Submitted:
22 July 2024
Posted:
22 July 2024
You are already at the latest version
Abstract
Keywords:
1. Lassa Fever: Virus and Disease
2. A Devastating Disease in West Africa
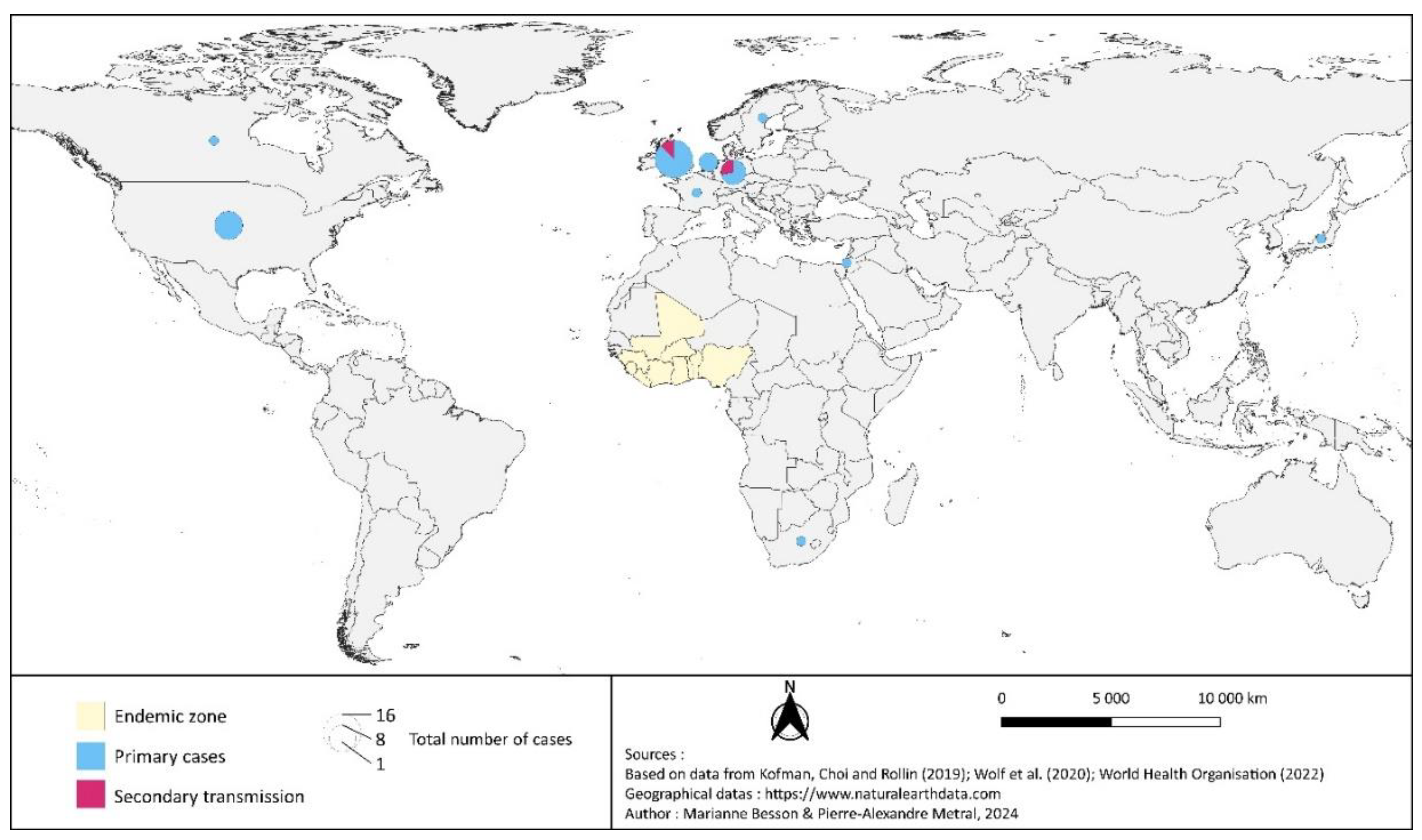
3. An Emerging Disease
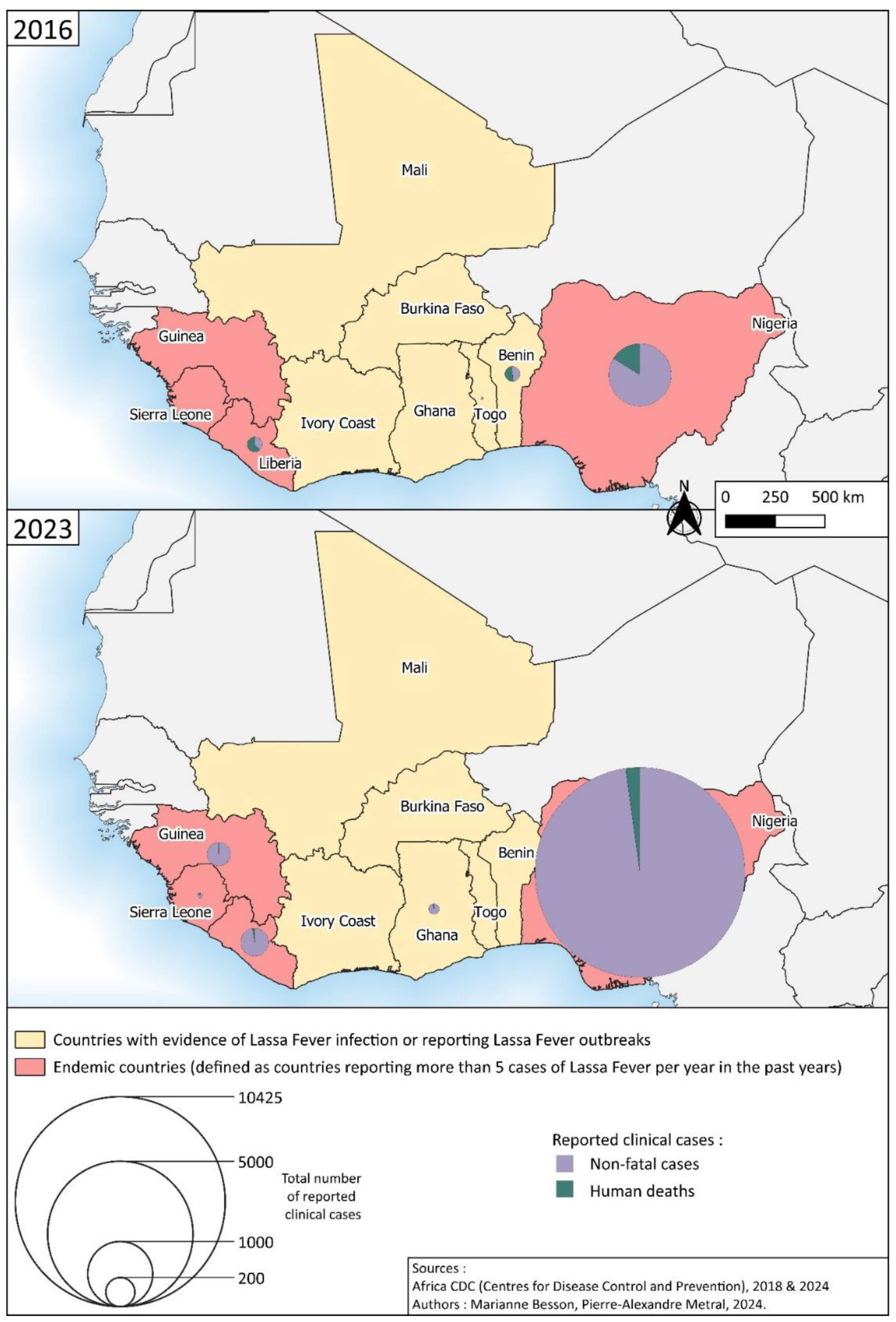
4. Mapping the Spatial Distribution of Lassa Fever
5. Why Lassa Fever Control Measures Are Failing
6. An Emerging Threat
7. Prospects for control of Lassa Fever
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Emonet, S.; Lemasson, J.-J.; Gonzalez, J.-P.; de Lamballerie, X.; Charrel, R.N. Phylogeny and evolution of old world arenaviruses. Virology 2006, 350, 251–257. [Google Scholar] [CrossRef]
- Zapata, J.C.; Salvato, M.S. Arenavirus Variations Due to Host-Specific Adaptation. Viruses 2013, 5, 241–278. [Google Scholar] [CrossRef]
- Ibukun, F.I. Inter-Lineage Variation of Lassa Virus Glycoprotein Epitopes: A Challenge to Lassa Virus Vaccine Development. Viruses 2020, 12, 386. [Google Scholar] [CrossRef]
- Baize, S.; Kaplon, J.; Faure, C.; Pannetier, D.; Georges-Courbot, M.-C.; Deubel, V. Lassa Virus Infection of Human Dendritic Cells and Macrophages Is Productive but Fails to Activate Cells. J. Immunol. 2004, 172, 2861–2869. [Google Scholar] [CrossRef]
- Russier, M.; Pannetier, D.; Baize, S. Immune Responses and Lassa Virus Infection. Viruses 2012, 4, 2766–2785. [Google Scholar] [CrossRef]
- Schaeffer, J.; Carnec, X.; Reynard, S.; Mateo, M.; Picard, C.; Pietrosemoli, N.; Dillies, M.-A.; Baize, S. Lassa virus activates myeloid dendritic cells but suppresses their ability to stimulate T cells. PLOS Pathog. 2018, 14, e1007430. [Google Scholar] [CrossRef]
- Dan-Nwafor, C.C.; Ipadeola, O.; Smout, E.; Ilori, E.; Adeyemo, A.; Umeokonkwo, C.; Nwidi, D.; Nwachukwu, W.; Ukponu, W.; Omabe, E.; et al. A cluster of nosocomial Lassa fever cases in a tertiary health facility in Nigeria: Description and lessons learned, 2018. Int. J. Infect. Dis. 2019, 83, 88–94. [Google Scholar] [CrossRef]
