1. Introduction
Wetlands are areas of land that are permanently or seasonally saturated with water, that provide numerous ecosystem services essential for supporting the lives of several organisms [
1]. These ecosystems act as natural filters that purify water, remove contaminants, and act as flood controllers [
2,
3]. Additionally, they play a crucial role in climate regulation, helping to maintain stable temperatures and humidity levels, and they store large amounts of carbon, contributing to the mitigation of climate change effects [
1,
4,
5].
Despite their environmental importance, wetlands have suffered dramatic declines. Since 1900, it is estimated that nearly 64% of the world’s wetlands have disappeared. This loss is due to various human activities, such as urbanization, intensive agriculture, and pollution, which have led to the degradation and disappearance of these ecosystems [
6,
7]. To address this crisis, the Ramsar Convention, signed in 1971, aims to conserve and wisely use wetlands through local and national actions and international cooperation [
8]. Chile joined the Ramsar Convention in 1981 and currently has 16 Ramsar sites. Additionally, in 2022, Chile enacted the Urban Wetlands Law (Law 21.202), aiming to protect these valuable ecosystems located in de urban radio. In Chile, wetlands cover approximately 6% of the national territory, which represents more than 40,000 wetlands, including around 1,400 urban. These wetlands not only offer ecological benefits but also provide crucial services to local communities, such as water provision, recreation, and biodiversity conservation [
1]. Regardless of its numerous benefits, Chilean urban wetlands are under continuous threats and constantly subjected to anthropogenic pressure. The lack of environmental awareness and education, together with economic activities and pollution, endangers the integrity of these ecosystems.
Zoonoses, defined as diseases transmitted from animals to humans [
9], are responsible for millions of cases and deaths annually worldwide. The Pan American Health Organization (PAHO) has warned about the serious threat zoonotic diseases pose to human health. It is estimated that around 60% of the known human pathogens have a zoonotic origin [
10]. In Chile, some zoonoses are monitored by government entities. For example, two of the most prevalent zoonoses in southern Chile are brucellosis and leptospirosis, caused by bacteria of the genus
Brucella and
Leptospira respectively [
11,
12]. Many of the etiological agents of zoonotic diseases can be dispersed through water, hence, it is necessary to maintain strict control of water quality and implement an interdisciplinary approach to avoid pathogen dispersion [
13]. Collaborative and interdisciplinary context seeks to sustainably balance and optimize human, animal, and ecosystem health. Recently, microbial surveillance using molecular approaches has been postulated as crucial for monitoring the presence of zoonotic pathogens in the environment [
14,
15]. In Chile, the Agricultural and Livestock Service (‘Servicio Agrícola Ganadero’, SAG), is the main governmental agency that monitors the health issues related to agricultural and livestock activities.
Several factors such as habitat fragmentation, environmental pollution, and climate change have increased the presence of emerging infectious diseases in the last century [
16]. The "Tripartite Guide to Address Zoonotic Diseases in Countries," published in 2021 in collaboration with the FAO, OIE, and WHO, offers guidance to address zoonotic diseases and other health emergencies, indicating that disease surveillance zoonotic infections in the environment should be one of the main tasks to prevent the dispersion of these [
17]. For example, Fresia et al., provided evidence of the prevalence of antimicrobial resistance genomics elements in coastal environments, located close to Montevideo, Uruguay, including beach and wastewater samples, characterizing pathogenic bacterial communities and their virulence repertoires. The evidence shows the presence of antibiotic-resistance genes in both wastewater and beach environments [
18]. Recent studies have identified several bacteria of sanitary interest in water bodies of the Los Lagos Region in Chile, highlighting the need to monitor and protect these ecosystems to prevent health risks [
19,
20]. For that purpose, the information recovered from DNA sequencing is an excellent tool, which provides data about taxonomic diversity and gene function [
21,
22]. In fact, the approach known as shotgun metagenomics, sequences all genetic material in a sample, enabling analysis of microbial diversity and functions at the same time. This improves the understanding of bacterial communities and reveals functional information such as the presence of antimicrobial resistance genes and genetic virulence mechanisms. Identifying and monitoring these genes is essential for managing and mitigating environmental biohazards reducing the risk of disease transmission. This work investigates the presence of antibiotic resistance genes (ARGs) and virulence factors (VFs) in various wetland environments. By employing shotgun metagenomic and bioinformatic analysis, we detected a wide range of ARGs and VFs in water recovered from urban wetlands. Our findings suggest that impaired wetlands can serve as reservoirs for these genetic elements, potentially facilitating the spread of antibiotic resistance and accumulating microbial pathogens. This research underscores the importance of monitoring wetlands to assess and mitigate public health risks associated with antimicrobial resistance and infectious diseases.
2. Materials and Methods
2.1. Area of Study and Sample Collection
A total of 13 urban wetlands were studied, located in the urban radio of the cities Osorno (wetlands namely Las Quemas and Ovejería), Llanquihue (namely El Loto, Baquedano, Las Ranas, and Teodosio Sarao), Puerto Varas (namely Marina and Quebrada Parque), and Puerto Montt (namely Luis Ebel, La Paloma, Antiñir, Rupallán, and Mirasol) of the Los Lagos region of Chile (
Figure Supplementary 1) were sampled. The wetlands namely Luis Ebel and Teodosio Sarao were sampled two times at different moments. A volume of 3 liters per sample site was taken using a sterile 1L glass jar. The samples were transported cold for subsequent processing at the Institutional Laboratory of Universidad San Sebastián, located in the city of Puerto Montt, Chile. The samples were individually processed through MCE (Mixed Cellulose Esters) filters with a pore size of 0.22 µm and a diameter of 47 mm. For this purpose, a negative pressure system was used, drawing the microorganisms through the filter. Subsequently, each filter was stored frozen at -80 °C until DNA extraction.
