation to the antenatal clinics at TUH. Smoking status, as with a history of using other substances during pregnancy, was self-reported as per the hospital practice, and women had the option to omit the smoking information. A urine specimen for cotinine measurements was collected at the same time to determine more objectively if they had been exposed to nicotine during pregnancy. Urine cotinine is not normally detected in non-smokers unless in an environment with much cigarette smoke [
13]. Once their infants were born, anthropometric parameters (weight, length and head circumference) measurements were carried out after birth. Birth weight was measured using an electronic infant weighing scale (Seca 727 Electronic baby scale, Seca Deutschland, Hamburg, Germany and were classified as small for gestational age (SGA; birth weight < 10th percentile for the gestational age (GA)) and appropriate for gestational age (AGA; birth weight being between 10th to 90th centile for GA) [
15]. In this report, we focused on singleton-term mother-infant dyads. Mother-infant dyads from premature (<37 completed weeks of GA) births and multiple pregnancies (twins or triplets) were excluded.
2.3. Urinary Cotinine Collection and Measurement
Urine was collected from women presenting for their antenatal visit. Urine cotinine is not normally detected in non-smokers unless in an environment with much cigarette smoke (nicotine exposure via passive smoking). The Human Cotinine Eliza kit (ABNOVA, Taiwan) had a detection limit of 1ng/mL and a calibration range of 5 to 100 ng/mL. Simultaneous urine creatinine levels were measured to take account of variations in urinary dilution between individuals [
16], and the maternal urine cotinine ratio was then calculated (UCCR)( ng/mmol). Urine cotinine concentrations below the assay's <5 ng/ml detection limit were recorded as 0 ng/ml (UCCR of 0 ng/mmol).
2.4. Statistical Analysis
The normality of the variables was determined by the D'Agostino-Pearson test [
17]. The results are expressed as the means [standard deviation (S.D.)] for continuous, normally distributed data and as median (interquartile range [IQR]) for continuous, non-normally distributed data. Comparisons of means of normally distributed data were made using t-tests, and the Mann-Whitney test was used for non-normally distributed data. Differences in categorical variables were analysed by the Chi-squared test or Fischer's exact test. Pearson correlation or Spearman's
rho correlation coefficient was used when appropriate. P value < 0.05 was considered statistically significant. Statistical analyses were performed using MedCalc® Statistical Software version 20.217 (MedCalc Software Ltd, Ostend, Belgium).
3. Results
We had 345 women who consented and participated in this study, and complete specimens were available from 303 women. We excluded 65 neonates for prematurity and multiple pregnancies and analysed data from 238 mother-term neonates' dyads. The mean GA for the women where the urine cotinine was collected was 33.4 (5.7) weeks. The mean birth weight for the whole cohort was 3375 (498) g, and the mean GA was 38.9 (1.2) weeks. Urine cotinine was detected in 50.4 % (120/238) women from the whole cohort, but only 16% (38/238) self-reported as smokers (chi-square 39.7, p<0.0001). The median UCCR was 0.07 [0.0-2.2] ng/mmol.
Table 1 compares neonates who were born to women without cotinine detected in their urine compared to those with cotinine.