- Marrama, E. Rapid Risk Assessment: Lassa fever in Nigeria, Benin, Togo, Germany and USA, 24 March 2016 [Internet]. 2016 Mar [cited 2023 Oct 22]. Available from: https://www.ecdc.europa.eu/en/publications-data/rapid-risk-assessment-lassa-fever-nigeria-benin-togo-germany-and-usa-24-march.
- Basler, C.F. Molecular pathogenesis of viral hemorrhagic fever. Semin. Immunopathol. 2017, 39, 551–561. [Google Scholar] [CrossRef]
- Africa Centres for Disease Control and Prevention. Lassa Fever [Internet]. Africa CDC. 2018 [cited 2023 Dec 10]. Available from: https://africacdc.org/disease/lassa-fever/.
- Yaro, C.A.; Kogi, E.; Opara, K.N.; Batiha, G.E.-S.; Baty, R.S.; Albrakati, A.; Altalbawy, F.M.A.; Etuh, I.U.; Oni, J.P. Infection pattern, case fatality rate and spread of Lassa virus in Nigeria. BMC Infect. Dis. 2021, 21, 1–9. [Google Scholar] [CrossRef]
- Nigeria Centre for Disease Control. Lassa fever situation report, epidemiological week 46. 24 November - 1 December, 2017 [Internet]. Abuja; 2017 [cited 2023 Dec 28]. Report No.: 46. Available from: https://ncdc.gov.ng/diseases/sitreps/?cat=5&name=An%20update%20of%20Lassa%20fever%20outbreak%20in%20Nigeria.
- Grace, J.-U.A.; Egoh, I.J.; Udensi, N. Epidemiological trends of Lassa fever in Nigeria from 2015-2021: A review. Ther. Adv. Infect. Dis. 2021, 8. [Google Scholar] [CrossRef]
- Nigeria Centre for Disease Control. Lassa Fever situation report, epidemiological week 52. 24 December - 31 December 2018. [Internet]. Abuja; 2018. Report No.: 52. Available from: https://ncdc.gov.ng/diseases/sitreps/?cat=5&name=An%20update%20of%20Lassa%20fever%20outbreak%20in%20Nigeria.
- UK Health Security Agency (UKHSA), Emerging Infections and Zoonoses (EIZ) team. Infectious disease surveillance and monitoring for animal and human health: summary January to June 2023 [Internet]. GOV.UK. 2023 [cited 2023 Dec 28]. Available from: https://www.gov.uk/government/publications/emerging-infections-monthly-summaries/infectious-disease-surveillance-and-monitoring-for-animal-and-human-health-summary-january-to-june-2023.