2.2. Genomic Material Recuperation and DNA Sequencing
The stored MCE filters were used for DNA extraction employing AccuPrep Genomic DNA Extraction Kit (Bioneer #K-3032, Korea), following manufacturer protocol, with few modifications. Filters were submerged in 500 μL DNA Extraction buffer and stirred to release microbial cells. An enzymatic digestion was used to facilitate microbial cell wall rupture, using 20 μL of lysozyme (20 mg/mL) and 20 μL of proteinase K (20 mg/mL) [
23], incubating the suspension for 1 h at 37 °C and 1 h at 55 °C respectively. After enzymatic digestion, the steps provided by the manufacturer for bacterial DNA extraction were followed. The obtained DNA was quantified by absorbance, and the ratios 260/280 nm were calculated to assess the purity of DNA obtained, and its integrity was evaluated through 1% agarose gel electrophoresis. Before DNA sequencing, we test the amplification capacity of DNA using 16S bacterial universal PCR. A total of 1 μg of DNA was sent to Novogene (USA) genomic service for shotgun metagenomic sequencing. DNA was sequenced by paired-end (2x150 335 bp) reads using the Illumina NovaSeq 6000 platform with an output of 6 GB per sample.
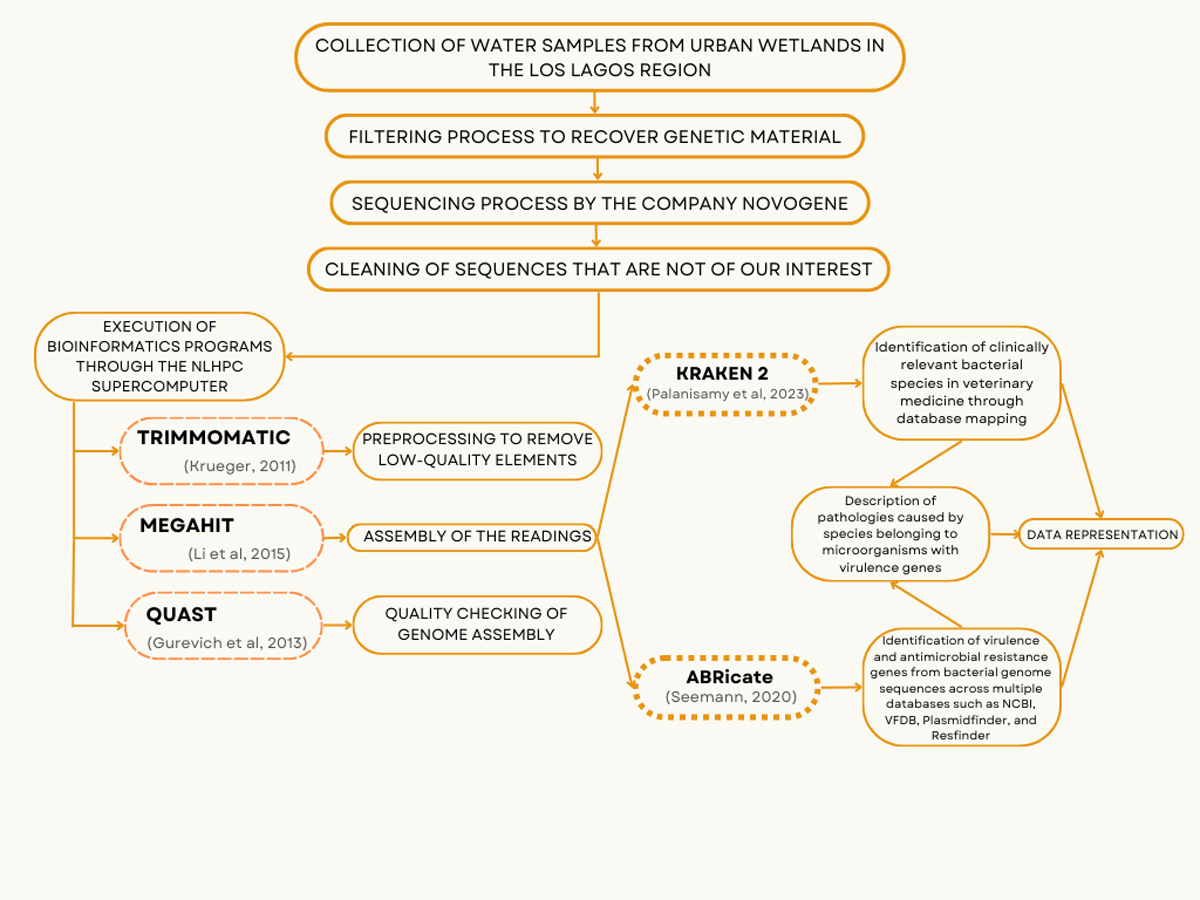
2.3. Metagenomic Data Analysis and Functional Characterization
Raw data obtained from the sequencing provider was initially inspected with FastQC [
24], and then reads were filtered and trimmed using Trimmomatic [
25] using the following parameters: LEADING:20, TRAILING:20, SLIDINGWINDOW:5:20, AVGQUAL:20, and MINLEN:90, followed by the application of Bowtie2 to screen out the contaminant DNA sequences from human and viruses [
26]. Filtered data were assembled using MegaHit [
27] and the quality of conting obtained was inspected using Quast [
28]. Taxonomic profiling was assessed using Kraken2 [
29], keeping the taxonomic assignation with over 50 hits by sample, while antimicrobial resistance and virulence genes were inspected using ABRicate [
30], utilizing the ResFinder [
31], NCBI AMRFinderPlus [
32] and VFdb [
33] databases. Data obtained was imported to R statistical language [
34] for further analysis and representation using ggplot2 package [
35].