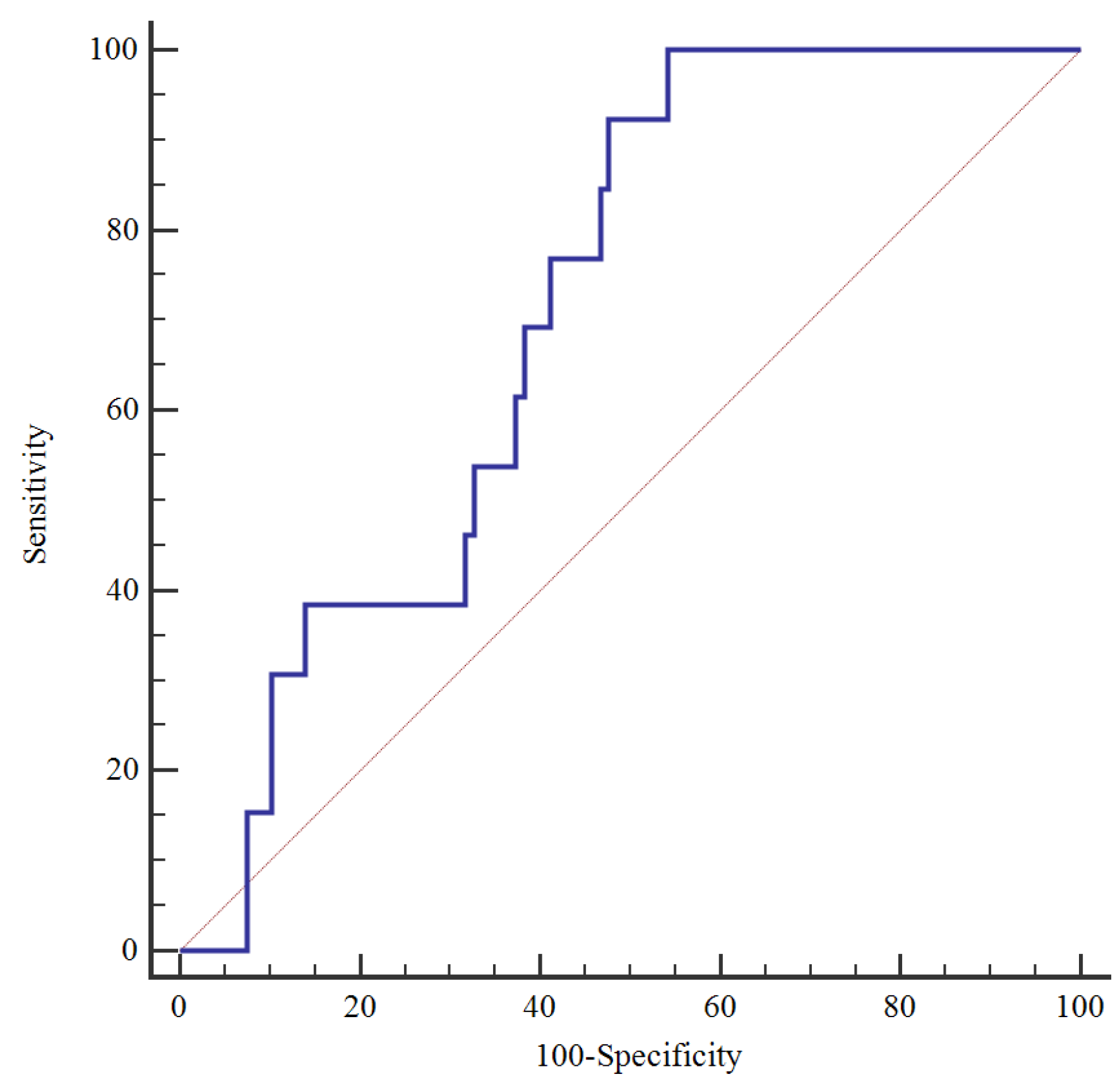
Neonates born to women with cotinine in urine had significantly smaller birth weights (3293(519) vs.3458(464) g, p=0.010) and birth weight Z scores (-0.16(1.04) vs.0.17(0.93), p=0.010). There were also significantly more neonates born SGA to women with cotinine in the urine. Using the ROC curve, we investigated the UCCR value in predicting SGA in the women's baby (