- European Centre for Disease Prevention and Control. Lassa fever in the Netherlands ex Sierra Leone. 2019; Available from: https://www.ecdc.europa.eu/sites/default/files/documents/RRA-Lassa-fever-in-the-Netherlands-ex-Sierra-Leone_0.pdf.
- McCormick, JB. Epidemiology and control of Lassa fever. Curr Top Microbiol Immunol. 1987;134:69–78.
- Richmond JK, Baglole DJ. Lassa fever: epidemiology, clinical features, and social consequences. BMJ. 2003 Nov 29;327(7426):1271–5.
- Eberhardt, K.A.; Mischlinger, J.; Jordan, S.; Groger, M.; Günther, S.; Ramharter, M. Ribavirin for the treatment of Lassa fever: A systematic review and meta-analysis. Int. J. Infect. Dis. 2019, 87, 15–20. [Google Scholar] [CrossRef]
- World Health Organisation. Lassa Fever, Fact sheets [Internet]. Geneva; 2017 Jul [cited 2020 Oct 15]. Available from: https://www.who.int/news-room/fact-sheets/detail/lassa-fever.
- Bannister, B. Viral haemorrhagic fevers imported into non-endemic countries: risk assessment and management. Br. Med Bull. 2010, 95, 193–225. [Google Scholar] [CrossRef]
- Warner, B.M.; Safronetz, D.; Stein, D.R. Current research for a vaccine against Lassa hemorrhagic fever virus. Drug Des. Dev. Ther. 2018, 12, 2519–2527. [Google Scholar] [CrossRef]
- Isaac, A.B.; Karolina, W.; Temitope, A.A.; Anuska, R.; Joanne, E.; Deborah, A.; Bianca, O.C.; Filip, T.; Zofia, P.; Oluwasegun, O.I.; et al. PROSPECTS OF LASSA FEVER CANDIDATE VACCINES. Afr J Infect Dis. 2022, 16, 46–58. [Google Scholar]
- Monath, T.P.; Newhouse, V.F.; Kemp, G.E.; Setzer, H.W.; Cacciapuoti, A. Lassa Virus Isolation from Mastomys natalensis Rodents during an Epidemic in Sierra Leone. Science 1974, 185, 263–265. [Google Scholar] [CrossRef]
- Oloniniyi, O.K.; Unigwe, U.S.; Okada, S.; Kimura, M.; Koyano, S.; Miyazaki, Y.; Iroezindu, M.O.; Ajayi, N.A.; Chukwubike, C.M.; Chika-Igwenyi, N.M.; et al. Genetic characterization of Lassa virus strains isolated from 2012 to 2016 in southeastern Nigeria. PLOS Neglected Trop. Dis. 2018, 12, e0006971. [Google Scholar] [CrossRef]
- Kafetzopoulou, L.E.; Pullan, S.T.; Lemey, P.; Suchard, M.A.; Ehichioya, D.U.; Pahlmann, M.; Thielebein, A.; Hinzmann, J.; Oestereich, L.; Wozniak, D.M.; et al. Metagenomic sequencing at the epicenter of the Nigeria 2018 Lassa fever outbreak. Science 2019, 363, 74–77. [Google Scholar] [CrossRef]
- World Health Organisation. Lassa fever - Nigeria [Internet]. WHO Disease Outbreak News. 2023 [cited 2023 Dec 10]. Available from: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON463.
- Fisher-Hoch, S.P.; Tomori, O.; Nasidi, A.; I Perez-Oronoz, G.; Fakile, Y.; Hutwagner, L.; McCormick, J.B. Review of cases of nosocomial Lassa fever in Nigeria: the high price of poor medical practice. BMJ 1995, 311, 857–859. [Google Scholar] [CrossRef]
- du Plessis J, Russo I, Child M. A conservation assessment of Mastomys spp. Child MF Roxburgh Linh San E Raimondo Davies-Most HT Ed Red List Mamm South Afr Swazil Lesotho. 2016.
- Schmaljohn, C.; Safronetz, D. Editorial overview: Lassa virus. Curr. Opin. Virol. 2019, 37, VII. [Google Scholar] [CrossRef]
- Simons, D. Lassa fever cases suffer from severe underreporting based on reported fatalities. Int. Heal. 2022, 15, 608–610. [Google Scholar] [CrossRef]
- Shaffer JG, Grant DS, Schieffelin JS, Boisen ML, Goba A, Hartnett JN, et al. Lassa Fever in Post-Conflict Sierra Leone. PLoS Negl Trop Dis. 2014 Mar 20;8(3):e2748.
- Price ME, Fisher-Hoch SP, Craven RB, McCormick JB. A prospective study of maternal and fetal outcome in acute Lassa fever infection during pregnancy. BMJ. 1988 Sep 3;297(6648):584–7.
- Cummins, D.; McCormick, J.B.; Bennett, D.; Samba, J.A.; Farrar, B.; Machin, S.J.; Fisher-Hoch, S.P. Acute Sensorineural Deafness in Lassa Fever. JAMA 1990, 264, 2093–2096. [Google Scholar] [CrossRef]
- Liao, B.S.; Byl, F.M.; Adour, K.K. Audiometric Comparison of Lassa Fever Hearing Loss and Idiopathic Sudden Hearing Loss: Evidence for Viral Cause. Otolaryngol. Neck Surg. 1992, 106, 226–229. [Google Scholar] [CrossRef]
- Mateer, E.J.; Huang, C.; Shehu, N.Y.; Paessler, S. Lassa fever–induced sensorineural hearing loss: A neglected public health and social burden. PLOS Neglected Trop. Dis. 2018, 12, e0006187. [Google Scholar] [CrossRef]
- McPherson B, Brouillette R. Audiology in developing countries. New York: Nova Science Publishers, Inc. Audiology in Developing Countries. 2008. 1 p.