3. Results
3.1. Bacterial Taxonomy in Urban Wetlands
Shotgun metagenomic and bioinformatic studies indicate a heterogeneous bacterial composition and abundance between the studied urban wetlands (
Figure 1 and
Supplementary Table 1). A total of 6493 bacterial species were identified (
Supplementary Table 1), including the genus
Pseudomonas,
Serratia,
Rahnella,
Shewanella, and
Aeromonas which has the most abundant species in all the wetlands analyzed. In the wetlands located in Osorno, the most abundant species were
Rahnella aceris,
Flavobacterium ammonificans,
Limnohabitans sp. TEGF004,
Stenotrophomonas rhizophila, and
Pseudomonas sivasensis, while the abundant species in Llanquihue wetlands were
Pseudomonas fragi,
Pseudomonas psychrophila,
Pseudomonas monsensis,
Serratia proteamaculans, and
Shewanella baltica. Moreover, in the Puerto Varas wetland, the bacterial species with the mots abundance were
Pseudomonas sp. B21-035,
Pseudomonas protegens,
Erwinia rhapontici, Rahnella aceris, and
Serratia proteamaculans, while in Puerto Montt wetlands were observed species namely
Serratia liquefaciens,
Serratia fonticola,
Pseudomonas fragi,
Pseudomonas monsensis and
Serratia grimesii (
Supplementary Table 1). Overall, in all the wetlands, the bacteria of the genus Pseudomonas were observed as the most abundant species.
3.2. Identification of Bacterial Microorganisms of Importance in Veterinary and Human Medicine
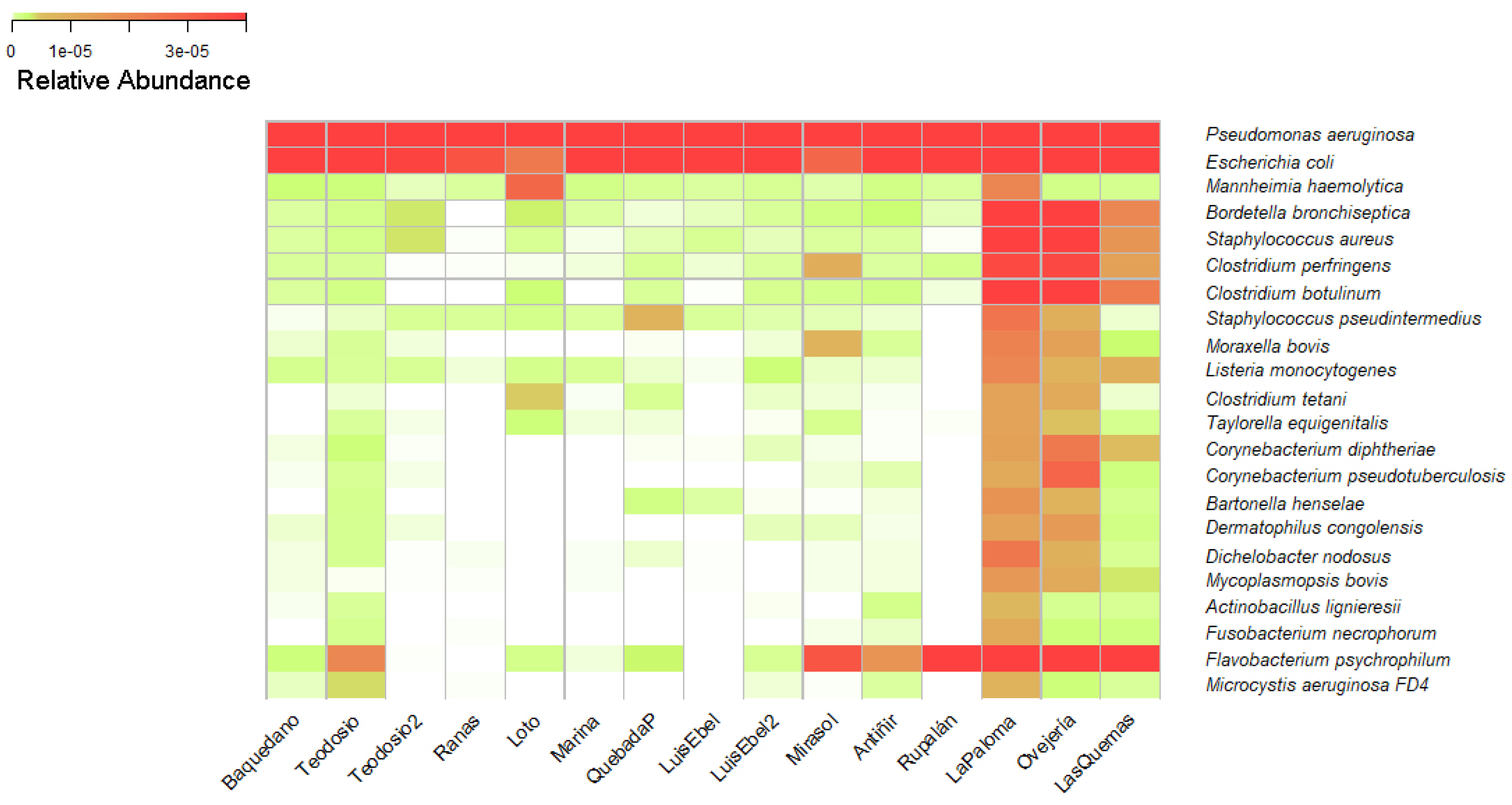
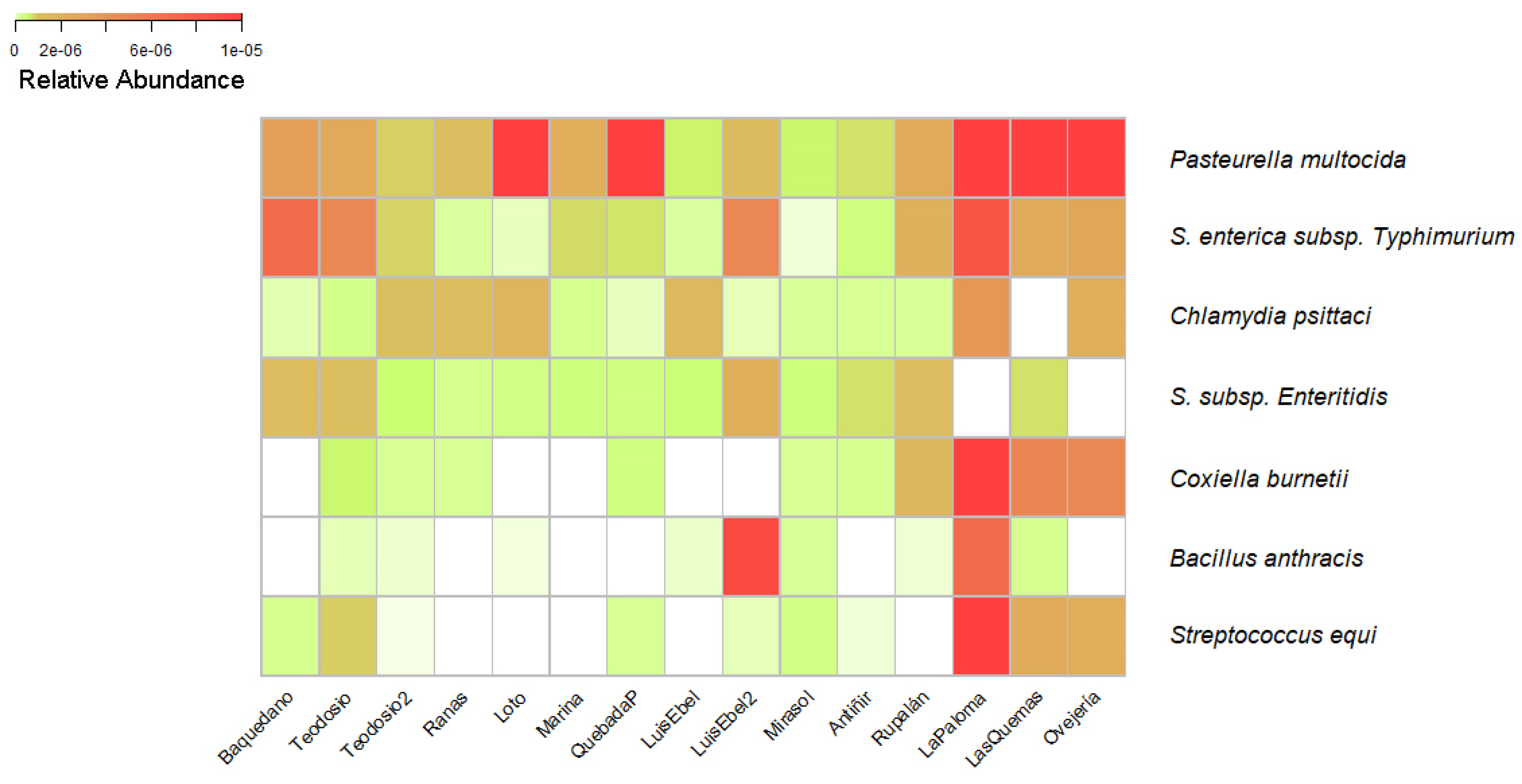
Bacterial species of importance in Veterinary Medicine were searched within the taxonomic data obtained, which also are included in the list of mandatory notifiable diseases monitored by the Agriculture Livestock Service of Chile (SAG) [
36]. From the mentioned list of diseases, some etiologic agents were identified at the species level, present in the water of the urban wetlands. Some of these bacteria were identified in more than one wetland, however, the abundance of these was different (
Figure 2). Among the identified species of importance to health and which are notifiable to the SAG were found the species
