Submitted:
22 July 2024
Posted:
23 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants
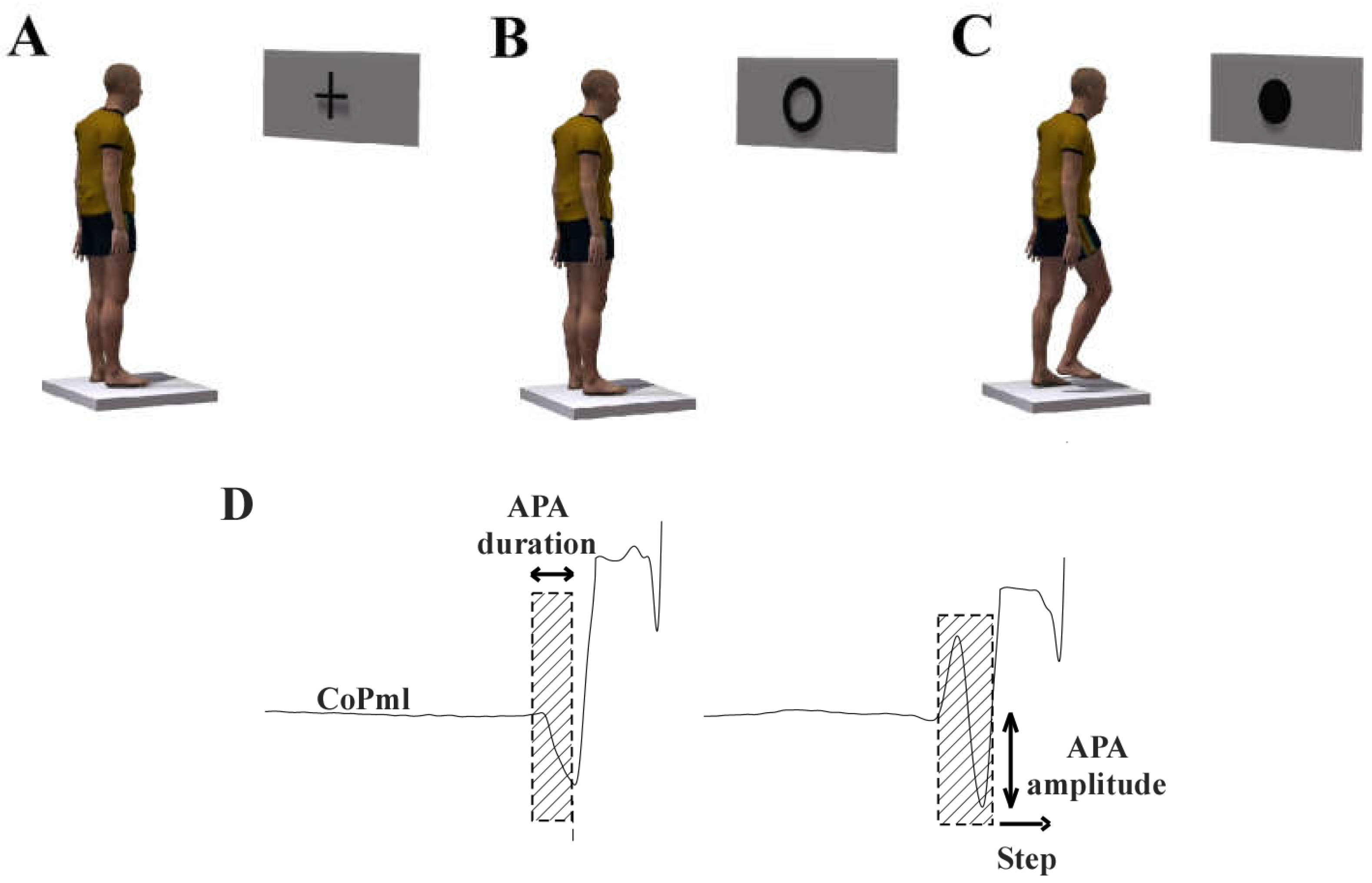
2.2. Behavioral Task: Step Initiation
2.3. Magnetic Resonance Imaging
2.4. Image Data Processing
2.5. Statistical Analyses
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lewis, S.J.; Barker, R.A. A Pathophysiological Model of Freezing of Gait in Parkinson’s Disease. Parkinsonism & Related Disorders 2009, 15, 333–338. [Google Scholar] [CrossRef]
- Nutt, J.G.; Bloem, B.R.; Giladi, N.; Hallett, M.; Horak, F.B.; Nieuwboer, A. Freezing of Gait: Moving Forward on a Mysterious Clinical Phenomenon. The Lancet Neurology 2011, 10, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Giladi, N.; Treves, T.A.; Simon, E.S.; Shabtai, H.; Orlov, Y.; Kandinov, B.; Paleacu, D.; Korczyn, A.D. Freezing of Gait in Patients with Advanced Parkinson’s Disease. Journal of Neural Transmission 2001, 108, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Heremans, E.; Nieuwboer, A.; Vercruysse, S. Freezing of Gait in Parkinson’s Disease: Where Are We Now? Curr Neurol Neurosci Rep 2013, 13, 350. [Google Scholar] [CrossRef]
- Moore, O.; Peretz, C.; Giladi, N. Freezing of Gait Affects Quality of Life of Peoples with Parkinson’s Disease beyond Its Relationships with Mobility and Gait. Mov Disord 2007, 22, 2192–2195. [Google Scholar] [CrossRef]
- Schaafsma, J.D.; Balash, Y.; Gurevich, T.; Bartels, A.L.; Hausdorff, J.M.; Giladi, N. Characterization of Freezing of Gait Subtypes and the Response of Each to Levodopa in Parkinson’s Disease. European Journal of Neurology 2003, 10, 391–398. [Google Scholar] [CrossRef]
- Giladi, N.; McDermott, M.P.; Fahn, S.; Przedborski, S.; Jankovic, J.; Stern, M.; Tanner, C. ; The Parkinson Study Group Freezing of Gait in PD: Prospective Assessment in the DATATOP Cohort. Neurology 2001, 56, 1712–1721. [Google Scholar] [CrossRef]
- Snijders, A.H.; Nijkrake, M.J.; Bakker, M.; Munneke, M.; Wind, C.; Bloem, B.R. Clinimetrics of Freezing of Gait. Mov. Disord. 2008, 23, S468–S474. [Google Scholar] [CrossRef] [PubMed]