- Macher, A.M.; Wolfe, M.S. Historical Lassa Fever Reports and 30-year Clinical Update. Emerg. Infect. Dis. 2006, 12, 835–837. [Google Scholar] [CrossRef]
- Okogbenin, E.O.; Obagaye, M.O.; Aweh, B.E.; Eriyo, W.O.; Okogbenin, S.A.; Okokhere, P.O. One-Year Retrospective Review of Psychiatric Consultations in Lassa Fever, Southern Nigeria. Emerg. Infect. Dis. 2020, 26, 3091–3093. [Google Scholar] [CrossRef]
- Monath, T.P. Lassa fever: review of epidemiology and epizootiology. Bull World Health Organ 1975, 52, 577–92. [Google Scholar]
- Frame JD, Jr JMB, Gocke DJ, Troup JM. Lassa Fever, a New Virus Disease of Man from West Africa. Am J Trop Med Hyg. 1970 Jul 1;19(4):670–6.
- Andersen, K.G.; Shapiro, B.J.; Matranga, C.B.; Sealfon, R.; Lin, A.E.; Moses, L.M.; Folarin, O.A.; Goba, A.; Odia, I.; Ehiane, P.E.; et al. Clinical Sequencing Uncovers Origins and Evolution of Lassa Virus. Cell 2015, 162, 738–750. [Google Scholar] [CrossRef]
- Manning, J.T.; Forrester, N.; Paessler, S. Lassa virus isolates from Mali and the Ivory Coast represent an emerging fifth lineage. Front. Microbiol. 2015, 6, 1037. [Google Scholar] [CrossRef]
- Lalis, A.; Leblois, R.; Lecompte, E.; Denys, C.; ter Meulen, J.; Wirth, T. The Impact of Human Conflict on the Genetics of Mastomys natalensis and Lassa Virus in West Africa. PLOS ONE 2012, 7, e37068. [Google Scholar] [CrossRef]
- Nigeria Centre for Disease Control. Lassa Fever situation report, epidemiological week 52. 27 December 2020 – 2 January 2021. [Internet]. Abuja; 2021 [cited 2023 Dec 10]. Report No.: 52. Available from: https://ncdc.gov.ng/diseases/sitreps/?cat=5&name=An%20update%20of%20Lassa%20fever%20outbreak%20in%20Nigeria.
- Nigeria Centre for Disease Control. Lassa Fever situation report, epidemiological week 52 [Internet]. Abuja; 2023. Report No.: 52. Available from: https://ncdc.gov.ng/diseases/sitreps/?cat=5&name=An%20update%20of%20Lassa%20fever%20outbreak%20in%20Nigeria.
- Africa Centres for Disease Control and Prevention. Africa CDC Weekly Event Based Surveillance Report, June 2024 [Internet]. 2024 Jun [cited 2024 Jul 21]. Report No.: Africa CDC Weekly Event Based Surveillance Report, 30 June 2024. Available from: https://africacdc.org/download/africa-cdc-weekly-event-based-surveillance-report-june-2024/.
- Whitmer SLM, Strecker T, Cadar D, Dienes HP, Faber K, Patel K, et al. New Lineage of Lassa Virus, Togo, 2016. Emerg Infect Dis. 2018 Mar;24(3):599–602.
- Emmerich, P.; Thome-Bolduan, C.; Drosten, C.; Gunther, S.; Ban, E.; Sawinsky, I.; Schmitz, H. Reverse ELISA for IgG and IgM antibodies to detect Lassa virus infections in Africa. J. Clin. Virol. 2006, 37, 277–281. [Google Scholar] [CrossRef]
- Dzotsi, E.K.; Ohene, S.-A.; Asiedu-Bekoe, F.; Amankwa, J.; Sarkodie, B.; Adjabeng, M.; Thouphique, A.M.; Ofei, A.; Oduro, J.; Atitogo, D.; et al. The first cases of Lassa fever in Ghana. Ghana Med J. 2012, 46, 166–70. [Google Scholar]
- Centres for Disease Control and Prevention. Outbreak Distribution Map | Lassa Fever | CDC [Internet]. 2014 [cited 2023 Dec 10]. Available from: https://www.cdc.gov/vhf/lassa/outbreaks/index.html.
- Atkin, S.; Anaraki, S.; Gothard, P.; Walsh, A.; Brown, D.; Gopal, R.; Hand, J.; Morgan, D. The first case of Lassa fever imported from Mali to the United Kingdom, February 2009. Eurosurveillance 2009, 14, 19145. [Google Scholar] [CrossRef]
- World Health Organisation. Weekly Bulletin on Outbreak and other Emergencies: Week 47: 18 - 24 November 2019. Weekly Bulletin on Outbreaks and other Emergencies World Health Organization. Regional Office for Africa. 2019; Available from: https://iris.who.int/handle/10665/329974 Revue Weekly Bulletin on Outbreaks and other Emergencies.
- Africa Centres for Disease Control and Prevention. Africa CDC Weekly Event Based Surveillance Report, Feb 2024. 2024 Feb. Report No.: Africa CDC Weekly Event Based Surveillance Report, 17 Feb 2024.
- Mylne, A.Q.N.; Pigott, D.M.; Longbottom, J.; Shearer, F.; Duda, K.A.; Messina, J.P.; Weiss, D.J.; Moyes, C.L.; Golding, N.; Hay, S.I. Mapping the zoonotic niche of Lassa fever in Africa. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 483–492. [Google Scholar] [CrossRef]
- Colangelo, P.; Verheyen, E.; Leirs, H.; Tatard, C.; Denys, C.; Dobigny, G.; Duplantier, J.-M.; Brouat, C.; Granjon, L.; Lecompte, E. A mitochondrial phylogeographic scenario for the most widespread African rodent,Mastomys natalensis. Biol. J. Linn. Soc. 2013, 108, 901–916. [Google Scholar] [CrossRef]
- Granjon, L. Mastomys natalensis. IUCN Red List Threat species. 2016.
- Redding, D.W.; Moses, L.M.; Cunningham, A.A.; Wood, J.; Jones, K.E. Environmental-mechanistic modelling of the impact of global change on human zoonotic disease emergence: a case study of Lassa fever. . Macro-mechanistic modelling of zoonotic disease emergence. 2016 Jun 13 [cited 2020 Apr 17]; Available from: https://www.repository.cam.ac.uk/handle/1810/256060. [CrossRef]
- Gibb, R.; Moses, L.M.; Redding, D.W.; Jones, K.E. Understanding the cryptic nature of Lassa fever in West Africa. Ann. Trop. Med. Parasitol. 2017, 111, 276–288. [Google Scholar] [CrossRef]
- Adetola OO, Adebisi MA. Impacts of Deforestation on the Spread of Mastomys natalensis in Nigeria. 2019;11.