Pasteurella multocida, Salmonella enterica subsp. Typhimurium, Salmonella enterica subsp. Enteritidis, Chlamydia psittaci, Coxiella burnetii, Bacillus anthracis, and
Streptococcus equi, which are responsible for the disease fowl cholera, salmonellosis, psittacosis, Q fever, carbuncle, and equine adenitis, respectively.
Additionally, in the taxonomic assignation tables (
Supplementary Table 1), the presence of microorganisms potentially infectious for animals and humans was found. Each wetland studied contains a different relative abundance of these bacteria. Remarkably, a high diversity of
Pseudomonas aeruginosa and
Escherichia coli was found in all the studied wetlands. The next top bacteria observed belong mainly to
Bordetella,
Staphylococcus, and
Clostridium genus, with high relevance in public health because
Bordetella species can lead to respiratory illnesses like whooping cough, while
Staphylococcus species can cause skin infections, while
Clostridium species are associated with foodborne illnesses such as botulism and tetanus.
Figure 3.
Heatmap of bacterial species relevant in veterinary and human medicine. The relative abundance of bacterial species in wetlands of the cities of Osorno, Llanquihue, Puerto Varas, and Puerto Montt is presented. The wetland with the highest relative abundance of infectious bacterial species, represented by an intense red color, is the La Paloma wetland in the city of Puerto Montt. Conversely, the wetlands with the lowest abundance are Las Ranas in the city of Llanquihue, Luis Ebel in the city of Puerto Montt, and La Marina in the city of Puerto Varas.
Figure 3.
Heatmap of bacterial species relevant in veterinary and human medicine. The relative abundance of bacterial species in wetlands of the cities of Osorno, Llanquihue, Puerto Varas, and Puerto Montt is presented. The wetland with the highest relative abundance of infectious bacterial species, represented by an intense red color, is the La Paloma wetland in the city of Puerto Montt. Conversely, the wetlands with the lowest abundance are Las Ranas in the city of Llanquihue, Luis Ebel in the city of Puerto Montt, and La Marina in the city of Puerto Varas.