- Fonoff, E.T.; De Lima-Pardini, A.C.; Coelho, D.B.; Monaco, B.A.; Machado, B.; Pinto De Souza, C.; Dos Santos Ghilardi, M.G.; Hamani, C. Spinal Cord Stimulation for Freezing of Gait: From Bench to Bedside. Front. Neurol. 2019, 10, 905. [Google Scholar] [CrossRef]
- Brugger, F.; Abela, E.; Hägele-Link, S.; Bohlhalter, S.; Galovic, M.; Kägi, G. Do Executive Dysfunction and Freezing of Gait in Parkinson’s Disease Share the Same Neuroanatomical Correlates? Journal of the Neurological Sciences 2015, 356, 184–187. [Google Scholar] [CrossRef]
- Fling, B.W.; Cohen, R.G.; Mancini, M.; Carpenter, S.D.; Fair, D.A.; Nutt, J.G.; Horak, F.B. Functional Reorganization of the Locomotor Network in Parkinson Patients with Freezing of Gait. PLoS One 2014, 9, e100291. [Google Scholar] [CrossRef] [PubMed]
- Kostić, V.S.; Agosta, F.; Pievani, M.; Stefanova, E.; Ječmenica-Lukić, M.; Scarale, A.; Špica, V.; Filippi, M. Pattern of Brain Tissue Loss Associated with Freezing of Gait in Parkinson Disease. Neurology 2012, 78, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Herman, T.; Rosenberg-Katz, K.; Jacob, Y.; Giladi, N.; Hausdorff, J.M. Gray Matter Atrophy and Freezing of Gait in Parkinson’s Disease: Is the Evidence Black-on-white? Movement Disorders 2014, 29, 134–139. [Google Scholar] [CrossRef]
- Tessitore, A.; Amboni, M.; Cirillo, G.; Corbo, D.; Picillo, M.; Russo, A.; Vitale, C.; Santangelo, G.; Erro, R.; Cirillo, M.; et al. Regional Gray Matter Atrophy in Patients with Parkinson Disease and Freezing of Gait. AJNR Am J Neuroradiol 2012, 33, 1804–1809. [Google Scholar] [CrossRef] [PubMed]
- Canu, E.; Agosta, F.; Sarasso, E.; Volonte, M.A.; Basaia, S.; Stojkovic, T.; Stefanova, E.; Comi, G.; Falini, A.; Kostic, V.S.; et al. Brain Structural and Functional Connectivity in Parkinson’s Disease with Freezing of Gait. Hum Brain Mapp 2015, 36, 5064–5078. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.-L.; Ng, K.-M.; Rumpel, H.; Fook-Chong, S.; Li, H.-H.; Tan, E.-K. Transcallosal Diffusion Tensor Abnormalities in Predominant Gait Disorder Parkinsonism. Parkinsonism & Related Disorders 2014, 20, 53–59. [Google Scholar] [CrossRef]