- Wulff, H.; Fabiyi, A.; Monath, T.P. Recent isolations of Lassa virus from Nigerian rodents. Bull World Health Organ. 1975, 52, 609–13. [Google Scholar]
- Demby, A.H.; Inapogui, A.; Kargbo, K.; Koninga, J.; Kourouma, K.; Kanu, J.; Coulibaly, M.; Wagoner, K.D.; Ksiazek, T.G.; Peters, C.; et al. Lassa Fever in Guinea: II. Distribution and Prevalence of Lassa Virus Infection in Small Mammals. Vector-Borne Zoonotic Dis. 2001, 1, 283–297. [Google Scholar] [CrossRef]
- Fichet-Calvet, E.; Becker-Ziaja, B.; Koivogui, L.; Günther, S. Lassa Serology in Natural Populations of Rodents and Horizontal Transmission. Vector-Borne Zoonotic Dis. 2014, 14, 665–674. [Google Scholar] [CrossRef]
- Olayemi, A.; Oyeyiola, A.; Obadare, A.; Igbokwe, J.; Adesina, A.S.; Onwe, F.; Ukwaja, K.N.; Ajayi, N.A.; Rieger, T.; Günther, S.; et al. Widespread arenavirus occurrence and seroprevalence in small mammals, Nigeria. Parasites Vectors 2018, 11, 416. [Google Scholar] [CrossRef]
- Gryseels, S.; Baird, S.J.E.; Borremans, B.; Makundi, R.; Leirs, H.; de Bellocq, J.G. When Viruses Don’t Go Viral: The Importance of Host Phylogeographic Structure in the Spatial Spread of Arenaviruses. PLOS Pathog. 2017, 13, e1006073. [Google Scholar] [CrossRef]
- Lalis A, Wirth T. 11 - Mice and Men: an Evolutionary History of Lassa Fever. In: Grandcolas P, Maurel MC, editors. Biodiversity and Evolution [Internet]. Elsevier; 2018 [cited 2020 Feb 15]. p. 189–212. Available from: http://www.sciencedirect.com/science/article/pii/B9781785482779500115.
- Klitting, R.; Kafetzopoulou, L.E.; Thiery, W.; Dudas, G.; Gryseels, S.; Kotamarthi, A.; Vrancken, B.; Gangavarapu, K.; Momoh, M.; Sandi, J.D.; et al. Predicting the evolution of the Lassa virus endemic area and population at risk over the next decades. Nat. Commun. 2022, 13, 1–15. [Google Scholar] [CrossRef]
- Happi, A.N.; Ogunsanya, O.A.; Ayinla, A.O.; Sijuwola, A.E.; Saibu, F.M.; Akano, K.; Nwofoke, C.; Elias, O.T.; Achonduh-Atijegbe, O.; Daodu, R.O.; et al. Lassa virus in novel hosts: insights into the epidemiology of lassa virus infections in southern Nigeria. Emerg. Microbes Infect. 2024, 13, 2294859. [Google Scholar] [CrossRef]
- Arruda, L.B.; Free, H.B.; Simons, D.; Ansumana, R.; Elton, L.; Haider, N.; Honeyborne, I.; Asogun, D.; McHugh, T.D.; Ntoumi, F.; et al. Current sampling and sequencing biases of Lassa mammarenavirus limit inference from phylogeography and molecular epidemiology in Lassa fever endemic regions. PLOS Glob. Public Heal. 2023, 3, e0002159. [Google Scholar] [CrossRef]
- Li, Y. Genetic basis underlying Lassa fever endemics in the Mano River region, West Africa. Virology 2023, 579, 128–136. [Google Scholar] [CrossRef]
- Olayemi, A.; Cadar, D.; Magassouba, N.; Obadare, A.; Kourouma, F.; Oyeyiola, A.; Fasogbon, S.; Igbokwe, J.; Rieger, T.; Bockholt, S.; et al. New Hosts of The Lassa Virus. Sci. Rep. 2016, 6, 25280–25280. [Google Scholar] [CrossRef]
- Ehichioya, D.U.; Dellicour, S.; Pahlmann, M.; Rieger, T.; Oestereich, L.; Becker-Ziaja, B.; Cadar, D.; Ighodalo, Y.; Olokor, T.; Omomoh, E.; et al. Phylogeography of Lassa Virus in Nigeria. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Clegg, J.; Lloyd, G. VACCINIA RECOMBINANT EXPRESSING LASSA-VIRUS INTERNAL NUCLEOCAPSID PROTEIN PROTECTS GUINEAPIGS AGAINST LASSA FEVER. Lancet 1987, 330, 186–188. [Google Scholar] [CrossRef]
- Hallam, H.J.; Hallam, S.; Rodriguez, S.E.; Barrett, A.D.T.; Beasley, D.W.C.; Chua, A.; Ksiazek, T.G.; Milligan, G.N.; Sathiyamoorthy, V.; Reece, L.M. Baseline mapping of Lassa fever virology, epidemiology and vaccine research and development. npj Vaccines 2018, 3, 11. [Google Scholar] [CrossRef]
- Akokuwebe, M.E.; Idemudia, E.S. A Comparative Cross-Sectional Study of the Prevalence and Determinants of Health Insurance Coverage in Nigeria and South Africa: A Multi-Country Analysis of Demographic Health Surveys. Int. J. Environ. Res. Public Heal. 2022, 19, 1766. [Google Scholar] [CrossRef]
- Sasu. Nigeria: health insurance coverage, by area and gender | Statista [Internet]. 2022 [cited 2023 Dec 24]. Available from: https://www.statista.com/statistics/1124757/health-insurance-coverage-in-nigeria-by-area-and-gender/.
- World Bank. Nigeria Overview: Development news, research, data | World Bank [Internet]. The World Bank. 2023 [cited 2023 Dec 24]. Available from: https://www.worldbank.org/en/country/nigeria/overview.
- Okpani, A.I.; Abimbola, S. Operationalizing universal health coverage in Nigeria through social health insurance. Niger. Med J. 2015, 56, 305–310. [Google Scholar] [CrossRef]
- Eke, C. Nigeria - Healthcare [Internet]. Official Website of the USA International Trade Association. 2023 [cited 2023 Dec 24]. Available from: https://www.trade.gov/country-commercial-guides/nigeria-healthcare.