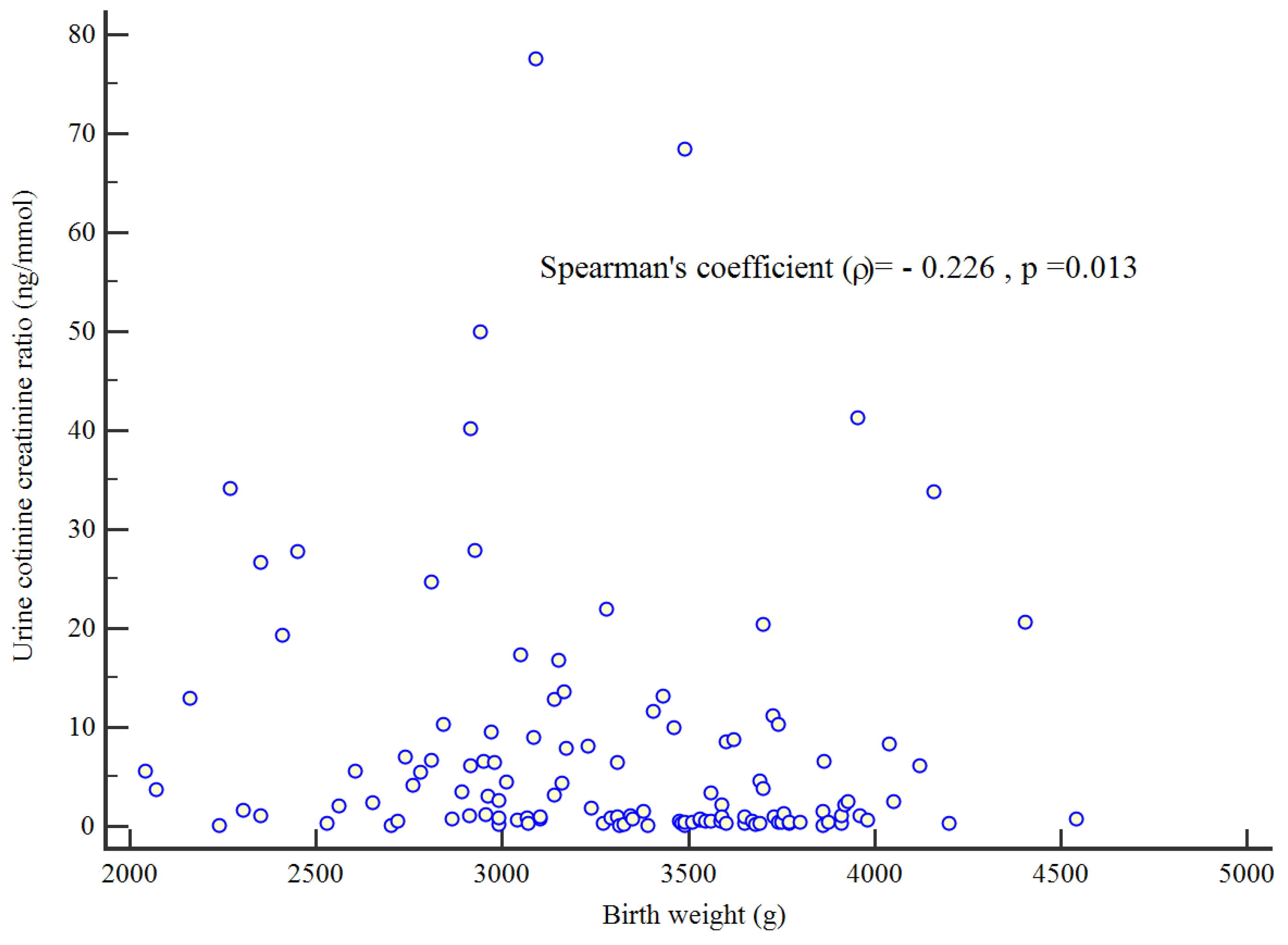
Figure 1). For a cut-off value of 1.06 ng/mmol, the area under the ROC curve was 0.71, with a sensitivity of 100% and a specificity of 45.8%. There was a significant negative correlation between birth weight and UCCR (Spearman's coefficient = -0.0226, p=0.013) (
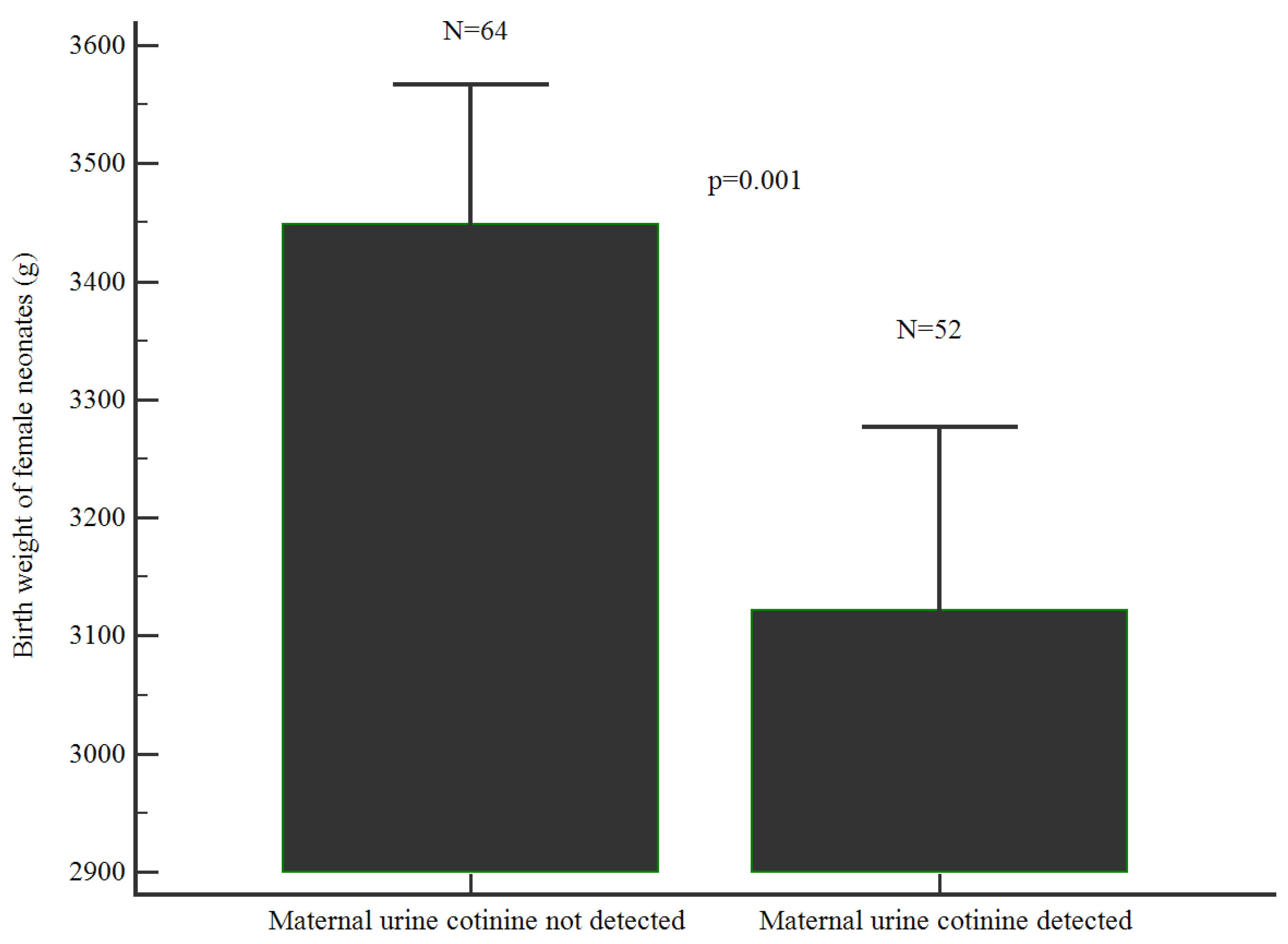
Figure 2). Female babies born to women with nicotine in the urine were significantly smaller compared to those without nicotine in the urine (3121(559) vs.3449(471); p= 0.001) (
Figure 3). However, there was no significant difference in the birth weight of male neonates born to women with nicotine in the urine compared to those without nicotine in the urine (3470(459) vs.3424 (448), p=0.580) (
Figure 4).