- Gu, Q.; Huang, P.; Xuan, M.; Xu, X.; Li, D.; Sun, J.; Yu, H.; Wang, C.; Luo, W.; Zhang, M. Greater Loss of White Matter Integrity in Postural Instability and Gait Difficulty Subtype of Parkinson’s Disease. Can. J. Neurol. Sci. 2014, 41, 763–768. [Google Scholar] [CrossRef]
- Schlenstedt, C.; Mancini, M.; Nutt, J.; Hiller, A.P.; Maetzler, W.; Deuschl, G.; Horak, F. Are Hypometric Anticipatory Postural Adjustments Contributing to Freezing of Gait in Parkinson’s Disease? Frontiers in Aging Neuroscience 2018, 10, 36. [Google Scholar] [CrossRef]
- de Lima-Pardini, A.C.; Coelho, D.B.; Nucci, M.P.; Boffino, C.C.; Batista, A.X.; de Azevedo Neto, R.M.; Silva-Batista, C.; Barbosa, E.R.; Cohen, R.G.; Horak, F.B.; et al. Brain Networks Associated with Anticipatory Postural Adjustments in Parkinson’s Disease Patients with Freezing of Gait. NeuroImage. Clinical 2020, 28, 102461. [Google Scholar] [CrossRef]
- Fling, B.W.; Cohen, R.G.; Mancini, M.; Nutt, J.G.; Fair, D.A.; Horak, F.B. Asymmetric Pedunculopontine Network Connectivity in Parkinsonian Patients with Freezing of Gait. Brain: A Journal of Neurology 2013, 136, 2405–2418. [Google Scholar] [CrossRef]
- Moreira-Neto, A.; Ugrinowitsch, C.; Coelho, D.B.; De Lima-Pardini, A.C.; Barbosa, E.R.; Teixeira, L.A.; Amaro, E.; Horak, F.B.; Mancini, M.; Nucci, M.P.; et al. Freezing of Gait, Gait Initiation, and Gait Automaticity Share a Similar Neural Substrate in Parkinson’s Disease. Human Movement Science 2022, 86, 103018. [Google Scholar] [CrossRef]
- Silva-Batista, C.; Lira, J.; Coelho, D.B.; De Lima-Pardini, A.C.; Nucci, M.P.; Mattos, E.C.T.; Magalhaes, F.H.; Barbosa, E.R.; Teixeira, L.A.; Amaro Junior, E.; et al. Mesencephalic Locomotor Region and Presynaptic Inhibition during Anticipatory Postural Adjustments in People with Parkinson’s Disease. Brain Sciences 2024, 14, 178. [Google Scholar] [CrossRef]
- Seto, E.; Sela, G.; McIlroy, W.E.; Black, S.E.; Staines, W.R.; Bronskill, M.J.; McIntosh, A.R.; Graham, S.J. Quantifying Head Motion Associated with Motor Tasks Used in fMRI. NeuroImage 2001, 14, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Moineau, B.; Boisgontier, M.P.; Barbieri, G.; Nougier, V. A New Method to Assess Temporal Features of Gait Initiation with a Single Force Plate. Gait & Posture 2014, 39, 631–633. [Google Scholar] [CrossRef]