- United Nations Development Programme. HDR21-22_Statistical_Annex_HDI_Table.xlsx [Internet]. 2022 [cited 2023 Dec 27]. Available from: https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fhdr.undp.org%2Fsites%2Fdefault%2Ffiles%2F2021-22_HDR%2FHDR21-22_Statistical_Annex_HDI_Table.xlsx&wdOrigin=BROWSELINK.
- Onwujekwe, O.; Agwu, P.; Orjiakor, C.; McKee, M.; Hutchinson, E.; Mbachu, C.; Odii, A.; Ogbozor, P.; Obi, U.; Ichoku, H.; et al. Corruption in Anglophone West Africa health systems: a systematic review of its different variants and the factors that sustain them. Heal. Policy Plan. 2019, 34, 529–543. [Google Scholar] [CrossRef]
- Wogu, J.O. Mass media awareness campaign and the prevention of the spread of Lassa fever in the rural communities of Ebonyi State, Nigeria: Impact evaluation. J. Public Heal. Afr. 2018, 9, 179–184. [Google Scholar] [CrossRef]
- Bonwitt, J.; Kelly, A.H.; Ansumana, R.; Agbla, S.; Sahr, F.; Saez, A.M.; Borchert, M.; Kock, R.; Fichet-Calvet, E. Rat-atouille: A Mixed Method Study to Characterize Rodent Hunting and Consumption in the Context of Lassa Fever. Ecohealth 2016, 13, 234–247. [Google Scholar] [CrossRef]
- Bonwitt J, Dawson M, Kandeh M, Ansumana R, Sahr F, Brown H, et al. Unintended consequences of the ‘bushmeat ban’ in West Africa during the 2013–2016 Ebola virus disease epidemic. Soc Sci Med. 2018 Mar 1;200:166–73.
- Woyessa, A.B.; Maximore, L.; Keller, D.; Dogba, J.; Pajibo, M.; Johnson, K.; Saydee, E.; Monday, J.; Tuopileyi, R.; Mahmoud, N. Lesson learned from the investigation and response of Lassa fever outbreak, Margibi County, Liberia, 2018: case report. BMC Infect. Dis. 2019, 19, 1–6. [Google Scholar] [CrossRef]
- Schroeder, L.F.; Amukele, T. Medical Laboratories in Sub-Saharan Africa That Meet International Quality Standards. Am. J. Clin. Pathol. 2014, 141, 791–795. [Google Scholar] [CrossRef]
- Boisen, M.L.; Uyigue, E.; Aiyepada, J.; Siddle, K.J.; Oestereich, L.; Nelson, D.K.S.; Bush, D.J.; Rowland, M.M.; Heinrich, M.L.; Eromon, P.; et al. Field evaluation of a Pan-Lassa rapid diagnostic test during the 2018 Nigerian Lassa fever outbreak. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Mazzola, L.T.; Kelly-Cirino, C. Diagnostics for Lassa fever virus: a genetically diverse pathogen found in low-resource settings. BMJ Glob. Heal. 2019, 4 (Suppl. 2), e001116. [Google Scholar] [CrossRef]
- Akpede GO, Asogun DA, Okogbenin SA, Dawodu SO, Momoh MO, Dongo AE, et al. Caseload and Case Fatality of Lassa Fever in Nigeria, 2001–2018: A Specialist Center’s Experience and Its Implications. Front Public Health [Internet]. 2019 Jun 25 [cited 2020 Apr 5];7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6603170/.
- Ijarotimi, I.T.; Ilesanmi, O.S.; Aderinwale, A.; Abiodun-Adewusi, O.; Okon, I.-M. Knowledge of Lassa fever and use of infection prevention and control facilities among health care workers during Lassa fever outbreak in Ondo State, Nigeria. Pan Afr Med J [Internet]. 2018 May 24 [cited 2019 Nov 12];30. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6125309/. [CrossRef]
- McKendrick, J.Q.; Tennant, W.S.D.; Tildesley, M.J. Modelling seasonality of Lassa fever incidences and vector dynamics in Nigeria. PLOS Neglected Trop. Dis. 2023, 17, e0011543. [Google Scholar] [CrossRef]
- Avenant, N.L.; Watson, J.P.; Schulze, E. Correlating small mammal community characteristics and habitat integrity in the Caledon Nature Reserve, South Africa. Mammalia 2008, 72, 186–191. [Google Scholar] [CrossRef]
- MacFadyen DN, Avenant NL, Merwe M van der, Bredenkamp GJ. The Influence of Fire on Rodent Abundance at the N’washitshumbe Enclosure Site, Kruger National Park, South Africa†. Afr Zool. 2012 Apr;47(1):138–46.
- Balogun, O.O.; Akande, O.W.; Hamer, D.H. Lassa Fever: An Evolving Emergency in West Africa. Am. J. Trop. Med. Hyg. 2021, 104, 466–473. [Google Scholar] [CrossRef]
- Wolf, T.; Ellwanger, R.; Goetsch, U.; Wetzstein, N.; Gottschalk, R. Fifty years of imported Lassa fever: a systematic review of primary and secondary cases. J. Travel Med. 2020, 27. [Google Scholar] [CrossRef]
- World Health Organisation. Lassa fever – United Kingdom of Great Britain and Northern Ireland [Internet]. Disease Outbreak News. 2022 [cited 2023 Dec 27]. Available from: https://www.who.int/emergencies/disease-outbreak-news/item/lassa-fever-united-kingdom-of-great-britain-and-northern-ireland.
- Brosh-Nissimov, T. Lassa fever: another threat from West Africa. Disaster Mil. Med. 2016, 2, 8. [Google Scholar] [CrossRef]
- Tuite, A.R.; Watts, A.G.; Kraemer, M.U.G.; Khan, K.; Bogoch, I.I. Potential for Seasonal Lassa Fever Case Exportation from Nigeria. Am. J. Trop. Med. Hyg. 2019, 100, 647–651. [Google Scholar] [CrossRef]
- World Health Organisation. Lassa Fever – Germany [Internet]. Disease Outbreak News. 2016 [cited 2023 Dec 28]. Available from: https://www.who.int/emergencies/disease-outbreak-news/item/23-march-2016-lassa-fever-germany-en.