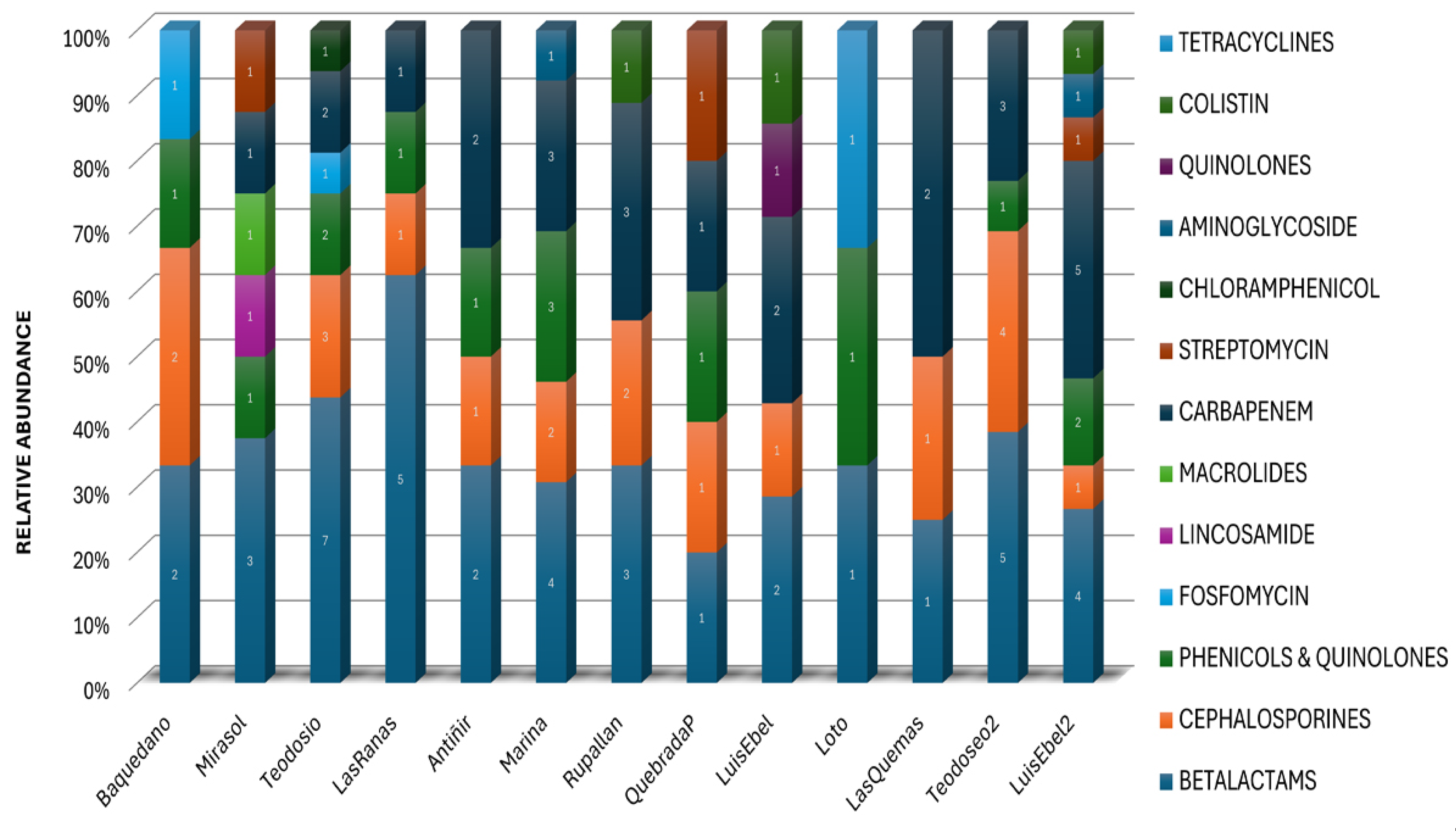
3.3. Detection of AMR Genes and Virulence Mechanism on DNA Recovered from Urban Wetlands
The metagenomic analysis revealed a diverse repertory of antimicrobial resistance genes in the environmental DNA recovered from the water of urban wetlands. The different genes identified according to the antimicrobial resistance family to which they belong were grouped and the resistance that confers were identified (
Figure 4,
Supplementary Table 2). These ARGs included genes that confer resistance to antibiotics commonly used in human clinical treatments, such as β-lactams, carbapenems, and chloramphenicol, as well as genes associated with resistance to antibiotics used in veterinary medicine such as macrolides, tetracyclines, and quinolones. The presence of these genes and the kind of the genes identified were different in each urban wetland (
Figure 4,
Supplementary Table 2), which may be related to the use of antibiotics based on compounds such as amoxicillin, ampicillin, ciprofloxacin, nalidixic acid, and imipenem.
Additionally, the data also show the presence of virulence mechanisms associated with the bacterial species namely
Yersinia enterocolitica subsp. enterocolitica,
Pseudomonas aeruginosa,
Escherichia coli O157, and
Salmonella enterica subsp.
enterica serovar
Typhimurium (
Table 1,
Supplementary Figure 2, Supplementary Table 3). Overall, the genes associated with
Y. enterocolitica subsp. were related to the flagellar motor proteins
fliG and
fliM, which are crucial proteins for the rotation of the flagellum, providing bacterial motility. Among the genes associated with
P. aeruginosa, the elevated expression of genes related to virulence, such as
pvdS sigma factor of extra-cytoplasmic function and the
flgC protein of the flagellar basal body, related to the capacity of the bacteria to move in the media to acquire nutrients, which is key for its survival and pathogenicity. Besides, the main virulence mechanism identified in
E.
coli O157 was mediated by the
yagZ/
ecpA genes, which encode the structural subunit EcpA of the pilus, which plays a crucial role in adherence to surfaces and interaction with host cells. Finally,
S. enterica subsp
. enterica serovar
Typhimurium showed that the main virulence mechanism was encoded by the gene csgF, related to the assembly of curli fibers that help bacteria to adhere surfaces and form biofilms [
37].