- Lindroth, H.; Nair, V.A.; Stanfield, C.; Casey, C.; Mohanty, R.; Wayer, D.; Rowley, P.; Brown, R.; Prabhakaran, V.; Sanders, R.D. Examining the Identification of Age-Related Atrophy between T1 and T1 + T2-FLAIR Cortical Thickness Measurements. Sci Rep 2019, 9, 11288. [Google Scholar] [CrossRef]
- Yendiki, A. Automated Probabilistic Reconstruction of White-Matter Pathways in Health and Disease Using an Atlas of the Underlying Anatomy. Front. Neuroinform. 2011, 5. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Winklewski, P.J.; Sabisz, A.; Naumczyk, P.; Jodzio, K.; Szurowska, E.; Szarmach, A. Understanding the Physiopathology Behind Axial and Radial Diffusivity Changes—What Do We Know? Front. Neurol. 2018, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wu, Z.; Long, J.; Li, W.; Wang, X.; Hu, N.; Zhao, X.; Sun, T. White Matter Changes in Parkinson’s Disease. npj Parkinsons Dis. 2023, 9, 150. [Google Scholar] [CrossRef]
- Iseki, K.; Fukuyama, H.; Oishi, N.; Tomimoto, H.; Otsuka, Y.; Nankaku, M.; Benninger, D.; Hallett, M.; Hanakawa, T. Freezing of Gait and White Matter Changes: A Tract-Based Spatial Statistics Study. J Clin Mov Disord 2015, 2, 1. [Google Scholar] [CrossRef]
- Kou, W.; Wang, X.; Zheng, Y.; Zhao, J.; Cai, H.; Chen, H.; Sui, B.; Feng, T. Freezing of Gait in Parkinson’s Disease Is Associated with the Microstructural and Functional Changes of Globus Pallidus Internus. Front. Aging Neurosci. 2022, 14, 975068. [Google Scholar] [CrossRef] [PubMed]
- Lench, D.H.; Keith, K.; Wilson, S.; Padgett, L.; Benitez, A.; Ramakrishnan, V.; Jensen, J.H.; Bonilha, L.; Revuelta, G.J. Neurodegeneration of the Globus Pallidus Internus as a Neural Correlate to Dopa-Response in Freezing of Gait. JPD 2022, 12, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.G.; Nutt, J.G.; Horak, F.B. Errors in Postural Preparation Lead to Increased Choice Reaction Times for Step Initiation in Older Adults. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 2011, 66A, 705–713. [CrossRef]