- World Health Organisation. Lassa Fever – Togo [Internet]. Disease Outbreak News. 2016 [cited 2023 Dec 28]. Available from: https://www.who.int/emergencies/disease-outbreak-news/item/23-march-2016-lassa-fever-togo-en.
- Njuguna, C.; Vandi, M.; Liyosi, E.; Githuku, J.; Wurie, A.; Njeru, I.; Raftery, P.; Amuzu, C.; Maruta, A.; Musoke, R.; et al. A challenging response to a Lassa fever outbreak in a non endemic area of Sierra Leone in 2019 with export of cases to The Netherlands. Int. J. Infect. Dis. 2022, 117, 295–301. [Google Scholar] [CrossRef]
- Ihekweazu, C. National Guidelines for Lassa Fever case management [Internet]. Nigeria: National Centre for Disease Control; 2018. Available from: https://ncdc.gov.ng/themes/common/docs/protocols/92_1547068532.pdf.
- Mehand, M.S.; Al-Shorbaji, F.; Millett, P.; Murgue, B. The WHO R&D Blueprint: 2018 review of emerging infectious diseases requiring urgent research and development efforts. Antivir. Res. 2018, 159, 63–67. [Google Scholar] [CrossRef]
- Prioritizing Diseases for Research and Development in Emergency Contexts. Available online: https://www.who.int/activities/prioritizing-diseases-for-research-and-development-in-emergency-contexts (accessed on 16 February 2021).
- Bernasconi, V.; Kristiansen, P.A.; Whelan, M.; Román, R.G.; Bettis, A.; Yimer, S.A.; Gurry, C.; Andersen, S.R.; Yeskey, D.; Mandi, H.; et al. Developing vaccines against epidemic-prone emerging infectious diseases. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz 2019, 63, 65–73. [Google Scholar] [CrossRef]
- CEPI. Testing the tests: Scientists seek out best on-the-spot diagnostics for deadly Nipah and Lassa | CEPI [Internet]. CEPI official website. 2024 [cited 2024 Jul 21]. Available from: https://cepi.net//testing-tests-scientists-seek-out-best-spot-diagnostics-deadly-nipah-and-lassa.
- Epidemic Preparedness Innovations. Enable Epi Study • Epidemic Preparedness Innovations [Internet]. The Global Health Network. 2024 [cited 2024 Jul 21]. Available from: https://epi.tghn.org/epidemiology/epi-studies/.
- Goios A, Varma A, Kagia C, Otiende M, Suykerbuyk P. Enable Lassa Research Programme Mid-term Workshop. Abuja, Nigeria; 2022.
- Mueller-Langer, F. Neglected infectious diseases: Are push and pull incentive mechanisms suitable for promoting drug development research? Health Econ Policy Law. 2013 Apr;8(2):185–208.
- Garry, RF. Lassa fever - the road ahead. Nat Rev Microbiol. 2023 Feb;21(2):87–96.
- Melnik, LI. Lassa Virus Countermeasures. Curr Top Microbiol Immunol. 2023;440:111–45.
- Oestereich, L.; Rieger, T.; Lüdtke, A.; Ruibal, P.; Wurr, S.; Pallasch, E.; Bockholt, S.; Krasemann, S.; Muñoz-Fontela, C.; Günther, S. Efficacy of Favipiravir Alone and in Combination With Ribavirin in a Lethal, Immunocompetent Mouse Model of Lassa Fever. J. Infect. Dis. 2015, 213, 934–938. [Google Scholar] [CrossRef]
- Rosenke, K.; Feldmann, H.; Westover, J.B.; Hanley, P.W.; Martellaro, C.; Feldmann, F.; Saturday, G.; Lovaglio, J.; Scott, D.P.; Furuta, Y.; et al. Use of Favipiravir to Treat Lassa Virus Infection in Macaques. Emerg. Infect. Dis. 2018, 24, 1696–1699. [Google Scholar] [CrossRef]
- Raabe, V.N.; Kann, G.; Ribner, B.S.; Morales, A.; Varkey, J.B.; Mehta, A.K.; Lyon, G.M.; Vanairsdale, S.; Faber, K.; Becker, S.; et al. Favipiravir and Ribavirin Treatment of Epidemiologically Linked Cases of Lassa Fever. Clin. Infect. Dis. 2017, 65, 855–859. [Google Scholar] [CrossRef]
- Cashman, K.A.; Wilkinson, E.R.; Posakony, J.; Madu, I.G.; Tarcha, E.J.; Lustig, K.H.; Korth, M.J.; Bedard, K.M.; Amberg, S.M. Lassa antiviral LHF-535 protects guinea pigs from lethal challenge. Sci. Rep. 2022, 12, 1–7. [Google Scholar] [CrossRef]
- Amberg, S.M.; Snyder, B.; Vliet-Gregg, P.A.; Tarcha, E.J.; Posakony, J.; Bedard, K.M.; Heald, A.E. Safety and Pharmacokinetics of LHF-535, a Potential Treatment for Lassa Fever, in Healthy Adults. Antimicrob. Agents Chemother. 2022, 66, e0095122. [Google Scholar] [CrossRef]
- Cross, R.W.; Heinrich, M.L.; Fenton, K.A.; Borisevich, V.; Agans, K.N.; Prasad, A.N.; Woolsey, C.; Deer, D.J.; Dobias, N.S.; Rowland, M.M.; et al. A human monoclonal antibody combination rescues nonhuman primates from advanced disease caused by the major lineages of Lassa virus. Proc. Natl. Acad. Sci. 2023, 120. [Google Scholar] [CrossRef]
- Cross, R.W.; Fenton, K.A.; Woolsey, C.; Prasad, A.N.; Borisevich, V.; Agans, K.N.; Deer, D.J.; Dobias, N.S.; Fears, A.C.; Heinrich, M.L.; et al. Monoclonal antibody therapy protects nonhuman primates against mucosal exposure to Lassa virus. Cell Rep. Med. 2024, 5, 101392. [Google Scholar] [CrossRef]
- ALIMA. First-ever global alliance of researchers, health workers, and humanitarians join forces to fight the deadly Lassa fever virus - ALIMA - The Alliance for Medical Action [Internet]. 2024 [cited 2024 Jul 21]. Available from: https://alima.ngo/en/press-releases/lassa-fever-alliance-virus/.