4. Discussion
Accelerated population growth, together with unplanned urbanization and extensive deforestation, has significantly modified the borders between human and animal populations, considerably altering the balance of ecosystems [
38]. These anthropogenic activities not only contribute to the loss of biodiversity and the destruction of natural habitats but also increase the incidence of zoonotic diseases by facilitating the impairment of the environment and stretching the contact between humans and wildlife [
9]. For these reasons, is needed to establish molecular surveillance programs, that allow the detection of biohazard elements in the environment [
39]. The characterization of microorganisms present in bodies of water is a research area of great relevance for public health and policy formulation. This information allows us to deeply understand local microbial diversity, identify patterns of antibiotic resistance, and establish relationships between human activity, urban infrastructure, and the well-being of nearby populations [
40]. Studies like those conducted by Fresia et al. (2019) and Campanini-Salinas et al. (2024) have demonstrated the prevalence of antibiotic resistance genes in coastal and urban environments in Uruguay and Chile, highlighting the need for comprehensive strategies to environmental surveil and avoid antimicrobial resistance transmission in both the veterinary and human health [
18,
20].
As mentioned by Ballesteros et al. (2023), which analyzed 7 wetlands with different anthropogenic loads using metagenomic amplicon sequencing, a high abundance of genera namely
Pseudomonas,
Flavobacterium,
Aeromonas, and
Mycoplasma was observed, similar to the observations performed in this study [
41]. These findings suggest that pathogenic microorganisms are present in high abundance in urban wetlands, which represents a potential risk to animal health, especially for those species that interact directly with these waterbodies. Farm animals, pets, and even wildlife can be exposed to pathogens through contact with contaminated water.
The present study identified the presence of microorganisms in urban wetlands that are also relevant to human health. Bacteria such as
V. cholerae,
P. aeruginosa,
E. coli O157, which can cause serious infections, also was presented in our observations. This suggests that water may act as a reservoir for these pathogenic bacteria, as has been demonstrated previously in other studies [
42,
43,
44]. Notably, in almost all the wetlands studied, bacteria belonging to the
Pseudomonas genus were at the top of the abundance. Moreover, the detection of antibiotic resistance genes and the characterization of virulence mechanisms in wetlands represents a tool with great potential to monitor public health risks. As mentioned by Y. Li et al. (2024) the presence of these genes can difficult the effective treatment of bacterial infections. Antimicrobial resistance is a growing global phenomenon, and its presence in aquatic environments such as non-conserved wetlands is particularly worrying due to its close connection with human and animal activity [
45].
The detection of ARGs in wetlands, coasts, and sewage indicates the presence of a reservoir of resistance that may affect marine and terrestrial fauna and ultimately human activities [
46]. This situation may be exacerbated in areas close to cities, with many water bodies and a climate characterized by rain, such as the Los Lagos region in Chile, which may contribute to the accumulation and spread of microorganisms via waterways. These reservoirs also can accumulate antibiotic compounds coming from various sources, such as agriculture, livestock, and human clinical treatments, promoting the apparition of resistant bacteria phenomena [
47]. Effective management of watersheds can significantly reduce the dangers to human health that arise from the presence of microbial biohazards in the environment [
46]. Reducing the use of antibiotics near water bodies during cattle raising or limiting the presence of aquaculture activities in freshwater are two examples of effective public politics on water management that can help to prevent ARG transference and pharmaceutical pollutant dispersion [
48,
49,
50]. In addition, monitoring and regulating the discharge of wastewater from urbanization and hospitals can help reduce the load of microbial biohazard and pharmaceutical pollutants in water systems [
51]. Furthermore, evidence-based decision-making on water quality, supported by monitoring data, can be critical for providing safe drinking water, optimizing water quality, and effectively managing water resources [
52,
53]. By preserving the water resource, will protect the health of the surrounding communities and the natural environment, but also help to maintain the long-term efficacy of antibiotics and sustainable public health management.
5. Conclusions
In this work, shotgun metagenomics was used to describe the bacterial and functional diversity present in urban wetlands, and serves as a demonstration of how metagenomics might help monitor microbial biohazards in the interface of urban emplacement that are closely connected to the natural environment. Several environmental and pathogenic bacteria were identified and a few of them are the etiological agent of disease listed on the mandatory notifiable diseases group, monitored by the Agriculture Livestock Service of Chile (SAG). Additionally, species with high prevalence relevant to human and animal health were detected. Functionally, ARGs and virulence mechanisms were identified. Our study demonstrated the utility of metagenomics in identifying genetic determinants and microbial biohazards in urban wetlands subjected to anthropogenic pressure.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org. Figure Supplementary 1: Map of sample sites; Figure Supplementary 2: Virulence mechanisms identified by bacterial species; Supplementary Table 1: Taxonomic identification and relative abundance obtained from Kraken2; Supplementary Table 2: ARGs identified by the NCBI and ResFinder databases; Supplementary Table 3: Virulence genes and biological processes identified by bacterial specie.
Author Contributions
Conceptualization, J.C.S. and D.A.M.; Methodology, J.C.S. and D.A.M; Validation J.C.S., D.A.M.; Formal Analysis, A.B., F.P., D.A.M. and C.O.R.; Investigation, A.B., F.P., C.O.R.; Resources, J.C.S. and D.A.M; Data Curation, D.A.M. and A.B.; Writing – Original Draft Preparation, C.O.R. and D.A.M; Writing – Review & Editing, C.O.R, D.A.M., J.C.S.; Visualization, J.C.S., C.O.R, and D.A.M.; Supervision, J.C.S. and D.A.M; Project Administration, C.O.R. and D.A.M.; Funding Acquisition, J.C.S. and D.A.M.
Funding
This research was funded by the Agencia Nacional de Investigación y Desarrollo de Chile by the Fondecyt Iniciación #11230295 (DAM) and by the Universidad San Sebastián by grants VRID_FAPPE21-07 and VRID_INTER23/02 (DAM). We appreciate the support from the National Laboratory for High-Performance Computing (NLHPC) of Chile, for facilitating their infrastructure for bioinformatic analysis. JCS thanks the VRID of Universidad San Sebastián for funding VRID_DocI22/06.
Institutional Review Board Statement
The study was conducted under the Declaration of Helsinki. Ethical review and approval were waived for this study because was not needed for environmental studies.