4. Discussion
In Australia in 2021, 8.7% of pregnant women reported smoking at any time during pregnancy (26,433 pregnant women according to AIHW 2022 report)(5). Even higher rates were reported in our cohort in regional Australia, where 16% of women self-reported smoking during pregnancy at their antenatal check-up (similar to 2021 statistics in inner regional (13.3%) and outer regional (15.5%) Australia) [
6]. Of concern, 50.7% of the entire cohort had presence of nicotine metabolites in their urine (detected via urine cotinine levels), well above the National percentage, including very remote Australia (35.5%). These findings highlight a major discrepancy between reported smoking habits and true exposure. It is well established that nicotine-induced vasoconstriction and placental dysfunction can lead to reduced birth weight in infants. Birth weight is a key indicator of infant health and a crucial determinant of survival and well-being. Currently, routine antenatal checks rely on self-reporting of nicotine exposure, which may lead to missed opportunities to identify infants at high risk of adverse effects. Indeed, our findings show that higher levels of nicotine exposure during pregnancy (measured from urine cotinine levels and urine cotinine when adjusted for creatinine) markedly reduced birth weight of the neonates, with more neonates born small for gestational age compared to women that did not smoke during pregnancy (evidenced by absences of cotinine in urine). Furthermore, female term neonates showed increased susceptibility to nicotine exposure, exhibiting significantly lower birth weights to mothers with cotinine in urine. Consequently, increasing their vulnerability to developing well documented long-term adverse effects of being born small for gestational age [
18]. Interestingly, this relationship was not evident in male neonates. Previous studies have also shown similar sex trends. Voigt et al. [
19] investigated the sex-specific risk of maternal smoking during pregnancy on the birth weight and the proportion of SGA newborns in 888,632 (49.9%) of 1,815,318 singleton births in Germany from 1995 to 1997 in whom data on maternal cigarette consumption were available. They reported that heavy smoking (> than 21 cigarettes/day) increased the risk of SGA 3.15-fold for a boy but 3.51-fold for a girl (p<0.001) and concluded that in heavy smokers, the negative effect of maternal smoking during pregnancy on the mean birth weight and risk of SGA is significantly more significant in the female neonate. On the other hand, a population-based study of 23 African countries investigating the impact of environmental tobacco smoking (ETS) involving 208,027 newborns showed ETS had a more negative effect on the male neonate than on the female neonate [
20]. These differences between studies could result from inaccuracies of smoking disclosure as highlighted in our findings. It is imperative to improve accuracy of smoking self-reporting, educating pregnant women on the importance of accurate reporting and considering incorporating biochemical verification by implementing non-invasive measurements of urine cotinine levels (as conducted in this study), as part of prenatal care to complement medical reporting.
UCCR remains a research tool and has not yet been recommended as part of routine clinical care by the professional bodies involved in determining clinical guidelines in obstetrics [
21,
22]. Cotinine assays have a lower detection threshold; hence, it is possible that the actual number of women with detectable urine cotinine could be higher should a more sensitive assay become available. In this regard, a limitation of our study is that urine cotinine measurement has the inability to determine the nature of nicotine exposure during pregnancy, i.e. smoking (or using e-cigarettes), passive smoking, smoking cannabis linked with tobacco or use of smokeless tobacco, whose increasing importance was highlighted recently in a large Swedish systematic review [
23]. Another limitation of our study is the urine cotinine concentrations below the assay's <5 ng/mmol detection limit were recorded as 0 ng/mmol; further highlighting the importance of improving record-taking. Together, considering our findings, we have some strategic suggestions to help improve medical reporting on smoking habits. 1) Training healthcare providers in more effective communication, including making patients aware that smoking habits include other forms of smoking (including passive smoking to cigarettes and/or e-cigarettes, use of e-cigarettes, use of non-tobacco cigarettes). 2) Reflecting on biases and creating a more non-judgemental environment, whilst considering cultural sensitivity. 3) Using motivational interviewing techniques to encourage honest communication about smoking habits, which involve open-ended questions, reflective listening, and affirmations. 4) To explore and implement more private and less confrontational strategies, including using anonymous questionnaires and use of digital tools (apps that allow women to report smoking habits, remote access to increase routine check-ups, and with embedded educational information). 5) Introducing smoking cessation programs or re-evaluate current smoking cessation programs to better attract women residing in regional and remote regions.