- Sulis, G.; Peebles, A.; Basta, N.E. Lassa fever vaccine candidates: A scoping review of vaccine clinical trials. Trop. Med. Int. Heal. 2023, 28, 420–431. [Google Scholar] [CrossRef]
- Tschismarov, R.; Van Damme, P.; Germain, C.; De Coster, I.; Mateo, M.; Reynard, S.; Journeaux, A.; Tomberger, Y.; Withanage, K.; Haslwanter, D.; et al. Immunogenicity, safety, and tolerability of a recombinant measles-vectored Lassa fever vaccine: a randomised, placebo-controlled, first-in-human trial. Lancet 2023, 401, 1267–1276. [Google Scholar] [CrossRef]
- Baden M, Kieh L, Fitz-Patrick D, Diemert D, Mutua G. First Safety & Immunogenicity Data from a FIH, Placebo-controlled, Dose-escalation Trial of a Recombinant Vesicular Stomatitis Virus-based Lassa Fever Vaccine in Healthy Adults [Internet]. 2023 [cited 2024 Jul 21]; Chicago. Available from: https://www.iavi.org/wp-content/uploads/2023/11/C102_ASTMH_Poster_Chicago2023.pdf.
- Abiola, A. Time to hope for a Lassa fever vaccine? [Internet]. GAVI, the Vaccine Alliance. 2024 [cited 2024 Jul 21]. Available from: https://www.gavi.org/vaccineswork/time-hope-lassa-fever-vaccine.
- Pan African Clinical Trials Registry. A Phase 1 Randomized, Blinded, Placebo Controlled, Dose-Escalation and Dosing Regimen Selection Study to Evaluate the Safety and Immunogenicity of rVSV-Vectored Lassa Virus Vaccine in Healthy Adults at Multiple Sites in West Africa [Internet]. Pan African Clinical Trials Registry. 2021 [cited 2024 Jul 21]. Available from: https://pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=14618.
- ClinicalTrials. No Study Results Posted | Safety, Tolerability and Immunogenicity of INO-4500 in Healthy Volunteers | ClinicalTrials.gov [Internet]. ClinicalTrials.gov (US government). 2020 [cited 2024 Jul 21]. Available from: https://clinicaltrials.gov/study/NCT03805984?tab=results.
- Fahrni, M.L.; Ismail, I.A.-N.; Refi, D.M.; Almeman, A.; Yaakob, N.C.; Saman, K.M.; Mansor, N.F.; Noordin, N.; Babar, Z.-U. Management of COVID-19 vaccines cold chain logistics: a scoping review. J. Pharm. Policy Pr. 2022, 15, 1–14. [Google Scholar] [CrossRef]
- Leach M, Fairhead J. Understandings of immunization: some west African perspectives. Bull World Health Organ. 2008 Jun;86(6):418.
- Michael, C.A.; Ogbuanu, I.U.; Storms, A.D.; Ohuabunwo, C.J.; Corkum, M.; Ashenafi, S.; Achari, P.; Biya, O.; Nguku, P.; Mahoney, F.; et al. An Assessment of the Reasons for Oral Poliovirus Vaccine Refusals in Northern Nigeria. J. Infect. Dis. 2014, 210, S125–S130. [Google Scholar] [CrossRef]
- World Health Organisation. Report of the SAGE Working Group on Vaccine Hesitancy [Internet]. 2014 Nov [cited 2024 May 3]. Available from: https://www.asset-scienceinsociety.eu/sites/default/files/sage_working_group_revised_report_vaccine_hesitancy.pdf.
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Cutler, S.J.; Fooks, A.R.; van der Poel, W.H.M. Public Health Threat of New, Reemerging, and Neglected Zoonoses in the Industrialized World. Emerg. Infect. Dis. 2010, 16, 1–7. [Google Scholar] [CrossRef]
- Pepin M, Tordo N. Emerging and re-emerging animal viruses. Foreword. Vet Res. 2010;41(6):69.
- El Amri H, Boukharta M, Zakham F, Ennaji MM. Emergence and Reemergence of Viral Zoonotic Diseases: Concepts and Factors of Emerging and Reemerging Globalization of Health Threats. Emerg Reemerging Viral Pathog. 2020;619–34.
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.-F.; et al. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef]
- Sinclair, JR. Importance of a One Health approach in advancing global health security and the Sustainable Development Goals. Rev Sci Tech Int Off Epizoot. 2019 May;38(1):145–54.
- Cunningham, A.A.; Scoones, I.; Wood, J.L.N. One Health for a changing world: new perspectives from Africa. Philos. Trans. R. Soc. B: Biol. Sci. 2017, 372, 20160162. [Google Scholar] [CrossRef]
- Abubakar, I.R. Access to Sanitation Facilities among Nigerian Households: Determinants and Sustainability Implications. Sustainability 2017, 9, 547. [Google Scholar] [CrossRef]
- Yaya, S.; Hudani, A.; Udenigwe, O.; Shah, V.; Ekholuenetale, M.; Bishwajit, G. Improving Water, Sanitation and Hygiene Practices, and Housing Quality to Prevent Diarrhea among Under-Five Children in Nigeria. Trop. Med. Infect. Dis. 2018, 3, 41. [Google Scholar] [CrossRef]
- Akokuwebe, M.E.; Idemudia, E.S. Fraud within the Nigerian health system, a double threat for resilience of a health system and the response to the COVID-19 pandemic: a review. Pan Afr. Med J. 2023, 45, 116. [Google Scholar] [CrossRef]
- Kalbarczyk, A.; Davis, W.; Kalibala, S.; Geibel, S.; Yansaneh, A.; Martin, N.A.; Weiss, E.; Kerrigan, D.; Manabe, Y.C. Research Capacity Strengthening in Sub-Saharan Africa: Recognizing the Importance of Local Partnerships in Designing and Disseminating HIV Implementation Science to Reach the 90–90–90 Goals. AIDS Behav. 2019, 23, 206–213. [Google Scholar] [CrossRef]
- Dalhat, M.M.; Olayinka, A.; Meremikwu, M.M.; Dan-Nwafor, C.; Iniobong, A.; Ntoimo, L.F.; Onoh, I.; Mba, S.; Ohonsi, C.; Arinze, C.; et al. Epidemiological trends of Lassa fever in Nigeria, 2018–2021. PLOS ONE 2022, 17, e0279467. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