Informed Consent Statement
No applicable
Data Availability Statement
Acknowledgments
We are grateful for the funding of “Agencia Nacional de Investigación y Desarrollo” of Chile. This work would not have been possible without the support of the “Vicerrectoría de Investigación y Doctorados” and the “Vicerrectoría de Vinculación con el Medio” of Universidad San Sebastián. Finally, we are grateful for the collaboration of the National Laboratory for High Performance Computing of Chile to facilitate its computational infrastructure.
Conflicts of Interest
The authors declare no conflicts of interest
References
- Mitsch, W.J.; Bernal, B.; Hernandez, M.E. Ecosystem Services of Wetlands. Int. J. Biodivers. Sci. Eco. Srvcs. Mgmt. 2015, 11, 1–4. [Google Scholar] [CrossRef]
- Xing, W.; Han, Y.; Guo, Z.; Zhou, Y. Quantitative Study on Redistribution of Nitrogen and Phosphorus by Wetland Plants under Different Water Quality Conditions. Environ. Pollut. 2020, 261, 114086. [Google Scholar] [CrossRef]
- Qu, Y.; Yang, Y.; Sonne, C.; Chen, X.; Yue, X.; Gu, H.; Lam, S.S.; Peng, W. Phytosphere Purification of Urban Domestic Wastewater. Environ. Pollut. 2023, 336, 122417. [Google Scholar] [CrossRef] [PubMed]
- Nahlik, A.M.; Fennessy, M.S. Carbon Storage in US Wetlands. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Zamora, S.; Zitácuaro-Contreras, I.; Betanzo-Torres, E.A.; Herazo, L.C.S.; Sandoval-Herazo, M.; Vidal-Álvarez, M.; Marín-Muñiz, J.L. Carbon Pool in Mexican Wetland Soils: Importance of the Environmental Service. Life (Basel) 2022, 12, 1032. [Google Scholar] [CrossRef] [PubMed]
- Davidson, N.C.; Fluet-Chouinard, E.; Finlayson, C.M. Global Extent and Distribution of Wetlands: Trends and Issues. Mar. Freshw. Res. 2018, 69, 620. [Google Scholar] [CrossRef]
- Salimi, S.; Almuktar, S.A.A.A.N.; Scholz, M. Impact of Climate Change on Wetland Ecosystems: A Critical Review of Experimental Wetlands. J. Environ. Manage. 2021, 286, 112160. [Google Scholar] [CrossRef] [PubMed]
- Royal C., Gardner; Davidson, N.C. The Ramsar Convention. In Wetlands; Springer Netherlands: Dordrecht, 2011; pp. 189–203. ISBN 9789400705500. [Google Scholar]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging Infectious Diseases of Wildlife-- Threats to Biodiversity and Human Health. Science 2000, 287, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E.J. Risk Factors for Human Disease Emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 983–989. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Bonilla-Aldana, D.K.; Idarraga-Bedoya, S.E.; Garcia-Bustos, J.J.; Cardona-Ospina, J.A.; Faccini-Martínez, Á.A. Epidemiology of Zoonotic Tick-Borne Diseases in Latin America: Are We Just Seeing the Tip of the Iceberg? F1000Res. 2019, 7, 1988. [Google Scholar] [CrossRef]
- Barros, M.; Sáenz, L.; Lapierre, L.; Nuñez, C.; Medina-Vogel, G. High Prevalence of Pathogenic Leptospira in Alien American Mink (Neovison Vison) in Patagonia. Rev. Chil. Hist. Nat. 2014, 87. [Google Scholar] [CrossRef]
- Gibbs, E.P.J. The Evolution of One Health: A Decade of Progress and Challenges for the Future. Vet. Rec. 2014, 174, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, J.R. Importance of a One Health Approach in Advancing Global Health Security and the Sustainable Development Goals. Rev. Sci. Tech. 2019, 38, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Riley, L.W.; Blanton, R.E. Advances in Molecular Epidemiology of Infectious Diseases: Definitions, Approaches, and Scope of the Field. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global Trends in Emerging Infectious Diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Taking a Multisectoral, One Health Approach: A Tripartite Guide to Addressing Zoonotic Diseases in Countries. Available online: https://www.who.int/publications/i/item/9789241514934 (accessed on 16 July 2024).
- Fresia, P.; Antelo, V.; Salazar, C.; Giménez, M.; D’Alessandro, B.; Afshinnekoo, E.; Mason, C.; Gonnet, G.H.; Iraola, G. Urban Metagenomics Uncover Antibiotic Resistance Reservoirs in Coastal Beach and Sewage Waters. Microbiome 2019, 7. [Google Scholar] [CrossRef]
- Rocha, J.D.; Opitz, C.; Cárdenas, V.; Mella, C.; Medina, D. Identification of Potentially Harmful Bacterial Genera of Veterinary Relevance in the Llanquihue Urban Wetlands. Austral J. Vet. Sci. 2024, e560106. [Google Scholar] [CrossRef]
- Campanini-Salinas, J.; Opitz-Ríos, C.; Sagredo-Mella, J.; Contreras S., D.; Gimenez, M.; Paez, P.A.; Tarifa, M.C.; Concha-Rubio, N.D.; Medina, D.A. Antimicrobial Resistance Elements in Coastal Water Identified from Llanquihue Lake, Chile. Preprints 2024.
- Loman, N.J.; Pallen, M.J. Twenty Years of Bacterial Genome Sequencing. Nat. Rev. Microbiol. 2015, 13, 787–794. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Ravel, J. The Vocabulary of Microbiome Research: A Proposal. Microbiome 2015, 3. [Google Scholar] [CrossRef]
- Bemer-Melchior, P.; Drugeon, H.B. Inactivation of Mycobacterium Tuberculosis for DNA Typing Analysis. J. Clin. Microbiol. 1999, 37, 2350–2351. [Google Scholar] [CrossRef]
- Babraham Bioinformatics - FastQC A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 16 July 2024).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, C.-M.; Luo, R.; Sadakane, K.; Lam, T.-W. MEGAHIT: An Ultra-Fast Single-Node Solution for Large and Complex Metagenomics Assembly via Succinct de Bruijn Graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality Assessment Tool for Genome Assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.E.; Lu, J.; Langmead, B. Improved Metagenomic Analysis with Kraken 2. Genome Biol. 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Abricate: :Mag_right: Mass Screening of Contigs for Antimicrobial and Virulence Genes; Github.