As with many other clinical studies worldwide [
24], our study was impacted significantly by the Covid 19 pandemic due to public health measures, which inadvertently resulted in barriers around requirements for in-person contact in enrolment and assessment for the families in our study. This resulted in some participants not attending the follow-up required in this study.
5. Conclusions
Urine cotinine was detected in half of the women in our cohort, and there was a significant negative correlation between birth weight. Neonates born to women with cotinine in urine had significantly smaller birth weights, and significantly more neonates born to SGA. Female babies born to women with cotinine in the urine were significantly smaller compared to those without. Whilst currently a research tool, the introduction of routine urine cotinine:creatinine ratio measurements in clinical practice might be beneficial to determine more accurately the impact of nicotine during pregnancy on neonatal outcome.
Author Contributions
Conceptualisation, DV, DR, RS, Y.K.; Methodology, DV, DR, RS, YK; Validation, D.R. Y.K; Formal Analysis, D.R, YK; Investigation, D.R, YK; Writing – Original Draft Preparation, DR, YK.; Writing – Review & Editing, DV, YK; Project Administration, DV, DR, YK.; Funding Acquisition, DR, YK, RS. All authors have read and agreed to the published version of the manuscript.
Funding
The research grant was provided by the National Health and Medical Research Council, Australia (Grant APP1159616). .
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Townsville Hospital's Human Research Ethics Committee approved the study (HREC/2018/QTHS/48326).
Informed Consent Statement
Informed and written consent was obtained from all women participating in the study.
Data Availability Statement
Dataset available on request from the authors-The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors would like to thank the research nurses and midwives who assisted with the recruitment of the participants.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 2008, 359, 61-73. [CrossRef] [PubMed]
- Rogers, J.M. Smoking and pregnancy: Epigenetics and developmental origins of the metabolic syndrome. Birth Defects Res 2019, 111, 1259-1269. [CrossRef]
- Bianco-Miotto, T.; Craig, J.M.; Gasser, Y.P.; van Dijk, S.J.; Ozanne, S.E. Epigenetics and DOHaD: from basics to birth and beyond. J Dev Orig Health Dis 2017, 8, 513-519. [CrossRef]
- De Smidt, J.J.A.; Odendaal, H.J.; Nel, D.G.; Nolan, H.; Du Plessis, C.; Brink, L.T.; Oelofse, A. The effects of in utero exposure to teratogens on organ size: a prospective paediatric study. J Dev Orig Health Dis 2021, 12, 748-757. [CrossRef]
- Health, A.I.o.; Welfare. Australia's mothers and babies; AIHW: Canberra, 2023.
- AIHW. Australian Institute of Health and Welfare 2024. Aboriginal and Torres Strait Islander Health Performance Framework: summary report March 2024. Canberra: AIHW. Accessed [16/7/2024]. 2024.