- Coelho, D.B.; Ribeiro De Souza, C.; De Lima-Pardini, A.C.; Treza, R.D.C.; Shida, T.K.F.; Silva-Batista, C.; Teixeira, L.A. Is Freezing of Gait Correlated with Postural Control in Patients with Moderate-to-severe Parkinson’s Disease? Eur J of Neuroscience 2021, 53, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.S.; Van Liew, C.; Stuart, S.; Carlson-Kuhta, P.; Horak, F.B.; Mancini, M. Relating Parkinson Freezing and Balance Domains: A Structural Equation Modeling Approach. Parkinsonism & Related Disorders 2020, 79, 73–78. [Google Scholar] [CrossRef]
- Bartels, A.L.; Leenders, K.L. Brain Imaging in Patients with Freezing of Gait. Mov. Disord. 2008, 23, S461–S467. [Google Scholar] [CrossRef] [PubMed]
- Pietracupa, S.; Suppa, A.; Upadhyay, N.; Giannì, C.; Grillea, G.; Leodori, G.; Modugno, N.; Di Biasio, F.; Zampogna, A.; Colonnese, C.; et al. Freezing of Gait in Parkinson’s Disease: Gray and White Matter Abnormalities. J Neurol 2018, 265, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.; Lee, J.-M.; Kwon, H.; Kim, J.S.; Son, T.O.; Cho, J.W. Alterations of Mean Diffusivity of Pedunculopontine Nucleus Pathway in Parkinson’s Disease Patients with Freezing of Gait. Parkinsonism & Related Disorders 2015, 21, 12–17. [Google Scholar] [CrossRef]
- Bartels, A.L.; De Jong, B.M.; Giladi, N.; Schaafsma, J.D.; Maguire, R.P.; Veenma, L.; Pruim, J.; Balash, Y.; Youdim, M.B.H.; Leenders, K.L. Striatal Dopa and Glucose Metabolism in PD Patients with Freezing of Gait. Movement Disorders 2006, 21, 1326–1332. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Tsapanou, A.; Qolamreza, R.R.; Gazes, Y. White Matter Integrity Mediates Decline in Age-Related Inhibitory Control. Behavioural Brain Research 2018, 339, 249–254. [Google Scholar] [CrossRef]
- Almeida, Q.J.; Lebold, C.A. Freezing of Gait in Parkinson’s Disease: A Perceptual Cause for a Motor Impairment? J Neurol Neurosurg Psychiatry 2010, 81, 513–518. [Google Scholar] [CrossRef]
- Monaghan, A.S.; Ofori, E.; Fling, B.W.; Peterson, D.S. Associating White Matter Microstructural Integrity and Improvements in Reactive Stepping in People with Parkinson’s Disease. Brain Imaging and Behavior 2024. [CrossRef] [PubMed]