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of Acquired Antimicrobial Resistance Genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef] [PubMed]
- Feldgarden, M.; Brover, V.; Haft, D.H.; Prasad, A.B.; Slotta, D.J.; Tolstoy, I.; Tyson, G.H.; Zhao, S.; Hsu, C.-H.; McDermott, P.F.; et al. Validating the AMRFinder Tool and Resistance Gene Database by Using Antimicrobial Resistance Genotype-Phenotype Correlations in a Collection of Isolates. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, D.; Liu, B.; Yang, J.; Jin, Q. VFDB 2016: Hierarchical and Refined Dataset for Big Data Analysis—10 Years On. Nucleic Acids Res. 2016, 44, D694–D697. [Google Scholar] [CrossRef]
- R Core Team. (2021) R A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. - References - Scientific Research Publishing. Available online: https://www.scirp.org/reference/referencespapers?referenceid=3131254 (accessed on 27 May 2024).
- Irizarry, R.A. Ggplot2. In Introduction to Data Science; Chapman and Hall/CRC: Boca Raton, 2024; pp. 107–125. ISBN 9781003220923. [Google Scholar]
- Enfermedades de denuncia obligatoria (EDO) al SAG. Available online: https://www.sag.gob.cl/ambitos-de-accion/enfermedades-de-denuncia-obligatoria-edo-al-sag (accessed on 16 July 2024).
- Nenninger, A.A.; Robinson, L.S.; Hultgren, S.J. Localized and Efficient Curli Nucleation Requires the Chaperone-like Amyloid Assembly Protein CsgF. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 900–905. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Jeggo, M. The One Health Approach—Why Is It so Important? Trop. Med. Infect. Dis. 2019, 4, 88. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Castro, R.; Romero, A.; Aranguren, R.; Pallavicini, A.; Banchi, E.; Novoa, B.; Figueras, A. High-Throughput Sequencing of Environmental DNA as a Tool for Monitoring Eukaryotic Communities and Potential Pathogens in a Coastal Upwelling Ecosystem. Front. Vet. Sci. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Salazar, C.; Giménez, M.; Riera, N.; Parada, A.; Puig, J.; Galiana, A.; Grill, F.; Vieytes, M.; Mason, C.E.; Antelo, V.; et al. Human Microbiota Drives Hospital-Associated Antimicrobial Resistance Dissemination in the Urban Environment and Mirrors Patient Case Rates. Microbiome 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, N.; Páez, L.; Luna, N.; Reina, A.; Urrea, V.; Sánchez, C.; Ramírez, A.; Ramirez, J.D.; Muñoz, M. Characterization of Microbial Communities in Seven Wetlands with Different Anthropogenic Burden Using Next Generation Sequencing in Bogotá, Colombia. Sci. Rep. 2023, 13. [Google Scholar] [CrossRef]
- Zhang, Q.; Alter, T.; Strauch, E.; Eichhorn, I.; Borowiak, M.; Deneke, C.; Fleischmann, S. German Coasts Harbor Non-O1/Non-O139 Vibrio Cholerae with Clinical Virulence Gene Profiles. Infect. Genet. Evol. 2024, 120, 105587. [Google Scholar] [CrossRef] [PubMed]
- Mena, K.D.; Gerba, C.P. Risk Assessment of Pseudomonas Aeruginosa in Water. In Reviews of Environmental Contamination and Toxicology Vol 201; Reviews of environmental contamination and toxicology; Springer US: Boston, MA, 2009; pp. 71–115. ISBN 9781441900319. [Google Scholar]
- Wang, G.; Doyle, M.P. Survival of Enterohemorrhagic Escherichia Coli O157:H7 in Water. J. Food Prot. 1998, 61, 662–667. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Li, Q.; Mao, S. Fluorescence Analysis of Antibiotics and Antibiotic-Resistance Genes in the Environment: A Mini Review. Chin. Chem. Lett. 2024, 35, 109541. [Google Scholar] [CrossRef]
- Pruden, A.; Larsson, D.G.J.; Amézquita, A.; Collignon, P.; Brandt, K.K.; Graham, D.W.; Lazorchak, J.M.; Suzuki, S.; Silley, P.; Snape, J.R.; et al. Management Options for Reducing the Release of Antibiotics and Antibiotic Resistance Genes to the Environment. Environ. Health Perspect. 2013, 121, 878–885. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the Aquatic Environment – A Review – Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Katusiime, J.; Schütt, B. Integrated Water Resources Management Approaches to Improve Water Resources Governance. Water (Basel) 2020, 12, 3424. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [PubMed]
- Pepi, M.; Focardi, S. Antibiotic-Resistant Bacteria in Aquaculture and Climate Change: A Challenge for Health in the Mediterranean Area. Int. J. Environ. Res. Public Health 2021, 18, 5723. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. The Presence of Pharmaceuticals in the Environment Due to Human Use – Present Knowledge and Future Challenges. J. Environ. Manage. 2009, 90, 2354–2366. [Google Scholar] [CrossRef] [PubMed]
- Amjad, U.Q.; Dalcanale, F.; Kayser, G.; Bentley, P.; Bartram, J. Evidence-Based Decision-Making on Water Quality in Domestic Water Supply in Malawi, Ecuador, and Brazil. Water Policy 2018, 20, 530–545. [Google Scholar] [CrossRef]
- Guidelines for Drinking-Water Quality, 4th Edition, Incorporating the 1st Addendum. Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 17 July 2024).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).