- He, B.; Zhang, Q.; Guo, Y.; Ao, Y.; Tie, K.; Xiao, H.; Chen, L.; Xu, D.; Wang, H. Prenatal smoke (Nicotine) exposure and offspring's metabolic disease susceptibility in adulthood. Food and Chemical Toxicology 2022, 168, 113384. [CrossRef] [PubMed]
- Lv, J.; Mao, C.; Zhu, L.; Zhang, H.; Pengpeng, H.; Xu, F.; Liu, Y.; Zhang, L.; Xu, Z. The effect of prenatal nicotine on expression of nicotine receptor subunits in the fetal brain. Neurotoxicology 2008, 29, 722-726. [CrossRef]
- Aoyagi, Y.; Momoi, N.; Kanai, Y.; Go, H.; Abe, Y.; Miyazaki, K.; Tomita, Y.; Hayashi, M.; Endo, K.; Mitomo, M.; et al. Prenatal nicotine exposure affects cardiovascular function and growth of the developing fetus. J Obstet Gynaecol Res 2020, 46, 1044-1054. [CrossRef]
- Suter, M.A.; Aagaard, K.M. The impact of tobacco chemicals and nicotine on placental development. Prenat Diagn 2020, 40, 1193-1200. [CrossRef] [PubMed]
- Kataoka, M.C.; Carvalheira, A.P.P.; Ferrari, A.P.; Malta, M.B.; de Barros Leite Carvalhaes, M.A.; de Lima Parada, C.M.G. Smoking during pregnancy and harm reduction in birth weight: a cross-sectional study. BMC Pregnancy and Childbirth 2018, 18, 67. [CrossRef]
- Kunutsor, S.K.; Spee, J.M.; Kieneker, L.M.; Gansevoort, R.T.; Dullaart, R.P.F.; Voerman, A.-J.; Touw, D.J.; Bakker, S.J.L. Self-Reported Smoking, Urine Cotinine, and Risk of Cardiovascular Disease: Findings From the PREVEND (Prevention of Renal and Vascular End-Stage Disease) Prospective Cohort Study. Journal of the American Heart Association 2018, 7, e008726. [CrossRef]
- Abdullah, B.; Muadz, B.; Norizal, M.N.; Ismail, N.; Kornain, N.K.; Kutty, M. Pregnancy outcome and cord blood cotinine level: A cross-sectional comparative study between secondhand smokers and non-secondhand smokers. European Journal of Obstetrics and Gynecology and Reproductive Biology 2017, 214, 86-90. [CrossRef] [PubMed]
- Health, Q. Queensland Health. Townsville Hospital and Health Service Annual Report 2020-2021 [Internet]: State of Queensland; 2021 [cited 2022 Sept 15].
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatrics 2013, 13, 59. [CrossRef] [PubMed]
- Thompson, S.G.; Barlow, R.D.; Wald, N.J.; Van Vunakis, H. How should urinary cotinine concentrations be adjusted for urinary creatinine concentration? Clin Chim Acta 1990, 187, 289-295. [CrossRef] [PubMed]
- D'agostino, R.B.; Belanger, A.; D'Agostino Jr, R.B. A suggestion for using powerful and informative tests of normality. The American Statistician 1990, 44, 316-321. [CrossRef]
- Ashorn, P.; Ashorn, U.; Muthiani, Y.; Aboubaker, S.; Askari, S.; Bahl, R.; Black, R.E.; Dalmiya, N.; Duggan, C.P.; Hofmeyr, G.J.; et al. Small vulnerable newborns-big potential for impact. Lancet 2023, 401, 1692-1706. [CrossRef]
- Voigt, M.; Hermanussen, M.; Wittwer-Backofen, U.; Fusch, C.; Hesse, V. Sex-specific differences in birth weight due to maternal smoking during pregnancy. Eur J Pediatr 2006, 165, 757-761. [CrossRef]
- Owili, P.O.; Muga, M.A.; Kuo, H.W. Gender Difference in the Association between Environmental Tobacco Smoke and Birth Weight in Africa. Int J Environ Res Public Health 2018, 15. [CrossRef] [PubMed]
- Guerby, P.; Garabedian, C.; Berveiller, P.; Legendre, G.; Grangé, G.; Berlin, I. Tobacco and Nicotine Cessation During Pregnancy. Obstet Gynecol 2020, 136, 428-429. [CrossRef]
- Mendelsohn, C.; Gould, G.S.; Oncken, C. Management of smoking in pregnant women. Aust Fam Physician 2014, 43, 46-51.
- Brinchmann, B.C.; Vist, G.E.; Becher, R.; Grimsrud, T.K.; Elvsaas, I.-K.Ø.; Underland, V.; Holme, J.A.; Carlsen, K.C.L.; Kreyberg, I.; Nordhagen, L.S.; et al. Use of Swedish smokeless tobacco during pregnancy: A systematic review of pregnancy and early life health risk. Addiction 2023, 118, 789-803. [CrossRef]
- McDermott, M.M.; Newman, A.B. Remote Research and Clinical Trial Integrity During and After the Coronavirus Pandemic. JAMA 2021, 325, 1935-1936. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).