- Richmond, S.B.; Peterson, D.S.; Fling, B.W. Bridging the Callosal Gap in Gait: Corpus Callosum White Matter Integrity’s Role in Lower Limb Coordination. Brain Imaging and Behavior 2022, 16, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Dale, M.L.; Mancini, M.; Curtze, C.; Horak, F.B.; Fling, B.W. Freezing of Gait Associated with a Corpus Callosum Lesion. J Clin Mov Disord 2016, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, S.; Yuan, Y.; Zhang, L.; Ding, J.; Wang, J.; Zhang, J.; Zhang, K.; Wang, J. Alterations of Functional and Structural Connectivity of Freezing of Gait in Parkinson’s Disease. J Neurol 2016, 263, 1583–1592. [Google Scholar] [CrossRef]
- Tan, S.Y.Z.; Keong, N.C.H.; Selvan, R.M.P.; Li, H.; Ooi, L.Q.R.; Tan, E.K.; Chan, L.L. Periventricular White Matter Abnormalities on Diffusion Tensor Imaging of Postural Instability Gait Disorder Parkinsonism. AJNR Am J Neuroradiol 2019, ajnr;ajnr.A5993v1. [CrossRef]
- Wen, M.-C.; Heng, H.S.E.; Lu, Z.; Xu, Z.; Chan, L.L.; Tan, E.K.; Tan, L.C.S. Differential White Matter Regional Alterations in Motor Subtypes of Early Drug-Naive Parkinson’s Disease Patients. Neurorehabil Neural Repair 2018, 32, 129–141. [Google Scholar] [CrossRef]
| FoG (n = 26) | nFoG (n = 17) | p-value | |
|---|---|---|---|
| Age (years) | 60.23 ± 11.21 | 67.35 ± 6.19 | 0.011 * |
| Disease duration (years) | 13.00 ± 5.72 | 6.55 ± 4.09 | 0.001 * |
| L-Dopa equivalent units (mg•day-1) | 760.71 ± 476.88 | 437.50 ± 291.07 | 0.014 * |
| MoCA | 18.07 ± 2.84 | 18.41 ± 2.27 | 0.709 |
| UPDRS-III (score) | 34.92 ± 9.74 | 27.53 ± 10.55 | 0.027 * |
| nFoGq (score) | 18.92 ± 5.56 | - | - |
| APA duration (ms) | 543.74 ± 186.02 | 488.60 ± 93.76 | 0.266 |
| APA amplitude | 0.44 ± 0.12 | 0.55 ± 0.14 | 0.009 * |
| Step length normalized | 1.53 ± 0.32 | 1.72 ± 0.33 | 0.074 |
| Group | Step initiation | DTI variables | Partial (r2) | p-value |
|---|---|---|---|---|
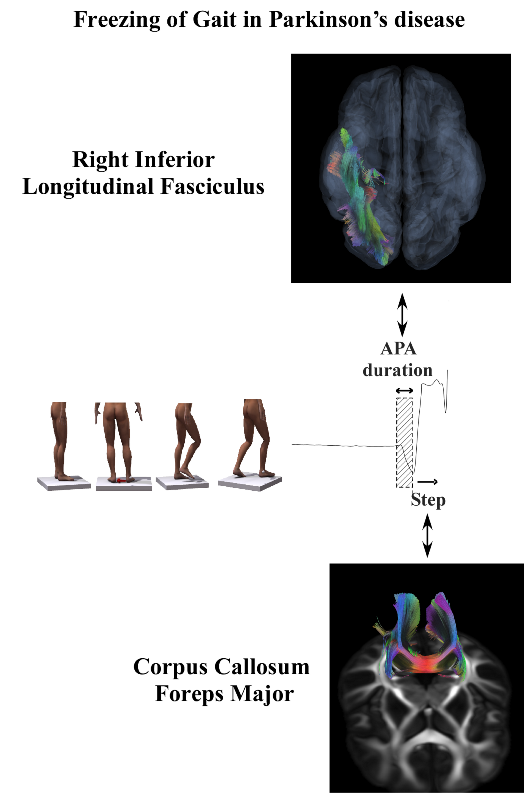
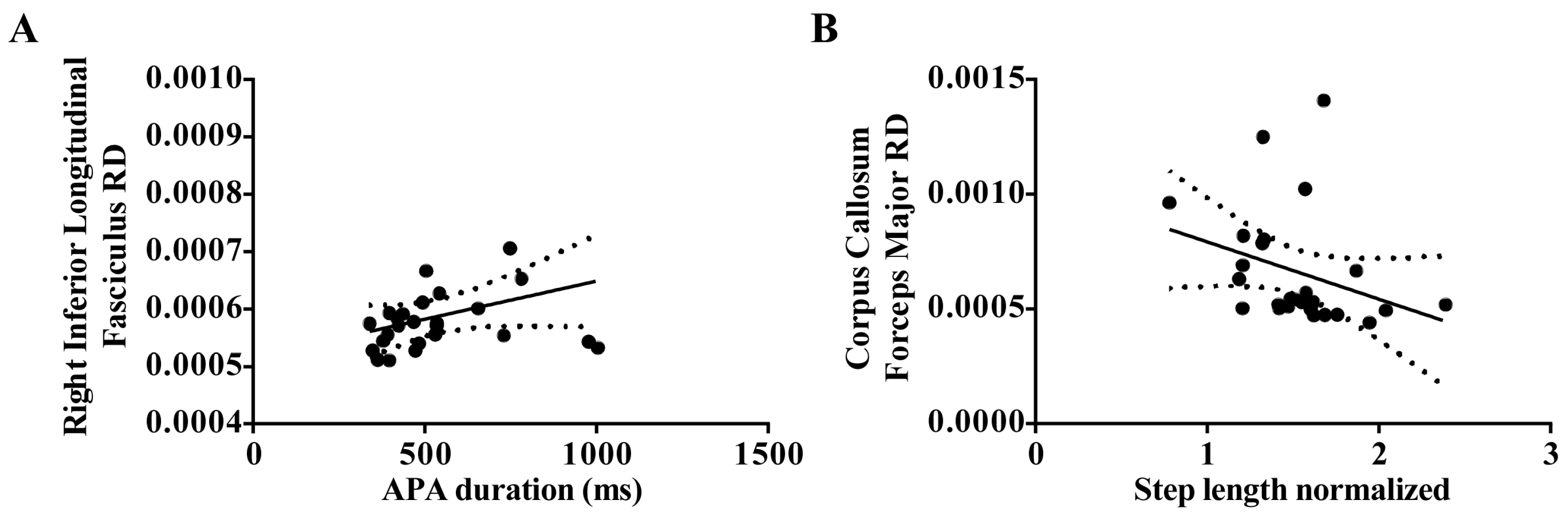
| FoG | APA duration | Right Inferior Longitudinal Fasciculus RD | 0.31 | 0.009 |
| FoG | Step length | Corpus Callosum Forceps Major RD | 0.25 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





